 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Catalytic hydrogenation of nitrocyclohexane as an alternative pathway for the synthesis of value-added products
Emil
Kowalewski
 * and
Anna
Śrębowata
* and
Anna
Śrębowata
 *
*
Institute of Physical Chemistry, Polish Academy of Sciences, ul. Kasprzaka 44/52, PL-01224 Warsaw, Poland. E-mail: ekowalewski@ichf.edu.pl; asrebowata@ichf.edu.pl
First published on 29th July 2022
Abstract
Catalytic hydrogenation of nitrocyclohexane could be an alternative source of various useful chemicals: cyclohexanone oxime, cyclohexanone, cyclohexanol, cyclohexylamine and dicyclohexylamine. Each one of these compounds found application in the modern chemical industry and has been produced on a large scale in multi-step processes. The possibility of direct synthesis via selective hydrogenation of nitrocyclohexane seems to be an excellent alternative. A thorough literature review shows broad research potential in this topic. Herein, we present the state of the art on nitrocyclohexane hydrogenation under batch and flow conditions and indicate possible directions for further studies in this topic.
1. Introduction
As a leading provider of materials for other industrial branches, the chemical industry is crucial to the global economy. Therefore, even a slight change in any chemical process may have a tremendous impact on many areas related to human activity. Hence, there is a strong need to search for novel, environmentally-friendly and inexpensive synthesis methods or modifications of the currently used ones. For instance, switching from batch to flow is one of the most often mentioned priorities for sustainable manufacture in the fine chemical sector.1A significant increase in interest in flow chemistry has been observed since the 2000s.2 Most of the previously conducted research was focused on batch processes.3 Wegner et al.4 suggested that the reason for that state of affairs in the past was related to the lack of flow equipment for laboratory-scale research. However, the systems for three-phase catalytic hydrogenation, for example developed by Kobayashi et al.,5 have opened entirely new perspectives for flow reaction studies. Among the many advantages of flow chemistry, easy scale-up and process intensification are the most interesting from the industrial point of view.6–8 Furthermore, a simple modification of the reaction conditions supports the procedure of the process optimisation, which can be performed even by automatic systems.9,10
The transition from batch to flow operation conditions is not the ultimate goal for all industrial syntheses, especially in the case of well-established manufacturing processes. The possibility and profitability of such transformations have been the subject of much research in the past two decades.11–15 Some researchers approached this issue in a very comprehensive and universal manner. For instance, Calabrese and Pissavini16 suggested a screening algorithm for batch reactions to determine the feasibility of transition processes. Similar work was performed by Teoh et al.17 by proposing practical assessment technology for converting fine chemicals production from batch to continuous. Nevertheless, the most important conclusion from these studies11–17 is the need for an in-depth analysis of already performed research and the use of developed solutions.
Nitrocyclohexane hydrogenation products
Catalytic hydrogenation of nitrocyclohexane is a promising source of various value-added chemical compounds. This reaction has been performed in batch reactors since the 1940s,18 and it has enormous and unfortunately still untapped application potential. The lack of laboratory-scale continuous flow equipment seems to be a reasonable explanation for the underestimation of this reaction.4 Despite the simplicity of nitrocyclohexane (NC), the process of its hydrogenation could be very challenging due to follow-up hydrogenation and competing reactions, which are presented in Scheme 1. Hence, easy control of reaction conditions and the usage of an appropriate catalyst are essential to achieve desired activity and selectivity to specific products. Among them are cyclohexanone oxime, cyclohexanone, cyclohexylamine, cyclohexanol and dicyclohexylamine. Each one of these compounds found application in modern industry.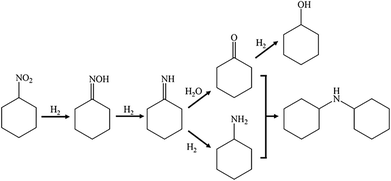 | ||
| Scheme 1 Mechanism of nitrocyclohexane hydrogenation proposed by Wang et al.19 Reprinted (adapted) with permission from Wang et al.19 Copyright {2013} Elsevier. | ||
Cyclohexylamine – is a colourless aliphatic amine compound in liquid form, which is miscible with water. It is used as a versatile intermediate in the production of herbicides, insecticides, artificial sweeteners (sodium/calcium cyclamate), corrosion inhibitors, rubber vulcanising additives, plasticisers and a lot more.20,21 Currently, cyclohexylamine is produced on an industrial scale by several different methods. The most commonly used is catalytic hydrogenation of aniline under elevated pressure. In this process, satisfactory efficiency is obtained with Ni and Co catalysts treated with basic oxides,22–24e.g. RANEY®-Co treated with sodium carbonate and calcium oxide gives more than 96% yield at 503 K and 60 bar.25 The other method of cyclohexylamine production is based on the synthesis from cyclohexanol and ammonia with a Co catalyst in a continuous reactor. Cyclohexanol reacts with at least 3 mol of ammonia in the presence of circulating hydrogen over a fix-bed catalyst at 493 K and 20 bar.25 Alternatively, Ni or Co catalysts can be used for the hydrogenation of cyclohexanone in the process carried out at 1–20 bar. Finally, cyclohexylamine can be obtained from the hydrogenation of phenol in the presence of ammonia over a rhodium catalyst.26 Most of these processes require high temperature and pressure, hydrogen excess and a large amount of ammonia to prevent side reactions, which cause waste formation. The commercially available cyclohexylamine purity is over 99.5%, in which the impurities are ammonia and water.
Dicyclohexylamine – is a colourless liquid secondary amine, sparingly soluble in water. It is used as a valuable chemical intermediate in the production of rubber vulcanisation accelerators, textiles, commercial insecticides, varnishes and corrosion inhibitors.27 Currently, it is produced by catalytic hydrogenation of aniline at elevated pressure and temperature over a Ru/Pd catalyst.28 This process leads to the formation of the cyclohexylamine/dicyclohexylamine mixture, which is hard to separate.27 Dicyclohexylamine as a main product can be formed by the amination of cyclohexanone under hydrogenation conditions29 or from cyclohexanone and cyclohexylamine at 4 bar with a Pd/C catalyst.29 Alternatively, it can also be obtained by the reaction of phenol and aniline over Pd/C under hydrogenation conditions at 5 bar.30–32
Cyclohexanol – is a secondary alcohol. This colourless substance creates crystalline needles with a camphor-like odour. Mild oxidation or catalytic dehydrogenation leads to cyclohexanone, whereas stronger oxidation gives adipic acid.33 Cyclohexanol is an essential intermediate in the production of Nylon-6. The mixture of cyclohexanol and cyclohexanone together is called KA oil (ketone-alcohol oil) – necessary for the production of adipic acid. Most of cyclohexanol is produced by liquid phase air oxidation of cyclohexane,33,34 which gives a cyclohexanone/cyclohexanol mixture. The ratio between these compounds could be modified by changing the production parameters or the application of the specific metal catalyst. The presence of anhydrous boric acid in the process leads to a higher yield of cyclohexanol, thanks to the trapping of the intermediate product.33 An alternative synthesis procedure involves hydrogenation of phenol over metal catalysts, e.g. the RANEY® nickel catalyst under specific conditions gives 99.9% selectivity to cyclohexanol.35 However, the only remaining large-phenol based plants belong to DSM in Holland and Allied in the Unites States.36
Cyclohexanone – is a cyclic ketone in the form of colourless liquid with an acetone-like odour. It can be hydrogenated to cyclohexanol, but also under more reductive conditions, it could be transformed into cyclohexane.33 The mixture of cyclohexanone and cyclohexanol could be used in the synthesis of adipic acid.33 It is also used to synthesise pharmaceuticals, plasticisers, herbicides and growth regulators for plants. However, the most important application, from the industrial point of view, is the synthesis of cyclohexanone oxime in the reaction with hydroxylamine. Cyclohexanone is synthesised similarly to cyclohexanol, mainly by the liquid phase air oxidation of cyclohexane.33 The addition of chromium(III) to the air oxidiser promotes the formation of cyclohexane.37 Cyclohexanone can be also prepared by dehydrogenation of cyclohexanol.36
Cyclohexanone oxime – is a solid colourless compound. The main application of cyclohexanone oxime is the role of an intermediate in the enormous industry of Nylon-6 production. It is transformed into ε-caprolactam via Beckman rearrangement. There are several commercial processes for the production of cyclohexanone oxime, which could be categorized into two groups. The first one is based on the most commonly applied process – the reaction of cyclohexanone with hydroxylamine (in the form of sulfate or phosphate38–40). The second group involves a more recent technology, in which cyclohexanone reacts with ammonium and a peroxide compound with the presence of a heterogeneous catalyst (known as the ammoximation process). For example, BASF and Inventa produce cyclohexanone oxime in a two-step process: a) formation of hydroxylamine sulfate solution from nitric oxide hydrogenation with a Pt catalyst, and b) oximation of cyclohexanone in weak acidic solution at 353 K.41,42 However, the main drawback in this solution is the production of a large amount of ammonium sulfate. In general, currently used methods involve multi-step processes, which demand specific conditions to prevent waste formation.40–44
As shown above, the production of these compounds is carried out with various technological processes with their advantages and disadvantages. The biggest problem for most of them is waste generation, which affects cost-effectiveness and negatively impacts the environment. Hence, the possibility to synthesise each one of them in one reaction makes catalytic hydrogenation of nitrocyclohexane extremely interesting.
2. Nitrocyclohexane hydrogenation
Despite the enormous versatility of nitrocyclohexane hydrogenation, a thorough literature investigation showed only limited data related to this process. Most of these research studies were focused on batch conditions at elevated pressure and temperature18,45–52 (Table 1). Nevertheless, these studies can serve as a guide for further experiments carried out in flow reactors (Table 2).| Literature | Catalyst | Catalyst amount [mg] | Solvent | NC amount [ml] | T [K] | p [bar] | Time [min] | Conversion [%] | Selectivity [%] | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cyclohexylamine | Dicyclohexylamine | Cyclohexanol | Cyclohexanone | Cyclohexanone oxime | Other | |||||||||
| a Estimated from the data available in the article. | ||||||||||||||
| 18 | RANEY®-Ni | 1500 | MeOH | 50 | 313 | 30 | 180 | n/a | 100 | — | — | — | — | — |
| CuCo3 | 1500 | MeOH | 50 | 423 | 100 | 120 | n/a | 100 | — | — | — | — | — | |
| Cu–Zn–Mn–Cr–O | 1500 | MeOH | 50 | 408 | 100 | 540 | n/a | n/a | n/a | n/a | n/a | 26 | n/a | |
| Ag–Zn–Cr–O | 1500 | MeOH | 50 | 368 | 50 | 1080 | n/a | n/a | n/a | n/a | n/a | 64 | n/a | |
| 47 | 5 wt% Pd/C | 15 | EtOH | 0.25a | 413 | 15 | 195 | 97.0 | 84.9 | — | — | 4.7 | 4.9 | 5.5 |
| 0.2 wt% Pt/C | 150 | EtOH | 0.25a | 413 | 15 | 100 | 85.0 | 70.0 | — | — | 5.0 | 18.0 | 7.0 | |
| 0.2 wt% Pt/TiO2 | 150 | EtOH | 0.25a | 413 | 15 | 100 | 97.0 | 31.8 | 40.6 | — | 14.2 | 5.4 | 8.0 | |
| 0.2 wt% Pt/TiO2 | 200 | — | 1a | 383 | 4 | 880 | 95.0 | 3.4 | — | — | 8.6 | 84.5 | 3.5 | |
| 48 | 1 wt% Au/Al2O3 | 200 | C2H8N2 | 1.2a | 373 | 6 | 720 | 100 | — | — | — | — | 83 | 17 |
| 1.5 wt% Au/TiO2 | 200 | C2H8N2 | 1.2a | 373 | 6 | 720 | 100 | — | — | — | — | 9 | 91 | |
| 49 | 5 wt% Pd/C | 200 | C2H8N2 | 1.2a | 323 | 2 | 180 | 100 | 6.2 | — | 7.9 | — | 55.1 | — |
| 5 wt% Pd/CNTs | 200 | C2H8N2 | 1.2a | 323 | 2 | 360 | 97.6 | 5 | — | 2.9 | — | 85.9 | — | |
| 53 | 5 wt% Pd/LAC | 200 | C2H8N2 | 1.2a | 323 | 3 | 360 | 98.9 | 5 | — | 4.6 | — | 55.0 | — |
| 5 wt% Pd/CAC | 200 | C2H8N2 | 1.2a | 323 | 3 | 360 | 99.2 | 5.6 | — | 3.2 | — | 60.5 | — | |
| 5 wt% Pd/CSAC | 200 | C2H8N2 | 1.2a | 323 | 3 | 360 | 91.0 | 4.6 | — | 2.9 | — | 38.9 | — | |
| 5 wt% Pd/SWCNTs | 200 | C2H8N2 | 1.2a | 323 | 3 | 360 | 97.3 | 0.3 | — | 2.9 | — | 94.6 | — | |
| 5 wt% Pd/DWCNTs | 200 | C2H8N2 | 1.2a | 323 | 3 | 360 | 98.1 | 0.2 | — | 4.5 | — | 83.5 | — | |
| 5 wt% Pd/MWCNTs | 200 | C2H8N2 | 1.2a | 323 | 3 | 360 | 99.9 | 3.4 | — | 4.6 | — | 87.9 | — | |
| 54 | 5 wt% Pd/SWCNTs | 100 | C2H8N2 | 0.6a | 323 | 3 | 360 | 98.9 | 6.3 | — | — | — | 85.9 | — |
| 5 wt% Pd/SWCNTs | 100 | C2H8N2 | 0.6a | 323 | 3 | 360 | 98.5 | 4.2 | — | — | — | 93.5 | — | |
| 5 wt% Pd/SWCNTs | 100 | C2H8N2 | 0.6a | 323 | 3 | 360 | 96.0 | 3.2 | — | — | — | 96.4 | — | |
| 50 | 5 wt% Pd/HTMC | 100 | C2H8N2 | 0.53a | 323 | 3 | 360 | 71.4 | 1.3 | — | — | 0.3 | 82.9 | — |
| 51 | 15 wt% Cu/SiO2 | 20 | C2H8N2 | 6 × 10−6a | 373 | 10 | 180 | 74 | 3 | — | — | 5 | 92 | — |
| 15 wt% Ni/SiO2 | 20 | C2H8N2 | 6 × 10−6a | 373 | 10 | 180 | 19 | 20 | — | — | 21 | 59 | — | |
| 15 wt% Fe/SiO2 | 20 | C2H8N2 | 6 × 10−6a | 373 | 10 | 180 | 8 | — | — | — | 75 | 25 | — | |
| 15 wt% Co/SiO2 | 20 | C2H8N2 | 6 ×a 10−6 | 373 | 10 | 180 | 4 | — | — | — | 88 | 12 | ||
| 52 | 1% Cu–20% Ni/AC | 120 | C2H8N2 | 0.47a | 373 | 3 | 480 | 99.6 | n/a | n/a | n/a | n/a | 87.8 | n/a |
| Literature | Catalyst | Reaction conditions | TOF [h−1] | Activity [μmol min−1] | Selectivity [%] | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cyclohexylamine | Dicyclohexylamine | Cyclohexanol | Cyclohexanone | Cyclohexanone oxime | Other | |||||
| 19 | 0.8 wt% Au/ZrO2 | Gas flow reactor 353 K | 6.1 | — | 28 | — | — | 54 | 18 | — |
| 1.2 wt% Au/TiO2 | 9.1 | — | 34 | 20 | — | 40 | 6 | — | ||
| 1.1 wt% Au/Al2O3 | 16.4 | — | 18 | — | — | 2 | 80 | — | ||
| 3.0 wt% Au/CeO2 | 1.6 | — | 2 | — | — | 98 | — | — | ||
| 56 | 2.2 wt% Pd@Tentagel-S-NH2 | Liquid flow reactor 20.5 μmol NC min−1 10 bar, 313 K | — | 0.1 | — | — | — | — | 100 | — |
| 57 | CuZnAl(0.5–1–1) | Liquid flow reactor 20.5 μmol NC min−1 5 bar, 413 K | — | 9 | — | — | 9 | 89 | 2 | — |
| CuZnAl(1–1–1) | Liquid flow reactor 20.5 μmol NC min−1 10 bar, 403 K | — | 15 | 100 | — | — | — | — | — | |
Batch conditions
Du Pont performed a further investigation on nitrocyclohexane hydrogenation with a Pd catalyst at the beginning of the 1960s.45,46 The experiments conducted at 35 bar and 413 K with palladium supported on acetylene-black gave selectivity mainly to cyclohexylamine (63%). The other products were cyclohexanone oxime (24%) and cyclohexanone (13%). The introduction of lead to the reaction system completely changed the proportions between products. Interestingly, different results were induced by the mere presence of Pb, irrespective of the method of introduction. For instance, the hydrogenation catalyst may be treated with elemental lead, a catalyst may be supported on a lead-containing material, or the reaction medium may be enriched with the lead compound. Even the reactor lined with lead gave different selectivity than the pure Pd catalyst. In terms of selectivity to cyclohexanone oxime, the best results were obtained in the presence of lead oxide at 35 bar and 433 K – 80% selectivity. These results were quite promising and were patented by Du Pont.45,46 It should be noted here that the requirement to use Pb-containing compounds entails enormous environmental costs, which could be a possible reason why this process was not implemented on an industrial scale.
Recent studies on nitrocyclohexane hydrogenation
Serna et al.47 found the Au/Al2O3 catalyst to be inactive in this process. However, it was verified by Shimizu et al.46 In their research,46 they were focused on the Au clusters supported on various metal oxides (Al2O3, SiO2, MgO and TiO2).48 Surprisingly, such catalysts turned out to be active and selective in nitrocyclohexane hydrogenation in the experiments performed at 373 K and 6 bar with ethanol as a solvent. For instance, 1.5 wt% Au/TiO2 was selective to cyclohexylamine, cyclohexanone and cyclohexyl–cyclohexylidene at the beginning of the process conducted for 12 h (unfortunately specific values were not provided). However, with prolonged reaction time, these products decomposed into undetectable compounds. On the other hand, 1 wt% Au/Al2O3 gave selectivity to cyclohexanone oxime (83%) and cyclohexanone (7%) at 100% conversion. Moreover, in other studies, the researchers discovered various correlations between morphology and catalytic parameters, like the influence of the average particle size. Independent from the support material, smaller Au nanoparticles gave higher conversion. For example, in the case of the previously mentioned 1 wt% Au/Al2O3, increasing the size of nanoparticles from 2 nm to 6 nm resulted in a significant drop in conversion (from 100% to 4.5%) and changed selectivity to cyclohexanone oxime (from 83% to 0%) in the processes conducted for 6 h at 373 K. Beside the influence of the particle size, the properties of the support materials also have a massive impact on the catalytic behaviour of gold. Basic metal oxides (MgO) and the one with more acidic character (SiO2) gave worse results than the amphoteric Al2O3. Acid–base pair sites seem to be crucial for this reaction. Based on these observations, Shimizu et al.48 proposed a cooperative mechanism, in which dissociative adsorption of H2 at the gold–support interface produces Hδ− on the low coordinated Au and Hδ+ on the support. Both groups are transferred to the polar nitro group, preferably adsorbed on the Alδ+–Oδ− site.48 This phenomenon could explain the higher activity of the gold nanoparticles supported on Al2O3. Hence, the impact of reaction pressure and temperature was investigated for the best catalyst – 1 wt% Au/Al2O3 with 2.5 nm nanoparticles. Its activity increased with increasing pressure up to 6 bar, to achieve 100% conversion and 83% selectivity to cyclohexanone oxime. Above this pressure, the selectivity to oxime decreased, and the formation of cyclohexanone was observed up to maximum at 11 bar. After exceeding this value, cyclohexyl–cyclohexylidene amine and dicyclohexylamine appeared among the reaction products. The same compounds were observed for temperatures above 373 K. At this temperature the catalyst achieved its maximum activity and selectivity to cyclohexanone oxime (83%) and cyclohexanone (7%). Serna et al.47 proved that the combination of specific morphology parameters and reaction conditions makes gold catalysts active and selective in nitrocyclohexane hydrogenation.
The research group from Xiangtan University came back to Pd catalysts and thoroughly investigated the catalytic performance of palladium nanoparticles supported on various carbon materials.49,50,53,54 In general, these catalysts showed high activity and selectivity to cyclohexanone oxime in the processes performed in the temperature and pressure range of 313–353 K and 2–3 bar, respectively. Unlike the other studies, ethylenediamine was chosen as the reaction solvent. The researchers established the optimal temperature for this process – 323 K, but also the minimal time required to achieve satisfactory conversion – 85% after 2 h. The initial experiments were performed with palladium nanoparticles supported on microporous activated carbon and carbon nanotubes, marked respectively as 5 wt% Pd/C and 5 wt% Pd/CNTs.49 These tests, conducted at optimal temperature and under 2 bar, showed the superiority of the Pd/CNTs in terms of selectivity to cyclohexanone oxime – 85.9% after 6 h, in comparison to 55.1% obtained after 3 h by Pd/C. Pd/CNTs domination was explained by smaller Pd nanoparticle size and the positive influence of the CNT support.49
Despite the satisfying results, Liu et al.53 decided to investigate more carbon derivative materials. Hence, a series of Pd catalysts (5 wt%) supported on activated carbons made from lignin, coal and coconut shell (LAC, CAC and CSAC), but also on carbon nanotubes: single-wall, double-wall and multi-wall (SWCNTs, DWCNTs, MWCNTs) were synthesised.53 Due to previously performed experiments49 it was not very surprising that all of these catalysts were active in nitrocyclohexane hydrogenation conducted in batch mode at 3 bar and 323 K. However, the usage of carbon materials with different properties allowed the structure of the support to be established as crucial to catalyst selectivity. The reactant sizes are comparable with the micropore dimension. Hence, the microporous structure is unfavourable for its adsorption and diffusion.53 The comparison between materials with the lowest and highest pore size, Pd/CAC – 0.95 nm and Pd/SWCNTs – 2.73 nm, showed similar conversion for both catalysts ∼100%. However, Pd/CAC gave 55% selectivity to cyclohexanone oxime, and PD/SWCNTs – 94.6%.
The carbon nanotubes generally revealed better catalytic performance, but there were also differences among them. Various structures of CNTs affected the reduction state of palladium. The Pd+ content increased in the following order: Pd/DWCNTs → Pd/MWCNTs → Pd/SWCNTs. Simultaneously, the selectivity to cyclohexanone oxime also increased for these materials in the same order 83.5% → 87.9% → 94.6%. Therefore, it could be concluded that Pd+ favours the formation of cyclohexanone oxime.
The studies performed in 2015 (ref. 54) aimed at the optimisation of the synthesis procedure to achieve higher Pd+ content for Pd/SWCNTs catalysts. The researchers checked various preparation methods: wet impregnation in water, wet impregnation in methanol, ion exchange and chemical reduction, but also different pretreatment conditions. Among the obtained catalysts, the ones gained by wet impregnation in water showed the best catalytic performance. With increasing reduction temperature of the Pd precursor (523 K → 623 K → 723 K), the selectivity to cyclohexanone oxime also increased (85.9% → 93.5% → 96.4%). It was related to the increase in Pd+ content with the reduction temperature in the following order 33.0% → 33.8% → 38.7%.
Unfortunately, the preparation of carbon nanotubes on a large scale is extremely complicated and expensive.50 Hence, Yan et al.50 attempted to achieve better results with palladium nanoparticles supported on mesoporous activated carbons. The researchers tested different materials synthesised by soft templating, hard templating and hydrothermal synthesis from various raw materials. The best results were obtained for Pd (5 wt%) supported on mesoporous carbon obtained by the hard template method from mesoporous silica.55 In the case of this catalyst, the selectivity to cyclohexanone oxime was equal to 82.8% in the experiments performed for 6 h at 323 K and 3 bar. However, its catalytic performance was affected by the small surface area and pore volume, and hence the final conversion was only 71.4%. Nevertheless, due to the cost of activated carbons, it can be an interesting alternative for carbon nanotubes.
In the end, all of the results obtained by the research group from Xiangtan University led to the conclusion that smaller Pd nanoparticles with higher dispersion and greater Pd+ content (which are supported on carbon materials with adequate pore size) favour the formation of cyclohexanone oxime with high activity.49,50,53,54
Because Zhang et al.51 were focused on the selectivity to cyclohexanone oxime, the copper catalyst was selected for further studies. Modification of various factors, such as the support material, synthesis method and metal loading, led to the catalyst with 92% selectivity to cyclohexanone oxime in combination with 74% conversion. The process of characterisation of these catalysts revealed the coexistence of metallic Cu and Cu2O. The ratio between these two species seems to be crucial for catalytic performance. Zhang et al.51 postulated that Cu0 species dissociate H2 and Cu2+ sites function as electrophilic sites to polarise the nitro group. Additionally, the researchers also discovered that with the increase of the pressure from 10 to 40 bar, the time needed to obtain the same conversion shortened proportionally from 4 to 1 h, which can be crucial for designing processes on the industrial scale.
In general, apart from the used catalyst, the combination of time, temperature and pressure is crucial to the catalytic performance in the hydrogenation of nitrocyclohexane in batch reactors. In order to obtain products of partial hydrogenation, the reaction has to be conducted at a lower temperature and pressure. Higher values favour the formation of cyclohexanone, cyclohexanol, cyclohexylamine and dicyclohexylamine. A similar situation is observed for the reaction time, which also affects the selectivity of the catalysts. Prolonged time supports further hydrogenation and even the decomposition of the products into undetectable compounds. For the above reasons, easy control of the reaction conditions provided by flow reactors appears to be very attractive.
Flow conditions
A thorough literature investigation revealed only three papers related to the usage of flow conditions in this reaction, Wang et al. in the gas phase19 and Kowalewski et al. in the liquid phase.56,57Gas flow conditions
Inspired by the studies performed by Serna et al.,47 Wang and coworkers19 proved that gold catalysts could be as active under flow as under batch conditions. A series of Au catalysts were prepared by the deposition–precipitation method with various metal oxides: 0.8 wt% Au/ZrO2, 1.2 wt% Au/TiO2, 1.1 wt% Au/Al2O3 and 3.0 wt% Au/CeO2. Different support materials affected nanoparticles' size and their distribution; a decrease in the mean values was observed in the following order: Au/ZrO2 (7.0 nm) → Au/TiO2 (4.7 nm) → Au/Al2O3 (4.3 nm) → Au/CeO2 (3 nm). Similar to previous studies,47 Wang et al.19 observed a strong correlation between Au nanoparticle size and the overall activity of the catalyst; smaller particles are better in the activation of H2 which is a crucial step in the hydrogenation of nitrocyclohexane. These properties were reflected in the catalytic performance. In the experiments performed at 353 K each one of these catalysts was selective to different products: Au/ZrO2–cyclohexanone (54%), cyclohexylamine (28%) and cyclohexanone oxime (18%), Au/CeO2–cyclohexanone (98%) and cyclohexylamine (2%), Au/TiO2–cyclohexanone (40%), cyclohexylamine (34%), dicyclohexylamine (20%) and cyclohexanone oxime (6%), and Au/Al2O3–cyclohexanone oxime (80%), cyclohexylamine (18%) and cyclohexanone (2%). In general, selectivity to cyclohexanone oxime is sensitive to surface hydrogen. The formation of cyclohexanone and cyclohexylamine is favoured at low and high coverage, respectively.Liquid flow conditions
Kowalewski et al.56,57 also conducted catalytic reactions under flow conditions, but in the liquid phase. This approach allowed them to perform the catalytic process under conditions comparable with batch hydrogenation studies.48–53 The application of palladium nanoparticles grafted on the polymeric resin (TSNH2) led to the formation of two different products: cyclohexanone oxime and cyclohexylamine. In general, a higher pressure requires a higher temperature to achieve 100% selectivity to cyclohexanone oxime (298 K for 5 bar, 313 K for 10 bar). On the other hand, the selectivity to cyclohexylamine increases with increasing temperature and pressure. In comparison to previous results obtained under batch conditions,49,53,54 palladium nanoparticles grafted on polymeric resin gave lower conversion.However, considering that the flow conditions allow for a long-term process, inferior activity was reduced, and selectivity can be easily modified by changing the reaction conditions.56
Inspired by Zhang et al.,51 Kowalewski et al.57 applied in nitrocyclohexane hydrogenation catalysts based on low-cost transition metals – CuZnAl hydrotalcite derived materials. The activated catalyst gave an entirely different catalytic performance depending on the different molar ratios between Cu, Zn and Al in the parent hydrotalcite material. The alteration of the copper content completely modified the selectivity of the used materials, which was caused by the modification of catalyst morphology. Lower copper content led to the formation of the CuZn alloy. It steered the reaction into cyclohexanone formation, and higher Cu concentration resulted in the production of cyclohexylamine (thanks to the presence of Cu–ZnO core–shell structure).
Comparison of batch and flow nitrocyclohexane hydrogenation
The comparison of batch and flow catalytic hydrogenation of nitrocyclohexane is not straightforward (Tables 1 and 2). Reaction conditions applied by the research groups from all around the world are not consistent with each other. Hence, even the comparison of the results obtained in batch reactors could be problematic (Table 1). However, assuming that in the average batch catalytic reaction (performed for 6 h) the amount of the used nitrocyclohexane is 0.6 g (4.37 mmol) per 0.1 g which corresponds to the flow reaction performed for 6 h with the same amount of catalyst with an NC flow rate ∼12 μmol min−1, some general conclusions can be made. Catalysts under batch conditions show higher activity. On the other hand, the possibility to perform long-term processes in continuous-flow reactors eliminates to some extent the problem with yield in a short time.Mechanism of nitrocyclohexane hydrogenation
Most of the research on nitrocyclohexane hydrogenation presents the reaction pathway as it was presented in Scheme 1, which was proposed by Wang et al. for the reaction conducted under gas flow conditions. Direct hydrogenation of nitrocyclohexane leads to the formation of cyclohexanone oxime. Further hydrogenation involves only one intermediate product – imine. Its hydrogenation leads to cyclohexylamine. On the other hand, the formation of cyclohexanone requires the participation of water.The mechanism proposed by Liu et al. (Scheme 2) indicates the possibility of the formation of two intermediate products: N-cyclohexylhydroxylamine and imine. The former one is obtained directly from nitrocyclohexane and can be hydrogenated to cyclohexylamine. The latter one is produced from cyclohexanone oxime and can be transformed into cyclohexylamine or cyclohexanone. Moreover, further hydrogenation of cyclohexanone leads to cyclohexanol.
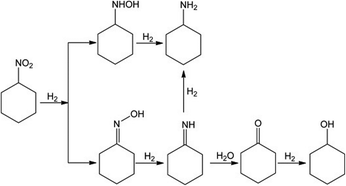 | ||
| Scheme 2 Nitrocyclohexane hydrogenation mechanism proposed by Liu et al.53 Reprinted (adapted) with permission from Liu et al.53 Copyright {2013} John Willey & Sons. | ||
More thorough studies on nitrocyclohexane hydrogenation mechanisms were performed by Shimizu et al.48 and Yao et al.52
Shimizu et al.48 exposed the beneficial role of coordinatively unsaturated Au atoms and the acid–base pair site (Alδ+–Oδ− site) coexistence (Scheme 3). In accordance with their work, the dissociation of H2 at the gold–support interface led to the formation of the Hδ− atom on the low coordinated Au atom and an H atom which releases an electron to the Lewis acid site of the support. The latter one is transformed to a proton stabilized at the oxygen atom (Lewis base) near the Lewis acid site. The nucleophilic Hδ− species on Au and electrophilic Hδ+ species on the support transfer to the polar nitro group. The Alδ+–Oδ− site also acts as the adsorption site of the nitro group.
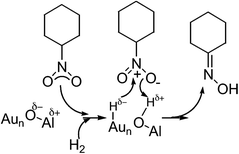 | ||
| Scheme 3 Nitrocyclohexane hydrogenation on the acid–base pair site on Au catalysts. Reprinted (adapted) with permission from Shimizu et al.48 Copyright {2011} Elsevier. | ||
Yao et al.52 proposed the nitrocyclohexane hydrogenation mechanism on the Cu–Ni/AC catalyst based on the classical Langmuir–Hinshelwood model (Scheme 4). Nitrocyclohexane and hydrogen molecules are adsorbed on the Cu–Ni catalyst surface, and hydrogen is dissociated to active hydrogen (H*). H* reacts with nitrocyclohexane to produce unstable intermediate N,N′-dihydroxycyclohexane (Scheme 4, (1)), and then it can be further dehydrated to form the intermediate (Scheme 4, (2)). Moreover, Knifton et al. by exchanging hydrogen for deuterium showed that the hydrogen atom in oxime does not come from hydrogen but from the reactants. It was suggested that the nitroso compound containing α-H can easily tautomerize to form oxime via α-H transfer, and the polar solvent environment is more favourable for this reaction. Hence, the produced intermediate (Scheme 4, (2)) can be isomerized by α-H transfer to form cyclohexanone oxime, which can be further hydrogenated and hydrolyzed to cyclohexylamine and cyclohexanone via intermediate cyclohexanimide (Scheme 4, (3)).47 Additionally, the intermediate (Scheme 4, (2)) can be hydrogenated to N-cyclohexylhydroxylamine, which can be further hydrogenated and deoxidized to cyclohexylamine.58 The results obtained by Yao et al.52 suggest that N-cyclohexylhydroxylamine can be quickly converted to cyclohexanone oxime. Additionally, they postulated that the decrease of cyclohexylamine content may be the effect of the formation of N,N-dicyclohexylamine by a condensation reaction between cyclohexylamine and N-cyclohexylhydroxylamine. Moreover, the reaction under hydrogenation conditions favors the conversion of N-cyclohexylhydroxylamine to cyclohexanone oxime as well as the condensation reaction between N-cyclohexylhydroxylamine and cyclohexylamine.
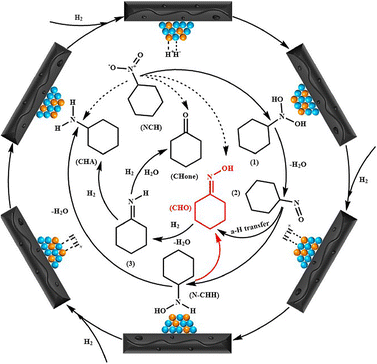 | ||
| Scheme 4 Mechanism involving the formation of cyclohexanone oxime over the Cu–Ni/AC catalyst. Reprinted (adapted) with permission from Yao et al.52 Copyright {2021} American Chemical Society. | ||
3. Conclusions
Based on the presented results, the seemingly simple catalytic hydrogenation of nitrocyclohexane can be very challenging due to the enormous number of crucial factors. However, the possibility to obtain numerous valuable compounds from the same substrate seems to be an exciting topic for further research. Among these chemicals, precursors for the production of pharmaceuticals, artificial sweeteners, insecticides, textiles, and many others can be found. Several very active and selective catalysts have been obtained so far (Table 1). Serna et al.47 synthesised 5 wt% Pd/C which converts nitrocyclohexane (97%) into cyclohexylamine with ∼85% selectivity. However, only 0.2 wt% Pt/TiO2 transforms NC into cyclohexanol (conversion – 97%, selectivity – 40.6%). Also, cyclohexanone could be efficiently obtained from NC with the 15 wt% Co/SiO2 catalyst offered by Zhang et al.51 (88% selectivity, but only 4% conversion). Moreover, Liu et al.53 proposed Pd nanoparticles supported on single-wall carbon nanotubes (5 wt% Pd/SWCNTs) which gave ∼95% selectivity to cyclohexanone oxime with 97% conversion. Until now, the catalyst for the efficient formation of cyclohexanol has not been proposed. Hence, more advanced studies on the influence of metal active phase support materials and reaction conditions should be carried out in the future. The already obtained results form the basis for future studies.Noteworthy is the lack of reactions conducted in flow reactors in the liquid phase. The application of the flow technique eliminates many problems of batch conditions and facilitates the performance of the nitrocyclohexane hydrogenation. Flow reactors were successfully applied in the hydrogenation of various nitro compounds,59–63 also in the hydrogenation of nitrocyclohexane. The thorough analysis of the already obtained results showed that 3 wt% Au/CeO2 is the best catalyst for the synthesis of cyclohexanone oxime (98% selectivity) under gas flow conditions.19 On the other hand, under liquid flow conditions, cyclohexanone oxime can be produced efficiently with palladium nanoparticles grafted on polymeric resin (2.2 wt% Pd@Tentagel-S-NH2, selectivity – 98%).56 Moreover, Kowalewski et al.57 also proposed catalysts based on hydrotalcite derived materials CuZnAl (0.5–1–1) and CuZnAl(1–1–1), which give possibility to produce cyclohexanone (selectivity – 89%) and cyclohexylamine (selectivity – 100%), respectively. Nevertheless, there are still not many publications concerning flow conditions. Hence, the hydrogenation of nitrocyclohexane under liquid flow conditions with readily available transition metals as catalysts should be a priority for further research on this topic.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was sponsored by the National Science Centre in Poland with the project UMO-2018/31/N/ST-5/02555.Notes and references
- K. Masuda, T. Ichitsuka, N. Koumura, K. Sato and S. Kobayashi, Tetrahedron, 2018, 74, 1705–1730 CrossRef CAS.
- M. Trojanowicz, Molecules, 2020, 25, 1434 CrossRef CAS PubMed.
- L. Vaccaro, D. Lanari, A. Marrocchi and G. Strappaveccia, Green Chem., 2014, 16, 3680–3704 RSC.
- J. Wegner, S. Ceylan and A. Kirschning, Chem. Commun., 2011, 47, 4583–4592 RSC.
- J. Kobayashi, Y. Mori, K. Okamoto, R. Akiyama, M. Ueno, T. Kitamori and S. Kobayashi, Science, 2004, 304, 1305–1308 CrossRef CAS PubMed.
- E. Riva, S. Gagliardi, C. Mazzoni, D. Passarella, A. Rencurosi, D. Vigo and M. Martinelli, J. Org. Chem., 2009, 74, 3540–3543 CrossRef CAS PubMed.
- Z. Yu, Y. Lv, C. Yu and W. Su, Org. Process Res. Dev., 2013, 17, 438–442 CrossRef CAS.
- Z. Amara, M. Poliakoff, R. Duque, D. Geier, G. Franciò, C. M. Gordon, R. E. Meadows, R. Woodward and W. Leitner, Org. Process Res. Dev., 2016, 20, 1321–1327 CrossRef CAS.
- J. P. Mcmullen and K. F. Jensen, Org. Process Res. Dev., 2010, 14, 1169–1176 CrossRef CAS.
- N. Cherkasov, Y. Bai, A. J. Expósito and E. V. Rebrov, React. Chem. Eng., 2018, 3, 769–780 RSC.
- C. V. Rode, A. A. Ghalwadkar, R. B. Mane, A. M. Hengne, S. T. Jadkar and N. S. Biradar, Org. Process Res. Dev., 2010, 14, 1385–1392 CrossRef CAS.
- Y. Shen, A. Maamor, J. Abu-Dharieh, J. M. Thompson, B. Kalirai, E. H. Stitt and D. W. Rooney, Org. Process Res. Dev., 2014, 18, 392–401 CrossRef CAS.
- A. D. M. Mendonça, A. V. B. De Oliveira and J. Cajaiba, Org. Process Res. Dev., 2017, 21, 1794–1800 CrossRef.
- J. G. Costandy, T. F. Edgar and M. Baldea, Ind. Eng. Chem. Res., 2019, 58, 13718–13736 CrossRef CAS.
- Y. Wang, P. Prinsen, K. S. Triantafyllidis, S. A. Karakoulia, A. Yepez, C. Len and R. Luque, ChemCatChem, 2018, 10, 3459–3468 CrossRef CAS.
- G. S. Calabrese and S. Pissavini, AIChE J., 2011, 57, 828–834 CrossRef CAS.
- S. K. Teoh, C. Rathi and P. Sharratt, Org. Process Res. Dev., 2016, 20, 414–431 CrossRef CAS.
- C. Grundmann, Angew. Chem., 1950, 62, 558–560 CrossRef CAS.
- X. Wang, N. Perret and M. A. Keane, Appl. Catal., A, 2013, 467, 575–584 CrossRef CAS.
- J. Wen, K. You, X. Liu, J. Jian, F. Zhao, P. Liu, Q. Ai and H. Luo, Catal. Commun., 2019, 127, 64–68 CrossRef CAS.
- S. Araki, K. Nakanishi, A. Tanaka and H. Kominami, J. Catal., 2020, 389, 212–217 CrossRef CAS.
- G. Poehler, H. Corr and W. Friedrichsen, DE Pat., DE1921467A1, 1969 Search PubMed.
- J. D. Seddon, GB Pat., GB969542A, 1961 Search PubMed.
- Methoden der organischen chemie (Houben-Weyl), ed. M. Eugene, Elsevier, 4th edn, 1958, vol. 11/1 Search PubMed.
- Y. Kobayashi, JP Pat., JP3180, 1968 Search PubMed.
- N. Getz, P. Jacobs, L. Hupfer, H. Toussaint and W. Reiss, DE Pat., DE3045719, 1984 Search PubMed.
- J. Wen, K. You, F. Zhao, J. Jian, P. Liu, Q. Ai and H. Luo, Catal. Commun., 2020, 145, 106112 CrossRef CAS.
- G. Darsow and R. Langer, US Pat., US6335470B1, 1997 Search PubMed.
- J. M. Solomon, B. Isbitsky and B. R. Bluestein, US Pat., US3551486, 1970 Search PubMed.
- F. H. Munster, US Pat., US3351661, 1967 Search PubMed.
- Abbott Laboratories, FR Pat., FR1343391, 1963 Search PubMed.
- L. J. Dankert and D. A. Permoda, US Pat., US2571016, 1949 Search PubMed.
- M. T. Musser, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2011 Search PubMed.
- D. Contreras, V. Melin, K. Márquez, G. Pérez-González, H. D. Mansilla, G. Pecchi and A. Henríquez, Appl. Catal., B, 2019, 251, 17–24 CrossRef CAS.
- J. F. Van Peppen, US Pat., US5015787, 1991 Search PubMed.
- M. T. Musser, Ullmann's Encycl. Ind. Chem., 2012, 11, 49–60 Search PubMed.
- D. C. Thomas and J. T. Adams, US Pat., US3404185A, 1964 Search PubMed.
- R. H. Belden and D. W. H. Roth, US Pat., US3338965A, 1967 Search PubMed.
- K. Kuma, US Pat., US4929756A, 1989 Search PubMed.
- G. Rapp, H. Fuchs and E. Thomas, US Pat., US4031139A, 1975 Search PubMed.
- J. Tinge, M. Groothaert, H. op het Veld, J. Ritz, H. Fuchs, H. Kieczka and W. C. Moran, Ullmann's Encycl. Ind. Chem., 2018, 1–31 Search PubMed.
- J. Ritz, H. Fuchs and H. G. Perryman, Ullmann's Encycl. Ind. Chem., 2000, 18, 503–509 Search PubMed.
- J. Damme, J. T. van Goolen and A. H. Derooij, Chem. Eng., 1972, 79, 54 CAS.
- P. Roffia, M. Padovan, E. Moretti and G. De Alberti, US Pat., US4745221A, 1985 Search PubMed.
- Du Pont, GB Pat., GB860340, 1961 Search PubMed.
- Du Pont, GB Pat., GB857902, 1958 Search PubMed.
- P. Serna, M. López-Haro, J. J. Calvino and A. Corma, J. Catal., 2009, 263, 328–334 CrossRef CAS.
- K. I. Shimizu, T. Yamamoto, Y. Tai and A. Satsuma, J. Mol. Catal. A: Chem., 2011, 345, 54–59 CrossRef CAS.
- H. G. Liao, Y. J. Xiao, H. K. Zhang, P. Le Liu, K. Y. You, C. Wei and H. Luo, Catal. Commun., 2012, 19, 80–84 CrossRef CAS.
- Y. Yan, S. Liu, F. Hao, P. Liu and H. Luo, Catal. Commun., 2014, 50, 9–12 CrossRef CAS.
- Q. Q. Zhang, J. Dong, Y. M. Liu, Y. Cao, H. Y. He and Y. D. Wang, Chem. Commun., 2017, 53, 2930–2933 RSC.
- F. Yao, S. Liu, H. Cui, Y. Lv, Y. Zhang, P. Liu, F. Hao, W. Xiong and H. Luo, ACS Sustainable Chem. Eng., 2021, 9, 3300–3315 CrossRef CAS.
- P.-L. Liu, H.-K. Zhang, S.-H. Liu, Z.-J. Yao, F. Hao, H.-G. Liao, K.-Y. You and H.-A. Luo, ChemCatChem, 2013, 5, 2932–2938 CrossRef CAS.
- S. Liu, F. Hao, P. Liu and H. Luo, RSC Adv., 2015, 5, 22863–22868 RSC.
- S. Jun, S. H. Joo, R. Ryoo, M. Kruk, M. Jaroniec, Z. Liu, T. Ohsuna and O. Terasaki, J. Am. Chem. Soc., 2000, 122, 10712–10713 CrossRef CAS.
- E. Kowalewski, B. Zawadzki, K. Matus, K. Nikiforow and A. Śrębowata, React. Kinet., Mech. Catal., 2021, 132, 717–728 CrossRef CAS.
- E. Kowalewski, M. Krawczyk, G. Słowik, J. Kocik, I. S. Pieta, O. Chernyayeva, D. Lisovytskiy, K. Matus and A. Śrębowata, Appl. Catal., A, 2021, 618, 118134 CrossRef CAS.
- S. M. Islam and C. R. Saha, J. Mol. Catal. A: Chem., 2004, 212, 131–140 CrossRef CAS.
- R. V. Jones, L. Godorhazy, N. Varga, D. Szalay, L. Urge and F. Darvas, J. Comb. Chem., 2006, 8, 110–116 CrossRef CAS PubMed.
- R. Javaid, S. I. Kawasaki, A. Suzuki and T. M. Suzuki, Beilstein J. Org. Chem., 2013, 9, 1156–1163 CrossRef CAS PubMed.
- S. S. Yakushkin, A. L. Nuzhdin, E. A. Artiukha, P. E. Plyusnin, G. A. Bukhtiyarova and O. N. Martyanov, Mendeleev Commun., 2018, 28, 536–537 CrossRef CAS.
- V. G. Dorokhov, G. F. Dorokhova and V. I. Savchenko, Russ. Chem. Bull., 2018, 67, 1412–1418 CrossRef CAS.
- A. L. Nuzhdin, G. A. Bukhtiyarova, T. Lin, E. Y. Gerasimov and V. I. Bukhtiyarov, Mendeleev Commun., 2019, 29, 553–555 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2022 |
