Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Structure–activity correlation in aerobic cyclohexene oxidation and peroxide decomposition over CoxFe3−xO4 spinel oxides†
Julia
Büker
a,
Steven
Angel
b,
Soma
Salamon
 c,
Joachim
Landers
c,
Joachim
Landers
 c,
Tobias
Falk
a,
Heiko
Wende
c,
Tobias
Falk
a,
Heiko
Wende
 c,
Hartmut
Wiggers
*b,
Christof
Schulz
b,
Martin
Muhler
c,
Hartmut
Wiggers
*b,
Christof
Schulz
b,
Martin
Muhler
 ad and
Baoxiang
Peng
ad and
Baoxiang
Peng
 *ad
*ad
aLaboratory of Industrial Chemistry, Ruhr University Bochum, 44780 Bochum, Germany. E-mail: baoxiang.peng@techem.rub.de
bIVG Institute for Combustion and Gas Dynamics – Reactive Fluids and CENIDE Center for Nanointegration, University of Duisburg-Essen, 47057 Duisburg, Germany. E-mail: hartmut.wiggers@uni-due.de
cFaculty of Physics, University of Duisburg-Essen, 47057 Duisburg, Germany
dMax Planck Institute for Chemical Energy Conversion, 45470 Mülheim an der Ruhr, Germany
First published on 21st April 2022
Abstract
Nanoparticulate CoxFe3−xO4 (0 ≤ x ≤ 3) catalysts were prepared by spray-flame synthesis and applied in liquid-phase cyclohexene oxidation with O2 as oxidant. The catalysts were characterized in detail using N2 physisorption, XRD, TEM, XPS, FTIR, Raman, and Mössbauer spectroscopy. A volcano plot was obtained for the catalytic activity in cyclohexene oxidation as a function of the Co content with a maximum at x = 1. Thus, CoFe2O4 achieved the highest degree of cyclohexene conversion and the fastest decomposition rate of the key intermediate 2-cyclohexene-1-hydroperoxide. Kinetic studies and a stability test were performed over CoFe2O4, showing that cyclohexene oxidation follows first-order kinetics with an apparent activation energy of 58 kJ mol−1. The catalytic hydroperoxide decomposition during cyclohexene oxidation was further investigated using H2O2 and tert-butyl hydroperoxide as simpler surrogates resulting in similar volcano-type correlations. The increase in catalytic activity with increasing Fe content with a maximum at x = 1 is ascribed to the increasing concentration of octahedrally coordinated Co2+ cations in the spinel structure leading to the presence of coordinatively unsaturated Co3c2+ surface sites, which are identified to be the most active sites for 2-cyclohexene-1-hydroperoxide decomposition in cyclohexene oxidation.
Introduction
The oxidation of olefins is a key technology in the chemical industry, as the formed products are important building blocks for the synthesis of fine and bulk chemicals, comprising agrochemicals, pharmaceuticals, fragrances, and polymers.1,2 In particular, the oxidation of cyclohexene provides access to valuable oxygenated products, such as 2-cyclohexene-1-one, 2-cyclohexene-1-ol, cyclohexane-1,2-diol, and cyclohexene oxide.2,3 Industrial processes are mainly conducted in the gas phase under harsh reaction conditions and operated at low conversion to retain high selectivity and to suppress total oxidation. In contrast, liquid-phase reactions can be carried out under milder reaction conditions leading to higher selectivities. Nevertheless, aerobic cyclohexene oxidation is subject to a complex reaction network because of the two active centres in the cyclohexene molecule (Scheme 1). When allylic oxidation takes place at the C–H bond, cyclohexene is oxidized to 2-cyclohexene-1-hydroperoxide, which can further react to 2-cyclohexene-1-ol, 2-cyclohexene-1-one and 7-oxabicyclo[4.1.0]-heptane-2-one. On the other hand, epoxidation can occur at the olefinic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, yielding cyclohexene oxide and cyclohexane-1,2-diol. Moreover, both reaction pathways cannot be clearly separated, because 2-cyclohexene-1-hydroperoxide formed within the allylic pathway can react with another cyclohexene molecule resulting in the formation of cyclohexene oxide, which is typically referred to the epoxidation pathway. Early studies identified 2-cyclohexene-1-hydroperoxide to be an unstable reaction intermediate of cyclohexene oxidation. Already in 1939, Criegee et al. detected 2-cyclohexene-1-hydroperoxide to be the main product of cyclohexene autoxidation and clarified its structure.4 Sridhar and coworkers investigated the uncatalyzed decomposition of 2-cyclohexene-1-hydroperoxide and found 2-cyclohexene-1-ol and 2-cyclohexene-1-one to be the main products even in the absence of O2.5 It can therefore be deduced that the aerobic oxidation of cyclohexene at least partially occurs autocatalytically forming 2-cyclohexene-1-hydroperoxide with high selectivity.
C bond, yielding cyclohexene oxide and cyclohexane-1,2-diol. Moreover, both reaction pathways cannot be clearly separated, because 2-cyclohexene-1-hydroperoxide formed within the allylic pathway can react with another cyclohexene molecule resulting in the formation of cyclohexene oxide, which is typically referred to the epoxidation pathway. Early studies identified 2-cyclohexene-1-hydroperoxide to be an unstable reaction intermediate of cyclohexene oxidation. Already in 1939, Criegee et al. detected 2-cyclohexene-1-hydroperoxide to be the main product of cyclohexene autoxidation and clarified its structure.4 Sridhar and coworkers investigated the uncatalyzed decomposition of 2-cyclohexene-1-hydroperoxide and found 2-cyclohexene-1-ol and 2-cyclohexene-1-one to be the main products even in the absence of O2.5 It can therefore be deduced that the aerobic oxidation of cyclohexene at least partially occurs autocatalytically forming 2-cyclohexene-1-hydroperoxide with high selectivity.
The autoxidation of cyclohexene renders the decomposition of the hydroperoxide intermediate to be the key step for selective cyclohexene oxidation. Thus, the use of a heterogeneous catalyst offers the possibility to selectively decompose 2-cyclohexene-1-hydroperoxide into one of the desired products, enabling the formation of high-value chemicals in high yields.
For this purpose, highly active catalysts are needed. Transition metal oxides have raised much interest because of their low cost and their potential to replace conventional noble metal catalysts. Especially, oxides with spinel structure were found to be highly stable under extreme reaction conditions and flexible with regard to the variety of metal ions with different valencies incorporated into their lattices.6 Spinel oxides consist of a close-packed cubic lattice of oxygen anions with the general formula AB2O4, where A can be a group IIA metal or a transition metal cation with the oxidation state 2+ and B is a group IIIA metal or transition metal cation in the 3+ oxidation state. In a normal spinel structure like ideal Co3O4, the A2+ ions occupy one-eighth of the tetrahedral holes and the 3+ species occupy half of the octahedral voids. In contrast, within an inverse spinel such as ideal CoFe2O4, the A2+ ions and half of the B3+ ions exchanged their positions.7
In addition to the occupation of octahedral and tetrahedral sites within the spinel structure, the particle size and the resulting facets of the particles also play an important role. While it is generally accepted that conversion increases with increasing specific surface area, our recent studies show that larger Co3O4 particles with consequently smaller surface areas can nevertheless lead to higher conversion due to the exposure of catalytically more active facets with, for example, multiple coordinatively unsaturated Cocus3+ sites.8
Because of their high stability, their large variety of materials and also semiconducting properties, spinel oxides are applied in many fields, such as Li-ion batteries,9 photodegradation of pollutants,10 water oxidation,11 and electrochemical oxygen evolution reaction.12 In addition, Co-based transition metal oxides have already been applied in liquid-phase oxidation reactions in our previous studies.13–17
Spinel oxides can be synthesized by several methods, such as mixed powder synthesis,7 co-precipitation,18 sol–gel synthesis,19 hydrothermal synthesis,20 and spray and freeze drying.21 Among these methods, the continuous and scalable spray-flame synthesis is a suitable method providing high specific surface areas, small nanoparticle sizes, and well-controlled catalyst compositions.13,22
In the present work, we report on the liquid-phase oxidation of cyclohexene in acetonitrile with molecular O2 over a series of spray-flame synthesized CoxFe3−xO4 spinel nanoparticles under mild conditions. To date, to the best of our knowledge, no comparable study has been reported on the liquid-phase cyclohexene oxidation over mixed Co–Fe spinel oxides. We aim at understanding the effect of Fe substitution into the Co3O4 spinel structure on the reaction mechanism of cyclohexene oxidation. The CoxFe3−xO4 nanoparticulate materials were characterized in detail, and their catalytic properties were systematically elaborated. Kinetic investigations, a reusability test and the influence of H2O were studied over the best-performing catalyst CoFe2O4. The decomposition of H2O2 and tert-butyl hydroperoxide (TBHP) was carried out to mimic the decomposition of the key intermediate 2-cyclohexene-1-hydroperoxide to correlate the rates of cyclohexene oxidation and peroxide decomposition and, thereby, identify the active sites.
Results and discussion
Catalyst characterization
A series of CoxFe3−xO4 (x = 0, 0.5, 1, 1.5, 2, 2.5, 3) nanoparticulate materials was synthesized by spray-flame synthesis using dissolved metal nitrates as precursors with a concentration of 0.2 M. The detailed synthesis procedure can be found elsewhere.13,23 After synthesis, the samples were heat-treated at 250 °C for 2 h in air to remove adsorbed organic and water residuals from the particles surface.The N2 physisorption-derived specific surface areas determined by applying the BET equation were high, ranging between 87 and 151 m2 g−1 (Table 1). Assuming monodisperse and spherical particles, average particle sizes of 8.3 to 12.0 nm were calculated (Table 1). The count mean diameters based on TEM images resulted in particle sizes between 8.4 and 11.1 nm with homogeneous particle size distributions, which are in agreement with the BET-based particle sizes (Table 1). Nevertheless, according to the analyzed TEM images, besides spherical particles, a reasonable number of faceted particles were identified (Fig. S1†). All catalysts show a single crystalline nanostructure with high crystallinity but crystallographically disordered structures (Fig. 1 and S1†). The EDX elemental mapping identified a homogeneous distribution of Co and Fe for all samples (Fig. S3†) and on average, the experimental Co/Fe ratios were similar to the desired nominal ones summarized in Table 1.
| Sample | Specific surface area | Particle sizea | Particle sizeb | Co/(Co + Fe)c | x(CoxFe3−xO4)c | Co/(Co + Fe)d | x(CoxFe3−xO4)d |
|---|---|---|---|---|---|---|---|
| [m2 g−1] | [nm] | [nm] | |||||
| a Estimated from the specific surface areas assuming spherical particles. b Determined by TEM. c Determined by EDX. d Determined by XPS. | |||||||
| Co3O4 | 118 | 8.3 | 11.1 | 1 | 3 | 1 | 3 |
| Co2.5Fe0.5O4 | 87 | 12.0 | 8.8 | 0.82 | 2.47 | 0.84 | 2.51 |
| Co2Fe1O4 | 128 | 8.3 | 8.8 | 0.67 | 2.01 | 0.68 | 2.04 |
| Co1.5Fe1.5O4 | 151 | 7.5 | 8.4 | 0.51 | 1.54 | 0.52 | 1.55 |
| Co1Fe2O4 | 129 | 8.8 | 8.7 | 0.33 | 0.99 | 0.36 | 1.07 |
| Co0.5Fe2.5O4 | 122 | 9.4 | 9.9 | 0.18 | 0.53 | 0.18 | 0.55 |
| γ-Fe2O3 | 111 | 10.5 | 9.2 | 0 | 0 | 0 | 0 |
The HAADF-TEM images of Co3O4 and CoFe2O4 were used to determine the lattice spacings of the spinel nanoparticles. For Co3O4, a distance of d = 0.234 nm was recorded in agreement with the theoretical lattice spacing of d100 = 0.24 nm (Fig. S2†).24 For CoFe2O4, a significantly higher number of hexagonal shaped particles was found, indicating (111) surfaces.25 The determined lattice spacings of 0.509, 0.476 and 0.480 nm agree with the theoretical value of d111 = 0.484 nm, suggesting that (111) surfaces are dominant for CoFe2O4.26 Whereas (100)-oriented surfaces expose coordinatively unsaturated metal cations, which are fivefold coordinated at the octahedral sites of the spinel (MO5c), those octahedrally coordinated metal cations are only threefold coordinated at the (111) surfaces (MO5c).25 Metal cations with a lower coordination number are assumed to be more catalytically active.
XRD patterns were recorded for the CoxFe3−xO4 catalysts (Fig. 2). A cubic spinel structure was identified for all samples. For Co3O4, the pattern fits better to the reference pattern of a cobalt-deficient spinel phase than to stoichiometric Co3O4. With increasing Fe content, the reflections start shifting to lower 2θ angles as expected. Based on the XRD patterns, the iron oxide sample (x = 0) cannot be clearly identified, because the magnetite (Fe3O4) and maghemite (γ-Fe2O3) phases have both almost identical lattice parameters. Crystal structure analysis was performed by Rietveld refinement resulting in lattice parameters, which amount to 8.08, 8.38 and 8.36 Å, well-fitting to the literature-reported values of 8.08,27 8.38,28 and 8.35 Å (ref. 29) for Co3O4, CoFe2O4 and γ-Fe2O3, respectively. The reported lattice constant of Fe3O4 is 8.39 Å,29 indicating the presence of γ-Fe2O3 instead of Fe3O4 (Fig. S4†).
Raman spectroscopy was applied to investigate the electronic structure and the cation occupation of the tetrahedral and octahedral sites in the spinel structure of the CoxFe3−xO4 samples (Fig. 3). The recorded spectra clearly show five bands, which can be assigned to A1g, Eg and three F2g phonon modes originating from lattice vibrations of the cubic spinel structure. With increasing Fe amount in the samples from Co3O4 to Co1.5Fe1.5O4, a redshift of the A1g mode was observed corresponding to the symmetric Co3+–O stretching vibration of the octahedral sites, which indicates a lower occupancy of Co3+ ions at octahedral sites and a starting phase transition towards an inverse spinel.30,31 The intensity of the F2g(3) mode at 195 cm−1 also decreased, indicating the lower occupation of tetrahedral voids by Co2+ ions.32 Similarly, the intensity of the Eg mode at 481 cm−1, corresponding to different motions of the AO4-tetrahedral unit,31 also decreased. The F2g(3) mode at 475 cm−1 of Co0.5Fe2.5O4 and CoFe2O4 corresponds to the vibration of Co2+ ions in octahedral voids,33 while the A1g(1) mode (688 cm−1) of both samples corresponds to the symmetric stretching of oxygen atoms with respect to Fe3+ cations in tetrahedral voids.34 The Raman spectrum of the iron oxide sample indicates the formation of the Fe3O4 and the γ-Fe2O3 phase,28 which is a Fe2+-vacant spinel structure. It is deduced that the samples CoFe2O4, Co0.5Fe2.5O4 and γ-Fe2O3 have a predominantly inverse spinel structure.
FTIR spectra were recorded in the frequency range between 800 and 400 cm−1, reflecting fundamental vibrations of metal–oxygen bonds in crystalline solids (Fig. S5†). The two bands for normal Co3O4 at 656 and 552 cm−1 fit to previously reported values corresponding to Co2+ in tetrahedral and octahedral voids, respectively.35 The band at 541 cm−1 for inverse γ-Fe2O3 is assigned to tetrahedrally coordinated Fe3+, while the band at 381 cm−1 originates from octahedral Fe3+.36,37 The shifting and vanishing of the recorded signals shows the transition from normal to inverse spinel with increasing Fe amount incorporated into the Co3O4 spinel structure.
The CoxFe3−xO4 samples were further characterized using X-ray photoelectron spectroscopy (XPS) to examine the oxidation states and the surface composition of the catalysts. The Co 2p region in Fig. 4 shows the Co 2p1/2 and Co 2p3/2 signals at 794.8 and 779.7 eV, respectively.38 The intense shake-up satellite peak at 787 eV can be assigned to Co2+, which was increasing with increasing Fe amount. Co3+only exhibits a weak satellite peak at 790 eV indicating a decrease in Co3+ concentration with increasing Fe amount.14 For the samples Co0.5Fe2.5O4 and CoFe2O4, only Co2+ was identified as expected, whereas the other samples contain mixed Co2+/Co3+ oxidation states. For Co3O4, a value of 60% Co2+ was determined with respect to the total Co amount on the catalysts' surface. Fig. 4 displays the Fe 2p1/2 and 2p3/2 peaks at 724.1 and 710.7 eV, respectively. The peak intensities gradually increased with increasing Fe amount in the CoxFe3−xO4 samples. The positions of the 2p3/2 peak and the satellite peak at 718.4 eV indicate the presence of Fe3+ for all samples. For Fe2+, both peaks would be shifted to lower binding energies.14,39 The C 1s region shows a distinct peak at 284.5 eV, which corresponds to C–C bonds and was used as a reference for calibration. The O 1s peak at 529.5 eV can be fitted by contributions of bulk and surface O (Fig. S6†). The surface composition of the CoxFe3−xO4 samples is summarized in Table S1.† The calculated surface Co/Fe ratios based on the XPS measurements agree with the bulk Co/Fe ratio determined by EDX, indicating similar bulk and surface compositions for all samples (Table 1).
Mössbauer spectroscopy measurements at 4.3 K were performed to additionally identify and verify the oxidation state of Fe in the CoxFe3−xO4 samples (Fig. S7†) with the applied methodology for evaluation of experimental spectra being illustrated in the ESI† in detail. A magnetically ordered sextet structure was recorded, which can be reproduced sufficiently for most of the samples by two subspectra representing the spinel tetrahedral A- and octahedral B-site. The sites were identified by their characteristic isomer shifts of δ ≈ 0.37 mm s−1 for the A-site and δ ≈ 0.47 mm s−1 for the B-site relative to α-Fe at room temperature.40,41 As no third subspectrum, which could be assigned to Fe2+ ions, was observed for any sample, only Fe3+ was identified in agreement with the XPS results. While for x ≥ 1 no Fe2+ is to be expected due to the presence of Co2+, the absence of Fe2+ for x = 0 indicates that this sample is oxidized to maghemite (γ-Fe2O3). Upon rising the Co-fraction x, a gradual change in the structure of the Mössbauer spectra can be observed, resulting in lower average hyperfine magnetic fields Bhf visible in Fig. S7A,† as well as increasing spin canting due to the enhanced magnetic anisotropy of CoFe2O4 and the trend towards antiferromagnetic ordering of Co3O4 for higher values of x (Fig. S7B†). Fe ion site occupation can be extracted directly from the relative A- and B-site subspectral intensities and thereby, the distribution of Co2+ ions on the different lattice positions can be inferred up to x = 3 when assuming that Co3+ is placed on octahedral positions exclusively, as illustrated in the ESI.† Following this approach, inversion parameters in the range of ca. 0.75 were found for x ≲ 1, pointing towards the tendency of Co2+ to be placed preferably on octahedral sites, corresponding more closely to the inverse spinel structure. For x > 1 the inversion parameter gradually decreases, as Co3+ is placed on B-site positions, reaching a minimum value of ca. 0.25 for x = 2.5 when approaching the normal spinel structure of Co3O4.42 Here, one has to consider that for x ≳ 1.5, due to the increasing spin frustration, applying the external field no longer yields better subspectral resolution. Still, even from zero field spectra, general trends in site occupancy can be extracted to some degree, displaying an evolution in the inversion parameter consistent with results from Raman spectroscopy.
In summary, we successfully synthesized a series of CoxFe3−xO4 catalysts with chemical compositions close to the nominal ones, high specific surface areas ranging from 87 to 151 m2 g−1, and small particle sizes between 8.3 and 12.0 nm. XRD measurements identified phase-pure samples of a cubic spinel structure. For x = 0, Rietveld refinement and Raman spectroscopy indicated the presence of the Fe2+-vacant structure γ-Fe2O3. XPS and Mössbauer measurements both demonstrated the absence of Fe2+ species in all samples. Raman and Mössbauer spectroscopy showed the tendency of Co3O4, Co2.5Fe0.5O4 and Co2FeO4 towards the normal spinel phase, whereas CoFe2O4, Co0.5Fe2.5O4 and γ-Fe2O3 were found to have a predominantly inverse spinel structure. The lattice spacing of CoFe2O4 indicates a dominant (111) surface, exposing coordinatively unsaturated MO5c metal sites.
Cyclohexene oxidation over CoxFe3−xO4
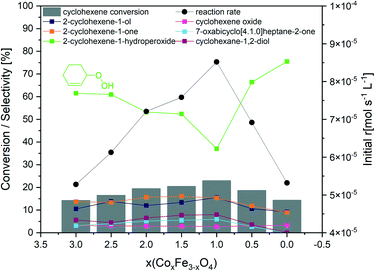
The catalytic properties of the CoxFe3−xO4 nanoparticulate spinel catalysts in cyclohexene oxidation were studied under mild reaction conditions in acetonitrile using molecular O2 as oxidizing agent. Initial experiments on the variation of the stirring speed showed that a speed of agitation of 600 rpm is suitable to exclude external mass transfer limitation (Fig. S8†).After 6 h, all catalysts revealed a moderate to high catalytic activity with degrees of conversion ranging from 44 to 69% (Fig. S9†), which is comparable to the degree of conversion (75%) over spray-flame synthesized LaCoO3 perovskite oxides.13,16 The comparison of Co3O4 and γ-Fe2O3 clearly shows the superior activity of the Co-containing catalyst after 6 h. In cyclohexene oxidation, Co3O4 achieved a significantly enhanced conversion as well as a lower selectivity to 2-cyclohexene-1-hydroperoxide. These results indicate a faster hydroperoxide decomposition, leading to an increase in 2-cyclohexene-1-one selectivity. In contrast, cyclohexene oxidation over γ-Fe2O3 was characterized by a slow hydroperoxide decomposition, so that the ketone selectivity is lowered by 12% in comparison to Co3O4.
The comparison of the CoxFe3−xO4 catalysts containing both Co and Fe clearly shows a positive effect of Fe substitution on the initial catalytic activity (Fig. 5). With increasing Fe amount up to CoFe2O4 (x = 1), the degree of conversion gradually increased from 14.3 to 23.0% after 0.5 h, whereas a blank reaction resulted in only 4.8% conversion (Fig. S10†). The increase in conversion was accompanied by a decreased hydroperoxide selectivity by 24.5%, indicating a faster decomposition, which results in higher product yields. However, the product selectivity was not affected, leading to the assumption that the catalyst is mainly involved in the formation and decomposition of the intermediate 2-cyclohexene-1-hydroperoxide. When the Fe content is further increased beyond CoFe2O4 (x < 1), the catalytic activity dropped as shown by the low degree of conversion of 14.4%, and the hydroperoxide selectivity became the highest within the whole catalyst series at 75.5%, identifying γ-Fe2O3 to be least active for cyclohexene oxidation.
The correlation of specific surface areas and particle sizes of the investigated catalysts with their catalytic activity excludes strong microstructural effects (Fig. S11†).
The comparison with the catalytic activity of spray-flame-synthesized LaCoO3 nanoparticles for cyclohexene oxidation we presented in a previous study indicates a comparable catalytic activity of the herein presented CoFe2O4 nanoparticles but a lower selectivity to 2-cyclohexene-1-hydroperoxide.13 Compared to other Co-based catalysts presented in literature reports, our CoFe2O4 achieves significantly higher degrees of conversion at similar or shorter reaction times (Table S2†).
The CoFe2O4 catalyst was chosen for further kinetic investigations, as it had the highest catalytic activity in cyclohexene oxidation with molecular O2. As expected, a strong influence of temperature on cyclohexene oxidation was observed (Fig. S12†). Cyclohexene conversion was limited to 38.7% at 60 °C, whereas at 100 °C nearly full conversion of 91.4% was recorded after 6 h. Additionally, hydroperoxide decomposition strongly increased at higher temperatures, resulting in its complete decomposition at 100 °C. Thus, product selectivity is enhanced and 2-cyclohexene-1-one becomes the main product of cyclohexene oxidation with a selectivity of 28.2%. Meanwhile, 2-cyclohexene-1-ol selectivity decreased, indicating the further oxidation of 2-cyclohexene-1-ol to the corresponding ketone. This led to strongly increasing ketone/alcohol ratios, which amount to 1.9 at 60 °C and 7.6 at 100 °C. However, the product distribution within the epoxidation products was not that strongly affected by temperature, as the cyclohexene-1,2-diol/cyclohexene oxide ratio only slightly decreased from 3.2 to 2.5. Nevertheless, higher temperatures shifted cyclohexene oxidation to the epoxidation pathway, as the ratio of allylic/epoxidation products decreased from 3.2 to 1.3. The catalytic data at different temperatures were used for the Arrhenius analysis. The degrees of cyclohexene conversion at different temperatures as a function of time are shown in Fig. 6A. Based on previous studies, cyclohexene oxidation over transition metal oxide catalysts is assumed to follow first-order reaction kinetics.13,16 The linearized plots based on first-order reaction kinetics were well suited for linear regression (R2 > 0.98), suggesting that cyclohexene oxidation over CoFe2O4 follows first-order reaction kinetics (Fig. S12D†).
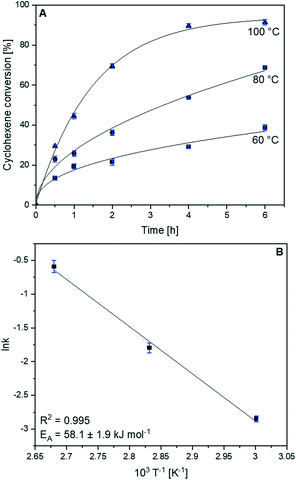 | ||
| Fig. 6 Kinetic investigations of cyclohexene oxidation over CoFe2O4. (A) Cyclohexene conversion as a function of time at different temperatures, and (B) the Arrhenius plot based on first-order kinetics. The rate constants k were determined according to Fig. S12D.† Reaction conditions: 20 mmol cyclohexene, 30 mL acetonitrile, 50 mg catalyst, 10 bar O2, 600 rpm, 6 h. | ||
The Arrhenius analysis resulted in an apparent activation energy of 58.1 kJ mol−1 (Fig. 6B). A reusability test was carried out to investigate the stability of the CoFe2O4 catalyst in terms of cyclohexene conversion and product selectivity (Fig. S13†). Four oxidation runs were performed, resulting in high and essentially equal degrees of cyclohexene conversion between 66.9 and 68.0%, which is in the range of experimental error and identifies CoFe2O4 to be a highly reusable catalyst under these mild oxidation conditions. Only a slight decrease in hydroperoxide decomposition by 5% was observed after the second run, resulting in a lower selectivity to 2-cyclohexene-1-one, indicating that the catalyst plays a crucial role in hydroperoxide decomposition. After the reusability test, a similar XRD pattern of phase-pure spinel structure of high crystallinity (Fig. S14†) and similar TEM images (Fig. S15†) were recorded, confirming the high stability of CoFe2O4 during cyclohexene oxidation.
As water is produced as a by-product in several reaction steps of cyclohexene oxidation, its influence on the catalytic activity was investigated by adding water to the initial reaction mixture (Fig. S16†). The initial reaction rate of cyclohexene oxidation was clearly suppressed. Nevertheless, cyclohexene conversion was only lowered by 5% after 6 h, and the hydroperoxide selectivity increased at low reaction times but reached a comparable value after 6 h. This was observed for almost all reaction products, indicating that H2O may block the active sites of CoFe2O4 at the beginning of the reaction, but the adsorption of the reactant is favored.
Mimicking 2-cyclohexene-1-hydroperoxide decomposition
Cyclohexene oxidation underlies an enormously complex reaction network, in which 2-cyclohexene-1-hydroperoxide plays a key role. The decomposition of 2-cyclohexene-1-hydroperoxide can lead to the formation of several reaction products (Scheme 1). Thus, the heterogeneously catalyzed decomposition of the hydroperoxide intermediate is the main target for selective cyclohexene oxidation. Within cyclohexene oxidation reactions, the hydroperoxide decomposition rate may be influenced by many factors, as the reaction mechanism and several correlations within the reaction network have not been fully clarified yet. Therefore, we implemented less complex peroxide decomposition reactions to mimic the hydroperoxide decomposition kinetics during cyclohexene oxidation over the CoxFe3−xO4 catalysts using H2O2 and TBHP as surrogates (Fig. S17†). Fig. S18† shows the evolved O2 volume as a function of time for both reactions. To avoid microstructural effects, the initial reaction rates normalized to the surface areas of the catalysts were calculated (Fig. 7). For H2O2 and TBHP decomposition, CoFe2O4 exhibited the highest reaction rates, whereas pure Co3O4 and γ-Fe2O3 showed a low catalytic activity, which is in agreement with the catalytic results of cyclohexene oxidation. Thus, these results indicate a synergistic effect of Co and Fe in CoxFe3−xO4 nanoparticles, which improves the decomposition rates of hydroperoxides such as 2-cyclohexene-1-hydroperoxide, TBHP and H2O2, resulting in the following activity ranking of the catalysts:| CHHP: | Co1> | Co1.5> | Co2> | Co2.5> | Co3> | Co0.5> | Co0 |
| H2O2: | Co1> | Co1.5> | Co2> | Co2.5> | Co3> | Co0.5> | Co0 |
| TBHP: | Co1> | Co1.5≈ | Co0.5> | Co2> | Co0> | Co2.5> | Co3 |
The CoxFe3−xO4 catalysts show an equal order of activity for 2-cyclohexene-1-hydroperoxide and H2O2 decomposition with CoFe2O4 as the most active sample. Both reactions exhibited a drastic decrease in activity over the samples containing a higher Fe amount than CoFe2O4 (x < 1). This activity drop was less pronounced in TBHP decomposition, resulting in a slightly different activity order, but the overall trend on TBHP decomposition is very similar to 2-cyclohexene-1-hydroperoxide and H2O2 decomposition (Fig. 7).
The rate constants for H2O2 decomposition over the CoxFe3−xO4 series are summarized in Table S3,† again identifying CoFe2O4 to be the most active catalyst with a rate constant of k = 0.679 s−1. The comparison with CoFe2O4 spinel samples from literature reports clearly shows a superior catalytic activity of the spray-flame synthesized CoFe2O4 sample (Table S4†). Cota et al.43 and Goldstein et al.,44 who both had a leading role in the research on peroxide decomposition in the last century, prepared CoFe2O4 samples enabling surface-area-normalized rate constants of ks = 0.071 s−1 m−2 and ks = 0.120 s−1 m−2 in alkaline solution, respectively, which are significantly lower compared with our catalyst (ks = 0.527 s−1 m−2). Onuchukwu et al.45 also studied H2O2 decomposition in alkaline solution over CoFe2O4 synthesized by co-precipitation and obtained a mass-normalized rate constant of km = 14 s−1 g−1, which is one fifth of the spray-flame synthesized CoFe2O4 (km = 67.9 s−1 g−1). Mimani et al.46 synthesized CoFe2O4 by a low-temperature decomposition approach and obtained a reaction rate constant of ks = 0.232 s−1 m−2, while CoFe2O4 materials synthesized by co-precipitation and sol–gel method led to significantly lower reaction rate constants of km = 0.037 s−1 g−1 and ks = 0.04 s−1 m−2, respectively.47,48
Interestingly, the H2O2 decomposition experiments using Co3O4 and CoFe2O4 synthesized by a precursor decomposition approach and direct co-precipitation revealed Co3O4 to be more active than CoFe2O4.17 This observation can be attributed to the platelet-like structure and the significantly smaller specific surface areas (∼30 m2 g−1) of these catalysts. Furthermore, unlike for the herein presented Co3O4, the surfaces of the Co3O4 sample in literature synthesized by co-precipitation were (111)-oriented.
Earlier studies on H2O2 decomposition over Co- and Fe-based spinels showed that it is not the incorporation of Fe, but rather the change of cation distribution within the spinel structure playing a crucial role in the high catalytic activity of Co–Fe mixed oxide catalysts. This assumption is supported by the low activity over γ-Fe2O3. Table S5† shows the theoretical cation valency distribution and spinel type of the prepared samples based on the crystal field stabilization energy of the transition metal ions.49 With increasing Fe amount incorporated in the spinel structure, a change from normal to inverse spinel and an increase of Co2+ ions located at octahedral voids is predicted. This was corroborated by our experimental results from Raman and Mössbauer spectroscopy, showing a clear trend towards a predominantly inverse structure with increasing Fe content.
Anthony and Onuchukwu50 investigated H2O2 decomposition over two series of NixFe3−xO4 and CuxFe3−xO4 catalysts. The authors highlighted the importance of the electron exchange by redox couples and were not able to demonstrate any significant influence of Fe present in the spinel samples. Similarly, Onuchukwu and Zuru45 prepared a CoxFe3−xO4 spinel series by co-precipitation and investigated its catalytic activity in H2O2 decomposition. They found Co1.5Fe1.5O4 to be the best catalyst and explained this observation by the presence of each 0.5 mol Co2+ and Co3+ per formula unit in the octahedral voids of the spinel, enabling an optimal electron exchange between the redox couple within the octahedral sites.45 However, H2O2 decomposition was carried out in a highly alkaline environment. The present study identifies CoFe2O4 to be the most active catalyst in acetonitrile, which does not contain the Co2+/Co3+ redox couple at the octahedral sites. Mimani and Patil46 found cobaltites to significantly better decompose H2O2 than ferrites, identifying the importance of Co species for H2O2 decomposition.
Goldstein and Tseung51 also investigated the complete series of CoxFe3−xO4 in H2O2 decomposition with 0 ≤ x ≤ 3 under basic conditions and proposed a ranking of redox couples:
| [Co2+–Co3+]O > [Co2+–Fe3+]O > (Co2+)T ≫ [Fe2+–Fe3+]O > (Fe2+)T |
| Co1 > Co1.5 > Co2 = Co2.5 = Co3 > Co0.5 > Co0 |
The reaction mechanism of H2O2 decomposition by ferrous and ferric salts was investigated by Haber and Weiss in 1932 and extended by Barb in 1949.52,53 They proposed H2O2 decomposition to be a radical chain reaction mechanism involving ˙OH and HO2˙ radicals as well as HO− and HO2− anions. Fe2+ is proposed to be the active site for H2O2 decomposition being oxidized to Fe3+ and forming OH− anions and ˙OH radicals, which initiate the radical chain reaction. The inferior activity of Fe3+ in H2O2 decomposition is confirmed by the lowest catalytic performance of γ-Fe2O3, which is a Fe2+-free structure. Similar to Fe2+ in the Haber–Weiss mechanism presented in Scheme S1,† we propose Co2+ to initiate a comparable reaction mechanism of H2O2 decomposition with a superior catalytic activity compared with Fe2+ and propose an analogous reaction mechanism for H2O2 decomposition over Co2+ (Scheme 2).
 | ||
| Scheme 2 Proposed reaction mechanism for H2O2 decomposition over Co2+ based on the Haber–Weiss mechanism.52,53 | ||
The presence of divalent Co cations at octahedral sites of the spinel structure is thus essential for the decomposition of peroxides. This observation is directly connected to the surface termination of particles, as different facets preferentially expose different cations. Sojka et al.25 investigated the surface structure of spinel nanocrystals by DFT + U calculations and TEM and found the (111) facet to be the most prominent facet with 67.5% of the overall surface area of truncated octahedral CoFe2O4 nanoparticles. This correlates with our findings, since TEM images of CoFe2O4 indicate a higher number of truncated octahedral particles with hexagonal and square surface planes (Fig. 1). Moreover, they calculated the surface composition of the (100), (110) and (111) facets by a slab model demonstrating the octahedral cations to be most pronounced on the surface of the (111) facet. For this reason, three-fold coordinated, coordinatively unsaturated Co3c2+ species in octahedral sites are highly exposed at CoFe2O4 nanoparticles acting as most active sites for peroxide decomposition reactions. In contrast, Co3O4 particles were reported to predominantly expose the (100) surface, which was also indicated for Co3O4 in this study. In cubic Co3O4 nanoparticles, five-fold coordinated divalent cations are exposed at the (100) facets, which can be regarded as significantly less active, since more strongly unsaturated cations allow higher catalytic activity. Overall, the coordinatively unsaturated Co3c2+ cations at octahedral sites on the (111) terminated surface of CoFe2O4 can be assumed to be the most active sites for peroxide decomposition.
Conclusions
A series of CoxFe3−xO4 nanoparticulate catalysts with x = 3, 2.5, 2, 1.5, 1, 0.5 and 0 was prepared by spray-flame synthesis and characterized in-depth by N2 physisorption, XRD, TEM, XPS, FT-IR, Raman and Mössbauer spectroscopy. The CoxFe3−xO4 samples were found to have chemical compositions close to the nominal ones, high specific surface areas ranging from 87 to 151 m2 g−1 and small particle sizes between 8.3 and 12.0 nm. The cation distribution was evaluated by Mössbauer spectroscopy, being consistent with trends also seen in theoretical estimates based on the crystal field stabilization energy. The application of CoxFe3−xO4 catalysts in cyclohexene oxidation resulted in a volcano plot with the highest catalytic activity at x = 1 (CoFe2O4). Cyclohexene conversion gradually increased with decreasing Co amount from x = 3 to x = 1. Simultaneously, the selectivity to the key intermediate 2-cyclohexene-1-hydroperoxide decreased, indicating its faster decomposition. Beyond this Co amount (x < 1), the catalytic activity strongly decreased again. The product selectivity was poorly affected by the Co and Fe amount incorporated into the CoxFe3−xO4 catalysts, identifying the catalysts to mainly accelerate the decomposition of the hydroperoxide. Kinetic investigations were performed using the most active catalyst CoFe2O4 revealing first-order reaction kinetics with an apparent activation energy of 58 kJ mol−1. A reusability test confirmed the excellent stability of the CoFe2O4 nanoparticles in cyclohexene oxidation. In addition, we were able to decouple 2-cyclohexene-1-hydroperoxide decomposition from the complex reaction network of cyclohexene oxidation by investigating the rates of H2O2 and TBHP decomposition. A very good agreement of the decomposition rates of 2-cyclohexene-1-hydroperoxide, H2O2 and TBHP was found, identifying CoFe2O4 as the most active catalyst for all three reactants, suggesting that 2-cyclohexene-1-hydroperoxide is also decomposed following the Haber–Weiss mechanism with octahedral Co3c2+ exposed at the (111) facets as the most active sites.Experimental
Materials
CoxFe3−xO4 nanoparticles were synthesized using the metal precursors of cobalt(II) nitrate (Co(NO3)2·6H2O, >99.0%, Honeywell) and iron(III) nitrate (Fe(NO3)3·6H2O, >99.9%, Sigma-Aldrich). As solvents, ethanol (>99.9%, VWR) and 2-ethylhexanoic acid (>99%, Alfa Aesar) were used.For cyclohexene oxidation, cyclohexene (99%), 2-cyclohexene-1-one (98%), 2-cyclohexene-1-ol (95%), cyclohexene oxide (98%), 7-oxabicyclo[4.1.0]heptane-2-one (98%), cyclohexane-1,2-diol (98%) and 1,2-dichlorobenzene (99%) were purchased from Sigma-Aldrich. Acetonitrile in analytical reagent grade was bought from Fisher Chemicals. All reagents were employed without further purification.
Catalyst synthesis
The synthesis of spinel nanoparticles was performed in a spray-flame reactor described previously in detail.54,55 Metal nitrates were used as precursors and dissolved in a mixture of 35 vol% ethanol and 65 vol% 2-ethylhexanoic acid. The precursor solutions contained a total metal-ion concentration of 0.2 M. The solutions were injected via syringe pumps to a capillary of an external-mixing atomizing nozzle at a constant flow rate of 2 mL min−1. Fine spray was formed by the interaction of the liquid flow with the dispersion O2 gas (6 slm, Air Liquide, technical). The spray is ignited by a continuously burning premixed pilot flame of methane (3 slm, Air Liquide, N25, 99.5%) and oxygen (5 slm). The pilot flame itself is surrounded by a sheath-gas flow (140 slm, compressed air) to stabilize the flame and shield it from the reactor walls. Downstream the reaction chamber, a quenching gas flow of compressed air was added to control the sintering of the particles and the off-gas temperature at values lower than 130 °C in order to prevent the damage of the filter membrane which is used to collect the particles during the synthesis process.Methods
X-ray diffraction patterns were recorded in a PANalytical X'Pert PRO operated with Cu-Kα radiation (0.15406 nm, 40 kV, 40 mA). The diffraction intensity was recorded at 2θ =10–60° with a step size of 0.05°.N2 physisorption measurements were performed at 77 K in a Quantachrome Nova2000. The specific surface areas were determined from the adsorption isotherm using the BET method.
High-resolution and high-angle annular dark-field scanning transmission electron microscopy (HR-TEM, HAADF-STEM), and energy dispersive X-ray spectroscopy (EDX) measurements were performed at a probe-side aberration-corrected JEM-2200FS (JEOL, Akishima, Japan) with an acceleration voltage of 200 kV.
Fourier-transform infrared spectroscopy was carried out in a Vertex 80 from Bruker using a KBr beam splitter, a DigiTect DLaTGS detector, and a mid-infrared light source from 400 to 4000 cm−1 in combination with an ATR sample holder.
Raman spectra were recorded using a Renishaw InVia confocal Raman microscope with a 633 nm laser operating at 1% of the total laser power (15 mW).
X-ray photoelectron spectra were obtained using a ULVAC-PHI device (Versaprobe II) using a XPS twin anode with Mg Kα & Al Kα, a spot size of 100 μm, and an energetic resolution of ∼0.5 eV. The calibration of the spectra was done using the C1s adventitious carbon C–C binding energy at 284.8 eV. The obtained data were fitted based on fixed positions, fixed contributions of the specific peaks to the overall signal as well as fixed FWHM. Using this procedure, a satisfactory overall fit was obtained in which nearly no area was left unfitted.
Mössbauer spectra were recorded in transmission geometry at 4.3 K with and without applying an external magnetic field, using a 57Co(Rh) radiation source, liquid helium (magnet) cryostats, 20–35 mg of each nanoparticle powder and a velocity transducer operating at constant acceleration.
Catalytic oxidation reactions
Oxidation reactions were carried out in a 100 mL autoclave reactor equipped with a Teflon liner (Parr Instruments). 50 mg catalyst were dispersed in 30 mL acetonitrile. 20 mmol cyclohexene and 4.5 mmol 1,2-dichlorobenzene as the internal standard for GC analysis were added. The autoclave was purged with oxygen for three times and pressurized to 10 bar. Subsequently, the reaction mixture was heated to 80 °C. The reaction was initiated by switching on the stirrer to 600 rpm. Samples were taken through an online sampling system after 0.5, 1, 2, 4, and 6 h, and analyzed by GC. The catalyst was separated by centrifugation.To test the reusability of the catalyst, four reaction runs were carried out under standard conditions. After each run, the catalyst was separated by centrifugation, washed three times with 5 mL acetonitrile, and dried overnight at room temperature.
For the investigation of cyclohexene oxidation in the presence of H2O, 3 mL H2O were added to the initial standard solution whereas all other reaction conditions remained unchanged.
Conversion (X), selectivity (S), the area-related reaction rate (r) normalized to the mass (mCat) and the specific surface area (SBET) of the catalyst were calculated, in which nCy, 0 and nP,0 denote the initial molar amount of cyclohexene and the respective product at t0, respectively, and nCy and nP denote the molar amount of cyclohexene and the respective product at a defined time t. The carbon balance was higher than 97% in all experiments.
Gas chromatography
Gas chromatography analysis was carried out in a 7820 A GC from Agilent Technologies. It was equipped with an Agilent DB-XLB column (30 m × 180 μm × 0.18 μm) and an FID detector. The injection volume was set to 0.5 μL with a split ratio of 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, a split flow of 30 mL min−1, and an inlet temperature of 260 °C. The column was first kept at 80 °C for 5 min. Subsequently, the oven was heated to 170 °C with a rate of 15 °C min−1. Afterwards, it was heated with a ramp of 30 °C min−1 up to 300 °C to avoid deposits of the PPh3 in the column. The end temperature was kept for 1 min.
1, a split flow of 30 mL min−1, and an inlet temperature of 260 °C. The column was first kept at 80 °C for 5 min. Subsequently, the oven was heated to 170 °C with a rate of 15 °C min−1. Afterwards, it was heated with a ramp of 30 °C min−1 up to 300 °C to avoid deposits of the PPh3 in the column. The end temperature was kept for 1 min.
TBHP decomposition
TBHP decomposition was carried out in a peroxide decomposition setup (Gasmess-5, Fig. S17†). 50 mg catalyst were dispersed in 30 mL acetonitrile. The solution was heated to 60 °C and stirred at 600 rpm. 500 μL TBHP were added and the gas volume measurement was started immediately. The decomposition reaction was run for 6 h.H2O2 decomposition
H2O2 decomposition was carried out in a peroxide decomposition setup (Gassmess-5, Fig. S17†). 10 mg catalyst were dispersed in 30 mL acetonitrile. The solution was kept at 30 °C and stirred at 600 rpm. 80 μL H2O2 (30 wt%) were added and the gas volume measurement was started immediately. The decomposition reaction was run for 30 min.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, TRR 247 “Heterogeneous Oxidation Catalysis in the Liquid Phase”, project number: 388390466). The work is supported by the “Center for Solvation Science ZEMOS” funded by the German Federal Ministry of Education and Research BMBF and by the Ministry of Culture and Research of Nord Rhine-Westphalia. We thank Dr. Hagemann and Dr. Heidelmann (Interdisciplinary Center for Analytics on the Nanoscale (ICAN), University of Duisburg-Essen) for XPS and TEM measurements, respectively.References
- I. M. Denekamp, M. Antens, T. K. Slot and G. Rothenberg, Selective Catalytic Oxidation of Cyclohexene with Molecular Oxygen: Radical versus Nonradical Pathways, ChemCatChem, 2018, 10, 1035–1041 CrossRef CAS PubMed.
- Y. Cao, H. Yu, F. Peng and H. Wang, Selective Allylic Oxidation of Cyclohexene Catalyzed by Nitrogen-Doped Carbon Nanotubes, ACS Catal., 2014, 4, 1617–1625 CrossRef CAS.
- B. G. Rao, P. Sudarsanam, P. R. Nallappareddy, M. Y. Reddy, T. V. Rao and B. M. Reddy, Selective Allylic Oxidation of Cyclohexene Catalyzed by Nanostructured Ce-Sm-Si Materials, Catal. Commun., 2017, 101, 57–61 CrossRef.
- R. Criegee, H. Pilz and H. Flygare, Zur Kenntnis der Olefinperoxyde, Berichte der deutschen chemischen Gesellschaft (A and B Series), 1939, 72, 1799–1804 CrossRef.
- S. M. Mahajani, M. M. Sharma and T. Sridhar, Uncatalysed Oxidation of Cyclohexene, Chem. Eng. Sci., 1999, 54, 3967–3976 CrossRef CAS.
- V. S. Kirankumar and S. Sumathi, A Review on Photodegradation of Organic Pollutants Using Spinel Oxide, Mater. Today Chem., 2020, 18, 100355 CrossRef CAS.
- P. Cousin and R. A. Ross, Preparation of Mixed Oxides: A Review, Mater. Sci. Eng., A, 1990, 130, 119–125 CrossRef.
- T. Falk, S. Anke, H. Hajiyani, S. Saddeler, S. Schulz, R. Pentcheva, B. Peng and M. Muhler, Influence of the Particle Size on Selective 2-Propanol Gas-Phase Oxidation over Co3O4 Nanospheres, Catal. Sci. Technol., 2021, 11, 7552–7562 RSC.
- S. Patoux, L. Daniel, C. Bourbon, H. Lignier, C. Pagano, F. Le Cras, S. Jouanneau and S. Martinet, High Voltage Spinel Oxides for Li-Ion Batteries: From the Material Research to the Application, J. Power Sources, 2009, 189, 344–352 CrossRef CAS.
- C. G. Anchieta, D. Sallet, E. L. Foletto, S. S. Da Silva, O. Chiavone-Filho and C. A. O. do Nascimento, Synthesis of Ternary Zinc Spinel Oxides and their Application in the Photodegradation of Organic Pollutant, Ceram. Int., 2014, 40, 4173–4178 CrossRef CAS.
- Y. Sun, H. Liao, J. Wang, B. Chen, S. Sun, S. J. H. Ong, S. Xi, C. Diao, Y. Du and J.-O. Wang, Covalency Competition Dominates the Water Oxidation Structure–Activity Relationship on Spinel Oxides, Nat. Catal., 2020, 3, 554–563 CrossRef CAS.
- S. Pal, U. P. Azad, A. K. Singh, D. Kumar and R. Prakash, Studies on Some Spinel Oxides Based Electrocatalysts for Oxygen Evolution and Capacitive Applications, Electrochim. Acta, 2019, 320, 134584 CrossRef CAS.
- J. Büker, B. Alkan, Q. Fu, W. Xia, J. Schulwitz, D. Waffel, T. Falk, C. Schulz, H. Wiggers and M. Muhler, Selective Cyclohexene Oxidation with O2, H2O2 and Tert-Butyl Hydroperoxide over Spray-Flame Synthesized LaCo1−xFexO3 Nanoparticles, Catal. Sci. Technol., 2020, 10, 5196–5206 RSC.
- T. Falk, E. Budiyanto, M. Dreyer, C. Pflieger, D. Waffel, J. Büker, C. Weidenthaler, K. F. Ortega, M. Behrens, H. Tüysüz, M. Muhler and B. Peng, Identification of Active Sites in the Catalytic Oxidation of 2-Propanol over Co1+xFe2-xO4 Spinel Oxides at Solid/Liquid and Solid/Gas Interfaces, ChemCatChem, 2021, 13, 2942–2951 CrossRef CAS.
- D. Waffel, E. Budiyanto, T. Porske, J. Büker, T. Falk, Q. Fu, S. Schmidt, H. Tüysüz, M. Muhler and B. Peng, Investigation of Synergistic Effects between Co and Fe in Co3-xFexO4 Spinel Catalysts for the Liquid-Phase Oxidation of Aromatic Alcohols and Styrene, Mol. Catal., 2020, 498, 111251 CrossRef CAS.
- J. Büker, B. Alkan, S. Chabbra, N. Kochetov, T. Falk, A. Schnegg, C. Schulz, H. Wiggers, M. Muhler and B. Peng, Liquid-Phase Cyclohexene Oxidation with O2 over Spray-Flame-Synthesized La1-xSrxCoO3 Perovskite Nanoparticles, Chem. – Eur. J., 2021, 27, 16912–16923 CrossRef PubMed.
- A. Rabe, J. Büker, S. Salamon, A. Koul, U. Hagemann, J. Landers, K. Friedel Ortega, B. Peng, M. Muhler, H. Wende, W. Schuhmann and M. Behrens, The Roles of Composition and Mesostructure of Cobalt-based Spinel Catalysts in Oxygen Evolution Reactions, Chem. – Eur. J., 2021, 27, 17038–17048 CrossRef CAS PubMed.
- Y.-I. Jang, H. Wang and Y.-M. Chiang, Room-Temperature Synthesis of Monodisperse Mixed Spinel (CoxMn1–x)3O4 Powder by a Coprecipitation Method, J. Mater. Chem., 1998, 8, 2761–2764 RSC.
- T. H. Dolla, K. Pruessner, D. G. Billing, C. Sheppard, A. Prinsloo, E. Carleschi, B. Doyle and P. Ndungu, Sol-Gel Synthesis of MnxNi1-xCo2O4 Spinel Phase Materials: Structural, Electronic, and Magnetic Properties, J. Alloys Compd., 2018, 742, 78–89 CrossRef CAS.
- Z. Chen, E. Shi, W. Li, Y. Zheng and W. Zhong, Hydrothermal Synthesis and Optical Property of Nano-Sized CoAl2O4 Pigment, Mater. Lett., 2002, 55, 281–284 CrossRef CAS.
- M. Seyedahmadian, S. Houshyarazar and A. Amirshaghaghi, Synthesis and Characterization of Nanosized of Spinel LiMn2O4 via Sol-Gel and Freeze Drying Methods, Bull. Korean Chem. Soc., 2013, 34, 622–628 CrossRef CAS.
- B. Alkan, D. Medina, J. Landers, M. Heidelmann, U. Hagemann, S. Salamon, C. Andronescu, H. Wende, C. Schulz and W. Schuhmann, Spray-Flame-Prepared LaCo1-xFexO3 Perovskite Nanoparticles as Active OER Catalysts: Influence of Fe Content and Low-Temperature Heating, ChemElectroChem, 2020, 7, 2564–2574 CrossRef CAS.
- S. Angel, J. Neises, M. Dreyer, K. Friedel Ortega, M. Behrens, Y. Wang, H. Arandiyan, C. Schulz and H. Wiggers, Spray-Flame Synthesis of La(Fe,Co)O3 Nano-Perovskites from Metal Nitrates, AIChE J., 2020, 66, e16748 CrossRef CAS.
- B. Varghese, C. H. Teo, Y. Zhu, M. V. Reddy, B. V. R. Chowdari, A. T. S. Wee, V. B. Tan, C. T. Lim and C.-H. Sow, Co3O4 Nanostructures with Different Morphologies and their Field-Emission Properties, Adv. Funct. Mater., 2007, 17, 1932–1939 CrossRef CAS.
- F. Zasada, J. Grybos, P. Indyka, W. Piskorz, J. Kaczmarczyk and Z. Sojka, Surface Structure and Morphology of M [CoM']O4 (M = Mg, Zn, Fe, Co and M′ = Ni, Al, Mn, Co) Spinel Nanocrystals: DFT+ U and TEM Screening Investigations, J. Phys. Chem. C, 2014, 118, 19085–19097 CrossRef CAS.
- S. Wang, Y. Zhao, H. Xue, J. Xie, C. Feng, H. Li, D. Shi, S. Muhammad and Q. Jiao, Preparation of flower-like CoFe2O4@ graphene composites and their microwave absorbing properties, Mater. Lett., 2018, 223, 186–189 CrossRef CAS.
- R. Al-Tuwirqi, A. A. Al-Ghamdi, N. A. Aal, A. Umar and W. E. Mahmoud, Facile Synthesis and Optical Properties of Co3O4 Nanostructures by the Microwave Route, Superlattices Microstruct., 2011, 49, 416–421 CrossRef CAS.
- L. J. Cote, A. S. Teja, A. P. Wilkinson and Z. J. Zhang, Continuous Hydrothermal Synthesis of CoFe2O4 Nanoparticles, Fluid Phase Equilib., 2003, 210, 307–317 CrossRef CAS.
- G. Gnanaprakash, S. Ayyappan, T. Jayakumar, J. Philip and B. Raj, Magnetic Nanoparticles with Enhanced γ-Fe2O3 to α-Fe2O3 Phase Transition Temperature, Nanotechnology, 2006, 17, 5851 CrossRef CAS.
- A. Diallo, A. C. Beye, T. B. Doyle, E. Park and M. Maaza, Green Synthesis of Co3O4 Nanoparticles via Aspalathus Linearis: Physical Properties, Green Chem. Lett. Rev., 2015, 8, 30–36 CrossRef CAS.
- B. Rivas-Murias and V. Salgueiriño, Thermodynamic CoO-Co3O4 Crossover Using Raman Spectroscopy in Magnetic Octahedron-Shaped Nanocrystals, J. Raman Spectrosc., 2017, 48, 837–841 CrossRef CAS.
- Y. Zheng, Y. Yu, H. Zhou, W. Huang and Z. Pu, Combustion of Lean Methane over Co3O4 Catalysts Prepared with Different Cobalt Precursors, RSC Adv., 2020, 10, 4490–4498 RSC.
- K. Gandha, K. Elkins, N. Poudyal and J. Ping Liu, Synthesis and Characterization of CoFe2O4 Nanoparticles with High Coercivity, J. Appl. Phys., 2015, 117, 17A736 CrossRef.
- V. Bartůněk, D. Sedmidubský, Š. Huber, M. Švecová, P. Ulbrich and O. Jankovský, Synthesis and Properties of Nanosized Stoichiometric Cobalt Ferrite Spinel, Materials, 2018, 11, 1241 CrossRef PubMed.
- R. N. Bhowmik and N. Naresh, Structure, ac Conductivity and Complex Impedance Study of Co3O4 and Fe3O4 Mixed Spinel Ferrites, International Journal of Engineering, Science and Technology, 2010, 2, 40–52 Search PubMed.
- R. Sagayaraj, S. Aravazhi and G. Chandrasekaran, Tuning of Ferrites (CoxFe3-xO4) Nanoparticles by Co-Precipitation Technique, Appl. Sci., 2019, 1, 1–11 CAS.
- A. M. Wahba and M. B. Mohamed, Structural and Magnetic Characterization and Cation Distribution of Nanocrystalline CoxFe3−xO4 Ferrites, J. Magn. Magn. Mater., 2015, 378, 246–252 CrossRef CAS.
- M. C. Biesinger, B. P. Payne, A. P. Grosvenor, L. W. Lau, A. R. Gerson and R. S. Smart, Resolving Surface Chemical States in XPS Analysis of First Row Transition Metals, Oxides and Hydroxides: Cr, Mn, Fe, Co and Ni, Appl. Surf. Sci., 2011, 257, 2717–2730 CrossRef CAS.
- H. Liu, G. Wei, Z. Xu, P. Liu and Y. Li, Quantitative Analysis of Fe and Co in Co-Substituted Magnetite Using XPS: The Application of Non-Linear Least Squares Fitting (NLLSF), Appl. Surf. Sci., 2016, 389, 438–446 CrossRef CAS.
- H. Le Trong, L. Presmanes, E. de Grave, A. Barnabé, C. Bonningue and P. Tailhades, Mössbauer Characterisations and Magnetic Properties of Iron Cobaltites CoxFe3−xO4 (1≤ x≤ 2.46) Before and After Spinodal Decomposition, J. Magn. Magn. Mater., 2013, 334, 66–73 CrossRef CAS.
- P. J. Murray and J. W. Linnett, Mössbauer studies in the spinel system CoxFe3− xO4, J. Phys. Chem. Solids, 1976, 37, 619–624 CrossRef CAS.
- G. M. Bancroft, T. K. Sham, C. Riddle, T. E. Smith and A. Turek, Ferric/Ferrous-Iron Ratios in Bulk Rock Samples by Mössbauer Spectroscopy—The Determination of Standard Rock Samples G-2, GA, W-1 and mica-Fe, Chem. Geol., 1977, 19, 277–284 CrossRef CAS.
- H. M. Cota, T. Katan, M. Chin and F. J. Schoenweis, Decomposition of Dilute Hydrogen Peroxide in Alkaline Solutions, Nature, 1964, 203, 1281 CrossRef CAS.
- J. R. Goldstein and A. C. Tseung, The Kinetics of Hydrogen Peroxide Decomposition Catalyzed by Cobalt-Iron Oxides, J. Catal., 1974, 32, 452–465 CrossRef CAS.
- A. I. Onuchukwu and A. B. Zuru, The Cobalt-Ferrite, CoxFe3−xO4, Activity in the Catalytic Chemical Decomposition of Hydrogen Peroxide, Mater. Chem. Phys., 1986, 15, 131–138 CrossRef CAS.
- T. Mimani, P. Ravindranathan and K. C. Patil, Catalytic Decomposition of Hydrogen Peroxide on Fine Particle Ferrites and Cobaltites, Springer, 1987, vol. 4 Search PubMed.
- P. Lahiri and S. K. Sengupta, Spinel Ferrites as Catalysts: A Study on Catalytic Effect of Coprecipitated Ferrites on Hydrogen Peroxide Decomposition, Can. J. Chem., 1991, 69, 33–36 CrossRef CAS.
- T. Tatarchuk, A. Shyichuk, I. Trawczyńska, I. Yaremiy, A. T. Pędziwiatr, P. Kurzydło, B. F. Bogacz and R. Gargula, Spinel cobalt (II) ferrite-chromites as catalysts for H2O2 decomposition: Synthesis, morphology, cation distribution and antistructure model of active centers formation, Ceram. Int., 2020, 46, 27517–27530 CrossRef CAS.
- U. Müller, Anorganische Strukturchemie, Vieweg+Teubner Verlag/GWV Fachverlage GmbH Wiesbaden, Wiesbaden, 6th edn, 2008 Search PubMed.
- A. I. Onuchukwu, Kinetics of the Decomposition of Hydrogen Peroxide Catalysed by Copper and Nickel Ferrites, J. Chem. Soc., Faraday Trans. 1, 1984, 80, 1447–1456 RSC.
- A. C. Tseung and J. R. Goldstein, The Preparation and Characterization of Ultrafine Cobalt-Iron Oxides, J. Mater. Sci., 1972, 7, 1383–1390 CrossRef CAS.
- F. Haber and J. Weiss, The Catalytic Decomposition of Hydrogen Peroxide by Iron Salts, Proc. R. Soc. London, Ser. A, 1934, 147, 332–351 CAS.
- W. G. Barb, J. H. Baxendale, P. George and K. R. Hargrave, Reactions of Ferrous and Ferric Ions with Hydrogen Peroxide. Part I.—The Ferrous Ion Reaction, Trans. Faraday Soc., 1951, 47, 462–500 RSC.
- S. Angel, F. Schneider, S. Apazeller, W. Kaziur-Cegla, T. C. Schmidt, C. Schulz and H. Wiggers, Spray-Flame Synthesis of LaMO3 (M= Mn, Fe, Co) Perovskite Nanomaterials: Effect of Spray Droplet Size and Esterification on Particle Size Distribution, Proc. Combust. Inst., 2021, 38, 1279–1287 CrossRef CAS.
- S. Hardt, I. Wlokas, C. Schulz and H. Wiggers, Impact of Ambient Pressure on Titania Nanoparticle Formation During Spray-Flame Synthesis, J. Nanosci. Nanotechnol., 2015, 15, 9449–9456 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cy00505k |
| This journal is © The Royal Society of Chemistry 2022 |