Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Crystal phase modified blue upconversion on Tm3+/Yb3+:BCZT ceramic phosphor benefits multifunctionality in white-light applications†
Prasenjit Prasad
Sukul
 * and
Hendrik C.
Swart
* and
Hendrik C.
Swart
 *
*
Department of Physics, University of Free State, Bloemfontein 9300, Republic of South Africa. E-mail: sukul.pp@ufs.ac.za; swarthc@ufs.ac.za
First published on 12th July 2022
Abstract
Rare earth (RE) doped perovskite oxide hosts especially titanates, are promising phosphor materials in terms of white-light emission owing to their extraordinary properties such as an exceptional hosting environment for RE-ions and a switchable crystal phase near the phase boundary. Here, we report a new strategy of crystal phase modification to enhance the blue upconversion (UC) efficiency to such an extent that the combinational mixing of blue and green/red-emitting phosphor gives intense white emission. The Lead free (Ba0.85Ca0.15)(Zr,Ti)O3 ceramics were synthesised at different sintering temperatures by incorporation of Tm3+/Yb3+ ions as dopants. The UC quantum efficiency of the Tm3+/Yb3+:BCZT sample sintered at 1300 °C was recorded at different excitation power densities. It was observed that the crystal phase transformation from tetragonal to rhombohedral symmetry in the sample near the phase boundary plays a cruicial role in improving the quantum efficiency. White-light emission applications were demonstrated by preparing biphasic samples with powder mixing of a BCZT:Tm3+/Yb3+ (blue-emitting) + BCZT:Er3+/Yb3+ (green/red-emitting) phosphor, and their composition were optimised at a mixed ratio. Thereafter, photometric characterization (CIE chromaticity, colour purity and corelated colour temperatures) was performed, and it indicated the suitability of the current biphasic samples in direct white-light (cooler) applications on an industrial scale. Crystal phase modified blue emission efficiency enhancement is a key feature of this work, which helps to generate approximately pure white-light with ideal chromacity (∼0.333, 0.343) emission when Tm3+/Yb3+:BCZT is mixed with a green emitting BCZT:Er3+/Yb3+ phosphor.
1 Introduction
The research on upconversion (UC) fluorescent materials has come a long way in terms of their wide applicability areas,1–7 and, in the last 61 years, significant contribution has been made by a wide community of researcher working day and night to explore more from the UC phenomena. Among the different applications of UC materials, a major research has been done on white LEDs8–11 due to their potentiality to replace traditional light sources because of their following advantages, less hazardous to the environment, low energy consumption,10 and longer lifespan.11 Commonly, UC based white-light are generated in two basic methods (1) combining three independently optimized monochromatic phosphors (red, green and blue) in a proper mixing ratio in the presence of a mercury atmosphere, which was first introduced in 1996 by Downing et al.,12 (2) direct synthesis of a single UC phosphor that converts infrared light into a mixture of RGB. The mercury, present inside the lamp, creates a UV light that excites the phosphor coating inside the walls of the bulb to emit visible light. The former method results into an unstable white-light due to difference in distinct degradation rates of each monochromatic phosphor. Noh et al. shown tridoped phosphors has biomedical and display device applications through upconversion in Y2O3 phosphors.13 The march toward more environmentally friendly lighting involves the development of new phosphors and alternative processes. Recently, research interest has grown to fabricate single white-light phosphors by several lanthanide ion combinations. It has been exploited in sol–gel derived thin films14 to avoid the intrinsic colour balance,1 device complexity15 and high cost associated with such multiple emitting components. The possible reason for the trend to switch into single white-light emitting phosphors can be realised through the fact, that although researcher have tried to modulate the Ln3+ ion concentration in different phosphor host materials and also tried various synthesis techniques, the obtained results are not satisfactory at this stage. As a majority of the synthesized phosphors has relevant limitations16 since blue- and white-light emitters are not as efficient as the green and red ones. Therefore, if we can concentrate in improving the efficiency of blue-emitting phosphors, it may solve the problem.The UC efficiency can be modified by changing the dopant or choosing an appropriate host material of low phonon energy, high transparency, and outstanding chemical and thermal stability. Host materials with low phonon energy may decrease the probability of nonradiative transitions, consequently leading to an increase in the UC efficiency. The UC luminescence (UCL) efficiency depends on the concentration and combination of dopants which define the number of luminescent centres as well as on their spatial distance in the host material lattice. It is reported that the UCL efficiency can be regulated by changing the crystal phase, size and morphology of the host matrix17 and the possible solution lies here. Rare earth (RE) ions in a crystal experience electrostatic interaction and it is dependent on the position of the ion in the crystal and also the structure of the crystal. Same ions in various crystal structures of a compound give different emission intensity, such as Er3+ ions in β-NaYF4 give very high UC emission compared to the α-NaYF4. Tailoring the crystal field of lanthanide ions could be an effective strategy for enhancing the UCL of a wide variety of host materials. Despite recent progress in the development of UC material synthesis, the development of UC with enhanced efficiency is still inefficient, especially for blue-emitting materials. In our previous reports18,19 it was observed that the crystal phase transformation in RE-ion doped perovskite phosphor not only changes its structural properties but also shows huge luminescence emission enhancement. Moreover, we have observed that the PL enhancements work as signature codes or spectroscopic probes to detect any crystal phase changes occurring in the sample, as predicted by Polman20 in his findings exactly 21 years ago. Our investigations led us to the conclusion that RE-ion doped perovskite Pb(Zr,Ti)O3(PZT) can be a good multifunctional host for observing crystal-phase-modified luminescence near the morphotropic phase boundary (MPB).18 This material was extensively investigated because of its ultrahigh piezoelectric properties near the phase transition region (MPB), that is, the concurrence of rhombohedral- and tetragonal phases. PZT is the most widely used and investigated multifunctional material which possesses dielectric, piezoelectric, and electro-optic ceramics with diverse applications in industrial manufacturing, power production, transportation, consumer electronics, communication, medicine, and health care. However, in 2003, the European Union imposed a ban on the use of hazardous compounds in consumer items.21 Because lead zirconate titanate ceramics contain 60% harmful PbO, it is recommended to substitute PbO with similar nontoxic materials because of these environment legislations.22 Although serious work has been done on lead substitutes, very few of the reported Pb-free materials can compete with the PZT framework. As a consequence, a number of lead-free perovskite ceramics gathered considerable attention among researchers in recent years because of their eco-friendly behaviour.
BNT–BT (Bi1/2Na1/2TiO3–BaTiO3), KNN (K1−xNaxNbO3), BCZT [(Ba,Ca)(Zr,Ti)O3] and their co-doped counterparts are not only environment friendly but at the same time they also project good piezoelectric properties such as hard and soft PZT.21,22 Among these alternatives, BaTiO3 (BT)-based materials have been investigated because of their high piezoelectric constant d33 of pure BT which is ∼190 pC N−1 with very high permittivity (∼104–106). BT can be easily modified by the addition of appropriate dopants at different sites of the ABO3 lattice or by varying the sintering temperatures.23,24 Recently, large temperature tuning of RE ion-doped BT phosphors by PL and Raman studies was reported by De et al.25 At room temperature, BCZT ceramics, which is a solid solution BT with BaZrO3 or CaTiO3, exhibits interesting phase transition behaviour and piezoelectric properties26e.g. a phase boundary between rhombohedral (BZT side) and tetragonal (BCT side) phases similar to solid state solutions of PbZrO3 and PbTiO3 to form PZT18 at room temperature. Moreover, it also shows MPB boundary27 and is also free from any volatile impurities such as the PZT framework discussed in our previous report.18 In 2016, Wang et al.28 reported strong luminescence and Piezo properties were observed from praseodymium (Pr) replacing BCZT ions at the A-site. Ramovatar et al.29 also reported PL emission and structural property enhancement on Pr-doped BCZT. However, reports on UC luminescence on BCZT correlating with crystal phase studies have not yet reported. Exploring the crystal phase on multifunctional hosts such as BCZT will be of great interest, and there is a need to study optical tailoring properties to observe high UCL efficiency which is expected from BCZT. As our aim was to obtain white-light emission from a combinational phosphor material without the help of several RGB phosphors to avoid degrading loss, we have considered focusing on increasing the blue emission efficiency. Considering the probable high upconversion of the host and the broad range of colours achievable by proper RE dopant selection, attempts to produce white-light from this host are of significant technology interest.
The thulium (Tm3+) activator ion gives a strong blue emission at approximately 489 nm, but it is observed only in hosts with a lower phonon frequency. However, like the Er3+ activator ion the Tm3+ ion has a smaller absorption cross-section. Therefore, for efficient blue upconversion, we need Yb3+ ions as sensitisers along with Tm3+ ions, as the ytterbium ion has a large absorption at 980 nm and can efficiently transfer its energy from 2F5/2 level to the Tm3+ ion. There are very few reports where crystal-phase-tailored luminescence is observed in RE io-doped PZT, except for our previous reports.18,19 Similarly, crystal phase modified luminescence emission has not been reported for Tm3+/Yb3+ dopant combinations in BCZT hosts. These facts motivate us to study above combination for completing the objective of possible white-light generation using phase tailored blue upconversion.
In the present study, we report visible UC emission studies of Tm3+/Yb3+ co-doped BCZT ceramic phosphors, where the substitution of BCZT-A site (Host) is replaced by Yb3+ and Tm3+ ions The replacement of the RE ions in the host follows the rule of substitution, such as charge compatibility and similarity of ionic radii. The blue UC emission efficiency, which was influenced by the crystal phase transformation occurring in the sample, was tested. To generate white-light, the current blue-emitting phosphor is employed with a green/red-emitting phosphor of the same stoichiometry (BCZT:Er3+/Yb3+), which is optimised separately. Two powders, BCZT:Tm3+/Yb3+ (blue emission) and BCZT:Er3+/Yb3+ (green/red emission), were properly mixed using acetone-assisted grinding for 50 min in the second procedure. The biphasic samples generated by powder mixing are referred to as BCZT:Tm3+/Yb3+ + BCZT:Er3+/Yb3+ phosphor and their composition were optimised at a mixed ratio to generate white-light. Intense blue emission by using crystal phase modification was not studied in the past, especially for uniquely designed Tm3+/Yb3+ co-doped BCZT ceramic phosphors. The Tm3+/Yb3+:BCZT ceramic phosphor was synthesized using a novel modified microwave-assisted solid-state reaction. Moreover, the photometric characterization (CIE, CCT, colour purity) of the current biphasic sample in terms of industrial lighting applications is carried out for its use as a commercial cooler white-light source.
2 Experimental
2.1 Materials and methods
Highly efficient blue emissive phosphor was synthesised by using a modified conventional solid-state reaction method through a microwave (MW) assisted solid state reaction with simultaneous multistage heating and drying. The detailed MW assisted multistage synthesis process is shown in the flowchart in Fig. 1. Reagent grade pure raw powders of BaCO3, CaCO3, TiO2, ZrO2 Tm2O3 and Yb2O3 from Sigma Aldrich (Germany) were used for the sample preparation. The RE ion concentrations of Tm3+ and Yb3+ were taken as 0.15 mol% and 2.0 mol%, respectively, to get the optimum luminescence output.30 After being weighted in stoichiometric quantities, the starting materials were mixed with zirconia in isopropanol for 10 h in an agate mortar. The mixed slurry was then kept in a MW reactor oven (2.45 GHz frequency, 700 W power) for 5 h at 300 °C. This will create separate particles of the material that diffuse to the neighbouring powder particles, which is better than the calcination process. The advantage of MW heating over direct heat treatment is that the temperature profile of an MW material is the inverse of that seen in the conventional heat treatment in a furnace, resulting in the surface being cooler than the interior.31 The MW treated slurry was then placed in a furnace for 4 hours at 800 °C. The heat-treated batch was then wet mixed using a pure ethanol medium and again MW treated for another 2 h at 300 °C. After the second MW treatment, the dried slurry was sintered at four different temperatures to obtain powdered samples. Part of the mixed batch was mixed with a Polyvinylidene fluoride (PVDF) binding agent and subsequently pressed into a pellet form. In the final stage, the pellets were sintered at four different temperatures from a 1000 to 1300 °C for 10 h after the second MW treatment, and the samples were ready for measurements. | ||
| Fig. 1 Modified MW assisted multistage solid state synthesis flowchart of BCZT:Tm3+/Yb3+ ceramic phosphor. | ||
The synthesis technique of the green/red-emitting phosphor Er3+/Yb3+:BCZT can be found in the ESI.†
2.2 Characterization techniques
The crystal structure of the finely ground Tm3+/Yb3+:BCZT ceramic phosphor was recorded using a Bruker D8 advanced X-ray diffraction (XRD) measuring instrument with Cu-Kα target radiation (1.5405 Å) over a wide range of Bragg angles 2θ (10° ≤ 2θ ≤ 90°) with a scanning rate of 3 deg per min. The UV-Vis NIR absorption spectrum was taken in diffuse reflectance mode on a Lambda 950, UV-VIS-NIR spectrophotometer (PerkinElmer). The UC luminescence characteristics of the Tm3+/Yb3+:BCZT samples at room temperature were investigated on a compact spectrophotometer with a dual-mode F980, Edinburgh Instruments for different 979 nm laser excitation power densities.3 Results and discussion
3.1 Structural investigations
 | ||
| Fig. 2 (a) Powder X-ray diffraction pattern of Tm3+/Yb3+ doped BCZT ceramic sample sintered at 1300 °C. (b) Gaussian fitted diffraction peak (200) scanned separately in the 2θ region 43–46°. | ||
The (200) diffraction peak was broad enough to be fitted with a Gaussian distribution, which led us to two peaks individually representing (200)Tetragonal and (200)Rhombohedral. The results can be explained considering the co-existence of two crystal symmetries together near MPB, with phase transforming from tetragonal to rhombohedral symmetry in the sample sintered at 1300 °C. The occurrence of the two different crystal symmetries is also supported by Rietveld analysis.
The Rietveld refinement was implemented using the FullProf Suite program (Free version) for improved structural information. The diffraction peak experiences into (002)T and (200)T, indicating the presence of a tetragonal (T) phase (ICDD # 05-0626) with a space group of P4mm for the samples sintered at 1000–1200 °C. For sintering temperature 1300 °C, (002)T and (200)T these two peaks merges into a single one to indicate a rhombohedral symmetry. Moreover, in the rhombohedral symmetry the peak near 2θ = 66° splits into two (220) and (202) respectively. The structural phase transition from tetragonal to rhombohedral is also confirmed by Rietveld refinements as shown in Fig. 3.
 | ||
| Fig. 3 Structural investigation and Rietveld refinement results for the Tm3+/Yb3+ doped BCZT sample sintered at 1300 °C. | ||
Rietveld refinement was performed using (Ba, Ca) (Zr, Ti)O3 structure as a standard model, and the results of the refinements are shown in Table 1. The Rietveld refinement results for the samples sintered at 1000–1200 °C are shown in the ESI (see Fig. ESI 1).† The intermediate phases were also considered in the refinement process. The shifting of the diffraction peaks (002/200) towards lower 2θ values may be attributed to the larger ionic radius of Tm3+ (0.88 Å) compared to that of Ti4+ (0.68 Å), and Zr4+ (0.72 Å). The positions of the dopant ions (Tm3+/Yb3+) could not be determined directly through the refinement. The probable locations in the crystal structure can still be inferred. The most likely case is that the Tm3+ ions replaced the Ba2+ and another charge was compensated by the Ti4+ ion at the B-site. The reduced χ2 value of the refined data was found to be 1.4548 for the sample sintered at 1300 °C and it confirms that a rhombohedral crystal symmetry has formed in the sample. The crystal phase transformation (tetragonal to rhombohedral) occurring in the sample increased the dopant ion transiting capabilities. A phase change from lower crystal symmetry to higher crystal symmetry is expected to help RE ion-dopants to give much better optical emission property. For our reference we have also recorded the XRD pattern for samples sintered at 1150 °C and 1250 °C to see any intermediate phase change or any change in diffraction peaks. The related plots are shown in Fig. (ESI 2).† The result shows no change in the diffraction peaks which confirms that the phase change occurs exactly at the 1300 °C sintering temperature.
| Lattice and fitting parameters | BCZT:Tm3+/Yb3+ | |||
|---|---|---|---|---|
| 1300 °C | 1200 °C | 1100 °C | 1000 °C | |
| a (Å) | 4.0059 | 4.0008 | 4.0064 | 4.0061 |
| b (Å) | 4.0008 | 4.0008 | 4.0064 | 4.0064 |
| c (Å) | 4.0123 | 4.0154 | 4.0064 | 4.0064 |
| V (Å3) | 64.3 | 64.3 | 64.3 | 64.3 |
| Structure | Rhombohedral | Tetragonal | Tetragonal | Tetragonal |
| Space group | R3m | P4mm | P4mm | P4mm |
| ICSD No. | 85–0368 | 05–0626 | 05–0626 | 05–0626 |
| Reduced χ2 | 1.4548 | 1.7928 | 1.7926 | 1.7926 |
| R wp (%) | 7.71 | 7.55 | 9.59 | 9.28 |
| R p (%) | 6.33 | 4.23 | 5.41 | 6.23 |
The FESEM images of the Tm3+/Yb3+:BCZT samples sintered at four different temperatures are shown in ESI 3.† All the micrographs were taken with a 40.00 kX magnification with EHT at 5.00 kV and WD = 2.9 mm. All samples showed micrometer size particles. Since the samples were crushed for the measurements and dispersed on the sample holder, it is hard to comment about the porosity of the samples. As the sintering temperature was increased the smaller particles grew into larger particles. The sample sintered at 1300 °C has the largest particle size with well-defined grain boundaries. The average particle size increased from 0.4 to 1 μm.
3.2 Optical emission studies
980 nm = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 204 cm−1 204 cm−1 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 204 cm−1 + 10 204 cm−1 + 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 204 cm−1 = 20 204 cm−1 = 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 408 cm−1 = 490 nm 408 cm−1 = 490 nm |
 | ||
| Fig. 6 Schematic illustrations of the energy level diagram using possible UC mechanism responsible for optical emissions. | ||
The energy from this virtual level was radiatively transferred to the 1G4 level of the Tm3+ ion. The 1G4 level relaxes further to lower levels and populates them. Also, at a small probability the process 2F5/2 (Yb3+) + 3H6 (Tm3+) → 2F7/2 (Yb3+) + 3H5 (Tm3+) also happens. The 3H5 level quickly depopulates via multi-phonon relaxation to the lower lying 3F4 level. The 3F4 level absorbs 980 nm excitation and populates the 3F2 level. The radiative emission wavelengths and associated transitions are shown in Fig. 6.
 | (1) |
Here, qp.emission is the upconverted emission photon flux (in photons per sec) and qp.absorbed is the photon flux absorbed by the sensitizer species (in photons per sec):
 | (2) |
Here, Iuc(λ) is the emission intensity (in photons per sec per nm), and λ1 and λ2 represents the boundary wavelengths of the complete upconverted emission spectrum, or the 4f–4f transition of interest, respectively. qp.absorbed is calculated by integrating over the excitation wavelength range λ3 to λ4, and subtracting the intensity of the excitation source that has passed through the sample (Iexc-sample, in photons per s per nm) from the intensity of the excitation source that has passed through a blank sample (work as reference sample) (Iexc-blank). A dispersion of undoped BCZT sample of identical size distributed in the same solvent as the sample with the similar concentration was used as blank sample. Absence of optically active dopant makes it suitable for reference sample which doesn't absorb the excitation wavelength.
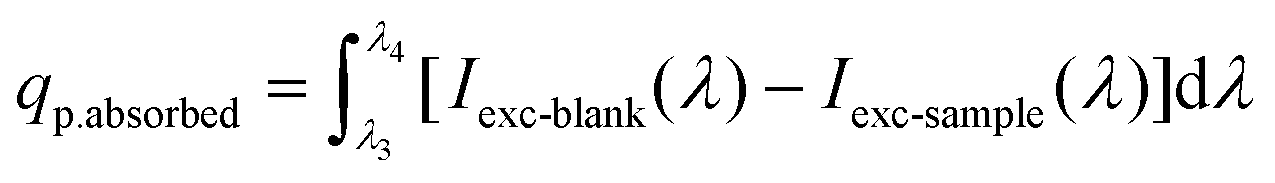 | (3) |
The eqn (2) can be expressed as,
 | (4) |
The spectrometer and the integrating sphere were calibrated such that the measured intensities are directly proportional to the photon flux, i.e. (λ) ∝ [mol of photons per s per nm]. As a result, integrating these values over the boundary wavelength range will directly count the flux of photons. Because the intensity of the upconverted light is lower than that of the stimulating laser source, the sample absorption and emission cannot be detected simultaneously, as the laser light saturates the spectrometer, preventing simultaneous measurement of UC. To overcome this, the absorption was measured using a neutral density (ND) filter with a specified transmittance (∼99.85% attenuation). To measure the absorbed photon flux, this filter was added between the integrating sphere and the spectrometer. To remove the excitation light before measuring the upconverted emission, this filter was replaced with an OD4 short pass filter (875 nm). Over the wavelength range of the laser (950–990 nm), the attenuation factor Fattn was averaged. The short pass filter used to detect the intensity of the upconverted emission was also employed to account for the light's minimal absorption. The UC luminescence intensity was divided by the short pass filter's transmission curve T(λ) in the wavelength range of the upconverted light. Due to the short pass filter's significant absorption of light with a wavelength shorter than 430 nm, this approach was unable to detect any UV emission. The accordingly corrected equation for ΦUC is eqn (5):
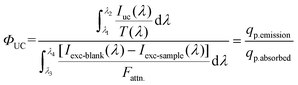 | (5) |
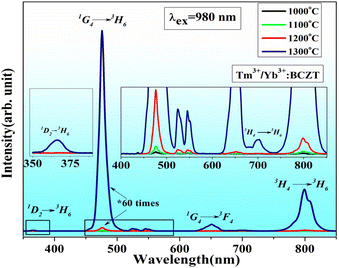
Fig. 7 shows the quantum yield for different samples measured based on eqn (1–5) using the above discussed setup. The quantum yield shown by the 479 nm blue UC emission was found to be the highest.
 | ||
| Fig. 7 Sample sintering temperature dependence of the absolute quantum yield ΦUC of the major and minor emission bands of Tm3+/Yb3+:BCZT ceramics. | ||
Moreover, the stability of the quantum yield can be determined by varying the pump power densities of the excitation pump. Quantum yields for a wide range of power dependencies were observed and plotted using the logarithmic relation shown in Fig. 8(a). The blue-emission band of Tm3+/Yb3+:BCZT exhibited the highest quantum yield over a wide range of pump power densities. These results also support the idea of crystal-phase-modified UC emission enhancement. To better understand the degradation and stability the quantum yield of the phosphors, we also investigated the QY in the Tm3+/Yb3+:BCZT thin films, as shown in Fig. 8(b). It is observed that the deposited phosphor thin film also shows the high yield values in the order of 1 × 10−3. The results predicted that the current phosphor material could also be individually used as a commercial blue-emitting phosphor in device fabrication with higher emissivity. The deposition technique of the Tm3+/Yb3+:BCZT thin film on a Si- substrate is described in the ESI.†
The UC emission studies of the Er3+/Yb3+:BCZT ceramic were performed using 980 nm CW laser irradiation. The ambient condition emission spectra of Er3+/Yb3+:BCZT ceramic samples sintered at 1300 °C are shown in figure (ESI 5†). Two intense green emission bands at 524 and 543 nm, assigned to the popular 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions of trivalent erbium, respectively, were observed in the sample.
The UC emission spectra of the white-emitting biphasic sample under 980 nm excitations are presented in Fig. 9. In the visible range, three primary emission bands centred at 479, 550, and 673 nm were observed. Based on the RGB emissions, it was plausible to obtain multicolour emissions, including white-light by appropriately adjusting the dopant concentrations. All the samples had high emissions in the near-infrared range, centred at 800 nm. The compositions, x and y calculated colour coordinates, excitation power densities, and room-temperature UC efficiencies of the selected biphasic sample under 980 nm stimulation are listed in Table 2.
 | ||
| Fig. 9 Typical emission spectra of white emitting biphasic sample (Tm3+/Yb3+:BCZT + Er3+/Yb3+:BCZT) under 980 nm excitation. | ||
| Powder 1 (mass m1) Tm3+/Yb3+:BCZT | Powder 2 (mass m2) Er3+/Yb3+:BCZT | m 2/m1 (sample acronyms) | P inc (w cm −2 ) | x | y | η UC |
|---|---|---|---|---|---|---|
| 0.15%, 2.0% | 0.2%, 2.0% | 10(S_1) | 35 | 0.329 | 0.343 | 0.30 |
| 15(S_2) | 45 | 0.331 | 0.344 | 0.35 | ||
| 10(S_3) | 40 | 0.348 | 0.341 | 0.40 | ||
| 10(S_4) | 45 | 0.313 | 0.343 | 0.35 | ||
| 5(S_5) | 45 | 0.313 | 0.333 | 0.33 | ||
| 10(S_6) | 30 | 0.311 | 0.333 | 0.27 | ||
| 5(S_7) | 30 | 0.321 | 0.333 | 0.25 |
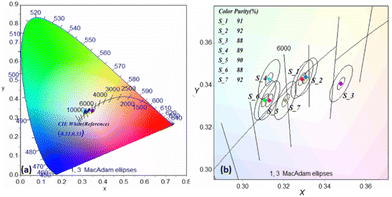
At low excitation power densities (as low as 35 W cm−2), biphasic materials generate white-light. Variations in the excitation power density had a little effect on the colour coordinates for a given sample composition (Table 2). The calculated chromaticity coordinates (x, y) are (0.331, 0.344), which are very close to the standard equal energy white-light co-ordinates (0.333, 0.333) according to the 1931 CIE diagram, (Fig. 11). Fig. 10 shows the images of the samples taken from a canon mirror-less camera with a longer exposure time of 5′′, ISO 200 aperture f6.3 and exposure +21/2, when the samples irradiated with a 980 nm diode laser at the referred power densities. From the images, it was found that the biphasic sample (S_2) showed the maximum efficiency and intense white-light. The images were captured using an NIR filter and a neutral density (ND) filter which blocked the NIR emission from the samples. Images without the NIR filter appears to be reddish and can be seen in the ESI 6.† In addition, ESI 6† contains the (a) biphasic sample emission image with longer NIR exposure (laser induced heating) (b) emission image from the sample without NIR filter, (c) top view of the sample holder, (d) horizontal view of the sample holder in normal light (e) full 980 laser setup for recording the white emission.
 | ||
| Fig. 10 Images of the biphasic samples with different combinational ratio with referred power densities. | ||
 | ||
| Fig. 11 The CIE 1931 chromaticity diagram of the biphasic samples with mixing ration referred at Table 1 with excitation of 980 nm lasers. | ||
The present biphasic material showed higher UC efficiencies of ∼0.30% at power densities as low as 35 W cm−2 (Table 2) and ∼0.40% at 40 W cm−2. Conventionally, it has been found that blue-emitting phosphors are an order of magnitude lower than green- or orange-emitting phosphor. However, the present blue-emitting phosphor (Tm3+/Yb3+:BCZT) showed exceptional quantum efficiency at different power densities (Fig. 8), which was due to the crystal phase transformation occurring in the sample discussed above. Taking advantage of the crystal symmetry change occurring in the sample, the blue-emitting phosphors can be efficiently employed in the white-light generation. Table 1 shows that to achieve white-light in biphasic materials, only 10 times more blue-emitting phosphors are required than green-emitting phosphors. Here, special emphasis is given to increase the efficiency of the blue-emitting phosphors via the crystal phase transformation occurring in the sample, which resulted in a significant increase in the overall white-light generation efficiency.
In three essential ways, we feel our samples are the best-reported white upconverting materials. First, as compared to materials for which UC efficiencies have been published; they exhibit greater blue UC emission efficiencies (Table 3). More importantly, the conventional blue-emission efficiency is significantly increased by using the crystal phase transformation from tetragonal to rhombohedral symmetry. This type of optical strategy has never been used to achieve white-light emissions. They also achieved high efficiencies when using lower excitation power densities than previously reported perovskite phosphor materials. It is worth noting that the power density for UC white-light generation and their efficiencies were not provided in many of the reports. For the reasons discussed above, the current biphasic ceramic phosphor (Tm3+/Yb3+:BCZT + Er3+/Yb3+:BCZT) is a promising candidates for a various applications, including display backlighting and WLED manufacturing.
| Host | Dopants | Excitations | CIE colour coordinate (x, y) | η UC |
|---|---|---|---|---|
| Oxyfluoride glass ceramic doped with YF3 nanocrystals37 | Yb3+,Er3+,Tm3+ | 976 nm pulsed laser (2 ps, 15 nJ, 2 W mm−2) | x = 0.310, y = 0.359 | 0.2 |
| BiPO4 submicron particles38 | Yb3+,Er3+,Tm3+,Ho3+ | 976 nm CW laser | x = 0.318, y = 0.356 | |
| Tridoped YBO3 phosphors39 | Dy3+,Eu3+,Tb3+ | 365 nm UV-LED laser | x = 0.30, y = 0.33 | |
| YNbO4 phosphors40 | Yb3+,Er3+,Tm3+ | 808 nm CW laser (2 W mm−2) | x = 0.340, y = 0.359 | |
| Y2O3 nanocrystals41 | Yb3+,Er3+,Tm3+ | 976 nm pulsed laser (down to 100 mW cm−2) | x = 0.310, y = 0.359 | |
| Lu3Ga5O12 nanocrystals42 | Yb3+,Er3+,Tm3+ | 976 nm pulsed laser (down to 34 mW cm−2) | x = 0.270, y = 0.338 | |
| Transparent oxyfluoride glass, ceramic embedded with YF3 nanocrystals43 | Yb3+,Ho3+,Tm3+ | 976 nm pulsed laser (2 ps, 15 nJ, 2 W mm−2) | x = 0.351, y = 0.306 | 0.1 |
| Fluorolead germanate glass44 | Yb3+,Ho3+,Tm3+ | 976 nm pulsed laser (2 ps, 15 nJ, 2 W mm−2) | x = 0.344, y = 0.364 | |
| Tellurite glass45 | Yb3+,Er3+,Pr3+ | 976 nm pulsed laser (2 ps, 15 nJ, 2 W mm−2) | x = 0.310, y = 0.335 |
We further checked the colour purity of the biphasic samples from S_1 to S_7 to support “colour consistency” factor, a term used by the lighting industry utilizes a colour tolerance system in conjunction with CCT. Fig. 12 shows the colour purity calculation diagram of the different biphasic samples on the locus of the blackbody curve in the CIE diagram. The inorganic emissions of narrow-band lights are characterized by the dominant wavelength and colour purity, whereas broadband lights are characterized by CCT. Colour purity was calculated using colour calculator software and the procedures were repeated as in our previous work.33 The colour purity for all the samples retained a value within 88–92%, which is higher than reported in the literatures.36 Therefore, biphasic sample combinations have the advantage of high colour purity and near-white colour coordinates with cooler white-light emissions, which can be employed readily for industrial lighting applications.
4 Conclusions
In pursuit of efficient upconverted white-light emission from RE ion-doped phosphors, two individual phosphors that are Tm3+/Yb3+:BCZT + Er3+/Yb3+:BCZT were mixed in a specific ratio (Table 2) and excited with a 980 nm CW diode laser. Tm3+/Yb3+ co-doped BCZT ceramic phosphors were synthesised using a novel microwave assisted multistage solid state reaction route. The synthesis route and sintering temperatures were specially designed to observe the crystal phase change in the Tm3+/Yb3+:BCZT near the MPB. The quantum efficiency of 479 nm blue emissions was found to be exceptionally good on the verge of the crystal phase transformation occurring in the sample. Moreover, the quantum efficiencies of the blue phosphor were high in a wide range of excitation power densities (from moderate to high), implying that the stability of the blue emission was also high. A strategy to enhance the blue UC emission efficiency through crystal phase modulation was achieved, and biphasic phosphor samples were characterised by the addition of an Er3+/Yb3+:BCZT phosphor, which was optimized separately. White-light was generated in all samples mixed at different ratios (Table 2). It was found that only 10 times bluer phosphor than green phosphor is required to produce ideal chromatic white-light emission. Moreover, the current biphasic phosphor shows higher UC efficiencies of ∼0.30% at power densities as low as 35 W cm−2 and ∼0.40% at 40 W cm−2 (white-light emission through frequency UC), which is the best result ever reported to our knowledge. The main advantage of generating white-light through the referred process is that two different phosphors are optimized separately with threshold efficiency values; only their mixing ratio will determine the ultimate desirable results. The present samples emitted white-light at moderate low power with almost ideal white chromaticity with analogous to the NTSC standards. The blue emission (479 nm) intensity significantly increased in comparison to NIR (808 nm) wavelengths almost (x 60 times) upon sintering temperature change in Tm3+/Yb3+:BCZT and the anomalous behaviour is explained using the crystal phase transformation occurring in the sample. This improved UC strategy can be used to enhance white-light applications through structural modulation. Photometric characterisations show that the emitted white-light can be used as a cooler white-light source because of its observed higher CCT value (∼5500 K) with a higher CRI value (90.56). The present biphasic phosphor has lab scale success in generating highly efficient (0.40%) upconverted white-light with a colour purity of ∼92%; it is also expected that the present phosphor will be fruitful at the industrial scale.Author contributions
The original idea, design, conceptualization of the work, experiments, Data analysis and interpretation and drafting of the article were done by PPS. Supervision, funding, resources, critical review, scientific suggestions and editing were done by HCS.Conflicts of interest
The authors have no conflicts of interest to declare. All co-authors have seen and agree with the contents of the manuscript and there is no financial interest to report. We certify that the submission is original work and is not under review at any other publication.Acknowledgements
The authors express their sincere thanks to the South African Research Chairs Initiative of the Department of Science and Technology and the National Research Foundation of South Africa (84415). The financial assistance from the University of the Free State, South Africa is highly recognized.References
- J. Wang and P. A. Tanner, J. Am. Chem. Soc., 2010, 132, 947–949 CrossRef CAS PubMed.
- C. Cao, Q. Liu, M. Shi, W. Feng and F. Li, Inorg. Chem., 2019, 58, 9351–9357 CrossRef CAS PubMed.
- K. Kim, S. K. Nam, J. Cho and J. H. Moon, Nanoscale, 2020, 12, 12426–12431 RSC.
- S. Asahi, H. Teranishi, K. Kusaki, T. Kaizu and T. Kita, Nat. Commun., 2017, 8, 14962 CrossRef CAS PubMed.
- J. Wang, N. He, Y. Zhu, Z. An, P. Chen, C. A. Grimes, Z. Nie and Q. Cai, Chem. Commun., 2018, 54, 591–594 RSC.
- K. Li, D. Zhu and H. Lian, J. Alloys Compd., 2020, 816, 152554 CrossRef CAS.
- K. Li, D. Zhu and C. Yue, J. Mater. Chem. C, 2022, 10, 6603–6610 RSC.
- Y. Li, J. Zhang, Y. Luo, X. Zhang, Z. Hao and X. Wang, J. Mater. Chem., 2011, 21, 2895–2900 RSC.
- B. Zhou, L. Tao, Y. H. Tsang and W. Jin, J. Mater. Chem. C, 2013, 1, 4313–4318 RSC.
- J. Li, Y. Long, Q. Zhao, S. Zheng, Z. Fang and B.-O. Guan, Opt. Express, 2021, 29, 21763–21772 CrossRef CAS PubMed.
- J. Wang, R. Deng, M. A. MacDonald, B. Chen, J. Yuan, F. Wang, D. Chi, T. S. A. Hor, P. Zhang, G. Liu, Y. Han and X. Liu, Nat. Mater., 2014, 13, 157–162 CrossRef CAS PubMed.
- E. Downing, L. Hesselink, J. Ralston and R. Macfarlane, Science, 1996, 273, 1185–1189 CrossRef CAS.
- H. M. Noh, J. H. Oh, J. H. Jeong, S. H. Park and B. C. Choi, Curr. Appl. Phys., 2022, 39, 190–195 CrossRef.
- S. Sivakumar, F. C. J. M. van Veggel and M. Raudsepp, J. Am. Chem. Soc., 2005, 127, 12464–12465 CrossRef CAS PubMed.
- C.-W. Yeh, W.-T. Chen, R.-S. Liu, S.-F. Hu, H.-S. Sheu, J.-M. Chen and H. T. Hintzen, J. Am. Chem. Soc., 2012, 134, 14108–14117 CrossRef CAS PubMed.
- I. Etchart, M. Bérard, M. Laroche, A. Huignard, I. Hernández, W. P. Gillin, R. J. Curry and A. K. Cheetham, Chem. Commun., 2011, 47, 6263–6265 RSC.
- F. Wang, Y. Han, C. S. Lim, Y. Lu, J. Wang, J. Xu, H. Chen, C. Zhang, M. Hong and X. Liu, Nature, 2010, 463, 1061–1065 CrossRef CAS PubMed.
- P. P. Sukul, K. Kumar and H. C. Swart, OSA Continuum, 2018, 1, 971 CrossRef CAS.
- P. P. Sukul, M. K. Mahata, U. K. Ghorai and K. Kumar, Spectrochim. Acta, Part A, 2019, 212, 78–87 CrossRef CAS PubMed.
- A. Polman, Physica B: Condens. Matter, 2001, 300, 78–90 CrossRef CAS.
- J. Rödel, W. Jo, K. T. P. Seifert, E.-M. Anton, T. Granzow and D. Damjanovic, J. Am. Ceram. Soc., 2009, 92, 1153–1177 CrossRef.
- E. Cross, Nature, 2004, 432, 24–25 CrossRef CAS PubMed.
- T. Karaki, K. Yan, T. Miyamoto and M. Adachi, Jpn. J. Appl. Phys., 2007, 46, L97–L98 CrossRef CAS.
- L. Dong, D. S. Stone and R. S. Lakes, J. Appl. Phys., 2012, 111, 84107 CrossRef.
- A. De and R. Ranjan, Mater. Horiz., 2020, 7, 1101–1105 RSC.
- I. Coondoo, N. Panwar, H. Amorín, V. E. Ramana, M. Algueró and A. Kholkin, J. Am. Ceram. Soc., 2015, 98, 3127–3135 CrossRef CAS.
- W. Liu and X. Ren, Phys. Rev. Lett., 2009, 103, 257602 CrossRef PubMed.
- Z. Wang, W. Li, R. Chu, J. Hao, Z. Xu and G. Li, J. Alloys Compd., 2016, 689, 30–35 CrossRef CAS.
- Ramovatar, I. Coondoo, S. Satapathy and N. Panwar, Ceram. Int., 2018, 44, 1690–1698 CrossRef CAS.
- P. P. Sukul, Amitabh and K. Kumar, OSA Continuum, 2018, 1, 1087–1096 CrossRef CAS.
- H. J. Kitchen, S. R. Vallance, J. L. Kennedy, N. Tapia-Ruiz, L. Carassiti, A. Harrison, A. G. Whittaker, T. D. Drysdale, S. W. Kingman and D. H. Gregory, Chem. Rev., 2014, 114, 1170–1206 CrossRef CAS PubMed.
- P. P. Sukul and K. Kumar, in Proceedings of SPIE - The International Society for Optical Engineering, 2017, vol. 10448.
- P. P. Sukul, K. Kumar and H. Swart, Dalton Trans., 2022, 51, 2827–2839 RSC.
- H. Dong, L. D. Sun and C. H. Yan, Nanoscale, 2013, 5, 5703–5714 RSC.
- M. W. Pin, E. J. Park, S. Choi, Y. Il Kim, C. H. Jeon, T. H. Ha and Y. H. Kim, Sci. Rep., 2018, 8, 2199 CrossRef PubMed.
- Y. N. Ahn, K. Do Kim, G. Anoop, G. S. Kim and J. S. Yoo, Sci. Rep., 2019, 9, 16848 CrossRef PubMed.
- D. Chen, Y. Wang, K. Zheng, T. Guo, Y. Yu and P. Huang, Appl. Phys. Lett., 2007, 91, 251903 CrossRef.
- Z. Wang, J. Feng, M. Pang, S. Pan and H. Zhang, Dalton Trans., 2013, 42, 12101–12108 RSC.
- K. Das, A. Marathe, X. Zhang, Z. Zhao and J. Chaudhuri, RSC Adv., 2016, 6, 95055–95061 RSC.
- F. F. do Carmo, J. P. C. do Nascimento, M. X. Façanha and A. S. B. Sombra, Mater. Lett., 2019, 65–68 CrossRef CAS.
- G. Y. Chen, Y. Liu, Y. G. Zhang, G. Somesfalean, Z. G. Zhang, Q. Sun and F. P. Wang, Appl. Phys. Lett., 2007, 91, 133103 CrossRef.
- V. Mahalingam, F. Mangiarini, F. Vetrone, V. Venkatramu, M. Bettinelli, A. Speghini and J. A. Capobianco, J. Phys. Chem. C, 2008, 112(46), 17745–17749 CrossRef CAS.
- D. Chen, Y. Wang, N. Yu, P. Huang and F. Weng, J. Solid State Chem., 2008, 181(10), 2763–2767 CrossRef CAS.
- A. S. Gouveia-Neto, L. A. Bueno, R. F. Do Nascimento, E. A. Da Silva, E. B. Da Costa and V. B. Do Nascimento, Appl. Phys. Lett., 2007, 91, 091114 CrossRef.
- S. B. Rai, Y. Dwivedi and A. Ray, J. Appl. Phys., 2008, 104, 043509 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2dt01962k |
| This journal is © The Royal Society of Chemistry 2022 |