Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Perilla frutescens seed oil combined with Anredera cordifolia leaf powder attenuates age-related cognitive decline by reducing serum triglyceride and glucose levels in healthy elderly Japanese individuals: a possible supplement for brain health†
Michio
Hashimoto
 *a,
Kentaro
Matsuzaki
*a,
Kentaro
Matsuzaki
 a,
Koji
Maruyama
b,
Eri
Sumiyoshi
a,
Koji
Maruyama
b,
Eri
Sumiyoshi
 a,
Shahdat
Hossain
a,
Shahdat
Hossain
 c,
Harumi
Wakatsuki
a,
Setsushi
Kato
d,
Miho
Ohno
d,
Yoko
Tanabe
a,
Yoko
Kuroda
e,
Shuhei
Yamaguchi
f,
Koji
Kajima
b,
Yasushi
Ohizumi
g and
Osamu
Shido
a
c,
Harumi
Wakatsuki
a,
Setsushi
Kato
d,
Miho
Ohno
d,
Yoko
Tanabe
a,
Yoko
Kuroda
e,
Shuhei
Yamaguchi
f,
Koji
Kajima
b,
Yasushi
Ohizumi
g and
Osamu
Shido
a
aDepartment of Environmental Physiology, Faculty of Medicine, Shimane University, Izumo, Shimane, Japan. E-mail: michio1@med.shimane-u.ac.jp; Fax: +81-853-20-2730; Tel: +81-853-20-2730
bSankyo Holdings Co., Ltd, Fuji, Shizuoka, Japan
cDepartment of Biochemistry and Molecular Biology, Jahangirnagar University, Savar, Dhaka, Bangladesh
dKato Hospital, Jinjukai Healthcare Corporation, Kawamoto, Shimane, Japan
eDepartment of Internal Medicine III, Faculty of Medicine, Shimane University, Izumo, Shimane, Japan
fShimane Prefecture Hospital Bureau, Izumo, Shimane, Japan
gKansei Fukushi Research Institute, Tohoku Fukushi University, Sendai, Miyagi, Japan
First published on 20th June 2022
Abstract
We have shown that Anredera cordifolia extract improves learning and memory in a senescence-accelerated mouse model, and that α-linolenic acid (ALA)-rich Perilla frutescens seed oil (PO) improves brain function in healthy Japanese adults and elderly individuals. Herein, we present a 12-month, randomised, double-blind, parallel-armed intervention trial examining the effects of PO supplementation alone or in combination with A. cordifolia leaf powder on brain function in healthy elderly Japanese individuals. Participants were randomly divided into two groups: the PO group received 1.47 mL PO (0.88 g ALA) daily via soft gelatine capsules, and the POAC group received 1.47 mL PO and 1.12 g A. cordifolia leaf powder (1.46 mg vitexin and 1.12 mg adenosine) daily. After 12 months of intervention, the POAC group showed generally higher cognitive index scores than the PO group. The beneficial effects of combined supplementation on cognitive function were associated with increased ALA and eicosapentaenoic acid levels in red blood cell plasma membranes, increased serum biological antioxidant potential, and decreased serum triglyceride, glucose, and N-(epsilon)-carboxymethyl-lysine (CML), an advanced glycation end-product and biochemical marker of oxidative stress levels. The effects of combined supplementation on cognitive function also showed a significant negative correlation with serum CML levels after 12 months of intervention. Our findings suggest that combined long-term supplementation with PO and A. cordifolia more effectively ameliorates age-related cognitive decline than PO alone. These findings may serve as a basis for the development of new supplements for brain health. Clinical Trial Registry, UMIN000040863.
1. Introduction
Aging is characterised by a progressive decline in brain function, evidenced by impaired learning and memory, along with changes in mental health. Concurrently, neuronal plasticity and synaptic function begin to decline with age, affecting cognition.1,2 These neuronal dysfunctions are associated with a decrease in plasma antioxidant power and an increase in neuronal oxidative stress.3 Observational studies have suggested a wide variety of potentially modifiable risk factors for cognitive impairment and dementia4,5 as targets for preventive care. For example, cardiovascular risk factors and lifestyle choices, including diet, exercise, tobacco use, and coffee consumption have been shown to directly affect cognitive decline. Interestingly, several nutrients have been shown to influence neural cell function,6 and several randomised clinical trials have proposed nutritional interventions as preventive or therapeutic trials to slow the progression of cognitive impairment or reduce the risk of Alzheimer's disease in the elderly. Although many efforts have been made to elucidate the pathogenesis of neuronal dysfunction, few effective therapies and interventions exist to target aging.Several naturally derived supplements have been suggested to influence mental health and cognitive function.7–10 The basellaceous perennial Anredera cordifolia (AC) has been used as a medicinal plant in East Asia for centuries. In preclinical studies, AC extracts have been shown to exert neuroprotective effects associated with antioxidant and anti-inflammatory functions and to improve N-methyl-D-aspartate receptor antagonist MK-801-induced memory impairment in mice.11–15 We recently found that the administration of AC extract enhances learning and memory in senescence-accelerated mouse-prone 8 (SAMP8) mice.16 The treatment resulted in increased levels of neuronal plasticity-related proteins, and caused no noticeable side effects.16 These findings suggest that long-term supplementation of AC ameliorates the age-related cognitive and mental health decline in healthy elderly individuals. However, no intervention studies have verified the effects of AC on cognitive function in this group.
Another plant-based supplement for cognitive function, Perilla frutescens seed oil (PO), is characterised by high levels of α-linolenic acid (ALA, C18:3; 54–64% by weight), an essential ω-3 polyunsaturated fatty acid (PUFA).17 ALA exhibits anti-inflammatory18 and neuroprotective19 properties, and long-term administration has been shown to improve spatial learning, memory, and synaptic plasticity in aging rats.20 ALA is converted to other ω-3 PUFAs, such as eicosapentaenoic acid (EPA, C20:5) and docosahexaenoic acid (DHA, C22:6) by the rate-limiting enzymes Δ5- and Δ6-desaturases in animals.21 DHA is well-known for its beneficial effects on brain function.21,22 Our previous randomised, double-blind, placebo-controlled studies revealed that the administration of DHA-rich foods prevents cognitive decline in elderly Japanese individuals.23,24 However, in humans, the rate of metabolic conversion from ALA to EPA and DHA is very low.25 Therefore, it is important to investigate whether dietary ALA can supply EPA and DHA to various tissues. Interestingly, flavonoids have been reported to modulate the expression of Δ5- and Δ6-desaturases and improve the conversion of ALA to EPA and DHA.26–28 However, a controversy exists regarding the effects of flavonoids on such conversion.
We previously reported that the long-term supplementation of dietary PO has beneficial effects on psychological conditions such as apathy, and on age-related cognitive decline in healthy Japanese elderly individuals by enhancing the antioxidant potential.29 In addition, we found that the administration of PO combined with nobiletin-rich ponkan powder improved cognitive function in healthy elderly Japanese individuals.30 Because of the high flavonoid levels in AC, the combined supplementation with PO and AC leaf powder may have a greater beneficial effect on cognitive function than PO supplementation alone. However, to the best of our knowledge, there have been no interventional studies on the combined effects of PO and AC leaf powder on brain health in the elderly. It is also unclear whether AC promotes the conversion of ALA to EPA or DHA in humans. Therefore, we aimed to investigate whether (1) PO + AC have a synergistic effect on brain health and (2) AC promotes the conversion of ALA to EPA and DHA, resulting in improved cognitive function in the elderly. In this study, two types of soft gelatine capsules (SGCs) containing either PO alone or combined with AC leaf powder (POAC) were prepared as easy-to-consume dietary supplements to prevent dementia and ameliorate age-related cognitive decline. We conducted a 12-month, randomised, double-blind, interventional trial in healthy elderly Japanese individuals to compare the effects of the two supplements on cognitive function and health. Our findings present POAC treatment as a beneficial and easy-to-consume dietary supplement to prevent and maintain cognitive function in the elderly population.
2. Materials and methods
2.1. Participants and study design
This study was approved by the Shimane University Ethics Committee (Study No. 3194, 3497) and was performed in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. All volunteers provided written informed consent before participating in the study. The intervention trial was conducted between 2018 and 2020.Details of the study design, intervention, recruitment of subjects, enrolment, and randomisation have been recently described.30 A total of 32 healthy elderly volunteers (17 women, mean age 67.7 ± 1.1; 15 men, mean age 68.5 ± 1.6) living in Shimane Prefecture, Japan, were recruited for this 12-month, randomised, double-blind, parallel-armed study. The volunteers were subjected to physical (e.g., body weight and height, blood pressure, and waist circumference), clinical (hepatic and renal function, serum lipids, glucose, and haematological parameters), cognitive, and mental assessments. Additionally, they were asked to respond to a self-reported lifestyle questionnaire regarding their medical/drug history.
Volunteers with a total Mini-Mental State Examination (MMSE; see section 2.4) score of 23 or less; medical disorders, including respiratory, hepatic, renal, and/or cardiac disease; diabetes mellitus; endocrine, metabolic, or haematological diseases; allergies or hypersensitivity; or a history of any psychotropic drug or supplement use that might significantly affect the results of the study were excluded.
Two types of SGCs were produced by Sankyo Holdings Co. Ltd (Fuji, Japan). Considering the ease of swallowing one capsule, the maximum amount of PO per capsule was set at 0.098 mL (58.8 mg ALA). Therefore, one SGC contained 0.098 mL PO alone, and the other contained both 0.098 mL PO and 0.075 g AC leaf powder (0.098 mg of vitexin). The nutrient composition of the AC leaf powder and PO is shown in Tables 1 and 2, respectively.
| Proximate analysis | Mineral and materials | ||
|---|---|---|---|
| Nutritional values per 100 g of dry powder. Data on proximate analysis were obtained from Japan Food Research Laboratories (JFRL, Tokyo, Japan). | |||
| Energy (kcal) | 287 | Sodium (mg) | 464 |
| Protein (g) | 26.3 | ||
| Fat (g) | 6.1 | Vitexin (mg) | 130 |
| Carbohydrate (g) | 18.0 | Adenosine (mg) | 100 |
| Fiber (g) | 27.5 | Polyphenol (mg) | 1560 |
| Moisture (g) | 3.4 | ||
| Ash (g) | 18.7 | ||
| Proximate analysis | N = 5 | Fatty acids (g per 100 g) | N = 5 |
|---|---|---|---|
| Values are means ± SE. Nutritional values per 100 g of perilla seed oil (O-san Farm Co., Kawamoto, Shimane, Japan). Data on proximate analysis were obtained from the Shimane Institute for Industrial Technology (Matsue, Japan), and data on fatty acids and vitamin E were obtained from Japan Food Research Laboratories (JFRL, Tokyo, Japan). N, number of samples analysed. | |||
| Energy (kcal) | 931 ± 22 | Palmitic acid (C16:0) (g) | 5.8 ± 0.1 |
| Protein (g) | 0 | Stearic acid (C18:0) (g) | 2.1 ± 0.1 |
| Fat (g) | 101 ± 2 | Oleic acid (C18:1 ω-9) (g) | 13.4 ± 0.6 |
| Carbohydrate (g) | 0 | Linoleic acid (C18:2 ω-6) (g) | 13.2 ± 0.4 |
| Fiber (g) | 0 | α-Linolenic acid (C18:3 ω-3) (g) | 62.9 ± 1.5 |
| Moisture (g) | 0 | ||
| Ash (g) | 0 | Vitamin E (mg) | 67.8 (N = 1) |
Thirty-two participants were randomly divided into two groups, and each received 15 SGCs in three even doses each day for 12 months, between or immediately after meals. The PO group (n = 15) received 1.47 mL of PO daily, and the POAC group (n = 17) received both 1.47 mL of PO and 1.12 g of AC leaf powder daily. Group allocation was performed by stratified random assignment according to the total MMSE score, sex, and age, as described previously.31–33 Randomised code lists were generated by the medical statistics advisor, and the investigators, participants, and sponsors were blinded to these codes. Neither the participants nor the researchers knew which capsules were consumed. Prior to the intervention trial, a blind sensory test was performed to confirm that no differences existed in the appearance or taste of the SGCs (data not shown).
2.2. Anthropometry, body composition, and intake analysis
The height, body weight, and waist circumference of all participants were measured. Body composition was determined using a bioelectrical impedance analyser, WB-150 (Tanita Co., Tokyo, Japan).To determine the interactions of PO or POAC supplementation with health status and lifestyle, all participants were asked to self-report their daily SCG intake and health/mental status during the entire 12-month intervention trial. Dietary intake before and after the trial was estimated using a brief-type self-administered diet history questionnaire (BDHQ) designed and validated for the Japanese population.34
2.3. Blood sampling
Before and after the trial, blood samples were collected either in the morning or afternoon, after confirming that the participants had not eaten breakfast or lunch. Blood samples were separated into serum and erythrocyte (red blood cell, RBC) aliquots by centrifugation. RBC samples were collected to monitor the fatty acid profiles of plasma membranes (RBC-PMs). Fresh serum samples were used to measure blood biochemistry, as well as N-(epsilon)-carboxymethyl-lysine (CML), the biological antioxidant potential (BAP), and brain-derived neurotrophic factor (BDNF) levels. The RBC and serum samples were stored at −80 °C within 8 h of collection.2.4. Cognitive function and mental health
Cognitive function was evaluated using three previously described methods.30 The first involved Hasegawa's Dementia Scale-Revised (HDS-R),35 which comprises nine simple questions and has been widely accepted for epidemiological screening of cognitive dysfunction in Asian populations. The second, the MMSE test,36 is commonly used to assess cognitive impairments associated with dementia status, especially in patients with Alzheimer's disease or mild cognitive impairment (MCI). The third method used the Japanese version of the Montreal Cognitive Assessment (MoCA-J),37 a reliable and valid cognitive screening test designed to assist health professionals in detecting MCI.Apathy and depression were assessed using the Japanese version of the apathy scale38 and the Zung Self-Rating Depression Scale (SDS),39 respectively. The apathy scale was developed as a tool for measuring apathy resulting from brain-related pathology, and the SDS is an established norm-referenced screening measure used worldwide to identify the presence of depressive symptoms in adults.38,39
All tests were performed before treatment (baseline) and again after the 12-month intervention.
2.5. Blood biochemical analysis, fatty acid profile, and apolipoprotein E (APOE) genotyping
Serum biochemical analyses included the evaluation of gamma-glutamyl transpeptidase, alanine aminotransferase, aspartate aminotransferase, albumin, total cholesterol, blood urea nitrogen, triglyceride (TG), creatinine, blood sugar, and high- and low-density lipoprotein cholesterol levels using a BiOLis 24i automatic analyser (Tokyo Boeki Medisys, Tokyo, Japan). Haemoglobin A1c (HbA1c) levels in blood were determined using a commercially available kit (MetaboLead HbA1c, TFB Inc., Tokyo, Japan).Haematological analyses included RBC, white blood cell, and platelet counts, as well as the evaluation of haemoglobin, mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), and mean corpuscular haemoglobin concentration (MCHC) using an automated haematology analyser, XS-1000i (Sysmex Corporation, Kobe, Japan).
Serum CML levels were measured using a CircuLex CML/Nε-(carboxymethyl) lysine ELISA kit (MBL Ltd, Tokyo, Japan). Serum was diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 in the included dilution buffer and the enzymatic reaction was stopped after 5 min. Serum CML concentrations were calculated using SoftMax Pro software (Molecular Devices).40 Serum BAP levels,29,30,41 RBC-PM fatty acid profiles,23,42 and (APOE) gene statuses24 were measured as previously described.30
4 in the included dilution buffer and the enzymatic reaction was stopped after 5 min. Serum CML concentrations were calculated using SoftMax Pro software (Molecular Devices).40 Serum BAP levels,29,30,41 RBC-PM fatty acid profiles,23,42 and (APOE) gene statuses24 were measured as previously described.30
2.6. Statistical analysis
Per-protocol analysis was performed as previously described.30 Data distribution was assessed by the Shapiro–Wilk test. Comparisons between the baseline and 12-month values for each group were assessed by paired t-tests or the Wilcoxon signed-rank tests. Comparisons between the two groups were performed by the independent t-test or Mann–Whitney U-test. Allelic distribution of the APOE gene was analysed by the Pearson chi-square test. Analysis of covariance was used to compare the differences between groups regarding cognitive outcomes and serum BDNF, CML, and BAP levels. Pearson partial correlation coefficients were used to assess associations between cognitive outcome (MoCA-J “total” and subscale “language”) scores and serum CML levels, and Spearman partial correlation coefficients were used to assess associations between changes (Δ) in MoCA-J total scores, and in ΔBAP and ΔRBC-PM ALA levels. Analyses were adjusted for age, sex, BMI, and educational level of the participants. All analyses were performed using PASW Statistics software (version 23.0, SPSS Inc., Chicago, IL, USA). All statistical tests were two-tailed, and significance was set at p < 0.05.3. Results
3.1. Demographic and clinical characteristics and assessment of dietary intake
Thirty-one participants completed the full 12-month intervention (Fig. 1). The one participant who dropped out of the study did so voluntarily, and not due to adverse effects, but due to moving to another area where he could not continue the intervention trial. Based on self-administration records, the protocol adherence of the final 31 participants to the study during 12 months was 93.4 ± 2.8% for the PO group and 94.5 ± 2.4% for the POAC group.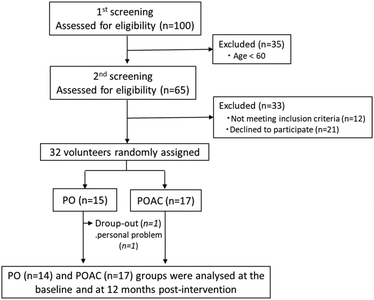 | ||
| Fig. 1 Flow diagram of participant selection. PO, perilla seed oil group; POAC, PO and Anredera cordifolia leaf powder group. | ||
No remarkable differences were observed between the PO and POAC groups regarding the general questionnaire on medical/medication history and lifestyle habits before or after the trial. The participants did not report adverse effects such as stomach irritation, palpitations, or allergic reactions that influenced their daily lives. No significant differences were observed between the groups regarding the baseline anthropometry, blood pressure, blood biochemical parameters, or haematological parameters (Table 3).
| PO (n = 14) | POAC (n = 17) | Change (12 months-baseline) | ||||
|---|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | PO | POAC | |
| Values are means ± SE. Significant differences from the baseline values, **p < 0.05, 0.05 < *p < 0.1. Significant differences from the PO group, ##p < 0.05. BUN, Blood urea nitrogen; GOT, glutamate oxaloacetate transaminase; GPT, glutamic pyruvic transaminase; γ-GTP, γ-glutamyl transpeptidase; HbA1c, haemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; MCV, mean corpuscular volume; NGSP, national glycohemoglobin standardization program; PO, perilla seed oil group; POAC, PO and Anredera cordifolia leaf powder group; RBC, red blood cell; WBC, white blood cell. | ||||||
| Anthropometry | ||||||
| Sex (male/female) | 14 (7/7) | — | 17 (8/9) | — | ||
| Age (years) | 68.9 ± 1.4 | 70.2 ± 1.4 | 67.0 ± 1.2 | 68.1 ± 1.2 | ||
| Height (cm) | 157.2 ± 1.9 | — | 158.4 ± 2.6 | — | ||
| Body Weight (kg) | 54.3 ± 2.6 | 54.1 ± 2.6 | 58.2 ± 3.3 | 58.2 ± 3.3 | −0.3 ± 0.3 | 0.0 ± 0.3 |
| Body Mass Index (kg m−2) | 21.9 ± 0.8 | 21.8 ± 0.8 | 22.9 ± 0.7 | 22.9 ± 0.7 | −0.1 ± 0.1 | 0.0 ± 0.2 |
| Waist Circumference (cm) | 81.5 ± 2.2 | 80.7 ± 2.1 | 84.6 ± 2.2 | 83.5 ± 1.9 | −0.8 ± 0.4 | −1.0 ± 0.5 |
| Body fat (%) | 26.1 ± 1.6 | 25.8 ± 1.6 | 28.1 ± 1.4 | 28.2 ± 1.4 | −0.4 ± 0.5 | 0.1 ± 0.4 |
| Blood pressure (BP) | ||||||
| Systolic BP (mmHg) | 146 ± 7 | 141 ± 6 | 141 ± 6 | 143 ± 5 | −4 ± 4 | 2 ± 6 |
| Diastolic BP (mmHg) | 90 ± 5 | 86 ± 4 | 82 ± 3 | 84 ± 4 | −4 ± 3 | 1 ± 3 |
| Education | ||||||
| ≦12 years (%) | 9 (64.3) | 10 (58.8) | ||||
| >12 years (%) | 5 (35.7) | 7 (41.2) | ||||
| Blood biochemistry | ||||||
| GOT (U L−1) | 24.9 ± 1.4 | 23.4 ± 0.8 | 23.5 ± 1.4 | 23.8 ± 1.6 | −1.5 ± 1.0 | 0.4 ± 0.7 |
| GPT (U L−1) | 20.5 ± 1.5 | 18.4 ± 1.6 | 20.8 ± 2.2 | 20.8 ± 2.6 | −2.1 ± 1.4 | 0.0 ± 0.7 |
| γ-GTP (IU L−1) | 23.7 ± 3.3 | 23.0 ± 3.1 | 38.4 ± 9.8 | 31.6 ± 5.0 | −0.7 ± 0.8 | −6.8 ± 5.8 |
| Albumin (g dL−1) | 4.2 ± 0.07 | 4.5 ± 0.07** | 4.3 ± 0.09 | 4.3 ± 0.11 | 0.21 ± 0.06 | −0.01 ± 0.10 |
| Total cholesterol (mg dL−1) | 218.7 ± 8.6 | 223.8 ± 10.0 | 205.6 ± 7.5 | 204.6 ± 7.1 | 5.1 ± 6.5 | −1.0 ± 7.0 |
| Triglyceride (mg dL−1) | 117.1 ± 11.5 | 90.9 ± 9.8* | 108.6 ± 6.9 | 79.9 ± 4.7** | −26.2 ± 13.0 | −28.8 ± 5.5 |
| Blood urea nitrogen (mg dL−1) | 16.2 ± 0.8 | 18.6 ± 1.0** | 16.0 ± 0.8 | 17.1 ± 1.0 | 2.4 ± 0.7 | 1.1 ± 0.7 |
| Creatinine (mg dL−1) | 0.7 ± 0.03 | 0.8 ± 0.03** | 0.8 ± 0.05 | 0.8 ± 0.06 | 0.03 ± 0.01 | −0.01 ± 0.02 |
| Blood sugar (mg dL−1) | 106.5 ± 7.8 | 90.6 ± 2.0** | 110.4 ± 4.1 | 102.2 ± 2.1** | −15.9 ± 7.1 | −8.1 ± 3.5 |
| HDL-C (mg dL−1) | 70.0 ± 4.5 | 69.4 ± 4.3 | 74.8 ± 4.3 | 73.6 ± 3.9 | −0.6 ± 2.5 | −1.2 ± 2.6 |
| LDL-C (mg dL−1) | 129.1 ± 6.7 | 135.7 ± 8.1 | 112.1 ± 6.1 | 114.4 ± 5.7 | 6.6 ± 5.5 | 2.2 ± 5.7 |
| HbA1c (NGSP) (%) | 5.6 ± 0.08 | 5.6 ± 0.09 | 5.6 ± 0.06 | 5.6 ± 0.08 | −0.04 ± 0.04 | −0.05 ± 0.05 |
| Haematological parameters | ||||||
| WBC (×103 μL−1) | 5.9 ± 0.6 | 5.8 ± 0.5 | 5.6 ± 0.3 | 5.5 ± 0.3 | −0.09 ± 0.3 | −0.12 ± 0.2 |
| RBC (×104 μL−1) | 442.9 ± 11.7 | 448.2 ± 12.0 | 439.3 ± 7.6 | 442.2 ± 10.2 | 5.2 ± 4.8 | 2.9 ± 4.8 |
| Haemoglobin (g dL−1) | 13.7 ± 0.3 | 13.9 ± 0.2 | 13.8 ± 0.3 | 13.9 ± 0.3 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| Haematocrit (%) | 41.3 ± 0.8 | 41.5 ± 0.7 | 41.3 ± 0.7 | 41.0 ± 0.9 | 0.2 ± 0.5 | −0.3 ± 0.4 |
| Platelet (×104 μL−1) | 21.3 ± 1.7 | 21.0 ± 1.7 | 21.0 ± 0.7 | 22.5 ± 1.5 | −0.3 ± 0.8 | 1.6 ± 1.5 |
| MCV (fL) | 93.5 ± 1.1 | 93.0 ± 1.2* | 94.1 ± 0.9 | 92.7 ± 0.9** | −0.5 ± 0.3 | −1.4 ± 0.2## |
| MCH (pg) | 31.1 ± 0.4 | 31.1 ± 0.4 | 31.4 ± 0.5 | 31.4 ± 0.5 | −0.0 ± 0.2 | −0.0 ± 0.1 |
| MCHC (g dL−1) | 33.2 ± 0.3 | 33.4 ± 0.2 | 33.3 ± 0.2 | 33.8 ± 0.2** | 0.2 ± 0.2 | 0.5 ± 0.1 |
The frequency of APOE2/2, APOE2/3, or APOE3/3 genotypes was 78.6% (11/14) in the PO group and 64.7% (11/17) in the POAC group, and that of APOE2/4 or APOE3/4 genotypes was 21.4% (3/14) in the PO group and 35.3% (6/17) in the POAC group. The APOE4/4 genotype was not detected in either group. These results indicated that significant differences in the distribution of APOE alleles were not observed between the two groups (p > 0.05).
The average dietary nutritional intake based on BDHQ reports was not significantly different between the baseline and 12-month responses for either the PO or POAC group (p > 0.05, Supplement 1). No overall change was detected for either group (Supplement 1), indicating that the intake of these supplements did not influence nutritional intake during the interventional trial.
When compared to the baseline levels, the serum albumin (p = 0.003), blood urea nitrogen (p = 0.005), and creatinine (p = 0.049) levels were significantly increased, and the blood sugar levels were significantly (p = 0.044) decreased in the PO group after 12 months of treatment, and serum TG levels (p = 0.064) tended to decrease over time (Table 3). Similarly, serum TG (p < 0.001), blood sugar (p = 0.035), and MCV (p < 0.001) levels were significantly lower, and MCHC levels were significantly higher (p = 0.001) in the POAC group at 12 months than at the baseline. The remaining parameters were unchanged in both the PO and POAC groups (Table 3). The mean change in the MCV levels was lower in the POAC group than in the PO group, and mean changes in the remaining parameters showed no significant differences between the two groups after 12 months of treatment.
Although serum TG, blood sugar, MCV, and MCHC levels at 12 months showed significant changes compared to the baseline levels in both groups, values remained within clinical standard ranges. Taken together, these results show that no adverse events were reported, and that neither PO alone nor POAC treatment altered lipid metabolism, hepatic or renal function, or haematopoiesis, suggesting the safety of long-term administration.
3.2. Cognitive function and mental health assessment
The mean HDS-R, MMSE, and MoCA-J total scores of the final 31 participants at the baseline of the interventional trial were (28.5 ± 0.2)/30, (28.8 ± 0.2)/30, and (25.8 ± 0.5)/30, respectively. After 12 months of treatment, the MoCA-J total scores were significantly increased in the POAC group (p = 0.049), but remained unchanged in the PO group (Table 4). Neither HDS-R nor MMSE total scores showed a significant change after 12 months of PO or POAC treatment.| PO (n = 14) | POAC (n = 17) | Change (12 months-baseline) | ||||
|---|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | PO | POAC | |
| Values are means ± SE. Significant differences from the baseline values, **p < 0.05, 0.05 < *p < 0.1. Tendency of significant differences from the PO, 0.05 < #p < 0.1. HDS-R, Hasegawa's dementia scale-revised; MMSE, mini-mental state examination; MoCA-J, Japanese version of Montreal cognitive assessment; PO, perilla seed oil group; POAC, PO and Anredera cordifolia leaf powder group. SDS, self-rating depression scale; SMRT, short-term memory recall task. | ||||||
| Cognitive index | ||||||
| MMSE | ||||||
| Total | 28.8 ± 0.3 | 29.2 ± 0.4 | 28.8 ± 0.3 | 29.2 ± 0.3 | 0.4 ± 0.5 | 0.5 ± 0.4 |
| HDS-R | ||||||
| Total | 28.6 ± 0.3 | 28.7 ± 0.4 | 28.5 ± 0.3 | 29.0 ± 0.3 | 0.1 ± 0.5 | 0.5 ± 0.4 |
| Subitem “recalling 5 objects” | 4.86 ± 0.10 | 4.93 ± 0.07 | 4.59 ± 0.15 | 4.88 ± 0.12* | 0.07 ± 0.13 | 0.29 ± 0.17 |
| MoCA-J | ||||||
| Total | 25.9 ± 0.7 | 26.8 ± 0.9 | 25.8 ± 0.8 | 27.5 ± 0.7** | 0.9 ± 0.7 | 1.8 ± 0.8 |
| Subscale “SMRT” | 2.50 ± 0.53 | 3.43 ± 0.45* | 2.59 ± 0.42 | 3.29 ± 0.45 | 0.93 ± 0.54 | 0.71 ± 0.49 |
| Subscale “language” | 4.00 ± 0.15 | 4.07 ± 0.22 | 4.06 ± 0.20 | 4.71 ± 0.11** | 0.07 ± 0.20 | 0.65 ± 0.21# |
| Emotional index | ||||||
| SDS | 35.0 ± 2.2 | 33.0 ± 1.6 | 35.5 ± 2.0 | 33.9 ± 1.7 | −2.0 ± 1.7 | −1.6 ± 1.6 |
| Apathy | 9.6 ± 1.5 | 9.6 ± 1.3 | 11.9 ± 1.2 | 12.5 ± 1.2 | 0.0 ± 0.9 | 0.6 ± 0.9 |
When analysing the scores for the HDS-R and MMSE sub-items and the MoCA-J subscales, the MoCA-J subscale “language” scores in the POAC group showed a significant increase over the 12 months (p = 0.007). The HDS-R sub-item “SMRT” score in the PO group and the “recalling 5 objects” score in the POAC group tended to increase over the 12 months (p = 0.100 and 0.096, respectively) (Table 4). The mean change in the MoCA-J subscale “language” score tended to be higher in the POAC group than in the PO group (p = 0.100) (Table 4). The remaining sub-item and subscale scores were not significantly different before and after intervention in either group.
No significant differences were observed between the baseline and 12-month apathy or SDS scores in either group (Table 4).
3.3. Fatty acid profiles of RBC-PMs
In the PO group, the RBC-PM ALA levels significantly increased from the baseline to 12 months (p < 0.001), whereas the levels of both lignoceric (p = 0.001) and nervonic (p < 0.001) acids significantly decreased; DHA and remaining fatty acid levels remained unchanged (Table 5). Similarly, in the POAC group, ALA (p = 0.001), EPA (p = 0.004), and docosapentaenoic acid (p = 0.01) levels were significantly higher at 12 months than at the baseline. Arachidonic acid levels were generally higher after treatment (p = 0.074), whereas lignoceric (p = 0.01) and nervonic (p = 0.01) acid levels were significantly lower. DHA and the remaining fatty acid levels did not change over time.| PO (n = 14) | POAC (n = 17) | Change (12 months – baseline) | ||||
|---|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | PO | POAC | |
| Values are means ± SE. Significant differences from the baseline values, **p < 0.05, 0.05 < *p < 0.1. AA: arachidonic acid; ALA: α-linolenic acid; C24:0: lignoceric acid; C24:1: nervonic acid; DHA: docosahexaenoic acid; DPA: docosapentaenoic acid; EPA: eicosapentaenoic acid; LLA: linoleic acid; OLA: oleic acid; PLA: palmitic acid; PO, perilla seed oil group; POAC, PO and Anredera cordifolia leaf powder group; STA: stearic acid. | ||||||
| PLA (C16:0) | 22.5 ± 0.42 | 22.4 ± 0.34 | 21.9 ± 0.26 | 21.8 ± 0.29 | −0.1 ± 0.45 | −0.1 ± 0.27 |
| STA (C18:0) | 17.1 ± 0.25 | 16.7 ± 0.36 | 17.3 ± 0.42 | 17.4 ± 0.53 | −0.4 ± 0.45 | 0.1 ± 0.65 |
| OLA (C18:1 ω-9) | 13.0 ± 1.43 | 15.3 ± 0.44 | 15.2 ± 0.29 | 15.2 ± 0.27 | 2.3 ± 1.39 | −0.0 ± 0.29 |
| LLA (C18:2 ω-6) | 12.9 ± 0.35 | 12.5 ± 0.39 | 12.2 ± 0.35 | 11.9 ± 0.36 | −0.4 ± 0.44 | −0.3 ± 0.25 |
| ALA (C18:3 ω-3) | 0.23 ± 0.01 | 0.32 ± 0.01** | 0.22 ± 0.02 | 0.29 ± 0.01** | 0.08 ± 0.01 | 0.07 ± 0.02 |
| AA (C20:4 ω-6) | 13.4 ± 0.45 | 13.2 ± 0.40 | 13.2 ± 0.29 | 13.8 ± 0.26* | −0.2 ± 0.48 | 0.6 ± 0.29 |
| EPA (C20:5 ω-3) | 1.9 ± 0.14 | 2.1 ± 0.20 | 1.7 ± 0.11 | 2.0 ± 0.13** | 0.1 ± 0.17 | 0.3 ± 0.08 |
| DPA (C22:5 ω-3) | 1.8 ± 0.04 | 1.8 ± 0.06 | 1.6 ± 0.06 | 1.7 ± 0.05** | 0.0 ± 0.05 | 0.1 ± 0.04 |
| C24:0 | 4.8 ± 0.13 | 4.2 ± 0.12** | 4.5 ± 0.13 | 4.2 ± 0.11** | −0.5 ± 0.12 | −0.3 ± 0.11 |
| DHA (C22:6 ω-3) | 8.2 ± 0.27 | 7.8 ± 0.25 | 8.0 ± 0.23 | 8.0 ± 0.21 | −0.4 ± 0.25 | 0.1 ± 0.20 |
| C24:1 | 3.8 ± 0.09 | 3.3 ± 0.09** | 3.7 ± 0.13 | 3.3 ± 0.10** | −0.5 ± 0.09 | −0.3 ± 0.11 |
| ω-6/ω-3 | 2.2 ± 0.08 | 2.2 ± 0.11 | 2.3 ± 0.10 | 2.2 ± 0.08 | 0.0 ± 0.10 | −0.1 ± 0.06 |
The mean changes in the RBC-PM fatty acid profiles were not significantly different between the two groups (Table 5).
3.4. Serum BAP, BDNF, and CML levels
No noticeable differences were observed between groups in the baseline serum BAP, BDNF, or CML levels (Table 6). The BAP levels significantly increased over 12 months in both the PO (p = 0.004) and POAC (p = 0.008) groups, whereas serum BDNF levels remained unchanged in both groups. The CML levels significantly increased over time in the PO group (p = 0.009) and tended to decrease in the POAC group (p = 0.076).| PO (n = 14) | POAC (n = 17) | Change (12 months-baseline) | ||||
|---|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | PO | POAC | |
| Values are means ± SE. Significant differences from the baseline values, **p < 0.05, 0.05 < *p < 0.1. Significant differences from the PO, ##p < 0.05. BAP, biological antioxidant potential; CML, N-(epsilon)-carboxymethyl-lysine; BDNF, brain-derived neurotrophic factor; PO, perilla seed oil group; POAC, PO and Anredera cordifolia leaf powder group. | ||||||
| BAP (μmol L−1) | 2759 ± 41 | 2964 ± 46** | 2820 ± 59 | 3007 ± 41** | 205 ± 58 | 187 ± 61 |
| CML (μg mL−1) | 9.5 ± 0.6 | 10.3 ± 0.4** | 10.1 ± 0.5 | 9.5 ± 0.4* | 0.8 ± 0.3 | −0.6 ± 0.3## |
| BDNF (pg mL−1) | 4104 ± 513 | 4398 ± 621 | 3616 ± 346 | 4083 ± 405 | 294 ± 399 | 466 ± 293 |
The mean change in serum CML levels over 12 months was significantly lower (p = 0.004) in the POAC group than in the PO group, whereas the mean changes in BAP and BDNF were not significantly different between the two groups (Table 6).
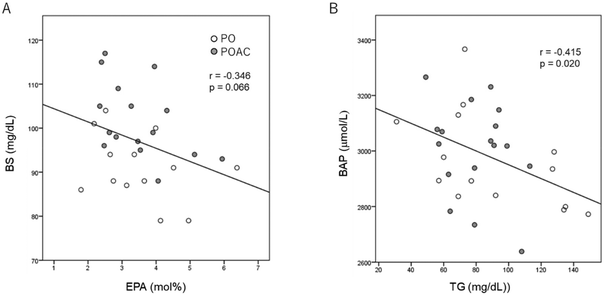
3.5. Correlation between cognitive function scores, serum characteristics, and serum BAP and CML levels
After 12 months of intervention, serum blood sugar levels tended to be negatively correlated (Fig. 2A) with RBC-PM EPA levels (r = −0.346, p = 0.066). At 12 months, serum BAP levels were negatively correlated (r = −0.415, p = 0.02) with serum TG levels (Fig. 2B). When adjusted for age, sex, and BMI, the MoCA-J total scores (r = −0.384, p = 0.043) and the MoCA-J subscale “language” scores (r = −0.397, p = 0.037) showed significantly negative correlations with serum CML levels (Table 7). Additionally, ΔMoCA-J total scores were positively associated with ΔRBC-PM ALA levels (r = 0.416, p = 0.038), whereas no significant correlations were observed between ΔMoCA-J subscale “language” scores and ΔRBC-PM ALA levels.| Associated variables | Coefficient r | p | |
|---|---|---|---|
| Adjusted by age, sex, BMI and education levels.a Values at the end of the study.b Differences between the extent of change (12-month-baseline values). CML, N-(epsilon)-carboxymethyl-lysine; ALA, α-linolenic acid; RBC-PM, red-blood cell plasma membrane. | |||
| Moca-J total | CMLa | −0.384 | 0.043 |
| RBC-PM ALAb | 0.416 | 0.038 | |
| Moca-J language | CMLa | −0.397 | 0.037 |
| RBC-PM ALAb | 0.099 | 0.630 | |
4. Discussion
In this study, we showed that a 12-month intervention with combined PO and AC leaf powder supplementation improved certain parameters of age-related cognitive decline in healthy elderly Japanese individuals more effectively than PO alone. This improvement was associated with an increase in RBC-PM ALA and EPA levels and serum BAP levels, as well as a decrease in serum TG, glucose, and CML levels. To the best of our knowledge, this is the first long-term, double-blind, randomised, parallel-armed study to demonstrate the combined effects of ALA-rich PO and AC leaf powder on cognitive ability and blood biochemical parameters in healthy elderly Japanese individuals.Participant compliance in consuming 15 SGCs daily for 12 months was excellent. This high compliance was evidenced by increased RBC-PM ALA levels in both the PO and POAC groups (Table 5), indicating the effective incorporation of ω-3 PUFAs into RBC-PMs by PO ingestion. This result is consistent with those of our recent intervention,29 in which the dietary intake of 7 mL of PO daily for 12 months increased RBC-PM ALA levels in association with ameliorated cognitive decline in healthy Japanese elderly participants. Similarly, a prospective study in Japan reported that serum ALA levels, but not EPA or DHA levels, showed a negative correlation with the risk of dementia.43 ALA is thought to enhance neuroprotection and brain plasticity.44 Interestingly, however, the increased RBC-PM ALA levels seen in the present study did not appear to affect cognitive performance in participants (Table 4). Similarly, it has been reported that PO does not influence cognitive performance in participants with mild to moderate dementia.45 The exact reason why the PO group in this study did not show the same changes in cognitive performance seen in our previous study29 remains unclear. However, the discrepancy may be related to the differences in PO dosage between the two interventions. The amount of PO administered in this study was 1.47 mL daily, compared to 7 mL daily in the previous study. The dose was reduced due to logistical reasons, as detailed in a recent article.30
AC contains bioactive compounds, such as flavonoids, saponins, tannins, and terpenoids.46 Flavonoids and saponins contain many bioactive substances, though it is not known which contribute to reducing cognitive decline. It has also been reported that adenosine and its derivative, cordysinin B (2′-O-methyl adenosine), abundant in AC, may be involved in cognitive improvement by activating the signalling pathway associated with memory formation (i.e., cAMP/PKA/CREB-pathway).47 Previous studies have suggested a link between flavonoid intake and cognitive function in humans and animals.48 Similarly, saponins have been reported to prevent synaptic loss and reverse learning-memory deficits in APP/PS1 transgenic mice, suggesting an improvement of cognitive function.49 It has been reported that AC extract administration improved MK-801-induced memory impairment in mice.15 Moreover, we recently reported that AC extract administration enhanced the levels of neuronal plasticity-related proteins such as hippocampal brain-derived neurotrophic factor (BDNF), PSD95, and NR2A in SAMP8 mice, suggesting benefits for learning and memory.16 After 12 months of treatment, the MoCA-J total scores and subscale “language” scores had significantly increased compared to the baseline levels in the POAC group, but not in the PO group (Table 4). Taken together, these findings suggest that POAC supplementation is more effective for reducing age-related cognitive decline than PO supplementation alone. Further studies are necessary to clarify the bioactive compounds of interest in AC leaves.
The brain is sensitive to changes in oxidative balance, and age-related declines in memory and cognition are associated with reduced plasma antioxidative power and increased oxidative stress.3 Cognitive impairment is remarkably correlated with oxidative damage, suggesting that enhancing antioxidant abilities can defend cognitive function in the elderly.3 In this study, serum BAP levels, which reflect the total antioxidant power,50 significantly increased compared to the baseline values after 12 months of both PO and POAC treatment (Table 6). We recently reported that dietary PO supplementation for 12 months enhances cognitive ability in healthy elderly Japanese individuals in association with raised serum BAP levels. This supports the idea that dietary antioxidants prevent cognitive decline in the elderly.29 PO contains vitamin E, a well-known antioxidant (Table 2), and AC leaves exhibit antioxidant activity via flavonoids and saponins.51,52 These compounds likely contributed to the increased serum BAP seen in both the PO and POAC groups. Furthermore, the AC leaf powder used in this study contained large amounts vitexin (Table 1). Vitexin has a variety of beneficial effects, including antioxidant, anti-inflammatory, and neuroprotective properties, and functions as an antioxidant in oxidative stress-related diseases, including memory impairment, cerebral ischaemia, and neurotoxicity.53 The difference in cognition-related outcomes between the PO and POAC groups observed in this study may have occurred due to the flavonoids, saponins, and polyphenols in AC leaf powder.
The formation of advanced glycation end-products, the irreversible end-products of non-enzymatic glycation, has been linked to neuronal diseases such as vascular dementia and Alzheimer's disease.54 CML is a well-studied advanced glycation end-product55 and biochemical marker of oxidative stress to represent early pathological changes in the brain during Alzheimer's disease.56 Ahmed et al.57 reported that cerebrospinal fluid CML levels were significantly higher in patients with Alzheimer's disease than in age-matched control individuals, with a clear link to cognitive impairment. Zhang et al.58 reported a negative correlation between plasma CML and cognitive function scores in older patients with type 2 diabetes and MCI, suggesting an important regulatory role of CML in the manifestation of diabetic cognitive impairment. In this study, serum CML levels after the 12-month interventional trial were significantly decreased in the POAC group, but increased in the PO group compared to the respective baseline values (Table 6). POAC supplementation also significantly increased the MoCA-J total scores and subscale “language” scores after 12 months of intervention, whereas the administration of PO alone did not affect these parameters (Table 4). Significant negative correlations were observed between serum CML levels and both MoCA-J total and “language” scores (Table 7). In addition, the mean changes in the MoCA-J total scores were positively correlated with the mean changes in RBC-PM ALA levels (Table 7). These results suggest that POAC supplementation may have improved the cognitive function in the elderly by reducing the CML levels. However, the reason why serum CML levels increased after PO intake alone remains to be explained, as this was not observed in previous studies.
ALA is a precursor molecule for EPA and DHA, which are thought to prevent cognitive decline with aging.59 However, their conversion ability in humans is low, with only 0.1–21% of ALA converted to EPA and 0.1–0.9% to DHA.25 In this study, the administration of ALA-rich PO for 12 months significantly increased the RBC-PM ALA, but not EPA or DHA, levels in participants (Table 5). This result was consistent with that of Hamazaki et al.,60 who reported that dietary PO administration did not influence EPA or DHA levels in the human serum phospholipid fraction. Similarly, we previously reported29 that dietary PO intake (7 mL daily) for 12 months did not change the RBC-PM EPA or DHA levels in healthy elderly Japanese participants. In contrast, our recent report30 showed that the administration of 1.47 mL of PO daily for 12 months significantly increased RBC-PM ALA and EPA levels in healthy elderly Japanese participants. This may be related to the difference in the number of participants (14 vs. 21 subjects), since this and the previous30 intervention trial were conducted at about the same time, and the participants were healthy elderly volunteers living in the same local area of Shimane Prefecture in Japan. If the number of participants increased, the RBC-PM EPA levels at 12 months may have increased in the PO group. In the present study, the administration of POAC, as opposed to PO alone, significantly increased RBC-PM ALA and EPA, but not DHA, levels in participants (Table 5). Generally, the RBC-PM EPA levels will increase if the levels of both the desaturation and elongation enzymes of the hepatic tissues increase upon AC administration, and/or if intestinal EPA absorption increases. Along with aging, sex, body weight, consumption of alcohol, smoking status, and genetics,61 research suggests that dietary polyphenols may influence the desaturase/elongase enzymes controlling the EPA or DHA concentration. Some studies with rodents and humans have suggested that polyphenols increase the levels of EPA or DHA, probably by stimulating the activities of hepatic Δ5 and/or Δ6 desaturases.28,62–65 Vauzour et al.66 reported that anthocyanins, polyphenols abundant in plants, do not influence the levels of EPA, mRNA of fatty acid synthesis genes (Fads1/2), or fatty acid synthesis proteins, such as Δ5- and Δ6-desaturases. Moreover, resveratrol, a stilbenoid polyphenol found in the skin and seeds of grapes,67 was found to decrease EPA levels with concurrent reduction or null effects on the mRNA levels of Δ5 and Δ6 desaturases. These reports thus suggest that in vitro effects of polyphenols do not reflect the in vivo effects mentioned above. However, the differences in the bioavailability of polyphenols in the body and the concentration of polyphenols used in these in vitro studies may be attributed to these discrepancies regarding the effects of polyphenols. The expression of Δ5- or Δ6-desaturase and elongase enzymes is regulated by transcription factors, such as peroxisome proliferator-activated receptor α (PPAR-α), sterol regulatory element binding protein 1c (SREBP-1c), retinoid X receptor (RXR), and carbohydrate response element binding protein (ChREBP);68,69 the effects of polyphenols on these regulatory proteins need to be clearly delineated. Whatever the exact mechanism, consistent with the in vivo reports is as mentioned above,62–65 POAC supplementation likely increased the RBC-PM EPA levels more than PO supplementation alone due to the flavonoids contained in AC.
Epidemiological studies have reported an association between serum BDNF levels and cognitive function,70,71 though the results have been inconsistent. The administration of EPA and DHA improved cognitive impairment and increased serum BDNF levels in a rat model of Alzheimer's disease.72 Furthermore, we recently found that the administration of AC extract enhanced learning and memory in association with increased hippocampal BDNF levels in SAMP8 mice.16 Håkansson et al.73 reported that a physical exercise-induced increase in serum BDNF levels was correlated with improved cognitive performance, concluding that the production of peripheral BDNF reflects the availability of BDNF in the brain. These results suggest that changes to serum BDNF levels in elderly individuals are associated with a decline in cognition during aging. In this study, however, neither PO nor POAC supplementation had an effect on serum BDNF levels in healthy elderly participants after 12 months. Further studies are needed to elucidate the relationship between serum BDNF levels and cognitive ability under PO and/or POAC treatment.
Several observational studies have suggested that cardiovascular risk factors such as hypertriglycaemia and hyperglycaemia are potential risk factors for the onset and/or progression of MCI and dementia.4 In the present study, serum TG and glucose levels significantly decreased in both the PO and POAC groups after 12 months of treatment (Table 3). This reduction in serum TG levels was associated with an increase in the RBC-PM EPA levels in the POAC group, but not in the PO group (Table 3). Many clinical studies have shown that ω-3 PUFAs, particularly EPA, lower TG levels. The administration of icosapent ethyl, a high-purity form of EPA, for 12 weeks significantly increased the RBC EPA levels and was associated with TG reduction in patients with hypertriglyceridaemia.74,75 Similarly, Egert et al.76 reported that serum TG levels significantly decreased after ALA intervention in normolipidemic individuals. Furthermore, Yamanaka et al.77 reported in a randomised, double-blind, controlled crossover study that a single oral ingestion of ALA-rich diacylglycerol oil suppressed postprandial serum TG levels in humans. These results suggest that the decrease in serum TG levels observed in both the PO and POAC groups after 12 months of treatment was caused by the increased ALA intake. However, no significant relationship was observed between serum TG levels and RBC-PM ALA levels in this study. Further studies are needed to elucidate the connection between ALA and serum TG levels as they relate to dementia and MCI.
AC leaves are traditionally used in Indonesia to reduce blood glucose.78,79 The AC leaf extract has been shown to reduce blood glucose levels in rats with high-fat diet-induced diabetes mellitus by regulating fatty acid metabolism.78,79 To our knowledge, no data have been published concerning the effects of AC intake on serum glucose levels in healthy elderly individuals. In this study, we observed significantly reduced serum glucose levels in association with increased RBC-PM ALA levels in both the PO and POAC groups after 12 months of treatment. After 12 months of POAC treatment, serum glucose levels tended to negatively correlate with RBC-PM EPA levels (r = −0.346, p = 0.066) (data not shown), but not with ALA levels. These findings suggest that chronic administration of PO or POAC supplements lowers serum glucose levels by increasing the RBC-PM EPA levels.
The APOE-ε4 allele is the strongest and most prevalent genetic risk factor for sporadic late-onset Alzheimer's disease due to its influence on amyloid-beta deposition, neuroinflammation, neurogenesis, synaptic function, and lipid metabolism.80,81 The potential of APOE-ε4 allele frequency to affect results of the present study made it meaningful to measure. The distribution of different APOE-ε4 alleles showed no significant differences between the PO and POAC groups, indicating that the outcome of this intervention trial was not influenced by allele frequency in the two groups.
This study has several limitations. First, because the volunteers were healthy elderly individuals without neuronal dysfunction, the observed cognitive and mental health decline was mostly related to age, rather than pathology. This is reflected in the total test scores for cognitive performance and mental health, which did not differ significantly in the PO or POAC groups. A wider range of baseline cognitive function scores could lead to more visible effects of intervention, and we plan to investigate the effects of PO and POAC on individuals with more diverse cognitive abilities. Second, to elucidate the exact mechanisms of ALA action, it is necessary to determine whether it is ALA alone, or its conversion to EPA and/or DHA, that yields effects. However, this distinction cannot be clarified by human intervention studies alone, nor by rodent model studies because the conversion efficiency of ALA to EPA and DHA in humans is much lower than that in rodents. Investigating human gene polymorphisms as they relate to conversion enzymes may provide insights into the mechanisms of ALA action. Third, the sample size of this study was relatively small, with participants from a single population, which limits the generalisability of the current results. Finally, this intervention study lacked two controls: a placebo group and a group taking only AC leaf powder. The small participant population limited our ability to divide patients into more than two groups, and study volunteers were unwilling to take 15 potentially ineffective (i.e., placebo) SGCs daily for a year. The lack of control groups may have decreased the power of this intervention trial, and larger trials are needed in the future.
5. Conclusions
Twelve months of POAC supplementation showed no adverse clinical effects and improved cognitive function in healthy Japanese elderly individuals, presumably by lowering the CML, TG, and glucose levels. Nutritional interventions that prevent cardiovascular risk factors, such as hypertriglyceridaemia and hyperglycaemia, are potential preventive agents against the onset and/or progression of MCI and dementia. Although further research is needed to elucidate the mechanisms underlying the beneficial effects of PO and POAC on cognitive performance, these effective and easy-to-consume supplements may be viable and accessible options for maintaining cognitive and cardiovascular function in the elderly. Future research will clarify the impact of PO alone or with AC on patients with clinical cognitive impairments and mental illness, including neurodegenerative disorders.Author contributions
Michio Hashimoto: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, visualization, writing – original draft, writing – review and editing. Kentaro Matsuzaki: data curation, investigation, methodology, software, formal analysis, visualization, writing-original draft, writing – review and editing. Koji Maruyama: conceptualization, project administration, funding acquisition, validation. Eri Sumiyoshi: validation, visualization, writing – review and editing. Shahdat Hossain: validation, writing – original draft, visualization, writing – review and editing. Harumi Wakatsuki: data curation, formal analysis, investigation, methodology. Setsushi Kato: methodology, resources, supervision, validation. Miho Ohno: investigation, methodology, resources. Yoko Tanabe: investigation. Yoko Kuroda: investigation, methodology. Shuhei Yamaguchi: supervision. Koji Kajima: conceptualization, funding acquisition, supervision. Yasushi Ohizumi: conceptualization, project administration, funding acquisition, writing – review and editing, supervision. Osamu Shido: supervision, validation.Conflicts of interest
Koji Maruyama and Koji Kajima, employees of Sankyo Holdings Co., Ltd, contributed to the experiments. Other authors have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was partly supported by the grant from Sankyo Holdings Co., Ltd (Shimane University Project code: E1D28018). We sincerely thank the participants of the study. We would also like to thank Editage for English language editing.References
- H. D. VanGuilder, J. A. Farley, H. Yan, C. A. Van Kirk, M. Mitschelen, W. E. Sonntag and W. M. Freeman, Hippocampal dysregulation of synaptic plasticity-associated proteins with age-related cognitive decline, Neurobiol. Dis., 2011, 43, 201–212 CrossRef CAS PubMed.
- J. H. Morrison and M. G. Baxter, The ageing cortical synapse: hallmarks and implications for cognitive decline, Nat. Rev. Neurosci., 2012, 13, 240–250 CrossRef CAS PubMed.
- N. T. Akbaraly, H. Faure, V. Gourlet, A. Favier and C. Berr, Plasma carotenoid levels and cognitive performance in an elderly population: Results of the EVA study, J. Gerontol., Ser. A, 2007, 62, 308–316 CrossRef PubMed.
- G. Livingston, A. Sommerlad, V. Orgeta, S. G. Costafreda, J. Huntley, D. Ames, C. Ballard, S. Banerjee, A. Burns, J. Cohen-Mansfield, C. Cooper, N. Fox, L. N. Gitlin, R. Howard, H. C. Kales, E. B. Larson, K. Ritchie, K. Rockwood, E. L. Sampson, Q. Samus, L. S. Schneider, G. Selbaek, L. Teri and N. Mukadam, Dementia prevention, intervention, and care, Lancet, 2017, 390, 2673–2734 CrossRef.
- V. Solfrizzi, C. Custodero, M. Lozupone, B. P. Imbimbo, V. Valiani, P. Agosti, A. Schilardi, A. D'Introno, M. La Montagna, M. Calvani, V. Guerra, R. Sardone, D. I. Abbrescia, A. Bellomo, A. Greco, A. Daniele, D. Seripa, G. Logroscino, C. Sabba and F. Panza, Relationships of Dietary Patterns, Foods, and Micro- and Macronutrients with Alzheimer's Disease and Late-Life Cognitive Disorders: A Systematic Review, J. Alzheimer's Dis., 2017, 59, 815–849 CAS.
- R. J. Wurtman, M. Cansev, T. Sakamoto and I. H. Ulus, Use of Phosphatide Precursors to Promote Synaptogenesis, Annu. Rev. Nutr., 2009, 29, 59–87 CrossRef CAS PubMed.
- Y. Ohizumi, A New Strategy for Preventive and Functional Therapeutic Methods for Dementia—Approach Using Natural Products, Yakugaku Zasshi, 2015, 135, 449–464 CrossRef CAS PubMed.
- M. Hashimoto, S. Hossain, K. Matsuzaki, O. Shido and K. Yoshino, The journey from white rice to ultra-high hydrostatic pressurized brown rice: an excellent endeavor for ideal nutrition from staple food, Crit. Rev. Food Sci. Nutr., 2022, 62(6), 1502–1520 CrossRef CAS PubMed.
- K. Matsuzaki and Y. Ohizumi, Beneficial Effects of Citrus-Derived Polymethoxylated Flavones for Central Nervous System Disorders, Nutrients, 2021, 13, 145 CrossRef CAS PubMed.
- M. Hashimoto, Y. Tanabe, S. Hossain, K. Matsuzaki, M. Ohno, S. Kato, M. Katakura and O. Shido, Intake of Alpha-Linolenic Acid-Rich Perilla frutescens Leaf Powder Decreases Home Blood Pressure and Serum Oxidized Low-Density Lipoprotein in Japanese Adults, Molecules, 2020, 25(9), 2099 CrossRef CAS PubMed.
- A. N. Garmana, E. Y. Sukandar and I. Fidrianny, Preliminary study of blood pressure lowering effect of Anredera cordifolia (Ten) steenis on Wistar rats, Int. J. Pharm. Pharm. Res., 2016, 8, 300–304 Search PubMed.
- D. R. Laksmitawati, A. Widyastuti, N. Karami, E. Afifah, D. D. Rihibiha, H. Nufus and W. Widowati, Anti-inflammatory effects of Anredera cordifolia and Piper crocatum extracts on lipopolysaccharide-stimulated macrophage cell line, Bangladesh J. Pharmacol., 2017, 12, 35–40 CrossRef.
- K. Maruyama, K. Arai, T. Sakakiyama, K. Yoshino and K. Kajima, Anredera cordifolia Water Extract Effectively Ameliorates Metabolic Syndrome Abnormalities in High Fructose-Fed Mouse Model, Pharmacometrics, 2014, 87(1/2), 21–24 Search PubMed.
- K. Maruyama, M. Yamada, K. Yamauchi, K. Yoshino and K. Kajima, Anredera cordiifolia Water Extract Effectively Decrease in Vivo Oxidative Stress in High Fructose-Fed Mouse Model, Pharmacometrics, 2015, 88(3/4), 47–51 Search PubMed.
- A. Nakajima, M. Hachiro, K. Kajima and Y. Ohizumi, Anredera cordifolia Extract Improves MK-801-Induced Memory Impairment in Mice, Pharmacometrics, 2020, 98, 27–30 Search PubMed.
- E. Sumiyoshi, M. Hashimoto, S. Hossain, K. Matsuzaki, R. Islam, Y. Tanabe, K. Maruyama, K. Kajima, H. Arai, Y. Ohizumi and O. Shido, Anredera cordifolia extract enhances learning and memory in senescence-accelerated mouse-prone 8 (SAMP8) mice, Food Funct., 2021, 12, 3992–4004 RSC.
- H. M. Ahmed, Ethnomedicinal, Phytochemical and Pharmacological Investigations of Perilla frutescens (L.) Britt, Molecules, 2019, 24, 102 CrossRef PubMed.
- G. X. Zhao, T. D. Etherton, K. R. Martin, P. J. Gillies, S. G. West and P. M. Kris-Etherton, Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects, Am. J. Clin. Nutr., 2007, 85, 385–391 CrossRef CAS PubMed.
- C. Nguemeni, B. Delplanque, C. Rovere, N. Simon-Rousseau, C. Gandin, G. Agnani, J. L. Nahon, C. Heurteaux and N. Blondeau, Dietary supplementation of alpha-linolenic acid in an enriched rapeseed oil diet protects from stroke, Pharmacol. Res., 2010, 61, 226–233 CrossRef CAS PubMed.
- H. Gao, P. P. Yan, S. Zhang, H. Huang, F. H. Huang, T. P. Sun, Q. C. Deng, Q. D. Huang, S. J. Chen, K. Q. Ye, J. Q. Xu and L. G. Liu, Long-Term Dietary Alpha-Linolenic Acid Supplement Alleviates Cognitive Impairment Correlate with Activating Hippocampal CREB Signaling in Natural Aging Rats, Mol. Neurobiol., 2016, 53, 4772–4786 CrossRef CAS PubMed.
- M. Hashimoto, S. Hossain, A. Al Mamun, K. Matsuzaki and H. Arai, Docosahexaenoic acid: one molecule diverse functions, Crit. Rev. Biotechnol., 2017, 37, 579–597 CrossRef CAS PubMed.
- G. Y. Sun, A. Simonyi, K. L. Fritsche, D. Y. Chuang, M. Hannink, Z. Z. Gu, C. M. Greenlie, J. K. Yao, J. C. Lee and D. Q. Beversdorf, Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2018, 136, 3–13 CrossRef CAS PubMed.
- M. Hashimoto, K. Yamashita, S. Kato, T. Tamai, Y. Tanabe, M. Mitarai, I. Matsumoto and M. Ohno, Beneficial effects of daily dietary omega-3 polyunsaturated fatty acid supplementation on age-related cognitive decline in elderly Japanese with very mild dementia: A 2-year randomized, double-blind, placebo-controlled trial., J. Aging Res. Clin. Pract., 2012, 1, 193–201 Search PubMed.
- M. Hashimoto, S. Kato, Y. Tanabe, M. Katakura, A. A. Mamun, M. Ohno, S. Hossain, K. Onoda, S. Yamaguchi and O. Shido, Beneficial effects of dietary docosahexaenoic acid intervention on cognitive function and mental health of the oldest elderly in Japanese care facilities and nursing homes, Geriatr. Gerontol. Int., 2017, 17, 330–337 CrossRef PubMed.
- G. Burdge, alpha-linolenic acid metabolism in men and women: nutritional and biological implications, Curr. Opin. Clin. Nutr. Metab. Care, 2004, 7, 137–144 CrossRef CAS PubMed.
- G. Kühn, K. Pallauf, C. Schulz and G. Rimbach, Flavonoids as putative modulators of Δ4-, Δ5-, and Δ6-desaturases: Studies in cultured hepatocytes, myocytes, and adipocytes, BioFactors, 2018, 44(5), 485–495 CrossRef PubMed.
- M. de Lorgeril, P. Salen, J. L. Martin, F. Boucher and J. de Leiris, Interactions of wine drinking with omega-3 fatty acids in patients with coronary heart disease: A fish-like effect of moderate wine drinking, Am. Heart J., 2008, 155, 175–181 CrossRef CAS PubMed.
- M. C. Toufektsian, P. Salen, F. Laporte, C. Tonelli and M. de Lorgeril, Dietary Flavonoids Increase Plasma Very Long-Chain (n-3) Fatty Acids in Rats, J. Nutr., 2011, 141, 37–41 CrossRef CAS PubMed.
- M. Hashimoto, K. Matsuzaki, S. Hossain, T. Ito, H. Wakatsuki, Y. Tanabe, M. Ohno, S. Kato, K. Yamashita and O. Shido, Perilla Seed Oil Enhances Cognitive Function and Mental Health in Healthy Elderly Japanese Individuals by Enhancing the Biological Antioxidant Potential, Foods, 2021, 10, 1130 CrossRef CAS PubMed.
- M. Hashimoto, K. Matsuzaki, K. Maruyama, S. Hossain, E. Sumiyoshi, H. Wakatsuki, S. Kato, M. Ohno, Y. Tanabe, Y. Kuroda, S. Yamaguchi, K. Kajima, Y. Ohizumi and O. Shido, Perilla seed oil in combination with nobiletin-rich ponkan powder enhances cognitive function in healthy elderly Japanese individuals: a possible supplement for brain health in the elderly, Food Funct., 2022, 13, 2768–2781 RSC.
- M. Hashimoto, K. Matsuzaki, S. Kato, S. Hossain, M. Ohno and O. Shido, Twelve-month Studies on Perilla Oil Intake in Japanese Adults-Possible Supplement for Mental Health, Foods, 2020, 9, 530 CrossRef CAS PubMed.
- E. Sumiyoshi, K. Matsuzaki, N. Sugimoto, Y. Tanabe, T. Hara, M. Katakura, M. Miyamoto, S. Mishima and O. Shido, Sub-Chronic Consumption of Dark Chocolate Enhances Cognitive Function and Releases Nerve Growth Factors: A Parallel-Group Randomized Trial, Nutrients, 2019, 11, 2800 CrossRef CAS PubMed.
- T. Ichinose, K. Matsuzaki, M. Kato, Y. Tanabe, N. Tachibana, M. Morikawa, S. Kato, S. Ohata, M. Ohno, H. Wakatsuki, S. Hossain, O. Shido and M. Hashimoto, Intake of Docosahexaenoic Acid-Enriched Milk Beverage Prevents Age-Related Cognitive Decline and Decreases Serum Bone Resorption Marker Levels, J. Oleo Sci., 2021, 70, 1829–1838 CrossRef CAS PubMed.
- S. Kobayashi, K. Murakami, S. Sasaki, H. Okubo, N. Hirota, A. Notsu, M. Fukui and C. Date, Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults, Public Health Nutr., 2011, 14, 1200–1211 CrossRef PubMed.
- Y. Imai and K. Hasegawa, The revised Hasegawa's dementia scale (HDS-R)---Evaluation of its usefulness as a screening test for dementia., J. Hong Kong Coll. Psychiatr., 1994, 4, 20–24 Search PubMed.
- M. F. Folstein, S. E. Folstein and P. R. McHugh, “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician, J. Psychiatr. Res., 1975, 12, 189–198 CrossRef CAS PubMed.
- Y. Fujiwara, H. Suzuki, M. Yasunaga, M. Sugiyama, M. Ijuin, N. Sakuma, H. Inagaki, H. Iwasa, C. Ura, N. Yatomi, K. Ishii, A. M. Tokumaru, A. Homma, Z. Nasreddine and S. Shinkai, Brief screening tool for mild cognitive impairment in older Japanese: validation of the Japanese version of the Montreal Cognitive Assessment, Geriatr. Gerontol. Int., 2010, 10, 225–232 CrossRef PubMed.
- K. Okada, S. Kobayashi, K. Aoki, N. Suyama and S. Yamagata, Assessment of moti-vational loss in poststroke patients using the Japanese version of Starkstein's Apathy Scale (in Japanese), Jpn. J. Stroke, 1998, 20, 318–327 CrossRef.
- W. W. Zung, A SELF-RATING DEPRESSION SCALE, Arch. Gen. Psychiatry, 1965, 12, 63–70 CrossRef CAS PubMed.
- I. F. Benzie and J. J. Strain, The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay, Anal. Biochem., 1996, 239, 70–76 CrossRef CAS PubMed.
- S. Reddy, J. Bichler, K. J. Wellsknecht, S. R. Thorpe and J. W. Baynes, N-epsilon-(carboxymethyl)lysine is a dominant advanced glycation end-product (age) antigen in tissue proteins, Biochemistry, 1995, 34, 10872–10878 CrossRef CAS PubMed.
- M. Hashimoto, K. Shinozuka, S. Gamoh, Y. Tanabe, M. S. Hossain, Y. M. Kwon, N. Hata, Y. Misawa, M. Kunitomo and S. Masumura, The hypotensive effect of docosahexaenoic acid is associated with the enhanced release of ATP from the caudal artery of aged rats, J. Nutr., 1999, 129, 70–76 CrossRef CAS PubMed.
- K. Yamagishi, A. Ikeda, C. L. Chei, H. Noda, M. Umesawa, R. Cui, I. Muraki, T. Ohira, H. Imano, T. Sankai, T. Okada, T. Tanigawa, A. Kitamura, M. Kiyama and H. Iso, Serum α-linolenic and other ω-3 fatty acids, and risk of disabling dementia: Community-based nested case-control study, Clin. Nutr., 2017, 36, 793–797 CrossRef CAS PubMed.
- C. Kamalashiran, K. Sriyakul, J. Pattaraarchachai and S. Muengtaweepongsa, Outcomes of Perilla Seed Oil as an Additional Neuroprotective Therapy in Patients with Mild to Moderate Dementia: A Randomized Control Trial, Curr. Alzheimer Res., 2019, 16, 146–155 CrossRef CAS PubMed.
- N. Blondeau, C. Nguemeni, D. N. Debruyne, M. Piens, X. Wu, H. Pan, X. Hu, C. Gandin, R. H. Lipsky, J. C. Plumier, A. M. Marini and C. Heurteaux, Subchronic alpha-linolenic acid treatment enhances brain plasticity and exerts an antidepressant effect: a versatile potential therapy for stroke, Neuropsychopharmacology, 2009, 34, 2548–2559 CrossRef CAS PubMed.
- S. M. Astuti, A. M. Sakinah and A. Risch, The triterpenoid saponin from binahong [Anredera cordifolia (Ten) Steenis] to potential using as antidiabetic activity in animal laboratory, in Int. Conf. Drug Develop. Nat. Resour., 2012, pp. 331–344 Search PubMed.
- Y. Ohizumi, M. Kawada, M. Kamada, A. Nakajima, K. Kajima, N. Uozumi, Y. Hara, Y. Guo and M. Ishibashi, Isolation of adenosine and cordysinin B from Anredera cordifolia that stimulates CRE-mediated transcription in PC12 cells, Planta Med. Int. Open, 2021, 8, e19–e24 CrossRef.
- J. P. E. Spencer, Flavonoids: modulators of brain function?, Br. J. Nutr., 2008, 99, ES60–ES77 CrossRef PubMed.
- G. Jin, L. Zhu, P. Liu, Q. Xu, Y. Qi, X. Y. Zhou, J. K. Xu, X. F. Ji, T. Y. Chi and L. B. Zou, Xanthoceraside prevented synaptic loss and reversed learning-memory deficits in APP/PS1 transgenic mice, J. Physiol. Sci., 2019, 69, 477–488 CrossRef CAS PubMed.
- E. H. Jansen and T. Ruskovska, Comparative Analysis of Serum (Anti)oxidative Status Parameters in Healthy Persons, Int. J. Mol. Sci., 2013, 14, 6106–6115 CrossRef CAS PubMed.
- E. Sutrisno, I. K. Adnyana, E. Y. Sukandar and d. L. T. Fidrianny, Study of wound healing and antibacterial activity of binahong (Anredera cordifolia (Ten.)) Steenis Centella asiatica (L.) Urban and their combination of Staphylococcus aureus and Pseudomonas aeruginosa from diabetic wound patients, Bionatura, 2014, 16, 78–78 Search PubMed.
- N. P. E. Leliqia, E. Y. Sukandar and I. Fidrianny, Overview of efficacy, safety and phytochemical study of Anredera cordifolia (Ten.) Steenis, Pharmacologyonline, 2017, 1, 124–131 CAS.
- F. Babaei, A. Moafizad, Z. Darvishvand, M. Mirzababaei, H. Hosseinzadeh and M. Nassiri-Asl, Review of the effects of vitexin in oxidative stress-related diseases, Food Sci. Nutr., 2020, 8, 2569–2580 CrossRef CAS PubMed.
- K. J. Bar, S. Franke, B. Wenda, S. Muller, R. Kientsch-Engel, G. Stein and H. Sauer, Pentosidine and N-epsilon-(carboxymethyl)-lysine in Alzheimer's disease and vascular dementia, Neurobiol. Aging, 2003, 24, 333–338 CrossRef CAS PubMed.
- K. Ikeda, T. Higashi, H. Sano, Y. Jinnouchi, M. Yoshida, T. Araki, S. Ueda and S. Horiuchi, N-epsilon-(carboxymethyl)lysine protein adduct is a major immunological epitope in proteins modified with advanced glycation end products of the Maillard reaction, Biochemistry, 1996, 35, 8075–8083 CrossRef CAS PubMed.
- R. J. Castellani, P. L. R. Harris, L. M. Sayre, J. Fujii, N. Taniguchi, M. P. Vitek, H. Founds, C. S. Atwood, G. Perry and M. A. Smith, Active glycation in neurofibrillary pathology of Alzheimer disease: N-epsilon-(carboxymethyl) lysine and hexitol-lysine, Free Radicals Biol. Med., 2001, 31, 175–180 CrossRef CAS PubMed.
- N. Ahmed, U. Ahmed, P. J. Thornalley, K. Hager, G. Fleischer and G. Munch, Protein glycation, oxidation and nitration adduct residues and free adducts of cerebrospinal fluid in Alzheimer's disease and link to cognitive impairment, J. Neurochem., 2005, 92, 255–263 CrossRef CAS PubMed.
- J.-H. Zhang, H.-Z. Xu, Q.-F. Shen, Y.-Z. Lin, C.-K. Sun, L. Sha, Y.-S. Ge, Y. Liu and C. Wang, Nε-(carboxymethyl)-lysine, White Matter, and Cognitive Function in Diabetes Patients, Can. J. Neurol. Sci., 2016, 43, 518–522 CrossRef PubMed.
- M. Kouba and J. Mourot, A review of nutritional effects on fat composition of animal products with special emphasis on n-3 polyunsaturated fatty acids, Biochimie, 2011, 93, 13–17 CrossRef CAS PubMed.
- K. Hamazaki, M. Itomura, T. Hamazaki and S. Sawazaki, Effects of cooking plant oils on recurrent aphthous stomatitis: a randomized, placebo-controlled, double-blind trial, Nutrition, 2006, 22, 534–538 CrossRef PubMed.
- R. H. M. de Groot, R. Emmett and B. J. Meyer, Non-dietary factors associated with n-3 long-chain PUFA levels in humans - a systematic literature review, Br. J. Nutr., 2019, 121(7), 793–808 CrossRef CAS PubMed.
- F. Ounnas, F. Privé, P. Salen, F. Hazane-Puch, F. Laporte, E. Fontaine, D. D. Rio, L. Calani, C. Melegari, M. A. Bianchi, C. Demeilliers and M. de Lorgeril, Wheat aleurone polyphenols increase plasma eicosapentaenoic acid in rats, Food Nutr. Res., 2014, 58 Search PubMed.
- R. di Giuseppe, M. de Lorgeril, P. Salen, F. Laporte, A. Di Castelnuovo, V. Krogh, A. Siani, J. Arnout, F. P. Cappuccio, M. van Dongen, M. B. Donati, G. de Gaetano and L. Iacoviello, European Collaborative Group of the IMMIDIET Project. Alcohol consumption and n-3 polyunsaturated fatty acids in healthy men and women from 3 European populations, Am. J. Clin. Nutr., 2009, 89(1), 354–362 CrossRef CAS PubMed.
- M. de Lorgeril, P. Salen, J. L. Martin, F. Boucher and J. de Leiris, Interactions of wine drinking with omega-3 fatty acids in patients with coronary heart disease: a fish-like effect of moderate wine drinking, Am. Heart J., 2008, 155(1), 175–181 CrossRef CAS PubMed.
- F. Ounnas, F. Privé, P. Salen, N. Gaci, W. Tottey, L. Calani, L. Bresciani, N. López-Gutiérrez, F. Hazane-Puch, F. Laporte, J. F. Brugère, D. Del Rio, C. Demeilliers and M. de Lorgeril, Whole Rye Consumption Improves Blood and Liver n-3 Fatty Acid Profile and Gut Microbiota Composition in Rats, PLoS One, 2016, 11(2), e0148118 CrossRef PubMed.
- D. Vauzour, N. Tejera, C. O'Neill, V. Booz, B. Jude, I. M. Wolf, N. Rigby, J. M. Silvan, P. J. Curtis, A. Cassidy, S. de Pascual-Teresa, G. Rimbach and A. M. Minihane, Anthocyanins do not influence long-chain n-3 fatty acid status: studies in cells, rodents and humans, J. Nutr. Biochem., 2015, 26(3), 211–218 CrossRef CAS PubMed.
- G. J. Soleas, E. P. Diamandis and D. M. Goldberg, Wine as a biological fluid: history, production, and role in disease prevention, J. Clin. Lab. Anal., 1997, 11(5), 287–313 CrossRef CAS PubMed.
- M. Miyazaki and J. M. Ntambi, Fatty acid desaturation and chain elongation in mammals, in Biochemistry of lipids, lipoproteins and membranes, ed. D. E. Vance and J. E. Vance, Elsevier, New York, 5th edn, 2008, pp. 191–211 Search PubMed.
- D. B. Jump, Fatty acid regulation of hepatic lipid metabolism, Curr. Opin. Clin. Nutr. Metab. Care, 2011, 14(2), 115–120 CrossRef CAS PubMed.
- J. Nettiksimmons, E. M. Simonsick, T. Harris, S. Satterfield, C. Rosano and K. Yaffe, The associations between serum brain-derived neurotrophic factor, potential confounders, and cognitive decline: a longitudinal study, PLoS One, 2014, 9, e91339 CrossRef PubMed.
- F. Angelucci, G. Spalletta, F. di Iulio, A. Ciaramella, F. Salani, L. Colantoni, A. E. Varsi, W. Gianni, G. Sancesario, C. Caltagirone and P. Bossù, Alzheimer's disease (AD) and Mild Cognitive Impairment (MCI) patients are characterized by increased BDNF serum levels, Curr. Alzheimer Res., 2010, 7, 15–20 CrossRef CAS PubMed.
- M. Hashimoto, R. Tozawa, M. Katakura, H. Shahdat, A. M. Haque, Y. Tanabe, S. Gamoh and O. Shido, Protective effects of prescription n-3 fatty acids against impairment of spatial cognitive learning ability in amyloid β-infused rats, Food Funct., 2011, 2, 386–394 RSC.
- K. Håkansson, A. Ledreux, K. Daffner, Y. Terjestam, P. Bergman, R. Carlsson, M. Kivipelto, B. Winblad, A. C. Granholm and A. K. Mohammed, BDNF Responses in Healthy Older Persons to 35 Minutes of Physical Exercise, Cognitive Training, and Mindfulness: Associations with Working Memory Function, J. Alzheimer's Dis., 2017, 55, 645–657 Search PubMed.
- H. E. Bays, C. M. Ballantyne, J. J. Kastelein, J. L. Isaacsohn, R. A. Braeckman and P. N. Soni, Eicosapentaenoic Acid Ethyl Ester (AMR101) Therapy in Patients With Very High Triglyceride Levels (from the Multi-center, placebo-controlled, Randomized, double-blInd, 12-week study with an open-label Extension MARINE Trial), Am. J. Cardiol., 2011, 108, 682–690 CrossRef CAS PubMed.
- R. A. Braeckman, M. S. Manku, H. E. Bays, W. G. Stirtan and P. N. Soni, Icosapent ethyl, a pure EPA omega-3 fatty acid: Effects on plasma and red blood cell fatty acids in patients with very high triglyceride levels (results from the MARINE study), Prostaglandins, Leukotrienes Essent. Fatty Acids, 2013, 89, 195–201 CrossRef CAS PubMed.
- S. Egert, F. Kannenberg, V. Somoza, H. F. Erbersdobler and U. Wahrburg, Dietary alpha-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans, J. Nutr., 2009, 139, 861–868 CrossRef CAS PubMed.
- N. Yamanaka, S. Saito, N. Osaki, S. Kamei, H. Nakamura and Y. Katsuragi, Alpha-Linolenic Acid-Enriched Diacylglycerol Oil Suppresses the Postprandial Serum Triglyceride Level-A Randomized, Double-Blind, Placebo-Controlled, Crossover Study, J. Nutr. Sci. Vitaminol., 2016, 62, 402–408 CrossRef CAS PubMed.
- E. Kusumanti and S. Sugiharto, Effect of dietary supplementation of binahong leaf meal, betel nut meal or their combination on serum albumin and globulin, fecal endoparasites and bacterial counts in milk of Saanen goats suffering from subclinical mastitis, Agric. Nat. Resour., 2017, 51, 415–419 Search PubMed.
- D. Dwitiyanti, Y. Harahap, B. Elya and A. Bahtiar, Binahong (Anredera cordifolia (Tenore) Steen.) Leaf Extract Modulates Fatty Acids and Amino Acids to Lower Blood Glucose in High-Fat Diet-Induced Diabetes Mellitus Rats, Adv. Pharmacol. Pharm. Sci., 2021, 2021, 8869571 CAS.
- W. Alata, Y. Ye, I. St-Amour, M. Vandal and F. Calon, Human apolipoprotein E epsilon 4 expression impairs cerebral vascularization and blood-brain barrier function in mice, J. Cereb. Blood Flow Metab., 2015, 35, 86–94 CrossRef CAS PubMed.
- V. Theendakara, C. A. Peters-Libeu, P. Spilman, K. S. Poksay, D. E. Bredesen and R. V. Rao, Direct Transcriptional Effects of Apolipoprotein E, J. Neurosci., 2016, 36, 685–700 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fo00723a |
| This journal is © The Royal Society of Chemistry 2022 |