Self-assembly of octanuclear Ln(iii)-based clusters: their large magnetocaloric effects and highly efficient conversion of CO2†
Abstract
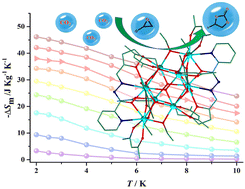
The design and construction of high-nuclear lanthanide clusters with fascinating topology and functional properties have been an active area of research, however, the development of an effective approach for obtaining high-nuclear lanthanide clusters with multifunctional properties is still extremely difficult. Up to now, a systematic approach for guiding the further expansion of Ln(III)-based clusters showing good functional properties is lacking. Herein, we design and synthesize a polydentate Schiff base ligand (HL), which reacts with β-diketonate salts Ln(acac)3·2H2O, and a series of Ln8 clusters [Ln8(acac)6(L)2(μ3-O)6(μ2-C2H5O)4(μ2-Hacac)2]·2CH3CN (Ln(III) = Gd (1), Dy (2), and Ho (3); HL = pyridine-2-carboxylic acid (5-hydroxymethyl-furan-2-ylmethylene)-hydrazide, Hacac = acetylacetone) have been successfully synthesized. Single-crystal X-ray diffraction studies reveal that clusters 1–3 are isostructural and can be viewed as a Ln8 core bridged by eighteen μ2-O atoms, six μ3-O atoms and two μ4-O atoms. Magnetic studies show that cluster 1-Gd8 displays a large magnetocaloric effect with −ΔSm = 46.14 J kg−1 K−1 (T = 2.0 K and ΔH = 7.0 T); cluster 2-Dy8 exhibits single-molecule magnet behavior under zero-field conditions. It is worth mentioning that the −ΔSm of cluster 1-Gd8 is larger than that of most reported polynuclear Gd(III)-based clusters; the 2-Dy8 cluster is one of the rare polynuclear Lnn SMMs (n ≥ 8) under zero dc field. Importantly, these Ln(III)-based clusters (1–3) can catalyze the cycloaddition of CO2 with epoxides with high efficiency under mild conditions; and cluster 1-Gd8 as a catalyst could be reused at least three times without obvious loss of catalytic performance.


 Please wait while we load your content...
Please wait while we load your content...