Open Access Article
Open Access ArticleRecovery of cobalt from lithium-ion battery cathode material by combining solvoleaching and solvent extraction
Nand
Peeters
 ,
Koen
Binnemans
,
Koen
Binnemans
 and
Sofía
Riaño
and
Sofía
Riaño
 *
*
KU Leuven, Department of Chemistry, Celestijnenlaan 200F, P.O. box 2404, B-3001 Leuven, Belgium. E-mail: sofia.riano@kuleuven.be
First published on 15th March 2022
Abstract
The recycling of cobalt from lithium-ion batteries (LIBs) is crucial for sustainability reasons. During hydrometallurgical recycling of LIBs, the cathode material is usually separated from the current collectors aluminium and copper at initial process stages. A common type of cathode material is lithium cobalt oxide (LCO) and recovery of cobalt from this source requires reduction of cobalt(III) to cobalt(II), often done by adding a separate reducing agent. This work aims to recover cobalt from LCO via a simple, Green and safe process whose novelty is based on using the current collectors themselves as reducing agents, and combining leaching and solvent extraction of cobalt into a single step. The acidic extractant di-(2-ethylhexyl)phosphoric acid (D2EHPA) was used to leach cobalt from LCO in the presence of metallic aluminium and copper. After optimisation, quantitative leaching of cobalt, copper and lithium was achieved, while aluminium remained unaffected. This observation demonstrates that copper can act as an effective reducing agent for cobalt(III) in LCO, which simplifies the process by avoiding the pre-separation of the current collectors. Compared to conventional sulphuric acid leaching, the proposed process was more selective and avoided the formation of explosive hydrogen gas. Furthermore, direct leaching with D2EHPA gave a cobalt-loaded organic phase from which cobalt was selectively stripped by controlling the equilibrium pH. This approach reduced the number of steps to recover cobalt compared to traditional methods, also decreased the volume of aqueous waste and could be a greener concept for future metal recovery processes.
Introduction
Lithium-ion batteries (LIBs) have been increasingly commercialized in the last three decades. Their high energy density and specific capacity make them suitable for electronic devices such as mobile phones, laptops and electric vehicles.1–7 Common LIBs contain metallic aluminium and copper as current collectors, and a lithium-intercalated metal oxide as cathode material. Some frequently used LIB cathode materials that contain cobalt are lithium cobalt oxide (LCO), lithium nickel manganese cobalt oxide (NMC) and lithium nickel cobalt aluminium oxide (NCA).5,8–11 Recycling of cobalt from spent LIBs is crucial from both an economic and environmental points of view.12,13Most hydrometallurgical LIB recycling routes comprise pre-treatment steps that remove the current collectors (i.e. metallic aluminium and copper) and other components, resulting in the relatively pure cathode material. Subsequently, cobalt and other metals are leached with mineral acids. Successful leaching is often achieved by adding reducing agents to reduce cobalt(III) to the more soluble and stable cobalt(II) in aqueous solutions. Finally, cobalt is purified and recovered from the pregnant leach solution (PLS) by precipitation and/or solvent extraction (SX).14–17 One of the most commonly used leaching systems for spent LIBs is the combination of sulphuric acid as lixiviant and hydrogen peroxide as reducing agent.1,4,14 Further downstream processing is usually done by removing impurities via precipitation or extraction with the acidic extractant di-(2-ethylhexyl)phosphoric acid (D2EHPA), followed by selective cobalt extraction with another acidic extractant bis(2,2,4-trimethylpentyl)phosphinic acid (Cyanex 272). Stripping of cobalt from the loaded Cyanex 272 phase is done with hydrochloric acid or sulphuric acid.18–20 Although these classical approaches are still preferred for industrial applications, they can also entail environmental and safety risks. These could be related with the emission of toxic or dangerous gasses during the mineral acid leaching, and the generation of considerable amounts of aqueous waste during the PLS purification.21
Recently, researchers have proposed alternative processes for the recovery of cobalt that are in some aspects greener, safer and cheaper than the established routes.2,22,23 Some of these approaches substitute mineral acids by organic acids in the recovery process of cobalt. Chen et al. proposed the use of mineral acids combined with organic reducing agents as a milder and safer approach to leach cobalt from spent LIBs.24 While Li et al. used organic acids as lixiviants (citric-, malic- and aspartic acid) combined with hydrogen peroxide to leach cobalt from real spent LIBs.25 In a different approach, some of the hydrometallurgical steps for the recovery of cobalt are replaced by solvometallurgical ones. In solvometallurgy, the aqueous medium is replaced by non-aqueous solvents, which can lead to higher selectivity and less aqueous waste generation.26 Some examples of non-aqueous media are deep-eutectic solvents (DESs) and certain organic substances, such as organic acids. Recently, we described the use of a choline chloride-citric acid DES for the solvoleaching of cobalt from LCO in presence of aluminium and copper current collectors,21 while Wang et al. used the choline chloride-urea DES to recover lithium and cobalt from LCO.27 Another solvometallurgical approach is to use commercially available organic extractants as leaching agents. For example, Gijsemans et al. used the chelating extractant LIX 984 N (equivolume mixture of 2-hydroxy-5-nonylacetophenone oxime and 5-dodecylsalicylaldoxime) as lixiviant to solvoleach copper from chrysocolla, after which the copper-loaded organic phase was directly stripped by a mineral acid.28 Using non-aqueous lixiviants during solvoleaching could avoid the formation of toxic and/or dangerous gasses compared to leaching with conventional mineral acids, which is a potential benefit.26
It has been demonstrated that process intensification by integration of unit operations not only reduces the operating costs but it also improves the safety, greenness and energy efficiency of processes.29–31 For example, in the process of Gijsemans et al., the number of solvent extraction steps was reduced.28 If their process would be up-scaled in counter-current mode, it would require fewer mixer-settler units and smaller volumes of chemicals, and would produce less aqueous waste. Furthermore, several recent studies on LIB recycling have shown that the aluminium and copper current collectors can also reduce cobalt(III) during the leaching of cathode material, which could avoid pre-separation steps and in turn reduce energy costs.21,32–34 Joulié et al. studied the reducing ability of aluminium or copper separately during leaching of cobalt from NMC material with sulphuric acid.32 Peng et al. described the reducing ability of copper when industrial untreated LIB waste streams were leached with sulphuric acid.33,34 Apart from using a DES as lixiviant, our recent work also described the combined reducing capacity of both aluminium and copper during leaching of cobalt from LCO by a choline chloride-citric acid-based DES.21 Hence, the direct solvoleaching of LCO with an organic extractant, while keeping the current collectors aluminium and copper together with the LCO material, could potentially intensify the conventional LIB recycling processes.
In this work, the acidic extractant D2EHPA is used for the solvoleaching of cobalt from LCO in presence of both aluminium and copper. Integrating the direct use of both the current collectors and D2EHPA as system to solvoleach cobalt is not yet exhaustively studied, and could be a greener alternative. The aim is to load cobalt directly into the organic D2EHPA phase (i.e. loaded D2EHPA phase) while aluminium and/or copper are used as reducing agent for cobalt(III). Furthermore, speciation studies are performed to investigate the coordination of the metals in the loaded D2EHPA phase. A cheap and easily accessible mineral acid could then be used to control the equilibrium pH in order to selectively strip cobalt from the metal-loaded D2EHPA phase. Stripping would exchange protons from the acid with cobalt cations from the loaded D2EHPA, regenerating D2EHPA as acidic lixiviant. The recyclability was evaluated for five successive cycles. The proposed leaching process is compared with the conventional leaching by sulphuric acid whereby the significant differences are mentioned. These differences show the ‘green’ nature and process intensification of our alternative approach.
Experimental
Products
Sulphuric acid (<95%) was purchased from Fisher Chemicals (Loughborough, UK). P507 (2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester) was delivered by Kopper Chemical Industry Corp. (Chongqing, China). Cyanex® 272 (85%) (bis(2,4,4-trimethylpentyl)phosphinic acid) was obtained from Cytec Industries (Vlaardingen, Netherlands). Aluminium powder (0.075 mm, 99%), 1-octanol (98%) and bis(2-ethylhexyl) phosphoric acid (D2EHPA, 95%) were purchased from Acros Organics (Geel, Belgium). Copper (0.075 mm, 99%) and lithium–nickel–manganese–cobalt–oxide (NMC 111, 0.5 μm, >98%) powders were obtained from Sigma-Aldrich (Overijse, Belgium). Lithium cobalt oxide (LiCoO2, LCO, 0.005 mm, 97%) was purchased from Alfa Aesar (Kandel, Germany). Nitric acid (65%) was obtained from Chem-Lab nv (Zedelgem, Belgium). Shell GTL GS270 (C8–C26 aliphatic hydrocarbon diluent) was obtained from Shell (Rotterdam, Netherlands). Both aqueous and oil ICP standards of aluminium, cobalt, copper, lithium and lanthanum were obtained from Merck (Overijse, Belgium). All the chemicals were used as received without any further purification. Ultrapure water (18.2 MΩ cm) provided by a Millipore Milli-Q Reference A+ system.Instrumentation
Metal concentrations in the loaded organic phase after leaching were determined by inductively coupled plasma-optical emission spectroscopy (ICP-OES) using an organic sample measurement set-up. This was done with a PerkinElmer Avio 500 spectrometer, equipped with an axial/radial dual plasma view, a High Solids GemCone™ nebulizer, a baffled cyclonic spray chamber and a quartz torch with a 2.0 mm internal diameter alumina injector. Samples, calibration solutions, and quality control solutions were diluted with 1-octanol. Calibration curves were made using solutions of 0.04, 0.2, 1, 5 and 25 mg L−1 of the corresponding metal from a standard oil solution. All samples were diluted 1000 times with 1-octanol and lanthanum(III) was added as internal standard. The metal concentrations in the aqueous phase, after stripping the organic extractant with different sulphuric acid concentrations, were analysed with a PerkinElmer Optima 8300 spectrometer equipped with an axial (AX)/radial (RAD) dual plasma view, a GemTip Cross-Flow II nebulizer, a Scott spray chamber and a Hybrid XLT ceramic torch with a 2.0 mm internal diameter sapphire injector. All aqueous samples were diluted 2000 times with 2 vol% nitric acid and aqueous standard ICP solutions were used. All metal concentrations were determined by first stripping the loaded D2EHPA phase with 3 mol L−1 sulphuric acid followed by aqueous ICP-OES measurements. This procedure was chosen because it allowed for a simpler sample preparation and a higher sample throughput since aqueous samples could be prepared by volumetrically and automatic micropipettes, whereas in the case of organic samples gravimetric sample preparation was required. Comparing the results obtained after direct measurement of the organic phase and measurement of the aqueous phase after stripping with 3 mol L−1 sulphuric acid confirmed hat 3 mol L−1 sulphuric acid efficiently striped all metals from the loaded D2EHPA phase.Qualitative analysis of the loaded extractant phase was done by UV/VIS absorption spectroscopy with an Agilent Cary 6000i spectrophotometer and Cary WinUV software. A Nemus Life Thermo Shaker TMS-200 was used for shaking the glass vials during the stripping experiments. A Heraeus Labofuge 200 centrifuge was used to accelerate phase separation after equilibration of the two phases. The solid starting material and the leaching residue were characterized by a X-ray diffraction analysis (XRD) (D2 Phaser, Bruker, Germany) with Cu Kα radiation (30 kV, 10 mA in the measurement range 2θ of 20–70°). Data processing was performed with the X'Pert HighScore software. The pH was measured by a S220 Seven compact pH-ion meter (Mettler Toledo, Belgium).
Solvoleaching experiments
Unless stated otherwise, a leaching experiment at optimised conditions was conducted by adding 237.5 mg of LCO or NMC, 28.5 mg of aluminium and 57.0 mg of copper into a glass vial of 10 mL. The added amount of aluminium and copper metal was based on their average mass ratios in LIBs and kept constant (i.e. 12 wt% and 24 wt% of the LCO amount for aluminium and copper, respectively).21,35,36 Subsequently, 9.5 mL of lixiviant (i.e. acidic extractant or diluted extractant) and a magnetic stirring bar were added. The vial was closed and then placed in a sand bath on a heating plate, equipped with a thermocouple for temperature control at 80 °C while stirring at 600 rpm. The leaching parameters were optimized by varying both the leaching time and temperature together with the amount of LCO, aluminium and copper. After solvoleaching, the loaded organic extractant phases were filtered with syringe filters (Chromafil® pore size 0.45 μm, diameter 25 mm). The metal concentrations in the loaded D2EHPA phase were determined with ICP-OES after stripping with 3 mol L−1 sulphuric acid. Recycling studies were performed by starting the first cycle in a 20 mL glass vial where 15 mL of acidic extractant was added and leaching was done at the optimised parameters. After leaching and filtration, 14.5 mL of the loaded extractant phase was stripped with 3 mol L−1 of sulphuric acid at equal Vo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Va ratio. After centrifuging, 14 mL of the stripped extractant phase was used to leach the next cycle at optimised parameters. This procedure was repeated until the fifth cycle, whereby 10 mL of stripped extractant was used to leach. All experiments were executed in duplicate. The percentage leaching (%L) was calculated via the following eqn (1):21,37
Va ratio. After centrifuging, 14 mL of the stripped extractant phase was used to leach the next cycle at optimised parameters. This procedure was repeated until the fifth cycle, whereby 10 mL of stripped extractant was used to leach. All experiments were executed in duplicate. The percentage leaching (%L) was calculated via the following eqn (1):21,37 | (1) |
Here, [Maq;ICP] denotes the metal concentration after stripping the loaded extractant phase with 3 mol L−1 sulphuric acid determined by aqueous ICP-OES analysis, and Vextractant represents the used volume of extractant. In the denominator, mweighed denotes the initial mass of solid starting material and wt%metal represents the fraction of the studied metal in the latter. The value of wt%metal is 0.07 for lithium and 0.60 for cobalt in LCO; and 1.00 for both aluminium and copper as they are weighed as powders in their elemental form.
Selective stripping experiments
The loaded organic phases after leaching were selectively stripped by aqueous solutions containing different sulphuric acid concentrations. Unless stated otherwise, 1 mL of loaded extractant was placed in a glass vial of 4 mL, together with 0.5 mL of sulphuric acid at the preferred Vo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Va ratio of 2
Va ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The vial was shaken at room temperature for 20 minutes at 1750 rpm and centrifuged for 5 minutes at 5000 rpm afterwards. The equilibrium pH was measured by inserting a pH electrode in the lower aqueous phase after stripping. The stripping efficiency (%S) was calculated viaeqn (2):38
1. The vial was shaken at room temperature for 20 minutes at 1750 rpm and centrifuged for 5 minutes at 5000 rpm afterwards. The equilibrium pH was measured by inserting a pH electrode in the lower aqueous phase after stripping. The stripping efficiency (%S) was calculated viaeqn (2):38 | (2) |
Here [Maq,strip] and [Morg,extractant] denote the metal concentrations in the final aqueous stripping solution and in the initial loaded organic phase, respectively. The terms Va and Vo are the volumes of the aqueous stripping phase and the loaded organic phase, respectively.
Results and discussion
Choice of extractant as lixiviant
Our recent work confirmed that the leaching of cobalt from LCO is mainly driven by three factors: (1) the reducing agent in the system (2) the acidity of the lixiviant and (3) the complexing anion that coordinates with the leached cobalt(II) species.21 Since the current collectors act as reducing agents, the choice of extractant is based on its acidity. There are several acidic extractants commercially available, which are mostly organophosphorus and carboxylic acids.39–41 The former are in general more acidic than the latter.42–44 Therefore, three of the most common organophosphorus acid based extractants were evaluated as lixiviants for the leaching of cobalt from LCO, in presence of aluminium and copper. These were bis(2-ethylhexyl) phosphoric acid (D2EHPA), 2-ethylhexyl phosphonic acid mono-2-ethylhexyl ester (P507) and bis(2,4,4-trimethylpentyl)phosphinic acid (Cyanex 272) (Fig. 1). A comparison of the leaching efficiencies with these extractants is shown in Table 1.| Extractant | %L Al | %L Co | %L Cu | %L Li |
|---|---|---|---|---|
a Leaching parameters: 80 °C, 8 hours, S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L = 20 g L−1, 600 rpm. Al L = 20 g L−1, 600 rpm. Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiCoO2 = 12 wt%, Cu LiCoO2 = 12 wt%, Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiCoO2 = 24 wt%. Extractants were used undiluted (100 vol%). LiCoO2 = 24 wt%. Extractants were used undiluted (100 vol%).
|
||||
| D2EHPA | 0.8 ± 0.2 | 97.3 ± 1.3 | 93.3 ± 2.6 | 78.7 ± 1.3 |
| P507 | 1.9 ± 1.7 | 93.9 ± 1.3 | 98.9 ± 1.6 | 74.6 ± 1.3 |
| Cyanex 272 | 4.9 ± 0.6 | 71.8 ± 0.2 | 92.9 ± 0.2 | 63.3 ± 0.2 |
Table 1 shows that D2EHPA leaches cobalt from LCO most efficiently. Additionally, D2EHPA is the cheapest extractant among the evaluated ones.45 Apart from cobalt, aluminium is not significantly leached by any extractant, while copper and lithium are considerably leached as well. The percentage leaching increases as the acidity of the extractant rises (i.e. D2EHPA > p507 > Cyanex 272).42–44 Furthermore, the studied extractants are relatively viscous when used in their pure forms (i.e. D2EHPA = 35 mPa s, P507 = 58 mPa s, Cyanex 272 = 142 mPa s, determined at room temperature).46–48 The viscosity further increases when metals are loaded, making operations such as filtration difficult after cooling down.44,49 Based on both leaching efficiency and viscosity values, D2EHPA was chosen for further studies such as optimisation of the D2EHPA concentration and more mechanistic investigations.
D2EHPA concentration
An aliphatic diluent (Shell GTL GS270) was used to dilute D2EHPA. Shell GTL diluents are biodegradable and show low toxicity.21,26,50 Both D2EHPA and the chosen diluent have flash points that are least 20 °C higher than the maximum applied leaching temperature (i.e. 170 °C for D2EHPA and 130 °C for the aliphatic diluent).50,51 The concentration optimisation of D2EHPA as lixiviant is shown in Fig. 2. | ||
Fig. 2 Percentage leaching by different concentrations of D2EHPA diluted in an aliphatic diluent. Leaching parameters: 80 °C, 8 hours, S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L = 20 g L−1, 600 rpm. Al L = 20 g L−1, 600 rpm. Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiCoO2 = 12 wt%, Cu LiCoO2 = 12 wt%, Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiCoO2 = 24 wt%. LiCoO2 = 24 wt%. | ||
Fig. 2 confirms that 75 vol% D2EHPA leaches ca. 97% cobalt, 99% copper and 87% lithium, while aluminium remains in the residue. This concentration was used in further experiments because it ensured quantitative cobalt leaching and reduced the viscosity compared with the undiluted form.
Solid-to-liquid ratio
The S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratio determines the concentration of the leached metal in the organic D2EHPA phase and is therefore an important parameter to optimise (Fig. 3).
L ratio determines the concentration of the leached metal in the organic D2EHPA phase and is therefore an important parameter to optimise (Fig. 3).
 | ||
Fig. 3 Percentage leaching by 75 vol% (diluted in an aliphatic diluent) D2EHPA at different S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratios. Leaching parameters: 80 °C, 8 hours, 600 rpm. Al L ratios. Leaching parameters: 80 °C, 8 hours, 600 rpm. Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiCoO2 = 12 wt%, Cu LiCoO2 = 12 wt%, Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LiCoO2 = 24 wt%. LiCoO2 = 24 wt%. | ||
Apart from aluminium, the leaching efficiencies in general decrease at S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratios higher than 30 g L−1. These increasing ratios lower the accessible surface area per volume unit, which impedes the mass transfer and consequently decreases the leaching of metals.52 Furthermore, more viscous loaded D2EHPA phases were obtained after cooling when working at S
L ratios higher than 30 g L−1. These increasing ratios lower the accessible surface area per volume unit, which impedes the mass transfer and consequently decreases the leaching of metals.52 Furthermore, more viscous loaded D2EHPA phases were obtained after cooling when working at S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratios of 30 g L−1 or higher. A ratio of 25 g L−1 was therefore chosen for further experiments. Under these conditions the concentration of cobalt, copper and lithium in the loaded organic phase corresponded to 15.5 g L−1, 6.3 g L−1 and 1.7 g L−1, respectively.
L ratios of 30 g L−1 or higher. A ratio of 25 g L−1 was therefore chosen for further experiments. Under these conditions the concentration of cobalt, copper and lithium in the loaded organic phase corresponded to 15.5 g L−1, 6.3 g L−1 and 1.7 g L−1, respectively.
Leaching time and temperature
The percentage leaching of 75 vol% D2EHPA in an aliphatic diluent as a function of time was evaluated at four different temperatures (Fig. 4). D2EHPA leached copper quantitatively at all tested temperatures, whereas only less than ca. 3% of aluminium was leached. Temperatures of 40 °C and 60 °C were not sufficient to quantitatively leach cobalt and lithium after relatively short leaching times. This was achieved after 6 hours at 80 °C and 5 hours 100 °C.Leaching of oxide materials with acidic protons produces water.21 This water starts to evaporate when leaching is performed at 100 °C, which was evident from a pressure build-up in the vial. Additionally, lower leaching temperatures are usually preferred for the cost-effectiveness of the process. Therefore, leaching at 80 °C for 6 hours was chosen as optimised condition for successful leaching of cobalt, copper and lithium from LCO in presence of the current collectors.
Leaching mechanism
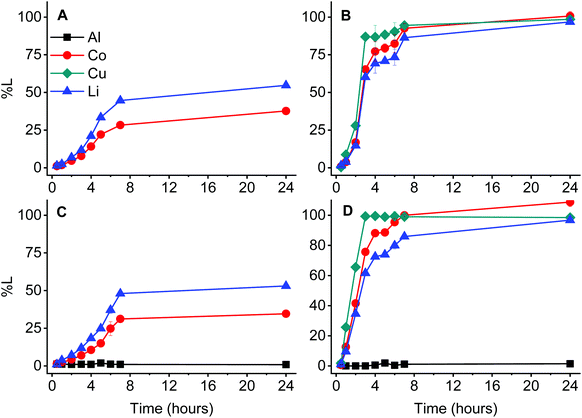
Mechanistic studies were carried out by comparing the leaching efficiency during time for each possible combination of LCO with/without aluminium and/or copper (Fig. 5).Complete leaching of cobalt and lithium from LCO in absence of copper was not achieved. This requires harsher conditions such as higher acidity, higher temperatures and longer leaching times.53,54 The presence of copper allows the reduction of cobalt(III) to cobalt(II) and therefore causes the quantitative leaching of cobalt, together with lithium. This effective reducing capacity of copper has been previously reported in the literature.21,32,34 Lithium occupies interstices in the crystal lattice of LCO and the acidic attack of D2EHPA partially opens the crystal lattice. Furthermore, the reduction of cobalt to its divalent state also distorts the LCO crystal lattice. This implies that the increasing leaching of cobalt is concomitant with the release of lithium.21,55 Interestingly, aluminium is not leached in any situation, showing no effect on the leaching behaviour. This is in contrast with our recent work, in which aluminium mainly gets dissolved by reducing protons of water and/or acids to form hydrogen gas.21 This was also observed by Joulié et al.32 and Peng et al.33,34
Structural analysis and comparison of the solid material before and after leaching was done by XRD and the results are presented in Fig. 6. The comparison between Fig. 6A and B shows that the diffraction peak intensities of LCO and copper diminish during leaching.
The relative peak intensity of aluminium increases after leaching, indicating that the leaching residue is rich in aluminium. Although these observations are in agreement with the trend observed in Fig. 5, it also shows that there is no significant presence of aluminium oxide in the residue. Therefore, it can be concluded that it seems that aluminium does not undergo structural changes and remains in its metallic state in the residue.
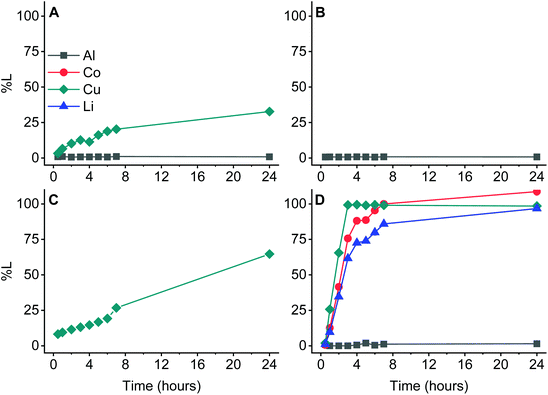
Corrosion studies of aluminium have shown that its activity in organic solvents differs strongly from that in water.56 This is most likely the reason that aluminium is not affected by D2EHPA in the studied system. As mentioned before, aluminium acts as a reducing agent towards water or mineral acids, with the tendency increasing as the acidity of the solution increases.21 D2EHPA is much less acidic than mineral acids and the water content in D2EHPA is neglibible.57,58 These factors reduce the undesired formation of hydrogen gas, leaving aluminium unaffected in the leaching residue.59–61 Hence, metals can show a remarkable different behaviour in organic media, which is observed when only the current collectors (without LCO) are leached and compared in Fig. 7.
The results in Fig. 7 confirm that aluminium is not leached under the studied conditions. It is known that aluminium dissolves in acidic aqueous media by reducing protons.62,63 Furthermore, copper ions normally accelerate the dissolution of aluminium in acidic aqueous conditions by taking part in a cementation reaction, especially at high temperatures.63,64 This causes the reduction of copper ions on the surface of aluminium while aluminium is partially oxidized and dissolved.63–65 This is not observed in any of the studied systems where aluminium and copper are combined Under the studied conditions copper does not undergo cementation at all. Moreover, copper by itself is readily dissolved by 75 vol% D2EHPA without addition of other oxidants, while it is considered to be more noble than aluminium in aqueous media.66–68 Mostafa et al. reported that copper(I/II) oxides were formed when copper was dissolved in weakly acidic organic media.69 This could partially explain the dissolution of copper by 75 vol% D2EHPA in absence of LCO. First, copper(I/II) oxides are formed, which are then dissolved by D2EHPA due to the attack of its acidic protons. This hypothesis is supported by the fact that the leaching efficiency of copper by itself dropped from 27% to 0% after 7 hours of leaching when the experiment was modified by continuously bubbling nitrogen gas through the solution. This again contrasts with observations in aqueous media, where aluminium oxide layers are generally more easily formed than copper oxides.70,71 However, when copper is combined with LCO, the reduction of cobalt(III) is most likely favoured over the formation of oxides. Furthermore, none of the latter oxide structures were detected with XRD (Fig. 6).
Eventually, there could be concluded that the selectivity of the leaching process is related to the redox couple copper(0)/cobalt(III) and the non-aqueous nature of D2EHPA as lixiviant. The former redox reaction solubilizes both metals in D2EHPA, cobalt(III) reduces to the soluble cobalt(II) and copper(0) oxidizes to the soluble copper(II). Consequently, the crystal lattice opens partially, which liberates lithium(I) and in turn assists its solubilisation. In contrast, aluminium has no significant redox capacity in the non-aqueous lixiviant D2EHPA, it is not involved in reducing cobalt(III), nor assisting the redox couple copper(0)/cobalt(III). Due to this inactivity, it remains unchanged in the residue.
Several loaded D2EHPA phases were collected at different time intervals during leaching at 80 °C, and qualitative analysis was done with UV-VIS absorption spectroscopy. Since lithium complexes are UV-VIS inactive, the focus is on the leached cobalt and copper complexes. This absorption spectra of the loaded cobalt complexes in the D2EHPA phase as a function of leaching time are given in Fig. 8.
This pattern with three peaks with the maximum absorbance located at ca. 625 nm is in accordance with literature data on cobalt(II) in D2EHPA, which confirms the tetrahedral structure the formed cobalt(II) complexes.72 This is also valid for the absorption maximum at ca. 822 nm for loaded copper(II) complexes. Unfortunately the latter peak suffers from peak broadening, as shown in Fig. 9.
The formed cobalt and copper complexes are widely described in the literature, whereby the divalent metal ion complexes with the deprotonated (anionic) D2EHPA dimer. This is simplistically summarized in Fig. 10, where the complex usually is presented as [M(L)2(HL)2].72–74
 | ||
| Fig. 10 Simplified reaction of the coordination between D2EHPA (present as dimer) and divalent metal cations such as cobalt(II) and copper(II) during leaching. | ||
A similar reaction is expected to occur for lithium, where only one proton would be exchanged, forming the [LiL(HL)3] complex.75 Since the main goal of this work was to propose a simplified reaction scheme, more detailed structural studies were omitted. For future studies, structural analytical techniques such as X-ray absorption fine structure or X-ray absorption near edge structure are recommended.
A schematic overview of the entire leaching process proposed in this work is highlighted in Fig. 11. As a summary, the acidic protons of D2EHPA partially open the crystal lattice of LCO, releasing the interstitial lithium(I) ions. Effective cobalt leaching is accompanied by that of metallic copper, which reduces cobalt(III) to cobalt(II), while being oxidised itself to copper(II), further opening the crystal lattice. Aluminium remains unaffected in its metallic state in the leaching residue. The leached metal cations lithium(I), cobalt(II) and copper(II) are coordinated with the deprotonated anion of D2EHPA.
 | ||
| Fig. 11 Schematic representation of the leaching of LCO in presence of aluminium and copper by D2EHPA. | ||
Comparison with conventional LCO leaching by sulphuric acid
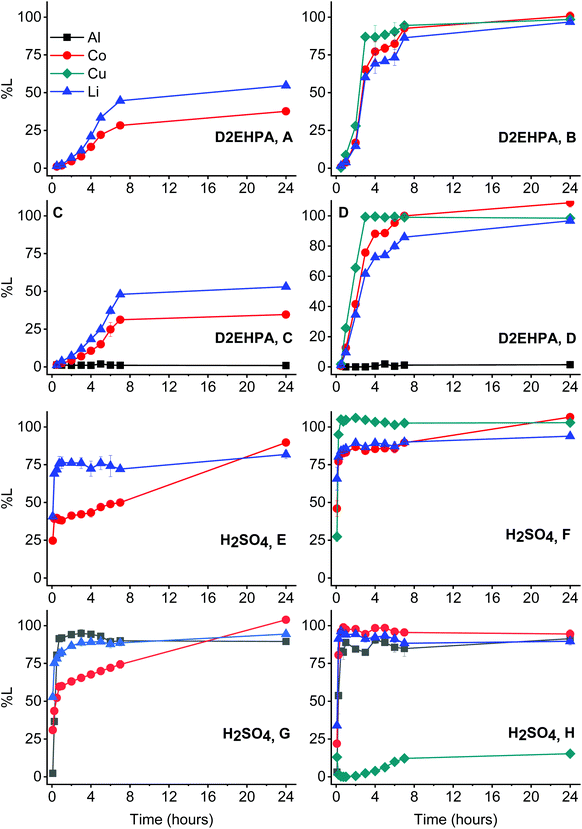
As mentioned, sulphuric acid is a common lixiviant for leaching of LCO.54,76 It was therefore compared with the solvoleaching system by D2EHPA in presence of aluminium and copper, under optimised conditions (Fig. 12). A sulphuric acid concentration of 2.25 mol L−1 was used, since this corresponds to the molar concentration of 75 vol% D2EHPA. The much higher acidity of sulphuric acid leads to faster leaching of LCO in general. This is one of the reasons that sulphuric acid is nowadays still the industrial preferred lixiviant.57,58In contrast to D2EHPA, sulphuric acid can leach lithium and cobalt quantitatively in absence of reducing agents. However, this is only achieved after 24 hours leaching. Copper still reduces cobalt(III) in both systems, which was also described by Joulié et al. and Peng et al.32–34 The reactivity of aluminium differs strongly when sulphuric acid is used as lixiviant. Comparison of Fig. 12E with Fig. 12G shows that the leaching of cobalt and lithium from LCO is not significantly affected by aluminium. Samples in which aluminium was treated with sulphuric acid (Fig. 12G and H) showed strong effervescence. Hence, the dissolution of aluminium in sulphuric acid is probably related to the reduction of water and/protons from sulphuric acid, producing hydrogen gas. This strong tendency of aluminium to produce hydrogen gas in sulphuric acid was also reported by Joulié et al.32 Furthermore, comparison of Fig. 12F with Fig. 12H shows that aluminium also reduces copper(II) species, hereby undergoing a cementation reaction. These copper(II) species are formed by reducing cobalt(III) in LCO as described above. The cementation reaction between copper(II) and metallic aluminium was also described in our previous work, producing an alloy of both metals that remains in the residue.21,62,64 The comparison of Fig. 12D with Fig. 12H is the most relevant to our study, since one of the main goals was to keep both aluminium and copper together with the LCO to avoid pre-treatment steps. It is clear from these figures that sulphuric acid leaches faster, but is less selective than D2EHPA. Only metallic aluminium stays in the residue after the leaching by D2EHPA, while the residue consists of an aluminium–copper alloy after leaching with sulphuric acid. When D2EHPA is used as lixiviant, no explosive hydrogen gas is formed. Moreover, the reducing capacity of copper and the relative inertness of aluminium are also an advantage in the D2EHPA system because there is no need for pre-treatment steps that separate these metals from the LCO material, which intensifies the process.
Furthermore, process intensification is also achieved by leaching directly with the extractant D2EHPA, or in other words by merging the leaching and the solvent extraction into one step. This means that the loaded cobalt, copper and lithium can be then directly stripped to the final aqueous phase. By contrast, leaching with sulphuric acid first forms an acidic PLS that contains aluminium, cobalt, copper and lithium. In a next step, this acidic PLS is purified by precipitation and/or extraction by acidic extractants, such as D2EHPA and Cyanex 272. These steps would then require the addition of relatively large amounts of base, in order to increase the pH to the preferred range for precipitation and/or extraction.19,44,77 Moreover, the produced loaded organic phases after extraction should be stripped as well.18–20 Both these extraction and stripping operations would require mixer-settler units, while the direct solvoleaching with D2EHPA only needs mixer-settlers for the stripping process. Hence, apart from avoiding the pre-treatment steps, solvoleaching with D2EHPA also avoids additional filtration set-ups and extra mixer-settlers units, which in turn reduces the generation of aqueous waste.45,78
Selective stripping of the loaded D2EHPA phase
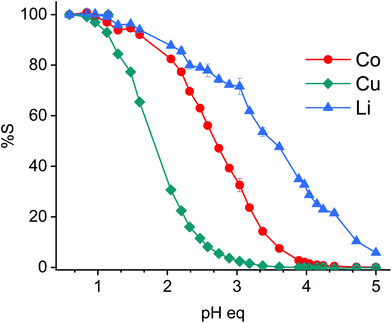
Metals are in general selectively stripped form loaded acidic extractants by controlling the equilibrium pH (pHeq).19,20 The stripping of cobalt, copper and lithium from the loaded D2EHPA was studied by controlling the pHeq and it is shown in Fig. 13. The highest selectivity between copper and cobalt is obtained at pHeq of ca. 2.5. This ensures stripping efficiencies of 11%, 70% and 90% for copper, cobalt and lithium, respectively. The highest selectivity between lithium and cobalt is obtained at pHeq of ca. 3.0; stripping 2%, 30% and 70% copper, cobalt and lithium, respectively.Sulphuric acid was chosen to control the pH due to its cheapness and the fact that it forms metal sulphate complexes in the aqueous stripped solution. These latter complexes are usually preferred electrolytes for further downstream electrowinning processes, since the sulphate anion provides high conductivity.79,80
On the one hand, lithium will not negatively affect the electrowinning process of cobalt, due to its lower standard reduction potential.81–83 Moreover, cobalt can be separated from lithium by producing cobalt oxalate precipitates.84–86 On the other hand, copper is a significant impurity that can affect the downstream processing of cobalt.87,88 These aspects make it important to focus more on the separation of cobalt and copper. In order to increase the selectivity and obtain the high purity of cobalt that is required for the production of LIBs,49,89 a continuous multistage counter-current set-up could be used.90–92 The number of mixer-settler stages could be estimated first by performing a multistage counter-current simulation, followed by the operation in the mixer-settler devices itself.74,93,94 This further optimization would require mixer settler units and pH controller devices, which are out of the scope of this work. Although more pure cobalt could be recovered by using mixer settlers, it should be mentioned that these devices are needed for stripping operations in any case at industrial scale. In conventional cases, copper is usually pre-separated or selectively extracted from the aqueous PLS, which again requires mixer settler units. While our approach only requires mixer settler units for the stripping, which are needed to strip cobalt anyway. Hence, our proposal could still reduce the amount of process steps compared to conventional cases while ensuring no emission of toxic and dangerous gasses. If more pure copper- and cobalt sulphate solutions are obtained via the abovementioned method, both metals could be recovered by electrowinning. This would reduce the environmental risk of copper(II) and cobalt(II) containing aqueous waste, and would also increase the economic impact of the process.
It has to be noted that Fig. 13 is constructed at Vo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Va ratio of two, which enriches final aqueous metal concentration. A pHeq of ca. 0.50 (i.e. an initial sulphuric acid concentration of 1.15 mol L−1) allowed complete striping of all three metals from the loaded D2EHPA phase and regenerates D2EHPA so that it can be reused in the next leaching series. Therefore, the recyclability of D2EHPA was studied after stripping with sulphuric acid of 1.15 mol L−1.
Va ratio of two, which enriches final aqueous metal concentration. A pHeq of ca. 0.50 (i.e. an initial sulphuric acid concentration of 1.15 mol L−1) allowed complete striping of all three metals from the loaded D2EHPA phase and regenerates D2EHPA so that it can be reused in the next leaching series. Therefore, the recyclability of D2EHPA was studied after stripping with sulphuric acid of 1.15 mol L−1.
Recycling studies of D2EHPA
The performance of D2EHPA after being used after several cycles was evaluated and the results are shown in Fig. 14. D2EHPA is still able to leach cobalt, copper and lithium quantitatively after five cycles. Although the small decrease in the cobalt leaching efficiency is still situated within the margin of error, a further decrease in leaching efficiency is expected after more cycles. This is mainly due to the solubility of D2EHPA in aqueous sulphuric acid media (e.g. ca. 120 to 250 mg L−1)95 and the loss of phosphoric acid impurities that are present in the commercially available D2EHPA.95–98 Fortunately, these losses are limited and could be solved by adding minor amounts of fresh D2EHPA when needed.Future work
In this work an alternative and simpler flowsheet for the recovery of cobalt, lithium and copper from LIBs was presented. The proposed solvometallurgical approach has several advantages: selective solvoleaching, avoiding hydrogen gas emission and achieving process intensification by omitting pre-treatment steps and merging solvoleaching with SX. Copper, cobalt and lithium can be recovered via selective stripping but further optimization of this step is required. Despite the advantages of the process presented here, it has to be mentioned that leaching with sulphuric acid remains attractive from an industrial perspective due to much faster leaching times, lower leaching temperature and the ability to leach at much higher S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratios.14 Obtaining these desired features with our method still remains challenging. A possible approach could rely on adding the electroplated metallic copper back to the solid material at the start of each subsequent leaching cycle, which could increase the effectiveness of our process in general. This was unfortunately not studied in this concept study due to the lack of upscaling facilities. However, sulphuric acid leaches less selectively, produces hydrogen gas and generates more waste water due to the requirement of extra chemicals to further treat the PLS. It needs to be noted that LCO cathode materials are progressively being replaced by NMC materials in order reduce the cobalt consumption.5,11,99 This study only focused on LCO cathode material, further investigations about the applicability of this solvometallurgical process on other cathode materials and real LIB waste streams are crucial to the transition towards a more sustainable and circular economy. As a proof of concept and to demonstrate the versatility of the process presented in this work, the optimized leaching process was applied to NMC powder. As it can be seen in Fig. 15, about 100% copper, 80% cobalt and lithium, 73% nickel, 63% manganese and no aluminium was leached by 75 vol% D2EHPA after seven hours at 80 °C. The current collectors behaved in the same way as they performed in the experiments with LCO (Fig. 4C). In the case of the leaching of the NMC powder, not only cobalt, copper and lithium came into the organic PLS but also nickel and manganese, as expected. The percentage of metal leached of lithium and cobalt was slightly lower than when leaching from the LCO. Further optimization of the leaching conditions such as D2EHPA concentration and S
L ratios.14 Obtaining these desired features with our method still remains challenging. A possible approach could rely on adding the electroplated metallic copper back to the solid material at the start of each subsequent leaching cycle, which could increase the effectiveness of our process in general. This was unfortunately not studied in this concept study due to the lack of upscaling facilities. However, sulphuric acid leaches less selectively, produces hydrogen gas and generates more waste water due to the requirement of extra chemicals to further treat the PLS. It needs to be noted that LCO cathode materials are progressively being replaced by NMC materials in order reduce the cobalt consumption.5,11,99 This study only focused on LCO cathode material, further investigations about the applicability of this solvometallurgical process on other cathode materials and real LIB waste streams are crucial to the transition towards a more sustainable and circular economy. As a proof of concept and to demonstrate the versatility of the process presented in this work, the optimized leaching process was applied to NMC powder. As it can be seen in Fig. 15, about 100% copper, 80% cobalt and lithium, 73% nickel, 63% manganese and no aluminium was leached by 75 vol% D2EHPA after seven hours at 80 °C. The current collectors behaved in the same way as they performed in the experiments with LCO (Fig. 4C). In the case of the leaching of the NMC powder, not only cobalt, copper and lithium came into the organic PLS but also nickel and manganese, as expected. The percentage of metal leached of lithium and cobalt was slightly lower than when leaching from the LCO. Further optimization of the leaching conditions such as D2EHPA concentration and S![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L ratio could lead to higher percentages of metal leached. Furthermore, aluminium was again not affected during the solvoleaching, while it is known that it can be leached by sulphuric acid.32 After leaching, the refining of cobalt is still necessary. D2EHPA has shown to have selectivity towards the extraction of copper and manganese over cobalt, nickel and lithium.100,101 Since stripping occurs via similar chemical equilibria as extraction, it can be expected that copper and manganese could be selectively stripped from cobalt, nickel and lithium. As a consequence; a sulphate solution enriched in cobalt, nickel and lithium would be obtained after stripping. From here, selective cobalt recovery could be easily achieved with Cyanex 272 as it is usually done in mixtures of cobalt and nickel. On the contrary, conventional leaching of NMC material by sulphuric acid would first result in an aqueous PLS rich in aluminium, copper, cobalt, manganese, nickel and lithium. The next step would require the separation of manganese and/or copper from cobalt, which could be achieved by extracting with D2EHPA and/or LIX 984 respectively followed by stripping with a mineral acid.102,103 Hereafter, cobalt could be separated from nickel, aluminium and lithium with Cyanex 272 as described above. This extra step in which manganese and/or copper would need to be extracted first by D2EHPA and/or LIX 984 will not only consume more chemicals but also require extra contactors such as mixer-settlers. In the process presented in this work, only cobalt, copper, manganese, nickel and lithium are directly loaded into D2EHPA and the process is intensified by merging steps. The results shown in Fig. 15 also corroborate that this process is not only valid for LCO but also for NMC material.
L ratio could lead to higher percentages of metal leached. Furthermore, aluminium was again not affected during the solvoleaching, while it is known that it can be leached by sulphuric acid.32 After leaching, the refining of cobalt is still necessary. D2EHPA has shown to have selectivity towards the extraction of copper and manganese over cobalt, nickel and lithium.100,101 Since stripping occurs via similar chemical equilibria as extraction, it can be expected that copper and manganese could be selectively stripped from cobalt, nickel and lithium. As a consequence; a sulphate solution enriched in cobalt, nickel and lithium would be obtained after stripping. From here, selective cobalt recovery could be easily achieved with Cyanex 272 as it is usually done in mixtures of cobalt and nickel. On the contrary, conventional leaching of NMC material by sulphuric acid would first result in an aqueous PLS rich in aluminium, copper, cobalt, manganese, nickel and lithium. The next step would require the separation of manganese and/or copper from cobalt, which could be achieved by extracting with D2EHPA and/or LIX 984 respectively followed by stripping with a mineral acid.102,103 Hereafter, cobalt could be separated from nickel, aluminium and lithium with Cyanex 272 as described above. This extra step in which manganese and/or copper would need to be extracted first by D2EHPA and/or LIX 984 will not only consume more chemicals but also require extra contactors such as mixer-settlers. In the process presented in this work, only cobalt, copper, manganese, nickel and lithium are directly loaded into D2EHPA and the process is intensified by merging steps. The results shown in Fig. 15 also corroborate that this process is not only valid for LCO but also for NMC material.
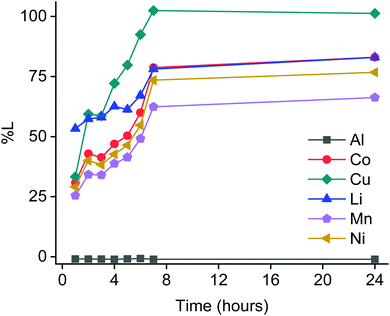 | ||
Fig. 15 The percentage leaching of NMC material during time by 75 vol% (diluted in an aliphatic diluent) leaching parameters: 80 °C, 25 g L−1, 600 rpm. Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NMC = 12 wt%, Cu NMC = 12 wt%, Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NMC = 24 wt%. NMC = 24 wt%. | ||
Finally, in this study the particle sizes of LCO, NMC, aluminium and copper were kept constant but it is known that properties such as redox capacity, leaching time and leaching temperature are influenced by the particle size.104–106 The used particle sizes are smaller than those used in industrial spent LIB recycling processes.33,34 It is therefore advisable to study the effect of larger particle sizes. We hope that the flowsheet developed here serves as an inspiration for future developments in the field where the critical points that are mentioned above are also considered.
Conclusions
The commercial available extractant D2EHPA is suitable as a non-aqueous lixiviant to recover cobalt from LCO in presence of metallic aluminium and copper. The applied solvometallurgical flowsheet makes use of process intensification by leaving aluminium and copper in the LIB without pre-treatment and by combining leaching with SX in one step, which are both innovative approaches. The former avoided the use pre-treatment steps, the latter eliminated the need of both extra mixer settler units and chemicals, and the formation of toxic and dangerous gasses. The optimized leaching conditions ensured the quantitative leaching of cobalt, copper and lithium with 75 vol% D2EHPA at 80 °C for six hours at a ratio of 25 g L−1. Hereby causing the direct loading of these three metals in the organic D2EHPA phase. The stripping of this loaded D2EHPA at an equilibrium pH of ca. 2.50 ensured 11% stripping of copper, while 70% and 90% of cobalt and lithium were stripped respectively in one contact. The selectivity of cobalt and lithium over copper can be increased by executing the stripping process in a continuous multistage counter-current circuit, which is recommended as future work. The reducing properties of the current collectors aluminium and copper were investigated. It became clear that copper acted as reducing agent during the reduction of cobalt(III) to cobalt(II) whereas aluminium remained in its metallic state in the residue. These consequences caused the our redox system together with D2EHPA as non-aqueous lixiviant is more selective compared to the commonly used aqueous lixiviant sulphuric acid. This difference was also confirmed by an experimental comparison, which also showed that hydrogen gas formation is avoided when using D2EHPA as non-aqueous lixiviant. This model-study could be implemented on real spent LIB waste or even other metallurgical recovery methods. As a summary, our intensified solvometallurgical process is compared with a conventional hydrometallurgical process in Fig. 16. | ||
| Fig. 16 Summarizing comparison of two processes to recover cobalt from spent LIBs. A conventional hydrometallurgical process above (A) and our proposed solvometallurgical process below (B). | ||
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research project has received funding from the European Union's EU Framework Programme for Research and Innovation Horizon 2020 under Grant Agreement No. 776473 (CROCODILE). This work reflects only the author's view, exempting the Community from any liability. Website: https://h2020-crocodile.eu/.References
- J. Lin, C. Liu, H. Cao, R. Chen, Y. Yang, L. Li and Z. Sun, Green Chem., 2019, 21, 5904–5913 RSC.
- Y. Shi, G. Chen and Z. Chen, Green Chem., 2018, 20, 851–862 RSC.
- X. Chen, J. Li, D. Kang, T. Zhou and H. Ma, Green Chem., 2019, 21, 6342–6352 RSC.
- H. Zou, E. Gratz, D. Apelian and Y. Wang, Green Chem., 2013, 15, 1183–1191 RSC.
- D. Di Lecce, R. Verrelli and J. Hassoun, Green Chem., 2017, 19, 3442–3467 RSC.
- Y. Yang, X. Meng, H. Cao, X. Lin, C. Liu, Y. Sun, Y. Zhang and Z. Sun, Green Chem., 2018, 20, 3121–3133 RSC.
- L. Chen, Y. Chao, X. Li, G. Zhou, Q. Lu, M. Hua, H. Li, X. Ni, P. Wu and W. Zhu, Green Chem., 2021, 23, 2177–2184 RSC.
- X. Zheng, Z. Zhu, X. Lin, Y. Zhang, Y. He, H. Cao and Z. Sun, Engineering, 2018, 4, 361–370 CrossRef CAS.
- Y. Yang, S. Xu and Y. He, Waste Manage., 2017, 64, 219–227 CrossRef CAS PubMed.
- X. Wang, H. Qiu, H. Liu, P. Shi, J. Fan, Y. Min and Q. Xu, Green Chem., 2018, 20, 4901–4910 RSC.
- A. Verma, G. H. Johnson, D. R. Corbin and M. B. Shiflett, ACS Sustainable Chem. Eng., 2020, 8, 6100–6108 CrossRef CAS.
- A. H. Tkaczyk, A. Bartl, A. Amato, V. Lapkovskis and M. Petranikova, J. Phys. D: Appl. Phys., 2018, 51, 1–26 CrossRef.
- C. Banza Lubaba Nkulu, L. Casas, V. Haufroid, T. De Putter, N. D. Saenen, T. Kayembe-Kitenge, P. Musa Obadia, D. Kyanika Wa Mukoma, J. M. Lunda Ilunga, T. S. Nawrot, O. Luboya Numbi, E. Smolders and B. Nemery, Nat. Sustainability, 2018, 1, 495–504 CrossRef PubMed.
- P. Meshram, B. D. Pandey and T. R. Mankhand, Chem. Eng. J., 2015, 281, 418–427 CrossRef CAS.
- H. Ku, Y. Jung, M. Jo, S. Park, S. Kim, D. Yang, K. Rhee, E. M. An, J. Sohn and K. Kwon, J. Hazard. Mater., 2016, 313, 138–146 CrossRef CAS PubMed.
- G. P. Nayaka, K. V. Pai, G. Santhosh and J. Manjanna, J. Environ. Chem. Eng., 2016, 4, 2378–2383 CrossRef CAS.
- L. Sun and K. Qiu, Waste Manage., 2012, 32, 1575–1582 CrossRef CAS PubMed.
- L. Chen, X. Tang, Y. Zhang, L. Li, Z. Zeng and Y. Zhang, Hydrometallurgy, 2011, 108, 80–86 CrossRef CAS.
- V. T. Nguyen, J. C. Lee, J. Jeong, B. S. Kim and B. D. Pandey, Met. Mater. Int., 2014, 20, 357–365 CrossRef CAS.
- S. H. Joo, D. J. Shin, C. H. Oh, J. P. Wang, G. Senanayake and S. M. Shin, Hydrometallurgy, 2016, 159, 65–74 CrossRef CAS.
- N. Peeters, K. Binnemans and S. Riaño, Green Chem., 2020, 22, 4210–4221 RSC.
- A. Sonoc, J. Jeswiet and V. K. Soo, Procedia CIRP, 2015, 29, 752–757 CrossRef.
- G. P. Nayaka, J. Manjanna, K. V. Pai, R. Vadavi, S. J. Keny and V. S. Tripathi, Hydrometallurgy, 2015, 151, 73–77 CrossRef CAS.
- X. Chen, C. Guo, H. Ma, J. Li, T. Zhou, L. Cao and D. Kang, Waste Manage., 2018, 75, 459–468 CrossRef CAS PubMed.
- L. Li, J. B. Dunn, X. X. Zhang, L. Gaines, R. J. Chen, F. Wu and K. Amine, J. Power Sources, 2013, 233, 180–189 CrossRef CAS.
- K. Binnemans and P. T. Jones, J. Sustainable Metall., 2017, 3, 570–600 CrossRef.
- S. Wang, Z. Zhang, Z. Lu and Z. Xu, Green Chem., 2020, 22, 4473–4482 RSC.
- L. Gijsemans, J. Roosen, S. Riaño, P. Tom and J. Koen, J. Sustainable Metall., 2020, 6, 589–598 CrossRef.
- R. Colin, Green Chem., 1999, 1, 15–17 RSC.
- E. Drioli, A. Brunetti, G. Di Profio and G. Barbieri, Green Chem., 2012, 14, 1561–1572 RSC.
- D. A. Waterkamp, M. Heiland, M. Schlüter, J. C. Sauvageau, T. Beyersdorff and J. Thöming, Green Chem., 2007, 9, 1084–1090 RSC.
- M. Joulié, E. Billy, R. Laucournet and D. Meyer, Hydrometallurgy, 2017, 169, 426–432 CrossRef.
- C. Peng, F. Liu, A. T. Aji, B. P. Wilson and M. Lundström, Waste Manage., 2019, 95, 604–611 CrossRef CAS PubMed.
- C. Peng, J. Hamuyuni, B. P. Wilson and M. Lundström, Waste Manage., 2018, 76, 582–590 CrossRef CAS PubMed.
- M. J. Lain, J. Power Sources, 2001, 97, 736–738 CrossRef.
- T. Georgi-Maschler, B. Friedrich, R. Weyhe, H. Heegn and M. Rutz, J. Power Sources, 2012, 207, 173–182 CrossRef CAS.
- T. Palden, B. Onghena, M. Regadío and K. Binnemans, Green Chem., 2019, 21, 5394–5404 RSC.
- S. Spathariotis, N. Peeters, K. S. Ryder, A. P. Abbott, K. Binnemans and S. Riaño, RSC Adv., 2020, 10, 33161–33170 RSC.
- M. Jun, R. R. Srivastava, J. Jeong, J. C. Lee and M. S. Kim, Green Chem., 2016, 18, 3823–3834 RSC.
- K. Wang, H. Adidharma, M. Radosz, P. Wan, X. Xu, C. K. Russell, H. Tian, M. Fan and J. Yu, Green Chem., 2017, 19, 4469–4493 RSC.
- B. Swain, C. Mishra, H. S. Hong and S. S. Cho, Green Chem., 2015, 17, 4418–4431 RSC.
- K. Omelchuk, P. Szczepański, A. Shrotre, M. Haddad and A. Chagnes, RSC Adv., 2017, 7, 5660–5668 RSC.
- A. Krzyzaniak, B. Schuur and A. B. De Haan, J. Chem. Technol. Biotechnol., 2013, 88, 1937–1945 CrossRef CAS.
- J. M. Zhao, X. Y. Shen, F. L. Deng, F. C. Wang, Y. Wu and H. Z. Liu, Sep. Purif. Technol., 2011, 78, 345–351 CrossRef CAS.
- Y. Q. Hu, T. Zhang, M. Q. Li, Y. Wang, Z. Zheng and Y. Z. Zheng, Green Chem., 2017, 19, 1250–1254 RSC.
- L. R. Koekemoer, M. J. G. Badenhorst and R. C. Everson, J. Chem. Eng. Data, 2005, 50, 587–590 CrossRef CAS.
- Y. E. Sishi, T. Qiao, Q. Junshuai and W. Yundong, Chem. Ind. Eng. Soc. China J., 2016, 67, 458–468 Search PubMed.
- V. N. H. Nguyen, T. H. Nguyen and M. S. Lee, Metals, 2020, 10, 1–19 Search PubMed.
- B. Swain, J. Jeong, J. C. Lee, G. H. Lee and J. S. Sohn, J. Power Sources, 2007, 167, 536–544 CrossRef CAS.
- Shell, Solvents: GTL fluids and solvents, http://www.shell.com/business-customers/chemicals/our-products/solvents-gtl-solvents-and-fluids.html, (accessed 1 May 2018).
- S. Acharya and A. Nayak, Hydrometallurgy, 1988, 19, 309–320 CrossRef CAS.
- S. M. Seyed Ghasemi and A. Azizi, J. Mater. Res. Technol., 2018, 7, 118–125 CrossRef CAS.
- P. Meshram, B. D. Pandey and T. R. Mankhand, Waste Manage., 2015, 45, 306–313 CrossRef CAS PubMed.
- J. Kang, G. Senanayake, J. Sohn and S. M. Shin, Hydrometallurgy, 2010, 100, 168–171 CrossRef CAS.
- I. L. Santana, T. F. M. Moreira, M. F. F. Lelis and M. B. J. G. Freitas, Mater. Chem. Phys., 2017, 190, 38–44 CrossRef CAS.
- S. Licht, G. Levitin, C. Yarnitzky and R. Tel-Vered, Electrochem. Solid-State Lett., 1999, 2, 262–264 CrossRef CAS.
- M. F. A. De Souza and M. B. Mansur, Braz. J. Chem. Eng., 2019, 36, 541–547 CrossRef CAS.
- Y. Alexeev, T. L. Windus, C. G. Zhan and D. A. Dixon, Int. J. Quantum Chem., 2005, 102, 775–784 CrossRef CAS.
- G. Levitin, C. Yarnitzky and S. Licht, Electrochem. Solid-State Lett., 2002, 5, 160–163 CrossRef.
- J. García-Serna, T. Moreno, P. Biasi, M. J. Cocero, J. P. Mikkola and T. O. Salmi, Green Chem., 2014, 16, 2320–2343 RSC.
- C. Schäfer, C. J. Ellstrom, H. Cho and B. Török, Green Chem., 2017, 19, 1230–1234 RSC.
- S. Licht, G. Levitin, R. Tel-Vered and C. Yarnitzky, Electrochem. Commun., 2000, 2, 329–333 CrossRef CAS.
- N. Demirkran and A. Künkül, Trans. Nonferrous Met. Soc. China, 2011, 21, 2778–2782 CrossRef.
- D. J. MacKinnon and T. R. Ingraham, Can. Metall. Q., 2014, 10, 197–201 CrossRef.
- I. Bakos and S. Szabó, Corros. Sci., 2008, 50, 200–205 CrossRef CAS.
- J. A. Sędzimir, Hydrometallurgy, 2002, 64, 161–167 CrossRef.
- O. Aschenbrenner, T. Fukuda, T. Hasumura, T. Maekawa, A. B. Cundy and R. L. D. Whitby, Green Chem., 2012, 14, 1196–1201 RSC.
- Z. Ke, Y. Zhang, X. Cui and F. Shi, Green Chem., 2016, 18, 808–816 RSC.
- S. N. Mostafa, M. Y. Mourad and S. A. I. Seliman, J. Electroanal. Chem., 1981, 130, 221–228 CrossRef CAS.
- V. G. Celante and M. B. J. G. Freitas, J. Appl. Electrochem., 2010, 40, 233–239 CrossRef CAS.
- K. Mutombo and M. Du, in Arc Welding, ed. W. Sudnik, InTech, Rijeka, 2011, pp. 177–218 Search PubMed.
- I. Van de Voorde, L. Pinoy, E. Courtijn and F. Verpoort, Solvent Extr. Ion Exch., 2006, 24, 893–914 CrossRef CAS.
- A. M. Wilson, P. J. Bailey, P. A. Tasker, J. R. Turkington, R. A. Grant and J. B. Love, Chem. Soc. Rev., 2014, 43, 123–134 RSC.
- L. Wang, X. Huang, Y. Yu, Y. Xiao, Z. Long and D. Cui, Green Chem., 2013, 15, 1889–1894 RSC.
- Y. Boukraa, Russ. J. Phys. Chem. A, 2020, 94, 1136–1142 CrossRef.
- G. Dorella and M. B. Mansur, J. Power Sources, 2007, 170, 210–215 CrossRef CAS.
- R. Torkaman, M. Asadollahzadeh, M. Torab-Mostaedi and M. Ghanadi Maragheh, Sep. Purif. Technol., 2017, 186, 318–325 CrossRef CAS.
- Y. Ding, D. Harvey and N. H. L. Wang, Green Chem., 2020, 22, 3769–3783 RSC.
- I. G. Sharma, P. Alex, A. C. Bidaye and A. K. Suri, Hydrometallurgy, 2005, 80, 132–138 CrossRef CAS.
- C. Chibwe and M. Tadie, Min., Metall. Explor., 2021, 38, 1225–1237 Search PubMed.
- M. B. J. G. Freitas and E. M. Garcia, J. Power Sources, 2007, 171, 953–959 CrossRef CAS.
- E. M. Garcia, J. S. Santos, E. C. Pereira and M. B. J. G. Freitas, J. Power Sources, 2008, 185, 549–553 CrossRef CAS.
- M. Vanitha and N. Balasubramanian, Environ. Technol. Rev., 2013, 2, 101–115 CrossRef CAS.
- Z. D. Xia, X. Q. Xie, Y. W. Shi, Y. P. Lei and F. Guo, Front. Mater. Sci. China, 2008, 2, 281–285 CrossRef.
- X. Chen and T. Zhou, Waste Manage. Res., 2014, 32, 1083–1093 CrossRef PubMed.
- S. M. Badawy, A. A. Nayl, R. A. El Khashab and M. A. El-Khateeb, J. Mater. Cycles Waste Manage., 2014, 16, 739–746 CrossRef CAS.
- J. Kang, J. Sohn, H. Chang, G. Senanayake and S. M. Shin, Adv. Powder Technol., 2010, 21, 175–179 CrossRef CAS.
- D. P. Mantuano, G. Dorella, R. C. A. Elias and M. B. Mansur, J. Power Sources, 2006, 159, 1510–1518 CrossRef CAS.
- C. Peng, F. Liu, Z. Wang, B. P. Wilson and M. Lundström, J. Power Sources, 2019, 415, 179–188 CrossRef CAS.
- C. Courson and K. Gallucci, in Substitute Natural Gas from Waste, ed. M. Materazzi and P. Foscolo Ugo, Elsevier, London, 2019, pp. 161–220 Search PubMed.
- C. Wiles and P. Watts, Green Chem., 2014, 16, 55–62 RSC.
- S. Wellens, R. Goovaerts, C. Möller, J. Luyten, B. Thijs and K. Binnemans, Green Chem., 2013, 15, 3160–3164 RSC.
- V. T. Nguyen, S. Rianõ and K. Binnemans, Green Chem., 2020, 22, 8375–8388 RSC.
- X. Huang, J. Dong, L. Wang, Z. Feng, Q. Xue and X. Meng, Green Chem., 2017, 19, 1345–1352 RSC.
- M. Azam, S. Alam and F. Khan, J. Chem. Eng., 2010, 25, 18–21 Search PubMed.
- N. M. Kocherginsky, Y. K. Zhang and J. W. Stucki, Desalination, 2002, 144, 267–272 CrossRef CAS.
- F. Principe and G. P. Demopoulos, Hydrometallurgy, 2003, 68, 115–124 CrossRef CAS.
- K. A. Rabie, Hydrometallurgy, 2007, 85, 81–86 CrossRef CAS.
- J. Heelan, E. Gratz, Z. Zheng, Q. Wang, M. Chen, D. Apelian and Y. Wang, JOM, 2016, 68, 2632–2638 CrossRef CAS.
- K. C. Sole, in Solvent Extraction and Liquid Membranes: Fundamentals and Applications in New Materials, CRC Press, Boca Raton, 2008, pp. 159–201 Search PubMed.
- C. Y. Cheng, Hydrometallurgy, 2000, 56, 369–386 CrossRef CAS.
- E. Rodríguez De San Miguel, J. C. Aguilar, J. P. Bernal, M. L. Ballinas, M. T. J. Rodríguez, J. De Gyves and K. Schimmel, Hydrometallurgy, 1997, 47, 19–30 CrossRef.
- X. Chen, Y. Chen, T. Zhou, D. Liu, H. Hu and S. Fan, Waste Manage., 2015, 38, 349–356 CrossRef CAS PubMed.
- B. Rufino, F. Boulc'h, M. V. Coulet, G. Lacroix and R. Denoyel, Acta Mater., 2007, 55, 2815–2827 CrossRef CAS.
- A. V. Parmuzina and O. V. Kravchenko, Int. J. Hydrogen Energy, 2008, 33, 3073–3076 CrossRef CAS.
- J. Chen, Q. Li, J. Song, D. Song, L. Zhang and X. Shi, Green Chem., 2016, 18, 2500–2506 RSC.
| This journal is © The Royal Society of Chemistry 2022 |