Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Preparation of a Mg2+-containing MOF through ion exchange and its high ionic conductivity†
Shintaro
Niwa
and
Masaaki
Sadakiyo
 *
*
Department of Applied Chemistry, Faculty of Science Division I, Tokyo University of Science, 1-3 Kagurazaka, Shinjuku-ku, Tokyo 162-8601, Japan. E-mail: sadakiyo@rs.tus.ac.jp
First published on 30th July 2022
Abstract
We report, for the first time, the preparation and ionic conductivity of a Mg2+-containing metal–organic framework (MOF) having type A features, i.e., an anionic framework containing Mg2+ as the counter cation. We prepared Mg3[(MnMo6O18)2L] (L12− = C{C6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC(CH2O)3}412−) (MOF-688-Mg) through a simple ion exchange reaction, and it showed high ionic conductivity above 10−5 S cm−1 at 25 °C under MeCN vapor.
NC(CH2O)3}412−) (MOF-688-Mg) through a simple ion exchange reaction, and it showed high ionic conductivity above 10−5 S cm−1 at 25 °C under MeCN vapor.
Metal–organic frameworks (MOFs) have recently been developed as a new class of ionic conductor because of their designable architectures and porous structures, which allow us to introduce ionic carriers and to construct efficient ion-conducting pathways in their pores.1,2 So far, a lot of ion-conductive MOFs have been created by introducing various ionic carriers, such as protons,3–6 hydroxide ions,7,8 and lithium ions.9,10 However, there is a lack of reports on magnesium ion (Mg2+)-containing MOFs and their conductive properties, although Mg2+ conductors are of importance for application as the electrolyte of next-generation secondary batteries that do not require the use of rare elements such as lithium.11
We have focused on creating novel Mg2+ conductors with MOFs by the introduction of Mg2+ carriers in their pores. Considering the charge compensation, there should be two possible ways to introduce Mg2+ carriers into the pores of MOFs.1 The first is to introduce Mg2+ as the counter ion of the anionic framework (type A). The second is to introduce Mg2+ together with the counter anions, i.e., the introduction of Mg2+ salts, which is applicable for charge-neutral or cationic frameworks (type B). So far, there are six reports of ionic conductivity of Mg2+-containing MOFs, which are prepared by the introduction of Mg2+ together with counter anions (i.e., type B features).12–17 However, there is no report of a Mg2+-containing MOF with type A features and thus their ionic conductivity is still unclear, even though type A features are expected to be the ideal structure for application as a battery electrolyte because of the high transport number and suppression of the side reaction of mobile anions. Here, in this study, we for the first time report the preparation of a Mg2+-containing MOF with type A features through a simple ion exchange reaction with an anionic mother framework, MOF-688,18 and its ion-conductive properties. We clarified that the Mg2+-containing MOF shows high ionic conductivity above 10−5 S cm−1 at room temperature (RT) under a vapor of organic guest molecules.
The mother framework, MOF-688, (NBu4)6[(MnMo6O18)2L] (L12− = C{C6H4CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NC(CH2O)3}412−, NBu4+ = tetrabutylammonium), was synthesized by imine bond formation between (NBu4)3[MnMo6O18{(OCH2)3CNH2}2] (abbreviated to NBu4–Mn–Mo6) and tetrakis(4-formylphenyl)methane (TFPM) (Fig. 1), according to a previous report (details are shown in the ESI†).18 The successful synthesis of MOF-688 was confirmed by X-ray powder diffraction (XRPD) measurements (Fig. 2) and elemental analysis (ESI†). The included cations, NBu4+, in MOF-688 were exchanged with Mg2+ through an ion exchange reaction; a powder sample of MOF-688 was immersed in a 1 M acetonitrile solution of Mg(TFSI)2 (HTFSI = bis(trifluoromethanesulfonyl)imide) at RT for 5 days, followed by careful washing with pure anhydrous acetonitrile (immersed for 2 days) to remove the remaining Mg(TFSI)2 from the sample (details are shown in the ESI†).
NC(CH2O)3}412−, NBu4+ = tetrabutylammonium), was synthesized by imine bond formation between (NBu4)3[MnMo6O18{(OCH2)3CNH2}2] (abbreviated to NBu4–Mn–Mo6) and tetrakis(4-formylphenyl)methane (TFPM) (Fig. 1), according to a previous report (details are shown in the ESI†).18 The successful synthesis of MOF-688 was confirmed by X-ray powder diffraction (XRPD) measurements (Fig. 2) and elemental analysis (ESI†). The included cations, NBu4+, in MOF-688 were exchanged with Mg2+ through an ion exchange reaction; a powder sample of MOF-688 was immersed in a 1 M acetonitrile solution of Mg(TFSI)2 (HTFSI = bis(trifluoromethanesulfonyl)imide) at RT for 5 days, followed by careful washing with pure anhydrous acetonitrile (immersed for 2 days) to remove the remaining Mg(TFSI)2 from the sample (details are shown in the ESI†).
 | ||
| Fig. 1 Schematic illustration of the synthetic scheme of the Mg2+-containing anionic MOF through an ion exchange reaction. | ||
To confirm the existence of Mg2+ in the sample after the ion exchange reaction (MOF-688-Mg), we performed inductively coupled plasma atomic emission spectroscopy (ICP-AES) and 1H-NMR measurements using a dissolved sample. From the results of the ICP-AES measurements, the number of Mg2+ ions included in MOF-688-Mg was estimated to be 2.9 per formula unit, confirming the stoichiometric ion exchange of NBu4+ with Mg2+ to form MOF-688-Mg, Mg3[(MnMo6O18)2L]. As shown in Fig. S1,† the results of 1H-NMR measurements clearly indicated the absence of NBu4+ in MOF-688-Mg, which is consistent with the ICP-AES results. The results of elemental analysis also confirmed that the fundamental chemical composition of the framework was not changed after ion exchange (see the ESI†).
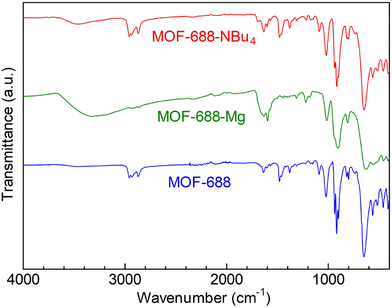
The XRPD pattern of MOF-688-Mg is shown in Fig. 2. The broadened peaks clearly indicated that MOF-688-Mg has an amorphous character. There should be two possibilities regarding the state of MOF-688-Mg. The first is that the framework structure of MOF-688-Mg completely collapsed during the ion exchange reaction, and thus it is not appropriate to call it a MOF sample. The second is that the ion exchange led to weakening of the long-range order of the framework, while the fundamental structure of the MOF still remained. To clarify this point, we reintroduced NBu4+ to MOF-688-Mg through an ion exchange reaction (MOF-688-NBu4) (see the ESI†). Reintroduction of NBu4+ was confirmed by ICP-AES and 1H-NMR measurements. From the results of the ICP-AES measurements, there was no detectable amount of Mg2+ after the reintroduction of NBu4+. In addition, there are apparent peaks from the reintroduced NBu4+ in the 1H-NMR spectra (Fig. S1†). These results clearly showed the successful ion exchange of Mg2+ with NBu4+. The XRPD pattern of the prepared MOF-688-NBu4 is shown in Fig. 2. The original patterns of MOF-688 were clearly recovered after the reintroduction of NBu4+, indicating that MOF-688-Mg indeed has a MOF structure with weakened long-range order even after the incorporation of Mg2+ (Fig. 1). This is similar to the case with the introduction of Li+ into MOF-688.18 We also measured the IR spectra of the samples (Fig. 3). The absence of C–H stretching bands from NBu4+ (at around 2870–2960 cm−1) in MOF-688-Mg and the presence of them in MOF-688-NBu4 confirmed the reversible ion exchange. Importantly, MOF-688-Mg showed characteristic peaks of the infinite framework of MOF-688, i.e., C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching (at around 1640 cm−1) and Mo–O–Mo stretching (at around 650 cm−1), clearly confirming the presence of the remaining MOF structure in MOF-688-Mg. To the best of our knowledge, this is the first example of a Mg2+-containing MOF having type A features, prepared by a simple ion exchange reaction.
N stretching (at around 1640 cm−1) and Mo–O–Mo stretching (at around 650 cm−1), clearly confirming the presence of the remaining MOF structure in MOF-688-Mg. To the best of our knowledge, this is the first example of a Mg2+-containing MOF having type A features, prepared by a simple ion exchange reaction.
To elucidate the ionic conductivity of the Mg2+-included anionic MOF, we performed alternating current (ac) impedance measurements (Fig. 4 and S2†). We previously reported that the ionic conductivity of MOFs containing Mg2+ in their pores deeply depends on the outer environment, i.e., gas or vapors of guest molecules.12,13 Thus, we measured the ionic conductivity under various environmental conditions, such as dry N2 or organic vapors using a home-made sealed cell.12 To eliminate the possibility of proton conduction, the measurements were performed after complete dehydration of the sample (see the ESI†). As shown in Fig. 4, the Mg2+-containing MOF did not show remarkable ionic conductivity under dry N2 at ambient temperature. However, an apparent increase in ionic conductivity was observed under organic vapors and the MOF showed high ionic conductivity of 1.3 × 10−5 S cm−1 at 25 °C under MeCN vapor as the optimal guest. Considering that the possible ionic carrier is only Mg2+ located in the pores of the MOF, the drastic increase of ionic conductivity under the guest vapor should be derived from the increase of the mobility of Mg2+. This is the first demonstration of vapor-induced ionic conduction of Mg2+ in a type A compound, although we previously reported vapor-induced ionic conduction in Mg2+ salt-containing MOFs (i.e., type B compounds).12,13 This result clearly indicated that the mobility of aprotic ions such as Mg2+ can be enhanced by including guest molecules. To acquire information about the guest adsorption ability of MOF-688-Mg, we performed adsorption isotherm measurements. As shown in Fig. S3,† MOF-688-Mg adsorbed a considerable amount of MeCN at high vapor pressure, which reaches 3.3 molecules per formula unit (at 0.92 P/P0), i.e., 1.1 MeCN molecules per one Mg2+. This clearly indicated that the MOF has the ability of MeCN vapor adsorption at RT, while the pore volume or pore size might be quite limited due to its interpenetrated structure,18 which was confirmed by almost no N2 adsorption in MOF-688-Mg at 77 K (Fig. S4†). Compared to Mg2+ salt-containing MOFs (i.e., Mg-MOF-74⊃{Mg(TFSI)2}0.15 and MIL-101⊃{Mg(TFSI)2}1.6),12,13 the adsorption amount of MOF-688-Mg for MeCN is relatively low and far from six molecules per Mg2+, which is considered as the important number for the formation of coordinated Mg2+ species showing high mobility.12,13 Considering the fact that the ionic conductivity of the Mg2+ salt-containing MOFs (Mg-MOF-74⊃{Mg(TFSI)2}0.15: 2.6 × 10−4 S cm−1 (25 °C); MIL-101⊃{Mg(TFSI)2}1.6: 1.9 × 10−3 S cm−1 (25 °C))12,13 under optimal conditions is much higher than that of MOF-688-Mg, there seem to be two reasons for the limited conductivity of MOF-688-Mg. The first is the limited pore volume or pore size as mentioned above, which did not allow the included Mg2+ to form highly mobile coordinated Mg2+ carriers due to the limited amount of guest adsorption. The second is the low crystallinity of MOF-688-Mg, which would prevent efficient migration of the formed carriers (i.e., insufficient ion-conducting pathways). Table S1 shows the estimated activation energy (Ea) values of the samples. The relatively high activation energy of MOF-688-Mg (0.40 eV under MeCN) compared to the Mg2+ salt-containing MOFs (Mg-MOF-74⊃{Mg(TFSI)2}0.15: 0.26 eV (under MeOH); MIL-101⊃{Mg(TFSI)2}1.6: 0.18 eV (under MeCN))12,13 is also suggestive that the highly crystalline pores are one of the important factors for constructing efficient ion-conducting pathways in MOFs. These results suggest that anionic MOFs containing Mg2+ (i.e., type A compounds) have great potential to be good Mg2+ conductors with optimal guests and that a highly crystalline anionic MOF with large-sized pores is one of the ideal structures for Mg2+-conducting MOFs.
Conclusions
In conclusion, we synthesized a Mg2+-containing MOF with type A features for the first time through a simple ion exchange reaction. This MOF showed high ionic conductivity of 1.3 × 10−5 S cm−1 at 25 °C under MeCN vapor because of the guest adsorption ability of the framework, while its crystallinity is low. We demonstrated that the Mg2+ located in the type A compound also showed a drastic increase of its mobility in the presence of organic guest vapors, which is similar to the case of Mg2+ salt-containing MOFs (type B compounds). These results should greatly contribute to the structural design and development of novel MOF-based Mg2+ conductors.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was partly supported by the Nippon Sheet Glass Foundation for Materials Science and Engineering, the Takano Science Foundation, the Shorai Foundation for Science and Technology, the Kurata Grants by the Hitachi Global Foundation, the Tokuyama Science Foundation, JSPS KAKENHI No. 21K05089, and JST FOREST No. JPMJFR211O.References
- M. Sadakiyo and H. Kitagawa, Dalton Trans., 2021, 50, 5385–5397 RSC.
- D.-W. Lim, M. Sadakiyo and H. Kitagawa, Chem. Sci., 2019, 10, 16–33 RSC.
- J. M. Taylor, K. W. Dawson and G. K. H. Shimizu, J. Am. Chem. Soc., 2013, 135, 1193–1196 CrossRef CAS PubMed.
- W. J. Phang, H. Jo, W. R. Lee, J. H. Song, K. Yoo, B. Kim and C. S. Hong, Angew. Chem., Int. Ed., 2015, 54, 5142–5146 CrossRef CAS PubMed.
- M. Sadakiyo, T. Yamada, K. Honda, H. Matsui and H. Kitagawa, J. Am. Chem. Soc., 2014, 136, 7701–7707 CrossRef CAS PubMed.
- D. Umeyama, S. Horike, M. Inukai, T. Itakura and S. Kitagawa, J. Am. Chem. Soc., 2012, 134, 12780–12785 CrossRef CAS PubMed.
- M. Sadakiyo, H. Kasai, K. Kato, M. Takata and M. Yamauchi, J. Am. Chem. Soc., 2014, 136, 1702–1705 CrossRef CAS PubMed.
- S. S. Nagarkar, B. Anothumakkool, A. V. Desai, M. M. Shirolkar, S. Kurungot and S. K. Ghosh, Chem. Commun., 2016, 52, 8459–8462 RSC.
- B. M. Wiers, M.-L. Foo, N. P. Balsara and J. R. Long, J. Am. Chem. Soc., 2011, 133, 14522–14525 CrossRef CAS PubMed.
- R. Ameloot, M. Aubrey, B. M. Wiers, A. P. G. Figueroa, S. N. Patel, N. P. Balsara and J. R. Long, Chem. – Eur. J., 2013, 19, 5533–5536 CrossRef CAS PubMed.
- H. D. Yoo, I. Shterengerg, Y. Gofer, G. Gershinsky, N. Pour and D. Aurbach, Energy Environ. Sci., 2013, 6, 2265–2279 RSC.
- Y. Yoshida, K. Kato and M. Sadakiyo, J. Phys. Chem. C, 2021, 125, 21124–21130 CrossRef CAS.
- Y. Yoshida, T. Yamada, Y. Jing, T. Toyao, K. Shimizu and M. Sadakiyo, J. Am. Chem. Soc., 2022, 144, 8669–8675 CrossRef CAS PubMed.
- S. Ma, L. Shen, Q. Liu, W. Shi, C. Zhang, F. Liu, J. A. Baucom, D. Zhang, H. Yue, H. B. Wu and Y. Lu, ACS Appl. Mater. Interfaces, 2020, 12, 43824–43832 CrossRef CAS PubMed.
- E. M. Miner, S. S. Park and M. Dincă, J. Am. Chem. Soc., 2019, 141, 4422–4427 CrossRef CAS PubMed.
- S. S. Park, Y. Tulchinsky and M. Dincă, J. Am. Chem. Soc., 2017, 139, 13260–13263 CrossRef CAS PubMed.
- M. L. Aubrey, R. Ameloot, B. M. Wiers and J. R. Long, Energy Environ. Sci., 2014, 7, 667–671 RSC.
- W. Xu, X. Pei, C. S. Diercks, H. Lyu, Z. Ji and O. M. Yaghi, J. Am. Chem. Soc., 2019, 141, 17522–17526 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2dt02166h |
| This journal is © The Royal Society of Chemistry 2022 |