Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The advent of thermoplasmonic membrane distillation
Sergio
Santoro
 a,
Ahmet H.
Avci
a,
Antonio
Politano
a,
Ahmet H.
Avci
a,
Antonio
Politano
 *b and
Efrem
Curcio
*a
*b and
Efrem
Curcio
*a
aUniversity of Calabria – Department of Environmental and Chemical Engineering, Cubo 44 A, Via Pietro Bucci, 87036 Rende CS, Italy. E-mail: efrem.curcio@unical.it
bDepartment of Physical and Chemical Sciences, University of L’Aquila, via Vetoio, 67100 L’Aquila (AQ), Italy. E-mail: antonio.politano@univaq.it
First published on 5th July 2022
Abstract
Freshwater scarcity is a vital societal challenge related to climate change, population pressure, and agricultural and industrial demands. Therefore, sustainable desalination/purification of salty/contaminated water for human uses is particularly relevant. Membrane distillation is an emerging hybrid thermal-membrane technology with the potential to overcome the drawbacks of conventional desalination by a synergic exploitation of the water–energy nexus. Although membrane distillation is considered a green technology, efficient heat management remains a critical concern affecting the cost of the process and hindering its viability at large scale. A multidisciplinary approach that involves materials chemistry, physical chemistry, chemical engineering, and materials and polymer science is required to solve this problem. The combination of solar energy with membrane distillation is considered a potentially feasible low-cost approach for providing high-quality freshwater with a low carbon footprint. In particular, recent discoveries about efficient light-to-heat conversion in nanomaterials have opened unprecedented perspectives for the implementation of sunlight-based renewable energy in membrane distillation. The integration of nanofillers enabling photothermal effects into membranes has been demonstrated to be able to significantly enhance the energy efficiency without impacting on economic costs. Here, we provide a comprehensive overview on the state of the art, the opportunities, open challenges and pitfalls of the emerging field of solar-driven membrane distillation. We also assess the peculiar physicochemical properties and synthesis scalability of photothermal materials, as well as the strategies for their integration into polymeric nanocomposite membranes enabling efficient light-to-heat conversion and freshwater.
Prof. Antonio Politano has a PhD in Physics and is an Associate Professor at the Department of Physical and Chemical Sciences of the University of L’Aquila (Italy). Previously, he was a post-doc at the Italian Institute of Technology, Genoa (Italy), the Autonomous University of Madrid (Spain), and Imdea Nanociencia, Madrid (Spain). He has coauthored >200 papers in peer-reviewed journals (H-Index: 41) and he is the principal investigator of several national and international projects. He was awarded with three prizes for his scientific activity, four international fellowships, and the APS Outstanding Referee. He was an invited speaker at about 20 conferences. He is involved in the evaluation of research projects in 11 countries. |
1. Introduction
Freshwater availability in adequate quality and quantity is one of the major societal challenges.1 Despite the significant technological progress made in the last few decades, demographic growth, climate change, intensive agriculture activities and rapid industrial development make water shortage one of the pressing global issues of this century.2 The global annual water demand is estimated to increase from 3500 km3 in 2000 to around 5500 km3 in 2050.3 Consistently, the World Health Organization (WHO) predicted an alarming scenario: about 50% of the world's population will live in water-stressed regions by the year 2025.4This drives the need to develop innovative technologies to be able to meet the growing water demand, while reducing the water footprint.5–7 Seawater desalination is considered the most reliable solution to the freshwater scarcity problem, considering that seawater constitutes more than 97% (ca.1.4 × 109 km3) of the total available water on Earth.8,9 Besides, the post-treatment of industrial wastewater has gained more attention in order to reduce the environmental impact in the logic of Circular Economy.10,11
Desalination is essentially defined as the process used to produce potable water by removing dissolved salts and minerals from seawater and brackish water.12 Existing desalination technologies are conventionally classified into two main categories: (i) thermal-based evaporative processes (with phase change) and (ii) filtration-based processes (without phase change).13 However, the widespread application of these processes, such as multi-stage flash distillation (MSF), multi-effect distillation (MED) and reverse osmosis (RO), requires significant energy power: the demand of conventional thermal desalination processes is 10–15 kW h m−3 to vaporize saline water,14 whereas 3–6 kW h m−3 is needed to overcome the osmotic pressure of seawater in RO.15,16
The concern about the water–energy nexus due to the urgency of the sustainable production of freshwater without stressing energy supplies has led to membrane filtration as the prominent technology for desalination. In 2021, ca. 78 million cubic meters of water were produced per day;17 RO covers about 60% of global desalination capacity,18 with an expected growth for the global market of major components of RO systems of $11.7 billion in 2020 and $19.1 billion by 2025 at a compound annual growth rate (CAGR) of 10.3% for the period of 2020–2025.19
Despite its success, the pitfall of RO is its maximum freshwater recovery factor which is typically limited to 40–50% due to the increase in the osmotic pressure of the hypersaline (>50 g L−1) rejected stream, known as brine.20,21 The effect of the osmotic phenomenon hampers the economic feasibility of brine desalination, since it involves severe risk of scaling and fouling in the membrane modules22 and requires hydraulic pressure exceeding the membrane burst pressure (typically 70–80 bar).23 As a result, RO desalination plants produce around 40 million m3 day−1 of brine as a by-product discharged to the sea, along with other chemicals involved in the pre-treatment steps including iron, copper, zinc, and cleaning agents (such as hydrochloric acid, sodium hexametaphosphate, anti-scalants etc.), severely impacting the marine habitat and the surrounding ecosystem.24,25
In recent years, membrane distillation (MD) has emerged as a complementary hybrid thermal/membrane technology not limited by either osmotic or concentration polarization phenomena, having the potential to produce desalted water at recovery factors close to 90% and at moderate operating temperatures (60–80 °C).26 Indeed, mass transfer in MD is driven by a partial pressure gradient across a hydrophobic microporous membrane; this promotes a net flux of water vapor from a warm saline feed contacting one side of the membrane, towards the opposite side.26 MD exhibits outstanding advantages with respect to conventional desalination processes: high quality of desalted water (theoretically, only volatile species evaporate, while dissolved salts are completely retained), small footprint, and low susceptibility of water vapor flux to feed concentration.27,28
Likewise, MD can be used in treating a large variety of industrial wastewater for the purification, extraction, concentration and final formulation of organic and inorganic species being a feasible route in order to implement the concept of Zero Liquid Discharge (ZLD).29 Beyond the benefits related to the remarkable reduction in the amount of disposed brine, the possibility to concentrate saline solutions up to supersaturation allows the implementation of the concept of membrane distillation-crystallization, opening unprecedented horizons in raw material recovery by mining desalination brine.30 To date, efforts have been focused on the development of innovative membranes and on process optimization, but MD is still far from technological maturity.
At the operational level, the performance of MD is negatively affected by the low thermal efficiency mainly due to temperature polarization (TP), a phenomenon intrinsically related to the removal of latent heat associated with water evaporation and – to a lesser extent – to the conductive heat flux through the membrane.31 Since evaporation takes place at the feed–membrane interface, a decreasing feed temperature profile throughout the adjacent boundary layer is observed. Consequently, evaporation occurs at a temperature lower than the bulk value, the net driving force to the mass transfer decreases and, ultimately, the overall efficiency of the process drastically falls (<50%).32,33
Despite the occurrence of TP, the low operative temperature of MD makes process integration with renewable energy or waste energy very attractive. Several attempts to exploit solar energy with MD for seawater desalination have been reported in the literature.34–37 In the last decade, efforts have been focused on the development of autonomous solar driven MD units for desalination in arid and remote regions by using solar thermal collectors for heating the seawater or brackish water, while the electricity is supplied by photovoltaic panels.38–40 Studies confirmed the viability of solar-driven MD, obtaining good quality of freshwater with a specific energy consumption of 200–300 kW h m−3.38–40
Although solar energy is renewable, “bulk” heating of feed saline water is energetically inefficient due to the occurrence of TP within the boundary layer adjacent to the membrane. Conversely, photothermal nanomaterials as “nano-heaters” incorporated on the membrane surface promote a localized light-to-heat conversion, leading to the withdrawal of TP for highly efficient water evaporation. In the last few years, intensive efforts have been devoted to the rational design of efficient photothermal nanoparticles (NPs) through the fine tuning of their chemical composition, surface functionalization and structural morphology.41,42 In particular, thermoplasmonics, i.e., the light-to-heat energy conversion associated with optically resonant plasmonic excitations in metal NPs, has been demonstrated to be beneficial for facilitating the vaporization of water with photothermal interface.43–45 Distinct from conventional intensive and inefficient heating in bulk water processes, interfacial vaporization boosted by photothermal surfaces drastically minimizes the heat loss by locally harvesting the heat at the water–vapor interface.46,47
Analogously, MD has received a new impulse due to the latest advances in thermoplasmonics that encourage the development of novel photothermal membranes for TP mitigation and improved MD performance.48–50 Moreover, next-generation materials exhibiting photothermal effects have the potential to exploit solar renewable energy for decentralized off-grid desalination by converting sunlight into heat for a unique and favorable water–energy nexus.51,52
Here, we review the recent progress in the development of photothermal membranes prepared by the immobilization of advanced functional nanomaterials in a polymeric network for efficient light-to-heat conversion in solar-driven low-energy water purification applications.
Moving beyond solar steam generation, which is the topic of some previously published review articles, we critically focus on technological potential, current challenges, and perspectives of photothermal membrane distillation (PMD). The review critically discusses the link between the morphological and physicochemical properties of MD membranes and the fundamentals of light-to-heat conversion in photothermal NPs (metals, semiconductors, carbon-based materials, and polymers), offering a comprehensive background for the development of the next generation of PMD membranes. In addition, this review systematically assesses the impact of the design criteria of membrane modules and optimization of the process parameters on the photothermal-driven freshwater production. Overall, the review highlights the need for a multidisciplinary approach to drive the practical implementation of thermoplasmonic MD, embracing fundamental studies of physics for understanding the mechanism of light-to-heat conversion, advanced chemical synthesis for the preparation of photothermal NPs, advances in materials science for the development of effective photothermal membranes, and a rational engineering of the PMD process.
2. Membrane distillation (MD)
2.1 Membrane distillation (MD): operational principles
MD is a non-isothermal process based on the evaporation and transport of volatile molecules (usually water vapor) through the macropores (typical pore size of 0.05–0.5 μm) of a hydrophobic membrane contacting an aqueous solution, whereas non-volatile components (i.e., ions, biomolecules, colloids, bacteria etc.) are rejected due to the hydro-repellent character of the membrane itself.53,54 In this sense, the membrane does not play an active role as a selective material in the separation but provides a support for the creation of a liquid–vapor interface at each pore mouth where phase transition takes place. Therefore, MD is categorized as “Membrane Contactors” technology. Its unique operational features make MD suitable for a wide spectrum of applications, such as desalination, wastewater decontamination and purification, and dehydration of aqueous solutions.55 When protracting the dehydration of the aqueous solution above the saturation limit – thus leading to the formation of crystals – MD is referred to as membrane crystallization (MCr), a versatile technology able to control the nucleation rate by modulating both the supersaturation degree and the physicochemical properties of the membrane surface where nuclei originate.56The productivity of MD is quantified in terms of transmembrane flux (J), which varies linearly with the partial pressure gradient (Δpi) of the i-th component transported in the vapor phase across the membrane:
| J = K·Δpi | (1) |
This gradient, acting as a driving force for the mass transfer in MD, is typically – but not exclusively – generated by a temperature difference (osmotic distillation57 is an exception); in the most common case of an aqueous feed solution, mass transfer includes three main steps:
(1) evaporation of water molecules at the interface of the warm aqueous feed solution in contact with the membrane (“feed” or “retentate” side);
(2) transport of water vapor across the membrane via Knudsen diffusion and/or molecular diffusion depending on the membrane pore size, the mean free path of diffusing molecules, and the eventual presence of trapped air in the pores;58,59 and
(3) removal or condensation of water vapor on the opposite side of the membrane (the “distillate” side).
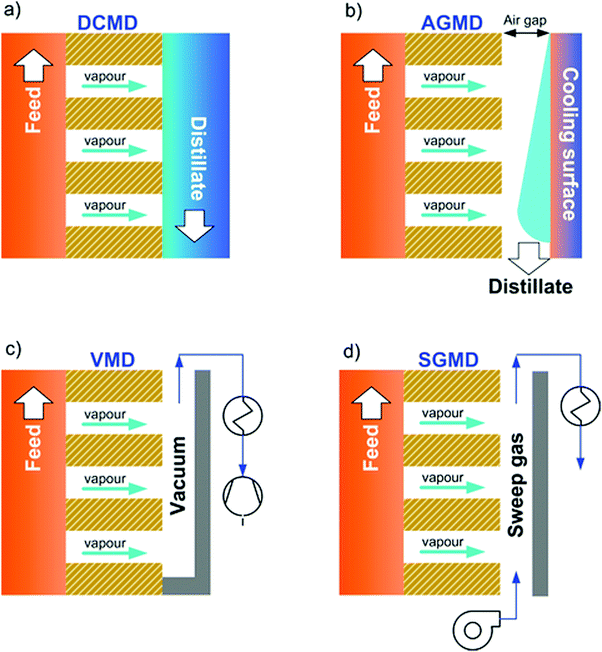
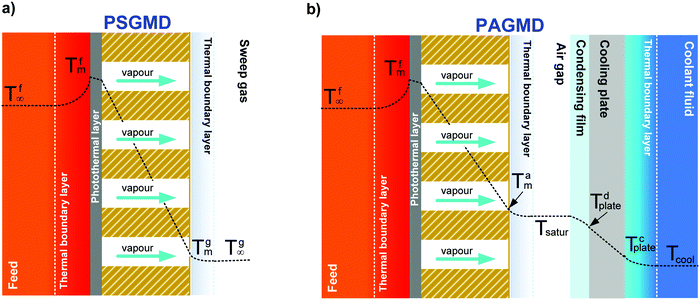
Depending on the methodology adopted to reduce the partial pressure on the distillate side, MD is classified into four basic configurations60 (Fig. 1):
(1) Direct contact membrane distillation (DCMD): the distillate side consists of pure water set at a lower temperature with respect to the feed solution;61
(2) Air gap membrane distillation (AGMD): the distillate compartment consists of: (i) a thin and stagnant air gap and (ii) a condensing cold surface made of a dense polymer or metal film;62
(3) Vacuum membrane distillation (VMD): a vacuum is applied at the distillate side at a level below the saturation pressure of water at the feed temperature, whereas the vapor is condensed in a subsequent step;64
(4) Sweep gas membrane distillation (SGMD): an inert sweep gas – which collects the vapor – flows at the distillate side, whereas condensation takes place in a subsequent step.63
Other configurations can be regarded as variants or hybrids of the four basic schemes previously described. For instance, permeate gap membrane distillation (PGMD) is a combination of AGMD and DCMD obtained by sandwiching the distillate channel between the membrane and the cooling surface.65 Vacuum-enhanced air gap membrane distillation (V-AGMD) is a combination of AGMD and VMD, where a weak vacuum is used to suck air from the gap (while allowing the condensation of the vapor) in order to decrease the mass transfer resistance.66
2.2 MD membranes
Fluoropolymers are particularly suitable for MD, owing to their high thermal and chemical stability, and low surface tension. These properties are mainly related to the low polarizability, strong electronegativity, and minimal van der Waals radius of the fluorine atom combined with the strength of the C–F bond.71 Among the above-mentioned polymers, PTFE has been widely used due its low surface energy (9–20 × 10−3 N m−1) and high crystallinity.72 The high degree of crystallinity (in general from 92% to 98%) depends on the long and unbranched PTFE molecules with CF2 groups along the polymer chain, twisted into a helix as in a length of rope whereas fluorine atoms prohibit a planar zigzag conformation.71 On the other hand, PTFE is poorly soluble being non-polar, thus hampering membrane preparation via phase-separation methods widely used in industrial practice, such as non-solvent induced phase separation (NIPS) or thermal induced phase separation (TIPS). Usually, PTFE membranes are prepared via the sintering process by mixing PTFE powders with volatile lubricating agents, resulting in a paste, subsequently extruded into flat sheets or hollow fibers.73 Alternatively, PTFE membranes are prepared via the polymer melt extrusion method followed by stretching.74
PVDF, consisting of long chain macromolecules containing 59.4 wt% fluorine and 3 wt% hydrogen,71 represents the most diffuse polymer used in the fabrication of membranes for MD,75 as a result of its superior solubility in a wide range of polar aprotic organic solvents, such as N-methyl-2-pyrrolidone (NMP), N,N-dimethylformamide (DMF), or dimethylacetamide (DMA),60 allowing membrane preparation using conventional phase inversion techniques. The Hansen solubility parameters, based on the assumption that cohesive energy can be expressed in terms of contributions from dispersive (δd), polar (δp) and hydrogen bonding (δh) forces, are effectively used to estimate the affinity between polymers and solvents.76,77 As shown in Table 1, the Hansen parameters of PVDF are close to the ones of a wide range of solvents complying with the chemist's “rule of thumb” that “like dissolves like”.77,78
| Chemical | δ h | δ d | δ p |
|---|---|---|---|
| PVDF | 9.2 | 17.2 | 12.5 |
| PTFE | 0 | 14 | 0 |
| TEP | 9.2 | 16.8 | 11.5 |
| DMF | 11.3 | 17.4 | 13.7 |
| DMA | 11.8 | 17.8 | 14.1 |
| NMP | 7.2 | 18.4 | 12.3 |
| Polar clean | 9.2 | 15.8 | 10.7 |
Consequently, PVDF enables high processability, flexible manufacturing in large-scale production, low cost and fine control on the membrane morphology.79 Moreover, recent studies have shown the feasibility of PVDF membrane preparation using green solvents (e.g., dimethyl sulfoxide (DMSO),80 triethyl phosphate (TEP),81 and methyl-5-(dimethylamino)-2-methyl-5-oxopentanoate known as Rhodiasolv Polarclean®79).
Macroporous PP membranes are also widely employed for MD applications, due to their superior elastic properties and crystallinity.60 PP membranes are fabricated either by dry-uniaxial stretching or by thermal phase separation after dissolving the polymer in diluents at a temperature above its melting point.85 Physicochemical properties of PP membranes, such as crystallinity, porosity and mechanical response, are easily tuned by post-stretching or annealing, since during membrane preparation the polymer is rapidly cooled down from the melt state to room temperature leading to imperfect packing density.86,87 Thus, post-treatment usually promotes polymer chain relaxation reaching a thermodynamically stable state causing ultimately significant changes in microstructure and physical properties.86,87
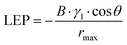 | (2) |
In MD, macroporous membranes exhibit a pore size typically in the range of 0.05–0.5 μm. Larger pore diameters, though favouring higher mass transport, increase the risk of wetting as per eqn (2).75,92 Membranes with elevated porosity (ε = 70–80%), maintaining sufficiently high mechanical strength (elastic modulus of 34–491 MPa, tensile strength of 3.4–57.9 MPa, and elongation at break of 41–710%91), secure high transmembrane flux.
Conversely, the membrane thickness is a key parameter exhibiting counteracting effects on the performance of MD: a low membrane thickness reduces the resistance to mass transport and improves the transmembrane flux, whereas reduction of conductive heat loss is favoured by a thick membrane. On the basis of this trade-off, the optimal membrane thickness lies between 10 and 60 μm.93
In general, membranes for MD require specific optimization of materials, structural and physicochemical properties.92 Different conventional and innovative techniques for membrane preparation (e.g. electrospinning94) and membrane modification (e.g. stretching, grafting and plasma treatment94), as well as novel polymers (e.g. ethylene chlorotrifluoroethylene (ECTFE)95,96 and Hyflon97) have been explored in the last few decades. Several studies have been focused on mixed matrix membranes, where different fillers play a key role in favoring the mass transport or enhancing the thermal efficiency of MD.94 A wide variety of nanomaterials have been also explored to provide antifouling,98 bactericidal,99 sensing,100,101 and catalytic102,103 properties.
Table 2 offers an overview of the main requirements of conventional membranes for MD applications.
| Parameters | Recommendations (range used in the literature) | Impacts |
|---|---|---|
| Membrane thickness (δ) | 10–60 μm (10–200 μm) | Trade-off between the thermal efficiency and the mass transfer |
| Contact angle (θ) | >90° (90°–160°) | High wetting resistance |
| Liquid entry pressure (LEP) | >250 kPa (50–460 kPa) | High wetting resistance |
| Porosity (ε) | 70–80% (40–90%) | High flux and energy efficiency |
| Reduces the mechanical strength of the membrane | ||
| Average pore size (rmax) | 0.3 μm (0.05–0.5 μm) | Low pore size reduces the risk of wetting but compromises the flux |
| Thermal conductivity (km) | As low as possible (0.1–0.3 W m−1 K−1) | Mitigate the temperature polarization |
| Tortuosity | As low as possible (1.0–3.9) | High pore tortuosity reduces the flux |
| Tensile strength | As high as possible (3.4–57.9 MPa) | High mechanical stability of the membrane |
2.3 Current challenges in energy efficiency
Since feed temperatures in MD typically range from 60 °C to 80 °C, low-energy waste heat or solar energy is suitable for the process. The specific thermal energy consumption (STEC) defined as the ratio of the rate of thermal energy added to the system (kW h s−1) to the total production of distillate (m3 s−1) ranges – for most commercially available MD modules – between 100 and 500 kW h m−3 depending on the operational conditions (mainly temperature difference and feed flowrate) and effectiveness of the heat recovery.104,105Regardless of the peculiar advantages and drawbacks related to a specific configuration (Table 3), energy efficiency in MD is severely affected by: (i) temperature polarization to a greater extent when the transmembrane flux is higher (about 50–80% reduction in driving force32,106); (ii) conduction heat losses through the membrane; and (iii) mass transfer resistance within the membrane pores.
| MD configuration | Advantages | Drawbacks |
|---|---|---|
| DCMD | – Technologically simple in design, easy scale-up | – High thermal conductive loss |
| – Moderate transmembrane flux | ||
| – Direct condensation of the distillate inside the module | ||
| AGMD | – Low thermal conductive loss | – Low transmembrane flux due to additional mass transfer resistance of the air gap |
| – Feed solution used as a cooling medium (pre-heating) | ||
| – Direct condensation of the distillate inside the module (heat recovery) | ||
| SGMD | – Moderate transmembrane flux | – High cost for condensing the distillate outside the module |
| – Low thermal conductive loss | ||
| VMD | – High transmembrane flux | – High cost for vacuum |
| – Very low thermal conductive loss | – High cost for condensing the distillate outside the module |
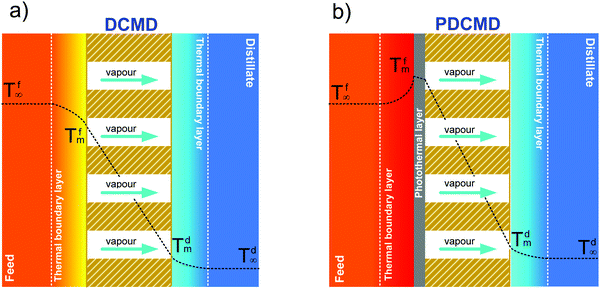
In MD, the term “temperature polarization” (TP) refers to the phenomenon whereby the temperature of the bulk feed solution is higher than the temperature at the feed side membrane interface, thus having a severe impact on transmembrane flux reduction. In this regard, Fig. 2a illustrates the temperature profiles within thermal boundary layers on both feed and distillate sides of the membrane for DCMD configuration. At the feed side, heat flux (Qf) is transferred from the bulk to the membrane surface by convection:
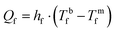 | (3) |
The heat flux transferred through the membrane (Qm), that is equal to Qf at the steady state, takes place by (i) conduction through the membrane (not contributing to water evaporation) and (ii) latent heat (ΔHv) associated with the transmembrane flux (J) of evaporating molecules:
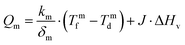 | (4) |
According to eqn (3) and (4), the overlapping effects of the removal of the latent heat of vaporization associated with the transmembrane flux and – to a lesser extent – of the conductive heat loss through the membrane, cause the TP phenomenon.
The reduction of the effective temperature difference (at the membrane interface) with respect to the theoretical driving force (across the bulks) is quantified by the temperature polarization coefficient (TPC):108,109
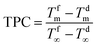 | (5) |
Recently, significant progress in the design and synthesis of advanced photothermal materials has allowed devising innovative nanotechnologies with excellent light-to-heat efficiency. Specifically, the incorporation of nanomaterials exhibiting thermoplasmonic effects in polymers has opened unprecedented scenarios related to radically innovative concepts enabled by UV- or sunlight-driven self-heating membranes. This is the premise for a radical change in the MD paradigm: from the conventional and highly energy-intensive approach of heating the bulk feed solution to efficient localized heating at the membrane surface – where water evaporation takes place – not limited by temperature polarization (Fig. 2b).
3. Photothermal materials
Recently, many classes of nanomaterials have emerged as promising candidates for light-to-heat conversion, whose efficiency can be tailored by tuning the physicochemical properties and atomic structure through bottom-up synthesis strategies.113 Specifically, photothermal materials can be classified into four categories: metals, inorganic semiconductors, carbon-based nanomaterials and polymers (see Table 4).| Photothermal materials | Advantages | Drawbacks |
|---|---|---|
| Metals | Size-dependent optical properties | Chemical instability for transition metals |
| Efficient light-to-heat conversion | Expensive raw materials in the case of noble metals | |
| Plasmonic excitations typically in the UV range | ||
| Inorganic semiconductors | Chemical stability | Modest efficiency of light-to-heat conversion |
| Moderate cost of raw materials | ||
| Carbon-based nanomaterials | Chemical stability | Complicated routes of synthesis for nanotubes |
| Efficient light-to-heat conversion | Poor scalability | |
| Polymers | Inexpensive materials | Poor efficiency of light-to-heat conversion |
| Processability |
3.1 From plasmons to thermoplasmonics
Plasmons are intrinsic collective charge oscillations coupled via the Coulomb interaction, which constitutes the restoring force. Specifically, bulk plasmons are excited at the plasma frequency, for which the real part of the dielectric permittivity is zero (epsilon-near-zero, ENZ, conditions114). Bulk plasmons are longitudinal waves and, generally, they cannot be excited with free-space light (transverse waves). However, it has been shown that the bulk plasmons (ENZ modes) are not completely longitudinal waves for ultrathin thickness,115,116 so that they can be excited with free-space light. Conversely, surface plasmon polaritons and localized surface plasmons can couple with light.117 These surface modes are collective charge oscillations at the interface between a dielectric and a conductor, with a resonance frequency smaller than that of bulk plasmons, as shown in Fig. 3.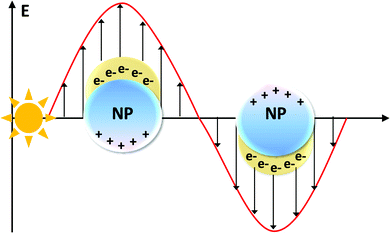 | ||
| Fig. 3 Light irradiation of a metal NP induces the oscillation of the conduction band electrons. This collective electronic excitation is named localized surface plasmon resonance (LSPR). | ||
The opportunity to excite and manipulate surface plasmon polaritons and localized surface plasmons has merged photonics and electronics at the nanoscale.118 However, to date, plasmons have been used mostly in optoelectronic119–123 or biomedical124–127 applications. The relatively high losses and the limited choice for materials represent serious hurdles for extending the application fields. Decay of plasmons into hot carriers is one of the main loss mechanisms,128–130 that, however, could be exploited for thermoplasmonic applications, where conversion into heat is beneficial.
In particular, different decay channels (electron-to-photon, electron-to-electron, and electron-to-phonon, see Fig. 4) permit the dissipation of plasmonic excitation in thermal energy.131 The absorption and the subsequent temperature increase around plasmonic nanostructures were long considered as side effects in plasmonic applications, mostly related to the optical properties. Only recently the scientific community has realized that the enhanced light absorption at plasmonic nanostructures could make them ideal nano-sources of heat. Thermoplasmonics,132i.e., the thermal heating associated with the excitation of plasmons in metal nanostructures, is based on the control of nanoscale thermal hotspots by light irradiation. Thus, thermoplasmonics allows for controlling thermal-related phenomena at the nanoscale. In particular, plasmonics offers new pathways and tools for chemical processes, through innovative or improved solutions to many important challenges in several subfields of chemistry, including NP chemistry,133 catalytic reactions,133,134 photovoltaics,133 sensing,135 biochemistry,136,137 therapeutics133 or membrane processes.48
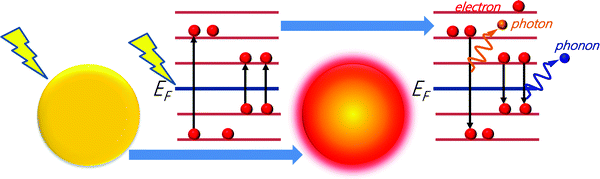 | ||
| Fig. 4 Decay channels for plasmonic excitation: electron-to-photon, electron-to-electron, and electron-to-phonon. | ||
The relative efficiency of scattering and absorption processes can be measured by introducing the photothermal efficiency:138,139
| μ = σabs/σsct | (6) |
The heat power Qj carried by each NP is directly related to σabs:
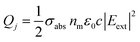 | (7) |
Whenever NPs are sufficiently far from each other, they can be considered as optically independent and, therefore, the heating power of each NP (QNP) is equal, so that:
| QNP = Qj = Iσabs | (8) |
| I = 1/2nmε0c|Eext|2 | (9) |
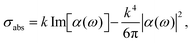 | (10) |
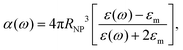 | (11) |
From eqn (11), one can infer a resonance condition for plasmonic NPs corresponding to ε(ω) ∼ −2εm.140,141 As an example, for a spherical Au NP whose diameter is less than 30 nm, the resonance condition corresponds to λ ∼ 530 nm.132
Therefore, the scattering cross section (σsct) scales as ∝RNP6 according to
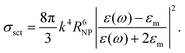 | (12) |
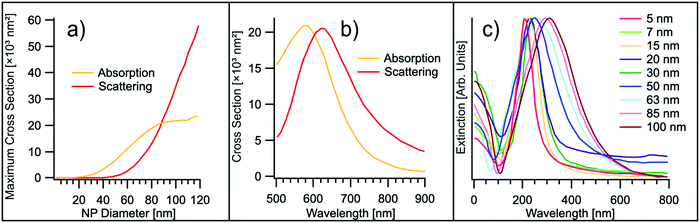 | ||
| Fig. 5 (a) Evolution of the maximum absorption and scattering cross-section as a function of the NP diameter d for the case-study example of gold. Experimental points are taken from ref. 132. (b) Absorption and scattering cross-section for a gold nanosphere in water, with d = 88 nm. Experimental points are taken from ref. 132. (c) UV-Vis extinction spectra of Ag NPs of different diameters. Experimental points are taken from ref. 142. | ||
The extinction cross section, σext, defined as the sum of absorption and scattering cross sections:
| σext = σabs + σsct, | (13) |
The increase of the temperature (ΔTNP) of a single NP irradiated by light is related to σabs:143
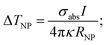 | (14) |
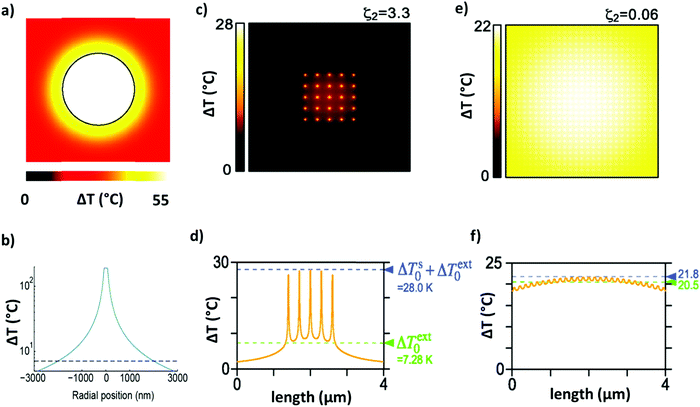 | ||
| Fig. 6 (a) Equilibrium distribution of the temperature increase around an Au NP with a diameter of 100 nm irradiated with λ = 530 nm. Adapted with permission from ref. 143. Copyright 2010 American Chemical Society. (b) Temperature profile around an irradiated Au NP with a diameter of 150 nm (adapted from ref. 147. This work is licensed under a Creative Commons Attribution 4.0 International License). (c and d) Temperature distribution throughout a finite-size square lattice of NPs, uniformly illuminated (p = 300 nm, d = 15 nm, N = 5 × 5, and I = 5.7 × 109 W m−2). Reprinted (adapted) with permission from ref. 146. Copyright 2013 American Chemical Society. (e and f) Temperature distribution throughout an infinite and periodic square lattice of NPs illuminated by a uniform circular beam (p = 150 nm, d = 40 nm, D = 6 μm, and P = 2 mW). Reprinted (adapted) with permission from ref. 146. Copyright 2013 American Chemical Society. | ||
Whenever the concentration of the NPs increases, the distance among NPs is definitely reduced with the activation of the thermal collective effects.144,145 Therefore, two different regimes are identified: (i) the “temperature confinement regime” related to the temperature distribution around the single hotspot and (ii) a “delocalization regime” consisting of a uniform temperature profile along the medium.146 In the latter case, the temperature increase ΔTdr is estimated to be:
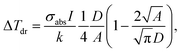 | (15) |
Indeed, for the case of an array of NPs with N elements, an additional contribution (ΔTextj) arises from the heat delivered by the other N − 1 elements (qk) of the array.146 In a region of radius rk around a NP, ΔTextj is estimated to be:
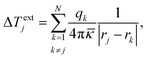 | (16) |
![[small kappa, Greek, macron]](https://www.rsc.org/images/entities/char_e0cb.gif) is the average conductivity of the interface between the NP and its environment.
is the average conductivity of the interface between the NP and its environment.
The regime can be identified by analyzing the dimensionless number ζ2, defined as the ratio between ΔTNP associated with the single particles and ΔText0 related to the contribution from the 2D array, as follows:146
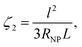 | (17) |
Overall, the contribution of an array of metallic NPs in a homogeneous medium is obtained by solving the equation for heat flow transfer, given by:
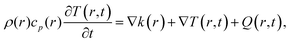 | (18) |
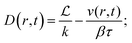 | (19) |
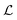 is the effective continuous heat production from NPs depending on the excitation energy, the intensity, the NP size and their concentration, and τ is a cooling time constant taking into account the dissipative phenomena.148 According to this model, the membrane steady-state temperature (ΔTSSm) in a spherical section of radius Rm is defined as:148
is the effective continuous heat production from NPs depending on the excitation energy, the intensity, the NP size and their concentration, and τ is a cooling time constant taking into account the dissipative phenomena.148 According to this model, the membrane steady-state temperature (ΔTSSm) in a spherical section of radius Rm is defined as:148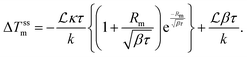 | (20) |
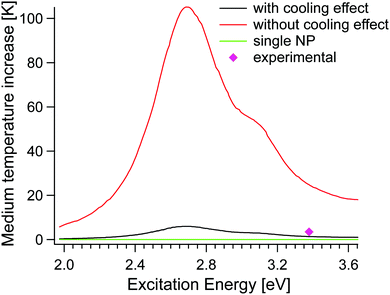 | ||
| Fig. 7 Membrane temperature increase as a function of the excitation energy, with (model by Elmaghraoui148) and without the cooling effect (model by Baffou et al.146). The heating generated from a single NP, represented by the green curve is practically invisible as the maximum temperature is too small. Experimental points are taken from ref. 148. | ||
The validation by direct measurements of the temperature at the nanoscale is technically challenging and macroscale experiments are often characterized by collective heating effects, which tend to make the actual temperature increase unpredictable. Moreover, IR thermal radiation measurement fails at the nanoscale since it implies the employment of micrometric wavelength (>10 μm). To date, several optical techniques have been explored for thermal microscopy,149–151 half of them based on the employment of luminophores with a strong temperature-dependent emission,151–153 limited by their poor emission and chemical stability at high temperature. Interestingly, luminophores have been recently exploited for the in situ monitoring of membrane processes and the experimental evaluation of the temperature on membrane surfaces, opening up interesting perspectives for the empiric description of TP and, eventually, of the effect of thermoplasmonics in the membrane process.32,33,100,154
3.2 Metals
Commonly, the plasma frequency of metals occurs in the UV region with energy between 5 and 15 eV, depending on their band structure.155 However, plasmonic modes in metals are affected by large losses related to interband transitions. These losses are detrimental to the performance of plasmonic devices, seriously limiting the feasibility of many plasmonic applications,156 but not those related to energy conversion, such as plasmon-assisted photocatalysis and thermoplasmonic MD.In general, d-electron metals (including noble metals) show strongly damped features in the excitation spectra, owing to efficient decay channels associated with interband transitions involving d-bands.157 For Ag and Au, interband transitions starting from d-bands induce a shift of the energy of the surface plasmon from 6.5 eV (as expected for a free-electron gas of density matching with Ag and Au) to 3.8157 and 2.5 eV,158 respectively. In particular, Ag plasmonic modes show large values of the propagation depth (up to 10 μm),159 due to the relatively low value of the imaginary part of the dielectric function around frequencies of the resonance conditions.157
Alkali metals, such as Na and K, show minimal loss compared to Ag but they are extremely reactive in the ambient atmosphere or in aqueous solutions, thus hindering their practical applications.160
In the ultraviolet range, Al represents a better choice for plasmonics, although Al is easily oxidized in the ambient atmosphere. Definitely, the formation of native Al2O3 oxide induces a shift of the surface plasmon resonance from 10.5 to 6.5 eV.161 Similarly, surface plasmon resonance in Mg is red-shifted from ∼7 to ∼5 eV upon air oxidation. Al NPs, able to absorb the solar spectrum (>96%), were found to be beneficial for solar-driven desalination.162
To quantify the relative efficiency of plasmonic metals, it was proposed to simply consider the ratio between the real part and the imaginary part of the permittivity of the material at the considered wavelength −ε′(λ)/ε′′(λ).163–165 Although this figure of merit was extensively adopted, it is inadequate for quantitative comparison between different materials, or between different wavelengths, and it does not account for the nature of the surrounding medium. Recently, the Joule dimensionless number (J0) has been proposed for quantifying the ability of a material to produce heat:166
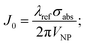 | (21) |
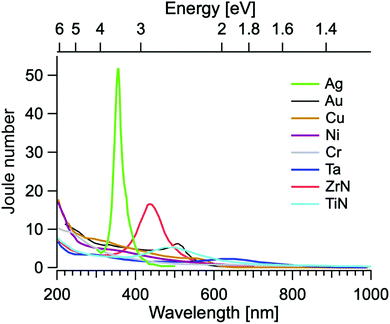 | ||
| Fig. 8 J 0 for different metals: Ag, Au, Cu, Ni, Cr, Ta, ZrN and TiN. Experimental points are taken from ref. 166. | ||
Table 5 reports the Joule dimensionless number along with the corresponding resonance wavelengths (λ) of several elements. Al shows the highest photothermal efficiency (J0 = 477), although in the ultraviolet, thus beyond the solar radiation spectrum. In the range of solar radiation, Ag displays the highest photothermal efficiency, with values ranging from 52 to 111, depending on the reference adopted from optical constants.167,168 As a matter of fact, usually numerical estimations for Ag difficulty match experimental results, mainly owing to sulfidation with the ultimate formation of AgS2 on the NP surface,169 which strongly damps Ag plasmonic excitations and it is difficult to control.
| Element | λ [nm] | J 0 |
|---|---|---|
| Au | 528 | 6.3 |
| Ag | 357 | 52.0; ∼111 |
| Cu | 585 | 2.6 |
| Al | 140 | ∼477 |
| Co | 366 | >12 |
| Cr | 289 | >11 |
| Fe | 337 | >11 |
| Mo | 154 | 41.3 |
| Mn | 380 | >9.2 |
| Ni | 218 | 21.4 |
| Pd | 223 | >21 |
| Pt | 323 | ∼12 |
The photothermal efficiency of Ag and Au was widely explored, considering their tunable absorption properties, morphology and size combined with chemical stability.170–173
Concerning Cu, its surface oxidation dramatically hinders its practical application in thermoplasmonics.174 Nevertheless, significant efforts were focused on the limitation of the oxidation of both Cu and Al NPs by the employment of an ultra-thin protective coating or corrosion inhibitors.175,176
Most recently, photothermal effects in bimetallic NPs (Fe–Au,177 Ag–Au176 or Pd–Au178) have been explored for exploiting the possibility to tune the plasmonic frequency through the stoichiometry of the bimetallic alloy.
3.3 Inorganic photothermal semiconductors
Inorganic semiconductors, exhibiting a bandgap between the valence band (VB) and the conduction band (CB), act as photothermal materials. Photo-excited electrons with incident light energy greater than the band gap energy first transfer to CB-bottom via non-radiative transition, then return to the VB recombining with holes and releasing energy in the form of light (dissipated as radiation) and heat.Limitations related to the narrow absorption wavelength of TiO2 (bandgap of 3.2 eV, only responsive in the UV region179) were mitigated through magnesium reduction to give black TiOx NPs.180 Besides, narrow-bandgap Ti-based semiconductors, such as nanosized titanium sesquioxide Ti2O3 (∼400 nm particle size, bandgap ∼0.1 eV) absorbed almost 94% of UV, 95% of visible, and 89% of infrared solar irradiation with a total absorption capacity of 92.5% over the whole solar spectrum;181 analogous broadband absorption properties were also detected for TiAlN black coatings with a vertically aligned columnar structure181 and layer-by-layer assembly of TiN plasmonic NPs with polyelectrolytes.182
In addition, Si and Ge are efficient photothermal materials as the largest part of their absorbed energy is thermally dissipated. For instance, water-dispersed Ge NPs with a diameter in the range of 3–5 nm irradiated with light (λ = 770 nm, P = 0.9 W cm−2) demonstrated efficient light-to-heat conversion, as indicated by the temperature increase of ca. 20 °C.183 Similarly, suspensions of porous Si in salty water are heated by ΔT = 27 °C upon irradiation with NIR light (P = 0.3 W cm−2).184
Unfortunately, synthesis routes for colloidal semiconductor NPs with uniform sizes and shapes are much less mature with respect to metal NPs. Explicitly, Ge requires high temperature of crystallization and, moreover, its salts need harsh reaction conditions and chemicals to be converted into elemental Ge;185 similar concerns exist for Si.
Degenerately doped semiconductors, such as copper chalcogenides Cu2−yX (X = S, Se, Te) are suitable candidates for thermoplasmonic applications, also considering the density of free carriers as high as 1021 cm−3.186 Recently, copper chalcogenides have been explored in biomedical applications. In particular, CuS NPs with an average size of ca. 3 nm, dispersed in an aqueous medium under light irradiation (λ= 808 nm, P = 24 W cm−2), increased the surrounding temperature by ca. 12.7 °C in 5 min, killing about 50% of the cancer cells.187 The presence of intermediate bands in the energy gap provides additional channels for light-to-heat-conversion, as it enables the relaxation of the photo-excited electrons via the non-emissive pathway,188 thus improving the photothermal efficiency. As an example, chalcopyrite (CuFeS2), empty Fe 3d orbitals generate an intermediate band activating an additional absorption at 0.5 eV,188 activate an additional absorption at 0.5 eV. Frequency tunability is achieved by changing the stoichiometry.189 However, the difficult scalability due to synthesis issues and the insufficient stability of copper chalcogenides inevitably limit their extensive use as photothermal agents.
Lately, nano-sized MoS2-based materials exploiting the excellent solar absorbing performance of the 2D transition metal dichalcogenide have also attracted attention in solar desalination applications.190
3.4 Carbon-based photothermal materials
Carbon-based photothermal materials can absorb light – efficiently converted into heat – like a black body, and exhibit the advantages of inexpensiveness, wide variety of optical and chemical properties and excellent ambient stability.191 The large number of conjugated π bonds present in carbon nanotubes (CNTs) and graphene results in a red-shift of the absorption light spectrum for high-efficiency solar energy utilization. In addition, a very low reflectance (<4% in UV-vis-IR regions) is observed for vertically aligned multiwall carbon nanotubes (MWCNTs).192,193 The combination of strong absorption capacity (up to 98% in the visible and NIR region194) and exceptional thermal conductivity (higher than 2000 W m K−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 195 although amorphous carbon-based nanostructures display inferior values due to phonon scattering196) guarantees both high light-to-heat conversion and remarkable heat transfer to the surrounding environment.197
195 although amorphous carbon-based nanostructures display inferior values due to phonon scattering196) guarantees both high light-to-heat conversion and remarkable heat transfer to the surrounding environment.197
Graphene-based derivatives, with easily tunable optical and surface properties, are promising photothermal nanofillers. Recently, it has been shown that dispersions of reduced graphene oxide (rGO) with concentration of 30 μg mL−1 under irradiation (t = 5 min, λ = 808 nm, P = 0.6 W cm−2) heated up from 25 to 70 °C,198 whereas an improvement of ca. 30 °C has been observed when using CNTs as photothermal materials under a solar illumination intensity of 10 kW m−2.199 Interestingly, the effect on the bulk fluid temperature was maximized at a CNT concentration of 4.76 × 10−4 vol% whereas, a higher amount of CNTs hindered the photothermal activity of nanofillers dispersed below the top layer directly exposed to the radiation.199 Despite their excellent photothermal properties, these materials still suffer from important limitations related to complicated synthesis procedure and high fabrication cost.
Another promising class of carbon-based nanomaterials is represented by Ti-based carbide nanosheets, i.e., MXenes, which display ∼100% light-to-heat conversion efficiency200 in the NIR region, although the mechanisms ruling photothermal effects are unclear yet. An aqueous suspension of Ti3C2 nanosheets with a concentration of 72 ppm resulted in a temperature enhancement of 57 °C in 6 min upon laser irradiation at 808 nm (P = 1.5 W cm−2).201 While some researchers claimed the high photothermal efficiency to be related to light trapping effects with multiple reflection between layers,202,203 a more reliable origin of the enhanced photothermal efficiency of MXenes is related to their plasmonic properties. Definitely, spatially-resolved electron energy loss spectroscopy experiments revealed that the plasmonic spectrum of MXenes exhibits both transversal (optically allowed) and longitudinal (optically forbidden) surface-plasmon modes ranging from visible down to 0.1 eV in the MIR region,204 whose frequency can be tuned with the shape, size, and thickness of Ti3C2Tx flakes (T = F, OH). Correspondingly, MXenes exhibit conductivity as high as 4600 S cm−1.202,205
Nevertheless, the state-of-the-art synthesis techniques for MXenes do not enable scalability for industrial applications, owing to high costs and insufficient crystalline quality for large-scale production.206,207 Furthermore, MXenes are hydrophilic,208 with subsequent unfeasibility in their application as nanofillers for PMD.
3.5 Polymeric photothermal materials
Polymers afford several possible advantages, including high flexibility and mechanical resistance, tunable morphological properties, and low-cost and scalable synthesis. Unfortunately, they suffer from a narrow absorption window and limited charge-carrier mobility, restricting their employment as photothermal materials.209 Strategies to overcome these drawbacks are based on the chemical modification of the polymers such as copolymerization of electron donor–acceptor complexes and oxidative doping.209Their possible use as photothermal materials was explored for conjugated polymers, such as polyaniline (PANi)210 and polypyrrole (PPy),211 owing to the presence of π-conjugated backbones of sp2-hybridized carbon atoms composed of aromatic heterocyclic rings.
PANi is characterized by light absorption in the visible and NIR regions and its Lewis basic character provides binding sites for dopants able to modulate the optical properties212 by inducing mid-gap states.213 Moreover, protonation in an acidic environment usually reduces the band gap causing a shift of the absorption toward the NIR region.214 For instance, the maximum of absorption of PANi NPs (115.6 ± 16.3 nm) shifted from 580 to 810 nm in phosphate buffer solution (pH 1).215 Correspondingly, irradiation by NIR light (λ = 808 nm, P = 2.45 W cm−2) increased the temperature of a dispersion of PANi (concentration of 0.5 mg L−1) by ca. 55 °C in 3 min.215
On the other hand, PPy has gained attention in photothermal ablation with promising results, as demonstrated by the efficient light-to heat conversion under NIR radiation (temperature increase from 21.3 to 55.8 °C) in a culture medium containing 30 μg mL−1 of Ppy NPs with a diameter of 46 nm.216
3.6 Outlook
Overall, extensive experimental and theoretical studies have been focused on identification of photothermal materials aiming to maximize the light-to-heat conversion. Tendentially, metal and carbon-based plasmonic nanostructures display superior performance, but economic issues related to the high cost of raw materials hinder the practical exploitation of the former, while the scalability of the routes of synthesis is the major drawback for the latter. In the case of semiconductors and polymers, the photothermal efficiency is insufficient and usually compensated by adapting highly concentrated systems (see Table 6).4. Photothermally-assisted evaporation and photothermal membrane distillation (PMD)
Conventional thermal desalination occurs by bulk heating of feed seawater to generate vapor. Recently, emergent photothermal materials with efficient light-to-heat conversion have enabled innovative capabilities related to heat harvesting, opening up new opportunities for water purification and desalination.218The efficacy of a photothermal material is usually evaluated in terms of the evaporation efficiency (η):
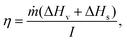 | (22a) |
| ΔHv(T) = 1.91846 × 106[T/(T − 33.91)]2, | (22b) |
Fig. 9 illustrates the evolutionary path of photothermal evaporation technology, starting from early investigations on nanofluids up to the most recent developments on photothermal membrane distillation. A critical discussion on each stage of advancement is proposed in the following sections.
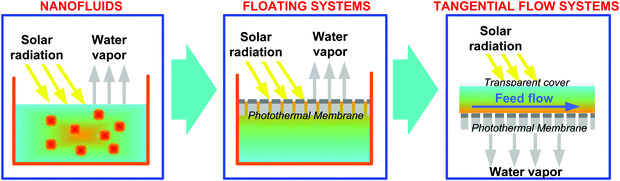
4.1 From photothermal nanofluids to floating photothermal membranes
Preliminary investigations on photothermal evaporation of aqueous solutions were mainly focused on suspensions of photothermal NPs dispersed in a bulk solution, named “nanofluids”, able to convert solar light to heat through the excitation of surface plasmon resonance. A practical problem of nanofluids is the need to separate the NPs from the solutions after the operation. In this respect, photothermal materials are often decorated with magnetic NPs, such as Fe3O4, to facilitate their recovery and recycle from the treated solution.220Nanofluids, as volumetric heating systems, are affected by severe energy loss to the surrounding aqueous medium by thermal conduction and convection, thus resulting in the undesired heating of the bulk liquid with a consequently modest evaporation efficiency. With the aim to overcome these limitations, several non-submersible solar absorbers have been developed in order to achieve localized heat harvesting at the water–air interface.221,222 Typically, the surface of the photothermal NPs is functionalized with hydrophobic moieties, such as alkyl or fluoroalkyl groups, in order to guarantee their self-floating capacity.223 However, the side effects related to the exposure to light, chemical oxidation or physical rubbing were found to compromise the functionalization and, ultimately, the opportunity for NPs to be suspended at the water interface.224
Table 7 summarizes the performance of some selected photothermal materials for volumetric and interfacial evaporation.
Localized photothermal heating at the water–air interface maximizes solar utilization by suppressing heat loss to the bulk water, and thus increases the efficiency of steam generation.225 On this premise, the immobilization of NPs on the surface of membranes is considered a promising approach to effective solar harvesting and conversion into thermal energy for a wide range of practical applications. Floating membranes directly placed on the feed seawater surface, exhibiting suitable sunlight absorption and persistent capillary flow of water to the heat source, are among the most investigated photothermal systems.228
In a static photothermal system, the water vapour released at the surface of the floating membrane exposed to air is typically condensed on a transparent cover (Fig. 10a and b).229 The housing design must promote fast dropwise condensation to prevent the head space from being saturated with water vapor, since a highly humid environment leads to a drastic reduction of the driving force for evaporation. As an additional drawback, the formation of droplets on the condensing surface causes light reflection and scattering that reduce the efficiency of the light absorber.
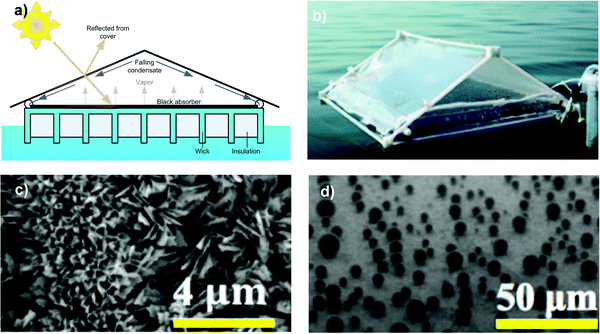 | ||
| Fig. 10 (a) Illustration of the floating evaporation structure in a fabricated polymer-film based condensation cover; (b) device operating in the ocean. Adapted from ref. 229 by permission of The Royal Society of Chemistry; (c) SEM image of the super-hydrophobic nanostructured condensing surface; (d) nucleation of water droplets. Reprinted (adapted) with permission from ref. 233. Copyright 2017 The American Society of Mechanical Engineers. | ||
In this regard, efforts have been focused on creating superhydrophobic nanotextured surfaces to limit droplet adhesion and increase water repellence (Fig. 10c).230 The roughness of a hydrophobic solid surface enhances its hydrophobicity according to the Wenzel model with the fluid wetting all of the rough surface area,231 or according to the Cassie model with the droplet resting on the tips of the surface asperities.232 Aili et al. (2017) attributed the increase of the nucleation rate of water droplets to the decreased energy barrier in confined cavities (Fig. 10d).233
In general, the adoption of floating membranes in static solar evaporation systems suffers from additional limitations due to the lack of fluid-dynamics control. The absence of tangential flow at the membrane surface drastically reduces the mass transfer coefficient within the boundary layer adjacent to the floating surface, thus exacerbating the concentration polarization phenomenon (i.e., the increase in concentration of rejected species at the membrane–feed solution interface due to the removal of water). As a consequence, sparingly soluble salts usually present in natural feed waters (mainly CaCO3, MgCO3 and CaSO4) can easily precipitate, resulting in a rapid incrustation and clogging of the membrane (a phenomenon known as “scaling”22,234). Static operational conditions also hinder the possibility to locally promote turbulence with the aim to mitigate the occurrence of biofouling due to adhesion, accumulation of dissolved organic matter and microorganisms on the supporting substrates. The impact of scaling and biofouling is expected to be particularly significant for substrates made of cellulose materials with an interconnected porous network, where the blockage of hydrophilic channels would stop the transport of water to the photothermal layer via the capillary force.235
4.2 Photothermal membrane distillation (PMD)
In order to overcome the limitations of floating photothermal evaporation systems, and to push lab-scale applications of photothermal membranes towards technology demonstration in an industrially relevant environment, R&D activities have recently focused on dynamic operations under tangential flow for continuous processing, and on modular systems implementing different configurations (see Section 2.1) for large productivity, easy scale-up and efficient heat recovery.236In PMD, the photothermal active layer provides a localized heating at the membrane surface that reduces or eliminates the external energy input required for heating the feed. The enhancement of temperature at the feed solution interface drastically reduces or reverses the temperature polarization phenomenon, leading to a highly efficient evaporation process (see Section 2.3).
At steady-state, the evaporative heat flux associated with the transport of water vapor through the membrane (transmembrane flux) is given by the difference between the absorbed radiative flux (Section 4.2.1) and heat fluxes across: (i) the boundary layer at the feed–membrane interface (Section 4.2.2); (ii) the membrane (Section 4.2.3); and (iii) the boundary layer at the distillate–membrane interface (Section 4.3).
The heat transfer mechanisms involved in PMD are:
(i) convection (qconv) through a moving fluid (liquid or gas), occurring because the surface temperature of the membrane (Tm) is different from that of the surrounding fluid (T∞):
| qconv = h(T∞ − Tm), | (23a) |
(ii) conduction (Qcond) through a medium under a temperature difference ΔT:
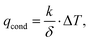 | (23b) |
(iii) radiation (qrad), i.e., the emission of electromagnetic waves from matter with a nonzero absolute temperature (i.e., the membrane):
| qrad = ε·σ(Tm4 − T∞4), | (23c) |
The specific configurations adopted to reduce the partial pressure on the distillate side (options listed in Section 2.1 for conventional MD) are discussed in Section 4.3 for PMD applications.
It is worth pointing out that a passive multistage system, realized using dead-end water or water wicking through the hydrophilic layer as the feed water supply, are here not categorized as PMD systems since they do not exhibit the peculiar requisites of modularity for a reliable process scale-up, neither tangential-flow operability for a feasible synergic integration with other membrane processes.
| Qabs = αI, | (24) |
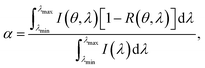 | (25) |
In this regard, noble-metal (Ag, Au) NPs immobilized in/on the membranes have been widely used for stream solar generation. Membranes with Au nanofillers deposited on their surface via vacuum-assisted flocculation exhibited sunlight absorption as high as ∼90% and an efficiency of∼ 62.5% under 1 sun (1 sun = 1 kW m−2);238 here, the anchoring of NPs was promoted by pre-treating the membrane with poly-diallyl dimethyl ammonium chloride (PDDA) solution, so that positively charged functional groups fixed on the polymeric support were able to attract the negatively charged Au NPs.238 Black Ag nanostructures are particularly efficient systems for absorbing and converting solar energy into heat.239 The assembly of Ag NPs in rod-shape with widely distributed interparticle distances via seeded growth confined on ellipsoidal iron oxyhydroxide nanorods led to broadband absorption in the visible and near-IR spectrum.239
Al–Ti–O (aluminum–oxygen–titanium) semiconductor NPs, easily produced by top-down approaches such as planetary milling of low-cost precursors (i.e., TiO2 and Al powder) and dispersed in a PVDF membrane, showed a solar absorption of ca. 90% with a water vapor flux of 0.5 kg m−2 h−1; it was also observed that the presence of Al positively affects the localized surface plasmon resonance of Ti.240 Moreover, an ultrathin porous photothermal film based on 2D transition metal dichalcogenide MoS2 nanosheets and SWCNTs exhibited an absorption higher than 82% over the whole solar spectrum range; with a temperature at the evaporative interface as high as 50 °C, the transmembrane flux reached 6.6 kg m−2 h−1 in air.190
With regard to carbon-based materials, single-wall CNTs deposited on a filter paper exhibited high broadband absorption (>90%) in the solar light spectra, resulting in a vapor generation rate of 3.6 kg m−2 h−1 under a solar power of 5 sun and evaporation efficiency above 40%.241
It is worth mentioning the promising strategy consisting of the exploitation of the synergistic benefits of different photothermal materials.
A photothermal layer resulting from the combination of 2D rGO and 1D MWCNTs, applied to a PVDF substrate, improved the evaporation rate by 79% and 8.9% with respect to that of bare rGO and MWCNT membranes, respectively.242 Membranes derived from carbonized egg-shell in combination with CNTs exhibited superior performance with respect to the functionalization with r-GO, showing an absorption of 99% in the IR-Vis-NIR region and a water vapour flux of ca. 1.3 kg m−2 h−1.243
The maximization of absorbance properties of photothermal materials can be complemented with the advanced design of morphological architectures at the photothermal surface with the aim to reduce the light reflection and the consequent energy loss. Intuitively, an increase of the optical path of the incident photons due to Lambertian scattering can lead to efficient recycling of reflected light for higher light harvesting efficiency. In this regard, main research lines include the design of photothermal devices based on macroscopic and microscopic structures. Practical interest of macroscopic structures, evolved from simple cylindrical and cone-like structures to more complex and elegant origami, is limited to static photothermal systems due to the relatively large size (in the order of centimeters) of the 3D assemblies. Shi et al.244 developed a cylindrical 3D cup-shaped photothermal structure (with the diameter and height of the wall as 4.7 and 5 cm, respectively) capable of recovering diffuse reflectance to ambient air; the composite, fabricated using CuFeMnO4 NPs – selected among mixed metal oxide (MMO)-type inorganic pigments – and a quartz glass fibrous filter membrane, reached near 100% energy efficiency with evaporation rate of 2.04 kg m−2 h−1 under one-sun illumination. According to Wang and coworkers,245 3D photothermal cones with a tunable apex angle were able to achieve an absorbance of up to 99.2% within the solar spectrum and evaporation rate of up to 1.70 kg m−2 h−1 under 1 sun illumination, corresponding to 93.8% solar conversion efficiency, which is about 1.7 times as high as the result obtained for a corresponding plane film. The light-trapping effect in a periodic concavity pattern was also observed for a 3D origami-based nanocarbon composite of GO and NTs designed on a Miura-ori tessellation, that reached a solar energy efficiency close to 100% under 1 sun illumination in a highly folded configuration.246
On the other side, the fabrication of micrometric anti-reflective structures on the surface of photothermal membranes – via hierarchical chemical functionalization or physical methods – can be potentially effective for improving their evaporation efficiency in PMD applications. 2D nanosheets of graphdiyne (GDY), a highly π-conjugated structure of sp- and sp2-hybridized C with a narrow band gap (0.46 eV) for an optical absorption window extending to ∼2700 nm, were fully decorated on the surface of 1D vertical CuO nanowires and supported on copper foam (CF). The ability of this 3D hierarchical architecture (Fig. 11a) to trap light by increasing its traveling distance inside the materials increased the solar absorption by 8%; moreover, under an irradiation density of 5 kW m−2, the temperature of GDY/CuO CF reached an equilibrium value of about 126 °C, i.e., 18 °C higher than that detected for the CuO nanowire substrate.247
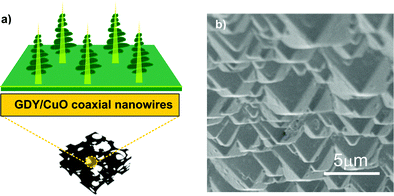 | ||
| Fig. 11 (a) Schematic illustration of graphdiyne/CuO-based multidimensional architecture. Reprinted (adapted) with permission from ref. 247. Copyright 2017 American Chemical Society. (b) The SEM images of the inverted pyramids of the photothermal membrane. Adapted from ref. 248 by permission of The Royal Society of Chemistry. | ||
Sun et al. fabricated an inverted pyramid structure with anti-reflective ability on the surface of a hydrogel loaded acetylene carbon black photothermal material by using a Si wafer template (Fig. 11b): as a result of its anti-reflective ability, the surface temperature of the photothermal membrane with inverted pyramids reached 49.4 °C after being irradiated under 1 sun for 30 min, that is 4.3 °C higher than the temperature measured on the flat surface.248
Moreover, a relevant aspect in maximizing light harvesting is that the light-to-heat conversion efficiency of photothermal membranes differs in the “dry” and “wet” states. The Fresnel equation,249 expressing the reflectivity (R) of light at the interface between two homogeneous media with refractive indices n1 and n2, provides a useful theoretical background to screen the optimal photothermal substrates:
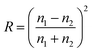 | (26) |
| Nu = a·Reb·Prc·(dh/L)d | (27a) |
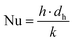 | (27b) |
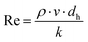 | (27c) |
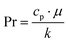 | (27d) |
Conversely, in PMD, the incorporation of photothermal NPs at the membrane interface determines, under light irradiation, an inverse temperature profile transversally to the boundary layer (see Fig. 2b), with Tfm > Tf∞. Therefore, this requires that the convective heat flux be minimized to reduce heat losses from the membrane surface towards the bulk of the feed solution. In this regard, low feed flow rates (Re in the order of 102) determine low values of h and, hence, higher evaporation rates, while guaranteeing an adequate control over concentration polarization and fouling phenomena. Experimental evidence of these deductions is offered by Wu et al., who observed an increase of the transmembrane flux from 0.43 kg m−2 h−1 to 0.57 kg m−2 h−1 when the feed flow rate decreased from 8.1 to 1.5 mL min−1.252
The extreme situation of zero feed flow rate reduces PMD to a static photothermal evaporation system (free convection), thus suffering from limitations previously discussed (Section 4.1). It is worth pointing out that, for free (natural) convection, the Nusselt number is expressed as a function of Prandtl number and Grashof number (Gr) – i.e., the ratio of buoyant to viscous forces – defined as:
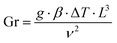 | (28) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 W m−2 K−1 depending on the operational conditions, typical intervals of h for air in free and forced convection are in the range of 2.5–25 and 10–500 W m−2 K−1, respectively.248
000 W m−2 K−1 depending on the operational conditions, typical intervals of h for air in free and forced convection are in the range of 2.5–25 and 10–500 W m−2 K−1, respectively.248
The idea of using spacers (usually employed in membrane modules to promote turbulence in feed channels) to induce photothermal conversion in proximity of the membrane surface, deserves a particular mention.253 Adoption of metal spacers made of Ni and coated with photocatalytic Pt led to a reduction of up to 28% in energy per unit volume of distillate under light irradiation with respect to DCMD carried out with conventional polymeric spacers.253
In addition to heat loss by convection, the amount of energy leaving the system by thermal radiation can represent a significant parasitic loss: considering the dependence on T4 in eqn (23c), the radiative heat loss is 680 W m−2 (one-sun illumination) assuming a black solar absorber (ε = 1) at 100 °C and an air temperature of 20 °C.
The electromagnetic solar radiation mostly extends from 290 to 3200 nm, with radiation energy distribution non-uniformly spread among ∼2% in the UV region, ∼47% in the visible region and ∼51% in the infrared (IR) region.254 On the other hand, the blackbody radiation spectrum extends over a broader wavelength region. For a nonselective thermal radiator, Kirchhoff's law states that – at any temperature and wavelength – the spectral directional emittance (ε) is equal to the spectral absorbance (σ) for radiation incident from the same direction.255 Therefore, an effective strategy to minimize thermal radiation heat loss is to use selective light absorbers (typically categorized as multi-layer thin film coatings, plasmonic nanostructures and carbon-based materials) that should ideally eliminate the transmittance and reflectance.
Lu et al.256 fabricated a patterned film absorber (Fig. 12a) comprising an ultrathin Ge film (Fig. 12b) and a reflective Au layer by laser interference lithography. In Fig. 12c, simulation results reveal that the optimized bilayer system (Ge/Au) can reach a total solar absorptance of 84.1% between 400 and 1100 nm.
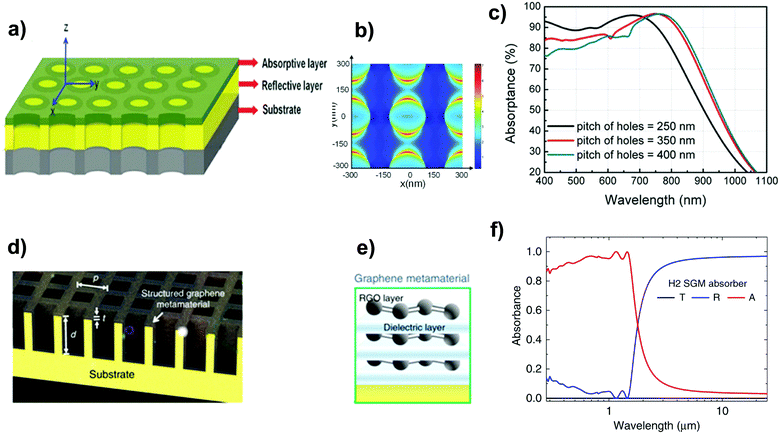 | ||
| Fig. 12 (a) Schematic representation of the plasmonic enhanced ultrathin film absorber with nanoporous patterns consisting of an absorptive layer and a reflective layer deposited on a substrate; (b) top view of energy flow distribution in the Ge layer; (c) simulated absorptance as a function of distance between nanoholes. Reprinted (adapted) with permission from,256 John Wiley and Sons. (d) Schematic representation of the 3D SGM absorber (d: depth of trenches, t: thickness of the graphene metamaterial layer, w: width of the hole, and p: period of the structure). (e) Structure of the graphene metamaterial. (f) Simulated spectral reflectance (R), transmittance (T), and absorbance (A) for the SGM absorber (d = 1 μm, t = 30 nm, w = 0.59 μm, p = 0.8 μm). Reproduced from ref. 257 (Creative Commons Attribution CC BY, Copyright 2020, Springer Nature). | ||
A spectrally selective solar absorber, consisting of a cermet (BlueTec eta plus) with a solar absorptance of 0.93 and an emittance at 100 °C of 0.07, was coated on a copper sheet; with respect to a blackbody absorber, the radiative heat loss was reduced by one order of magnitude.258
Lin et al.257 coated a 30 nm-thick structured graphene metamaterial (SGM) – consisting of alternating graphene and dielectric layers – on a 3D trench-like copper substrate (Fig. 12a). The resulting hybrid nanostructure, designed in such a way that it should have the C4 rotational symmetry to realize polarization-independent absorption, was able to achieve a near-unity absorption across the UV and NIR spectral ranges while suppressing almost completely the absorbance in the IR regime due to the strong interference phenomena (Fig. 12c). The SGM absorber reached a solar-to-vapor efficiency of 96.2% and a water evaporation rate of 1.5 kg m−2 h−1.257
In principle, radiative heat loss can be effectively reduced by decreasing the surface temperature exposed to light radiation. A study by Li et al.259 proved that, despite the lower surface temperature (32.7 °C) compared to the other configurations (39.5 °C for 2D direct contact evaporator, and 43.0 °C for 2D indirect contact evaporator), a 3D umbrella-like system exhibited the highest solar steam efficiency (85%) due to the consequent reduction of heat losses towards the environment.
However, the effectiveness of this approach is questionable since a low working temperature deteriorates the transmembrane water vapor flux (driven by a vapor pressure gradient, whose intensity depends exponentially on temperature). Beyond the interest of a comprehensive understanding of the heat transfer phenomena occurring in photothermal systems for solar evaporation, these creative solutions are of no practical use for PMD.
In order to determine the effective thermal conductivity of composite materials or layers (kc,eff) made by dispersing nanofillers within a matrix, several empirical models have been proposed in the literature.260 For filler volume fractions up to 40%, the Lewis–Nielsen mathematical model provides relatively good results for a wide range of NP shapes:
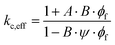 | (29a) |
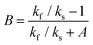 | (29b) |
 | (29c) |
| Shape | Type of packing | φm | NP aspect ratio (length/diameter) | A |
|---|---|---|---|---|
| Sphere | Face-centered cubic | 0.7405 | 1 | 1.5 |
| Body-centered cubic | 0.6 | |||
| Simple cubic | 0.524 | |||
| Random close | 0.637 | |||
| Random loose | 0.601 | |||
| Rods | Uniaxial hexagonal close | 0.907 | 2 | 1.58 |
| Uniaxial simple cubic | 0.785 | 4 | 2.08 | |
| Uniaxial random | 0.82 | 6 | 2.8 | |
| Three-dimensional | 0.52 | 10 | 4.93 | |
| Random | 15 | 8.38 |
Finally, the thermal conductivity of the membrane (km) can be estimated as a function of the effective thermal conductivity of the photothermal composite (kc,eff) and of the fluid (kfluid) filling the pores, according to the following relationship:66
| km = (1 − εp)·kc,eff + εp·kfluid | (30) |
Mitigation of conduction heat loss is critical in MD, where the required hydrophobicity of the membrane is achieved by using polymers (i.e., PFTE, PVDF, and PP) exhibiting a relatively high thermal conductivity (see Table 9). This situation is further exacerbated by the incorporation of photothermal materials.
| Photothermal material | Category | Thermal conductivity (W m−1 K−1) | Ref. |
|---|---|---|---|
| Graphene film | Carbon | 1000–3000 | 261 |
| Vertically aligned graphene | Carbon | 100–600 | |
| Graphene foam | Carbon | <2 (pristine) 90 (compressed) | |
| Ag | Metal | 429 | 262 |
| Au | Metal | 318 | 263 |
| MWCNT film | Carbon | 244–267 | 264 |
| MWCNT | Carbon | 12–17 | |
| MoS2 | Semiconductor | 34.5 | 265 |
| CuFeS2 | Semiconductor | 5.9 | 266 |
| Polypyrrole | Polymer | ∼0.7–1 | 267 |
| Support material | Thermal conductivity (W m−1 K−1) | Ref. |
|---|---|---|
| Mixed cellulose esters | 0.565 | 268 |
| Polyurethane | 0.5182 | 269 |
| PTFE | 0.25–0.27 | 85 |
| PVDF | 0.17–0.19 | |
| PP | 0.11–0.16 | |
| MWCNT array | 0.145 | 264 |
| Wood | 0.11-0.35 | 235 |
| Cellulose aerogel | 0.06 | 270 |
| Polymer foam | 0.057 | 271 |
| Cotton rod | ∼0.04 | 272 |
| Polystyrene foam | ∼0.04 | 273 |
| Air-laid paper | 0.03–0.05 | 274 |
| Cellulose paper | 0.031 | 275 |
| Cellulose-membrane | 0.02 | 181 |
| Polyacrylonitrile (PAN) electrospun nanocomposite fibers | 0.02 | 276 |
| Graphene oxide aerogel | 0.0047–0.035 | 277 |
An increase of the membrane thickness reduces the thermal conductivity flux across the membrane (eqn (23b)); however, this positive effect is counterbalanced by the higher resistance to mass transport. Therefore, the most effective option to reduce the conductive heat loss is to support the photothermal layer with a thermally insulating layer (low km in eqn (23b)). Recently, a large variety of materials have been explored for the realization of an additional thermally insulating layer in composite membranes, including polymers or carbon-based foam, cotton, wood, aerogels and hydrogels (Table 9).
On these premises, we focus our attention on homogeneous hydrophobic photothermal membranes (Section 4.2.4), with the photothermal material uniformly dispersed in the hydrophobic polymeric matrix, and on more energy-efficient composite hydrophilic/hydrophobic photothermal membranes (Section 4.2.5).
The first photothermal membrane for MD application was reported by Politano et al. (2017); here, Ag NPs were dispersed in a solution of PVDF in DMF. Localized thermal hotspots, activated under UV irradiation at a wavelength of 366 nm, resulted in an increase of temperature at the membrane surface even greater than 23 K with respect to the bulk value.278
Flat-sheet microporous membranes consisting of black Al–Ti–O nanohybrid structures240 showed an evaporation rate (with pure water as the feed) of 1.03 kg m−2 h−1 with a corresponding thermal efficiency of ∼77%.
In porous HCuPO (40%)–PDMS films in contact with 3.5% NaCl aqueous solution an evaporation rate of 1.01 kg m−2 h−1 and a thermal efficiency of 63.6% under one sun279 were observed.
The most critical drawback of homogeneous membranes is that a large part of the photothermal material, incorporated within the interior part of the membrane not exposed to light irradiation, is not effectively used. Moreover, the uniform dispersion of nanofillers increases the thermal conductivity of the whole membrane, leading to high conductive thermal losses.
To overcome these limitations, bi- and multi-layered membranes have been developed at the unavoidable cost of introducing complexity in the manufacturing procedure.280
Membranes made of metal-based photothermal materials on a polymeric support. Au NPs with a particle size of 30–40 nm were immobilized by filtration on a PVDF membrane with asymmetric wettability, prepared via the phase inversion method within a coagulation bath comprising hydrophilic copolymer poly-vinylpyrrolidone-vinyltriethoxysilane (PVP-VTES).281 The thermal conductivity of the hydrophobic side of the membrane was 0.026 W m−1 K−1, thus determining a low conductive loss (between 13.4 and 17.0% depending on the orientation of the device). The composite membrane exhibited opposite wettability, with the hydrophilic top layer with spherical structures and the bottom surface with a flower-like structure characterized by a contact angle of 142–145° (Fig. 14a–c).
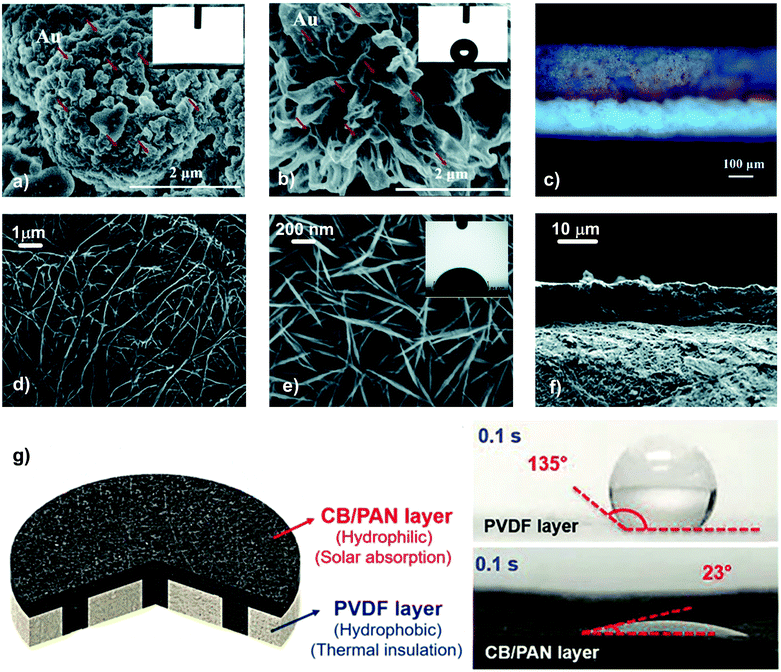 | ||
| Fig. 14 SEM micrographs of the Au NPs/PVDF composite membrane: (a) top layer with the spherulitic structure showing almost zero water contact angle; (b) bottom layer with a flower-like morphology exhibiting a contact angle of ∼140°; (c) cross-sectional image of the by-layered structure. Adapted from ref. 281 with permission from Royal Society of Chemistry. SEM micrographs of the W18O49@PDMS/PTFE composite membrane: (d) PTFE hydrophilic support; (e) W18O49@PDMS mesocrystals at the top layer; (f) cross-sectional image of the by-layered structure. Adapted from ref. 287 with permission from Royal Society of Chemistry. (g) Schematics of the bi-layered hydrophilic CB/PAN – hydrophobic porous PVDF membrane and contact angles of the PVDF and CB/PAN nanofiber layers. Reprinted (adapted) from ref. 288 with permission from John Wiley and Sons. | ||
Zhao et al. (2018) fabricated a composite MXene membrane by depositing 2D delaminated d-Ti3C2 nanosheets, and made it hydrophobic by chemical modification with trimethoxy(1H,1H,2H,2H-perfluorodecyl) silane (PFDTMS), on a mixed cellulose ester filter.282 A steady-state temperature of ca. 39 °C was measured on the membrane surface; the distillation performance was stable over 200 hours of continuous operation.
Nanowires represent another interesting category of photothermal materials, enabling the penetration of the exciting electric field in the confined geometries,283,284 with Green's dyadic function estimating a +60% change in the heating efficiency by shifting the Au NP morphology from spherical to elongated nanorods.285 Pt–Ni–S nanowires, having high light absorption capacity in the whole spectral region between 200 and 2500 nm, were poured onto a commercial PTFE membrane and then dried; after the chloroalkylsilane modification method, the membranes exhibited a water contact angle of 106°.286 The best performance, registered for a Pt3Ni–S NW solution concentration of 21.6 mg mL−1 deposited on PTFE membrane, resulted in a water evaporation rate of 1.27 kg m−2 h−1 and a thermal conversion efficiency of ∼80%.
Membranes made of semiconductor photothermal materials on a polymeric support. A bi-layered solar steam generator was developed by immobilizing non-stoichiometric W18O49 mesocrystals on a PTFE membrane using PDMS as a binder (Fig. 14d–f). The mesocrystals showed a broad absorption band of 550–2500 nm, whereas PDMS acted as a hydrophobic modifier guaranteeing self-floating properties; the system was able to generate 1.15 kg m−2 of steam after 1 h under one-sun irradiation, with efficiency reaching 82%.287
Beside their potential use for solar stream generation and desalination, photothermal membranes are of interest in a wide range of applications, such as wastewater treatment or pasteurization. The combination of plasmonic Au NPs with hedgehog-like ZnO particles acting as the photocatalyst on the surface of a cellulose ester hydrophilic support (contact angle of ca. 70°) resulted in a super-hydrophobic membrane (top layer water contact angle of ca. 155°) able to synchronize the process of stream generation with photodegradation. Specifically, the presence of Au NPs allowed the tripling of the rate of water evaporation whereas ZnO favoured the degradation under solar photocatalysis of 30% of an organic dye contaminant (i.e., Rhodamine B).289
Membranes made of carbon-based photothermal materials on a polymeric support. In MD and PMD, the non-wetting properties of a composite membrane are satisfied by the presence of a single hydrophobic layer. A Janus membrane fabricated via the electrospinning technique and formed by a hydrophobic layer made of carbon black NP (CB)-coated polymethylmethacrylate (PMMA) for light harvesting, supported by a low-thermally conductive bottom hydrophilic polyacrylonitrile (PAN) layer for water uptake, was proposed by Xu et al.290 After 5 min under 6 sun illumination, the temperature of the top layer increased from 27 to 60 °C, while that of the bottom water only increased by 0.4 °C, thus confirming the insulation ability of the PAN layer. Under one-sun irradiation, the measured transmembrane flux was 1.3 kg m−2 h−1 with an energy conversion efficiency of 72%.
Despite the good performance, the intrinsic detaching problem of electrospinning layers remains challenging, ultimately making the system unsuitable for robust and long-term desalination operation.
Based on a similar concept, but with inverted hydrophilic/hydrophobic properties of the solar absorption/support layers, Gao et al.288 proposed a Janus membrane composed of a hydrophilic carbon black/polyacrylonitrile (CB/PAN) composite nanofiber layer on an electrospun hydrophobic polyvinylidene fluoride (PVDF) support (Fig. 14g). The estimated thermal conductivity of the bilayer membrane under wet and dry conditions was 0.075 and 0.046 W m−1 K−1, respectively. As a consequence of the low conductive loss, the steady-state temperature of the top light absorbing layer increased up to 34.3 °C under one-sun irradiation, while the steady-state temperature of the bottom PVDF layer was significantly lower (20.2 °C); under these conditions, an efficiency of 82.0% was achieved.288
Membranes made of polymer-based photothermal materials on a polymeric support. Photothermal membranes based on conjugated polymers were developed using PPy as the coating photothermal layer deposited onto the substrates via CVDP.291 The preparation method consisted of dipping the membrane substrate in FeCl3 – used as an oxidizing agent to initiate polymerization – and pyrrole monomer solutions, thus obtaining a layer of PPy. PVDF/PPy-coated membranes exhibited an evaporation rate of 1.41 kg m−2 h−1 and a solar conversion efficiency of 81.9%.291
A Janus membrane was fabricated by coating hydrophobic PPy on the inner pore walls of the PVDF membrane via CVDP, and by deposition of hydrophilic polydopamine (PDA) on a single side of the membrane itself.292 While the PPy-coated sides exhibited a water contact angle (WCA) >120°, the WCA of the opposite membrane side decreased with increasing PDA coverage (a minimum WCA of ca. 50° was measured for the highest adopted deposition time of 80 min).
4.3. Design of the distillate compartment and PMD module configurations
As stated in Section 2.1, the driving force that activates the transmembrane flux in the vapor phase is a partial pressure difference between the feed and distillate sides. Moreover, under typical PMD conditions (feed temperature at the interface of photothermal membranes in the range of 60–80 °C), water vapor carries a latent heat of vaporization of about 2400 kJ kg−1. At the distillate side, the particular strategy adopted to lower the partial pressure, to condense the water vapor, to minimize conductive heat loss, and to recover the condensation heat determines the specific configuration of the PMD membrane module (Fig. 15). | ||
| Fig. 15 (a) Prototype equipped with a solar panel to provide electric power for the feed and permeate/vacuum pump in PVMD. Reprinted (adapted) with permission from ref. 293. Copyright 2019 American Chemical Society. Membrane modules employed in lab activities for experiments of (b) PVMD, and (c) PDCMD or PSGMD. (d) Scheme of a membrane module for PAGMD. | ||
The heat transfer mechanisms within the distillate side for photothermal direct contact membrane distillation (PDCMD), photothermal air gap membrane distillation (PAGMD), and photothermal sweeping gas membrane distillation (PSGMD), complementing energy balances in Sections 4.2.2 and 4.2.3, are detailed in Table 10. Temperature profiles reported in Fig. 2b (PDCMD) and Fig. 16a and b (PSGMD, PAGMD) clarify the meaning of the terms of the reported equations.
| Configuration | Heat flux at the distillate side | Equation |
|---|---|---|
| PDCMD | Convective heat flux within the distillate channel |
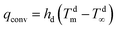
|
| PSGMD | Convective heat flux within the coolant channel |
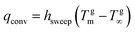
|
| PAGMD | Conductive heat flux across the air gap |
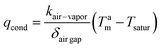
|
| Latent heat flux of water vapor across the air gap | q conv = J·ΔHv | |
| Conductive heat flux within the condensing film layer (Nusselt theory260) |
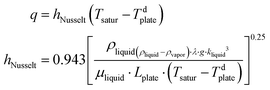
|
|
| Conductive heat flux across the cooling plate |

|
|
| Convective heat flux within the coolant channel |
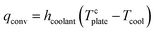
|
In the specific case of photothermal vacuum membrane distillation (PVMD), since low vacuum pressure is applied at the distillate side, no boundary layer is considered. Table 11 provides a selection of the most relevant contributions to PMD operated under different lab-scale module configurations; although still in its infancy, the growing number of studies demonstrates the enthusiasm of the scientific community towards this emerging technology.
| PMD configuration | Membrane | Membrane properties | Feed | PMD operational conditions | Flux (kg m−2 h−1) | Ref. |
|---|---|---|---|---|---|---|
| v f = flow velocity, n.a. = not available. | ||||||
| PDCMD | FTCS-PDA-PVDF | θ = 125° | T f = 20 °C, NaCl 0.5 M | P in = 0.75 kW m−2 | 0.5 | 252 |
| r = 0.46 μm | T fm = 35 °C (wet) | |||||
| ε = 73.2% | Q f = 16.2 mL min−1 | |||||
| CB-PVA-PVDF | n.a. | T f = 20 °C, NaCl 1% | P in = 0.7 kW m−2 | 0.2–0.5 | 294 | |
| T mf = 21–28 °C (wet) | ||||||
| Q f = 17 mL min−1 | ||||||
| Fe3O4-PVDF-HFP | θ = 140° | T f = 25 °C, NaCl 3.5% | P in = 1 kW m−2 | 1.0 | 295 | |
| r = 0.44 μm | T fm = 36.5 °C (wet) | |||||
| ε = 91.7% | Q f = 20 mL min−1 | |||||
| PDMS-MWCNTs-PVDF | θ = 154° | T f = 25 °C, NaCl 3.5% | P in = 1 kW m−2 | 0.6 | 296 | |
| r = 0.51 μm | T fm = 66 °C (wet) | |||||
| Q f = 1.5 mL min−1 | ||||||
| mXenes-PVDF | θ = 152° | T f = 65 °C, NaCl 1% | P in = 5.8 kW m−2 | 8–10 | 297 | |
| T fm = +49 °C (dry) | ||||||
| Q f = 250 mL min−1 | ||||||
| TiN-PVA-PVDF | LEP = 1.95 bar | T f = 23.1 °C, NaCl 3.5% | P in = 1 kW m−2 | 1.0 | 298 | |
| r = 0.20 μm | T fm = 39.4 °C (wet) | |||||
| ε = 70.3% | Q f = 25 mL min−1 | |||||
| PANI-PVDF | θ = 32° | T f = 25 °C, NaCl 1% | P in = 1 kW m−2 | 1.1 | 299 | |
| LEP = 2.7 bar | T fm = 52 °C (dry) | |||||
| Q f = 0, 01 mL min−1 | ||||||
| PVDF-HFP-PDA | θ = 152° | T f = 20 °C, NaCl 3.5% | P in = 1 kW m−2 | 1.3 | 300 | |
| r = 0.26 μm | T fm = 30 °C (wet) | |||||
| ε = 81% | Q f = 20 mL min−1 | |||||
| Ca10(PO4)6(OH)2-PDA-CS | θ = 126° | T f = 20 °C, 0.5M NaCl | P in = 1 kW m−2 | 0.9 | 301 | |
| r = 0.2 μm | T fm = 46 °C (dry) | |||||
| Q f = 3.6 mL min−1 | ||||||
| PVMD | ATO-PVDF | θ = 125° | T f = 70 °C, NaCl 3.5%, | P in = 0.1 kW m−2 | 27 | 302 |
| LEP = 1, 5 | T fm = 81 °C (wet) | |||||
| ε = 77% | Q f = 1500 mL min−1 | |||||
| TiC-TiO2-PVDF | θ = 162° | T f = 20 °C, NaCl 30 g L−1 | P in = 1 kW m−2 | 0.6 | 303 | |
| r = 0.41 μm | T fm = 33 °C (wet) | |||||
| ε = 67% | v f = 0.02 m s−1 | |||||
| Ag-PVDF | θ = 80° | T f = 30 °C, NaCl 0.5% | P in = 23 kW m−2 | 26 | 278 | |
| r = 0.4 μm | T fm = 54 °C (wet) | |||||
| ε = 22% | Q f = 330 mL min−1 | |||||
| PSGMD | Ag-PVDF-non-woven tissue | θ = 138° | T f = 25 °C, NaCl 3% | P in = 23 kW m−2 | 8.6 | 280 |
| r = 0.15 μm | T fm = 41 °C (wet) | |||||
| ε = 69% | Q f = 50 mL min−1 | |||||
| PAGMD | TiN-PVA-PVDF | θ = 23° | T f = 20 °C, NaCl 3.5% | P in = 1 kW m−2 | 0.9 | 304 |
| r = 0.79 μm | T fm = 58 °C (wet) | |||||
| Q f = 0 mL min−1 | ||||||
| FTCS-PDA-Graphene-PTFE | θ = 118° | T f = 20 °C, NaCl 0.5 M | P in = 0.75 kW m−2 | 1.2 | 305 | |
| r = 1.58 μm | T fm = 52 °C (dry) | |||||
| LEP = 1, 2 | Q f = 0 mL min−1 | |||||
Polydopamine (PDA), a mussel-inspired polymer characterized by broad light absorption and photothermal conversion behavior combined with hydrophilic character and adhesive properties, was employed for the preparation of a PDA-coated poly-vinylidene fluoride (PVDF) photothermal membrane, subsequently operated in the PDCMD configuration.252 The coating was prepared via self-polymerization, whereas the hydrophobicity of the membrane surface was guaranteed by fluoro-silanization with (tridecafluoro-1,1,2,2-tetrahydrooctyl)-trichlorosilane (FTCS). Using pure water as the feed, the sunlight-driven PDCMD system exhibited – under an irradiation of 0.75 kW m−2 – a transmembrane flux of 0.58 kg m−2 h−1, a 3.8 fold higher value with respect to the pristine PVDF membrane tested under the same operation conditions. When feeding the PDCMD system with saline water (NaCl 0.5 M), the water flux decreased to 0.49 kg m−2 h−1 due to the lower vapor pressure of the feed solution.
Analogously, a bilayer structure obtained by freeze-drying a hydrogel made of PDA particles and nanocellulose subsequently grafted with FTCS showed a permeate flux of 1 kg m−2 h−1 under 1 sun (efficiency of 68%) in the PDCMD process combined with antibacterial activities.307
As discussed in Section 3.4, carbon-based materials are attracting increasing attention in photothermal membrane manufacturing. The performance of CB deposited on a PVDF membrane via the evaporation method was compared with SiO2/Au anchored to the PVDF surface using polydopamine (PDA) as the binder.308 CB-PVDF membranes exhibited a flux of 0.5 kg m−2 h−1 in PDCMD, which is one order of magnitude higher than the value observed in the case of a SiO2/Au–PVDF membrane (0.07 kg m−2 h−1).308 The theoretical investigation ascribed the flux improvement under one sun irradiation to the remarkable increase (33%) of the TPC.308
Electrospinning was also employed to develop a bilayer structure consisting of a commercial PVDF membrane treated with polydopamine to ensure the adhesion of a thin (25 μm) optically absorbing, porous layer of electrospun polyvinyl alcohol (PVA) loaded with carbon black (CB).294 Although this method allowed increasing the concentration of the photothermal materials up to 20%, the optimal performance in terms of water flux was observed for a value of CB of 5.5 wt% that guaranteed higher light absorption and superior temperature increase at the PVDF membrane surface.294 Water vapor flux reached 0.22 kg m−2 h−1 with a solar efficiency of 21% at 20 °C, which increased to 0.55 kg m−2 h−1 with a solar efficiency of 54% when increasing the operating temperature to 40 °C; a pilot plant equipped with a membrane of 1 m2 active area and operated in the PDCMD configuration at a peak temperature of ∼35 °C was able to distillate 4 L day−1 under about 700 W m−2 illumination and 8 hours of sunlight in the summer.294
Similarly, PVDF membranes were modified to incorporate titanium nitride (TiN)301 or MXenes (Ti3C2Tx).297 MXene fillers were transferred to the membrane surface via vacuum filtering; the mechanical stability of the photothermal layer was guaranteed by a protective layer of PDMS subsequently deposited via dip-coating.297 Although the presence of the coating imposed an additional resistance to the mass transport, resulting in a flux decline of ca. 13%, the photothermal properties of MXenes allowed a decrease of ca. 12% of the energy input per unit volume of the distillate and provided remarkable antifouling properties to the membrane.297
Immobilization of Fe3O4 NPs on an electrospun polyvinylidene fluoride-co-hexafluoropropylene membrane (PVDF-HFP) by vacuum-assisted filtration resulted in a strong interfacial adhesion, owing to the coordination between inorganic NPs and polymeric nanofibers.295 Fe3O4/PVDF-HFP membranes showed a light-to-heat conversion efficiency of 53% and a water evaporation rate of ca 1 kg m−2 h−1 under static conditions. Fe3O4/PVDF-HFP membranes were tested in a pilot scale PDCMD system obtaining a flux of ca. 22 kg m−2 h−1 under the exposure to light irradiation with an intensity of 3 kW m−2, ca. 11% higher than the performance observed in absence of irradiation.295
The transition towards more eco-compatible approaches inspired the development of novel membranes based on biomaterials, such as hydroxyapatite (Ca10(PO4)6(OH)2,), the main inorganic mineral in the bones, or chitosan (CS), a biopolymer. Cao et al.296 developed a bilayer membrane by assembling a photothermal layer of hydroxyapatite NWs coated with PDA over a thermal isolator support of hydroxyapatite NWs. The bio-inspired membrane, operated in the PDCMD configuration, showed a photothermal efficiency of 62% and a water vapor flux of 0.89 kg m−2 h−1 under 1 sun.296
In a pioneering study on PVMD, Ag NPs were immobilized in PVDF membranes via NIPS, thus leading to a homogeneous photothermal membrane.278 The NIPS method, widely used in the industrial production of membranes, guarantees the stable embodiment of nanofillers into the membrane, avoiding their release as a crucial requirement in water purification operations.309,310
Notably, an increase of the temperature at the membrane surface under UV irradiation was revealed, withdrawing the thermal polarization in PVMD experiments and, consequently, dramatically improving the productivity of the process (Fig. 17). Surprisingly, plasmonic nanofillers reversed the temperature polarization effect under UV exposure (TPC increased up to 106.5% for the highest Ag NP loading), thus leading to a membrane surface temperature higher than that in the bulk and determining an unprecedented improvement of the process performance. In fact, the transmembrane water flux increased from 2.2 kg m−2 h−1 to 25.7 kg m−2 h−1, as a consequence of the thermoplasmonic effects induced by UV radiation.278 Heat balance evidenced an enhancement of the thermal efficiency of the PVMD process by 28%.311
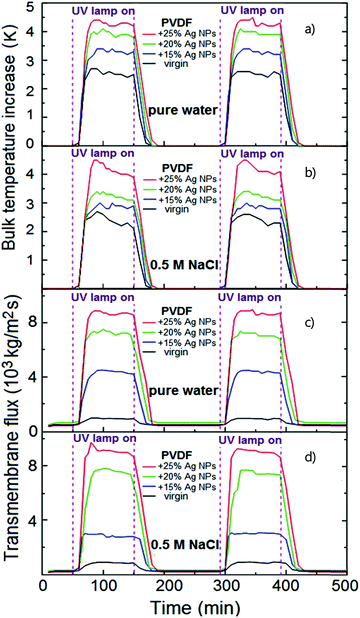 | ||
| Fig. 17 The effect of Ag NP concentration in PVDF dope solution on (a and b) the bulk feed temperature, and (c and d) water vapor flux in PVMD operated with pure water and 0.5 M NaCl solution. Copyright 2016 Wiley. Used with permission from ref. 278. | ||
Spraying NPs over a substrate is another option to localize the photothermal activity on the membrane surface. This strategy has been already explored for titanium carbide (TiC) using PDMS as an anchoring agent, securing a flux of 0.64 kg m−2 h−1 under one-sun at room temperature in PVMD.312 Alternatively, PDMS was also employed as a protective layer to prevent the leakage of MWCNTs sprayed on PVDF nanofibers.296
Recently, electrospinning has emerged as an innovative and attractive method for nano-composite membranes made of uncountable polymeric nanofibers,313 enabling the development of a nanostructured photothermal membrane through the effortless dispersion of the photothermal NPs in the polymeric solution submitted to electrospinning. For instance, antimony-doped tin oxide (ATO) nano-powder was directly dispersed in a PDVF solution and subsequently electrospun in order to obtain a PVDF membrane made of functional nanofibers loaded with inorganic ATO NPs.302 When compared to the unloaded PVDF nanofiber membrane, the hybrid PVDF-ATO membrane exposed to IR light presented a surface temperature higher by ca. 13 °C, which reduced the impact of temperature polarization by increasing the TPC from 98.2% to 116.1% in PVMD operation; as a result, a remarkable enhancement of the flux from 8 kg m−2 h−1 to 27 kg m−2 h−1 was observed.302
Avci et al. developed composite membranes consisting of a 20–25 μm photothermal layer of PVDF loaded with Ag NPs cast on the non-woven fabric of 110–115 μm by VIPS/NIPS routes; the inclusion of the photothermal layer led to a gain of 10-fold transmembrane flux under UV radiation in PSGMD operation.280 Experiments were performed by feeding a saline solution (0.5 M NaCl) to the membrane module at a flow rate of 3 L h−1 at 25 °C whereas air (RH = 50%, T = 20 °C) was flushed at 50 L h−1 for the removal of water molecules from the distillate compartment.280 The warm and hyper-concentrated retentate stream (from 1 to 4 M NaCl) was fed to a reverse electrodialysis (RED) stack to generate a maximum power density of up to 0.9 W mMP−2 (MP: RED membrane pair).
A dual-layer membrane with a PVA photothermal coating layer doped with TiN electrospun on a PVDF support layer was recently tested in PAGMD.304 With a stagnant feed-water, the embodiment of 10% of photothermal TIN NPs secured an improvement of the flux from 0.498 kg m−2 h−1 to 0.772 kg m−2 h−1 increasing to 0.940 kg m−2 h−1 when the depth of the feed was decreased from 15 mm to 2 mm.304 In fact, the feed water on the top of the membrane slightly scatters the radiation reducing the number of effective phonons reaching the membrane surfaces resulting in a diminution of the self-heating performance. As mentioned above, PAGMD offers the advantage to implement the heat recovery strategy, such as in the case of a multi-layer stacked module (1 mm of air gap) equipped with a photothermal FTSC/PDA/graphene/PTFE membrane.305 A four-layered module achieved a flux of 1.17 kg m−2 h−1 under the exposure to light irradiation with an intensity of 0.75 kW m−2; which consists of a remarkable gain with respect to the single layer module showing a flux of 0.55 kg m−2 h−1.305
5. Challenges in PMD
5.1 Reduction of membrane porosity
An optimized design of the photothermal layer requires a proper balance between an effective light harvesting enhanced by a highly dense distribution of NPs at the surface of modified membranes and high porosity for an efficient mass and heat transport through the microporous membrane. This aspect is particularly critical when the incorporation of NPs takes place by applying a functional coating, usually based on polydopamine (PDA),301,308,314 polypyrrole (PPy),292,315 polydimethylsiloxane (PDMS),316 and polyvinyl alcohol (PVA),294 that inevitably reduces membrane porosity. As discussed in Section 4.2.2, porous structures promote internal light scattering and minimize light reflection and the consequent energy loss.High membrane porosity also reduces the resistance to the diffusional transport of volatile molecules through the pores, promoting higher transmembrane fluxes. As established by the Dusty Gas Model,317 both effective free molecular diffusion coefficient 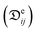 and effective Knudsen diffusion
and effective Knudsen diffusion 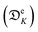 increase linearly with porosity (ε):
increase linearly with porosity (ε):
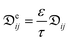 | (31a) |
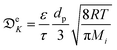 | (31b) |
5.2 Fouling and wetting
Fouling is a major challenge in all membrane separation processes, including MD and PMD. The crystallization of sparingly soluble salts – most commonly CaCO3 and CaSO4 in different polymorphs and hydrates – within membrane pores, enhanced by the concentration polarization phenomenon and heterogeneous nucleation mechanism234,319 (“scaling”), the deposition of colloids and particulate matter such as silica, clay, silt, debris, iron oxide generated from electrochemical corrosion etc. (“colloidal and particulate fouling”),320,321 the accumulation of natural organic matter (NOM) composed of a large spectrum of substances such as proteins, polyhydroxy aromatics, polysaccharides, amino sugars and humic substances322,323 (organic fouling), and the growth of bacteria and microorganisms excreting extracellular polymer substances (EPS) to adhere to the membrane surface324,325 (“biological fouling” or “biofouling”), degrade the performance of the membrane process by lowering rejection and transmembrane flux. An additional concern in PMD is that fouling dramatically affects the long-term stability against wetting, with major concerns for the crystallization of hydrophilic salts within the porous membrane matrix, and the presence of organics, synthetic surfactants and natural amphiphiles that reduce the surface tension of the feedwater.326 As discussed in Section 2.2, the primary parameter for evaluating membrane wettability is the liquid entry pressure (LEP) expressed by the Laplace (Cantor) eqn (2). Therefore, occurrence and prevention of wetting in PMD operation mainly depend on the interactions between feed components (especially those having low surface tension) and membrane (usually characterized by contact angle-based parameters). The correlation between contact angle and interfacial tension of phases in contact, ideally expressed by the Young equation for solid, homogeneous, smooth and flat surfaces,327 is not applicable to real membranes. In order to take into account the surface roughness, the Wenzel model231 (suitable at moderate hydrophobicity) and the Cassie–Baxter model232 (relevant at high roughness and superhydrophobic behavior, i.e., water contact angle >150°) were developed (Fig. 18a):cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θW = r·cos θW = r·cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ | (32a) |
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θC = fs(cos θC = fs(cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ + 1) − 1. θ + 1) − 1. | (32b) |
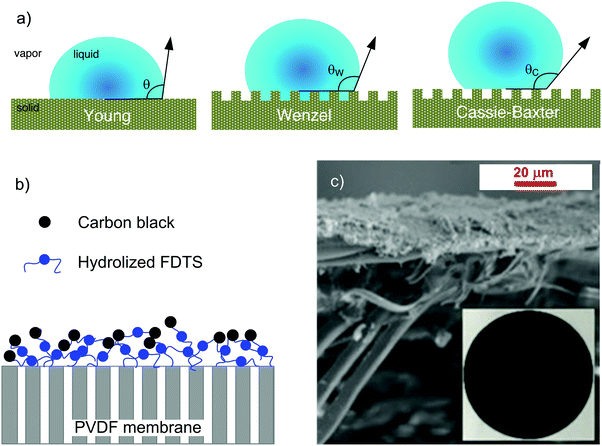 | ||
| Fig. 18 (a) Schematic representation of the Young, Wenzel and Cassie–Baxter models. (b) Schematics of a dual-layer FDTS-CB membrane structure. Reprinted (adapted) with permission from ref. 355 Copyright 2019 American Chemical Society. (c) SEM images of the cross-section of the FDTS-CB-membrane. Inset: Optical image of the membrane. Reprinted (adapted) with permission from ref. 355 Copyright 2019 American Chemical Society. | ||
From a practical point of view, the system is in the Cassie−Baxter state if the liquid, in contact with the re-entrant surface, is supported by the protrusions of the solid substrate and the air trapped in the grooves between these protrusions. In addition to contact angle and LEP, the surface free energy (SFE) of the membrane can provide useful information about the wetting potential of the feed solution with surface tension γl. In principle, wetting is predicted to occur if SFE > γl; however, the easy applicability of this indicator is counterbalanced by the complexity in SFE calculation, commonly made by the Lifshitz–van der Waals/Acid–base (LW-AB) method.328 Causes that can lead to membrane wettability are various and, in some cases, concomitant. Table 12 classifies those occurring more frequently in three main categories: feed characteristics,90,329 fouling,319,326,330 and operating conditions.331
| Category | Cause | Wetting mechanism | Explanation |
|---|---|---|---|
| Feed characteristics | High salt concentration | Surface free energy (SFE) of the membrane > feed surface tension (γl) | Decrease of the surface free energy of the membrane |
| Presence of organics, oil, and surfactants | Synthetic surfactants and natural amphiphiles decrease the feed surface tension: LEP is reduced proportionally to γl | ||
| Hydrophobic moieties allow organics/oil/surfactants to adsorb on the membrane surface through hydrophobic–hydrophobic interactions, increasing the SFE | |||
| Fouling | Scaling-induced wetting | Loss of membrane hydrophobicity | Overcoming the saturation limit of sparingly soluble salts (primarily CaCO3 and CaSO4) leads to the formation of hydrophilic crystals within the membrane pores |
| Organic fouling | Strong attractive interactions between membrane and humic acids/polysaccharides/proteins etc., decrease the surface free energy of the membrane | ||
| Biological fouling (or Biofouling) | Biological microorganisms (e.g. bacteria, fungi, algae etc.) grow at the membrane surface forming a gel-like structure termed “biofilm” | Pore wetting occurs due to the secretion of extracellular polymeric substances (EPS) with amphiphilic properties | |
| Operating conditions | High feed flow rate | Transmembrane pressure ΔP > LEP | High feed velocity increases the pressure of the feed solution; wetting risk is enhanced when a vacuum is applied at the distillate side (VMD/PVMD) |
| Chemical degradation during the long-term MD process | Loss of membrane hydrophobicity | Reduction of contact angle due do the formation of hydrophilic groups (e.g. hydroxyl (OH), carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and unsaturated (C O) and unsaturated (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) groups) by chemical oxidative degradation C) groups) by chemical oxidative degradation |
An efficient strategy to avoid or reduce the risk of wetting is to implement an appropriate pre-treatment of the feed solution. Typically, conventional membrane filtration (microfiltration, ultrafiltration) can be operated upstream of PMD to remove particulate and colloidal contaminants, while nanofiltration or softening via reactive precipitation can also be used to remove sparingly soluble compounds including divalent and multivalent ions (most commonly Ca2+, Mg2+).332,333 The use of anti-scalants (e.g., polyacrylic acid) can prolong the induction period for the nucleation of gypsum and calcite, although their organic nature increases the risk of membrane wetting.334,335 Beside coagulation/flocculation,336,337 nanofiltration is also effective to remove anionic surfactants336 and organic matter. However, the addition of a pretreatment stage increases capital and operating costs; for this reason, many research efforts are addressed to the development of wetting-resistant membranes as more versatile and affordable alternatives to sustain long-term stability of MD/PMD operations.
In this respect, preparation methods and/or surface modification protocols are oriented to the fabrication of superhydrophobic membranes (repellent to water), omniphobic membranes (repellent to both water and organic liquids), and Janus membranes composed of an omniphobic or superhydrophobic substrate and a fouling-resistant hydrophilic surface coating, all exhibiting superior wetting resistance.338
Membranes are conventionally classified as superhydrophobic when exhibiting a static water contact angle higher than 150° and a contact angle hysteresis (difference between the advancing and receding contact angles) or – alternatively – a sliding angle lower than 10°.339
Membrane superhydrophobicity is achieved by tuning surface roughness, thus minimizing the liquid–solid contact area to achieve a Cassie–Baxter state (eqn (32b)). From a practical point of view, surface roughening is usually achieved by attaching nanoparticles (e.g., CNTs,340,341 graphenes,342,343 carbon black,344,345 SiO2,346,347 TiO2,346,347 ZnO nanostructures348–350etc.), eventually grafted with perfluorinated agents to increase their hydrophobicity, via (electro)spray coating or electrospinning to form hierarchical or re-entrant structures at the nanoscale, or by patterning the skin layer of the membrane with micro-pillared arrays via the micro-molding phase separation method.351–353 The complex fabrication procedures involved in the last approach pose severe hurdles to the implementation at large scales. On the other side, the opportunity to simultaneously combine the light-to-heat conversion properties of photothermal nanoparticles with their ability to roughen the membrane surface makes the first option the one preferentially used in PMD. Liao et al. (2022) electrosprayed a PVDF/PDMS/MWCNT solution on an electrospun nanofiber PVDF membrane; the consequent formation of microspheres at the PVDF surface conferred the superhydrophobicity to the composite membrane, resulting in water contact angles greater than 150°.354 Moreover, mixing different typologies of nanoparticles can act synergistically to achieve multiple goals. Anti-wetting and anti-fouling abilities have been stimulated by spraying on PVDF membranes a mixture of titanium carbide (TiC) NPs – exhibiting photothermal properties – and titania (TiO2) NPs – having potential to degrade organic compounds; a final fluorination step was accomplished to enhance surface hydrophobicity (water contact angle of 154.7°).303
Whereas most of the superhydrophobic membranes are prone to wetting by organic solutions, omniphobic surfaces exhibit high repellency to both water and other low surface tension liquids such as surfactants, alcohols and oils. Typically, the preparation of omniphobic surfaces includes the creation of a single/multilevel re-entrant hierarchical texture that provides an energy barrier to the wetting transition from the metastable Cassie–Baxter state to the stable Wenzel state, and the functionalization (typically a fluorination treatment) of the coated substrates to lower the SFE.
A re-entrant topography is typically obtained by adding nanoparticles such as SiO2,356,357 ZnO,358,359 TiO2358,359etc., while chemical modification is prevalently carried out via fluorinated alkyl silane (FAS), 1H,1H,2H,2H-perfluorodecyltriethoxy-silane (17-FAS), 1H,1H,2H,2H-perfluorodecyltriethoxysilane (FTCS), 1H,1H,2H,2H-perfluorodecyltriethoxysilane (FDTES), perfluorodecyltrichlorosilane (FDTS), fluorinated-decyl polyhedraloligomeric silsesquioxane (F-POSS), trichloro (1H,1H,2H,2H-tridecafluoro-n-octyl) silane (FOTS), 1H,1H,2H,2H-perfluorooctyltrichlorosilane (PFTS), perfluorooctanoate (PFO), 1H,1H,2H,2H-perfluorooctyltrichlorosilane (PFOTS), and poly(perfluorodecyl acrylate) (PPFDA), as comprehensively reviewed.90 Focusing on specific PMD applications, omniphobic photothermal membranes were prepared by forming a hierarchical structure of 17-FAS modified carbon black (CB) NPs on the PVDF membrane surface. The functionalization resulted in an increase of the contact angle from 0° (pristine PVDF membrane) to 94.2° (0.2 wt% CB NPs) for an 80% v/v ethanol–water mixture (surface tension of 26 mN m−1), and from 132.1° to 140.8° for pure water (surface tension of 72 mN m−1). In addition, the membrane exhibited anti-wetting behavior in the presence of surfactants: no liquid penetration was observed for sodium dodecyl sulfate (SDS) concentration within the investigated range of 0.1–0.4 mM, while liquid intrusion through a pristine PVDF membrane was detected at 0.2 mM SDS.360 With a similar approach, FDTS-CB coated PVDF membranes (Fig. 18b and c) exhibited an increased water CA from 118.1° (pristine PVDF) to 142.3°, corresponding to an LEP of 424 kPa; moreover, FDTS-CB coated PVDF membranes showed effective repellent behavior the low-surface tension liquids, such as mineral oil (138.5°) and ethanol (126.2°) in air.355
In the perspective of PMD, the manufacturing of the hydrophilic face of Janus composite membranes resistant to fouling and wetting can potentially exploit the photothermal ability of polymers such as polypyrrole361 and polydopamine.300
5.3 Cost assessment of photothermal MD
The purpose of the implementation of photothermal membrane technology is to produce freshwater at the water–energy nexus by minimizing the environmental impact of the desalination and water treatment processes. Despite their superior performance in the light-to-heat conversion, there are serious concerns about the viability of photothermal membranes based on metallic NPs. Their synthesis commonly implies the employment of harmful chemicals (e.g. CTAB and NaBH4) employed as capping/reducing agents, hindering the industrial scale up of the production of metallic nanoparticles.172 Microbes have gained more attention as promising, inexpensive, energy-efficient and green nano-factories, whereas plant extracts (e.g., polyphenols, phenolic acid, alkaloids, proteins, and sugar) have been extensively studied in metal NP fabrication as a reducing or stabilizing agent of nanoparticles allowing an inexpensive scalable eco-friendly route to be followed.362 Nevertheless, the understanding of the molecular mechanism of biosynthesis is not still achieved. Moreover, the high cost of photothermal membranes loaded with noble metal NPs (typically above 100 $ g−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 363) is the main drawback of the commercialization of thermoplasmonics.
363) is the main drawback of the commercialization of thermoplasmonics.
From an energetic point of view, thermoplasmonics typically relies on UV radiation, characterized by a consequent remarkable specific energy consumption for the generation of artificial radiation,364 counterbalanced by outstanding performance. Taking as example the work of Politano et al. (2017),278 PVDF membranes loaded with Ag NPs guaranteed a flux of ca. 26 kg m−2 h−1 under UV radiation of 23 kW m−2 resulting in a specific energy consumption of 1.5 kW h L−1 which makes photothermal PMD based on noble metal NPs technically misleading.
Thus, the band-gap engineering for the exploitation of sunlight is a crucial strategy to overcome the severe energy impact of thermoplasmonics.364 In this framework, advanced carbon-based materials (e.g. graphene and CWNTs) emerged as the most viable solution acting as a black body achieving a photothermal efficiency above 95% under natural radiation. Unfortunately, the cost of graphene (150–250 $ g−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 365) still makes the preparation of the next-generation of photothermal carbon-based materials prohibitively expensive.
365) still makes the preparation of the next-generation of photothermal carbon-based materials prohibitively expensive.
On the other hand, commercially available and inexpensive carbonous NPs have the potential to guarantee economic and energetic viability to photothermal MD. Roughly, the natural radiation daily provides 4–8 kW h m−2 useful to vaporize 3–6 L m−2 day−1 (water evaporation heat is around 667 kW h m−3) assuming a photothermal efficiency of 50%,366 as confirmed by the stand-alone PMD system based on CB-PVA-PVDF developed by Dongare et al. (2017).294 Another interesting advantage is the opportunity to synthesize effective photothermal carbon-based NPs by the carbonization of organic compounds opening the door for the valorization of the biomass coherently with the circular economy paradigm.367
Beyond these two major categories, PDA has gained more attention as an ecofriendly and inexpensive photothermal material being a biocompatible and biodegradable polymer with a broad absorption of the solar radiation.368 Although PDA showed competitive performance with conventional carbon-based materials,252,307 the cost of a mussel-inspired membrane (33$ m−2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 369) and its long term stability under real conditions are two critical challenges to be addressed.370
369) and its long term stability under real conditions are two critical challenges to be addressed.370
6. Other applications based on photothermal membrane distillation
In recent years, membrane crystallization (MCr) has demonstrated the potential to replace the conventional crystallizers, considering its unique advantages. MCr enables accurate control of the transmembrane flux of the solvent driving the system through specific supersaturation pathways within the metastable zone, thus enhancing crystal formation in terms of particle shape and size distribution, polymorph/hydration form selection and impurity control. Moreover, the physicochemical properties of the polymeric membrane (surface energy/contact angle) and topography (roughness, porosity, pore size, etc.) act as exclusive tools to modulate the extent of the free Gibbs energy barrier to heterogeneous nucleation and, ultimately, to control the crystallization kinetics.371 Thermally- or osmotically-driven MCr can be considered as an evolution of the MD process since supersaturation is achieved through the removal of the solvent from the feed solutions.372 Among the most interesting applications, MCr has been studied as a feasible method for the post-treatment of the hypersaline retentate of desalination and wastewater treatment processes for the recovery of valuable minerals such as NaCl,30 Na2SO4,373 MgSO4·7H2O,373 LiCl374etc.A nature-inspired synthetic floating leaf made of a graphene oxide (GO) thin film has been studied for solar-driven MCr. Under 0.82 sun illumination, the system reported allowed the photothermal generation of steam at a rate of 1.1 L m−2 h−1 with a light-to-vapor energy conversion of 54%.375 A long-term evaporation experiment with a 15 wt% NaCl solution showed the possibility to crystallize the salt on the membrane surface.375
Inspired by the natural transpiration process in plants, GO-based membranes with over 85% solar steam efficiency under one-sun were employed for the treatment of heavy-metal-contaminated solutions (Cu2+, Cr3+, and Pb2+).376 Nevertheless, this strategy can potentially open the perspective of a sustainable recovery of valuable raw materials: a photothermal 3-D device based on a GO-filter paper membrane allowed the treatment of an aqueous solution mimicking the wastewater from electroplating industry and the simultaneous precipitation of copper sulfate salt (CuSO4 5H2O).259
Overall, from the perspective of ZLD, photothermal membranes can potentially guarantee a thermally efficient steam generation and a feasible recovery of valuable salt crystals to fulfil the goal of a sustainable brine management.42
The implementation of photothermal membranes for a simultaneous production of freshwater and energy with the goal to alleviate the water–energy nexus was investigated by using a photocatalytic hydrogel of TiO2/Ag nanofibers embedded into CS.377 Ag played the dual role of: (i) thermoplasmonic effects facilitating the vaporization of water at a rate of 1.49 kg m−2 h−1 under 1 sun and (ii) a cocatalyst to facilitate charge transfer and the light absorption for improved hole-scavenging activity for the photocatalytic H2 generation at a rate of 3260 μmol m−2 h−1 377. An emerging trend is to exploit the electrochemical potential of the brines to produce renewable blue energy (Salinity Gradient Power) via Reverse Electrodialysis (RED), an electromembrane-based process that harvests the Gibbs free energy of mixing of solutions with different salinity;378 feeding the hypersaline retentate from the photothermal SGMD unit to a RED stack enabled the recovery of 10% of the energy not used for water evaporation, thus improving the overall efficiency of the integrated system.280
Biofouling control is essential to extend the shelf-life of the membrane, to reduce the operation and maintenance cost, to sustain the long-term performance of the membrane process, and to ensure a stable quality of produced water. Carbon-based materials and metal NPs have been widely explored to enhance the anti-fouling properties of the membranes.379 Ag and Au NPs are able to inhibit biofouling because of their reactivity with the thiol groups present in bacteria, leading to the inactivation of the proteins.380 The synergistic coupling of the photothermal and bactericidal properties of GO and Au NPs allowed the development of multifunctional membranes: the presence of a nanoheater effectively inactivated the bacteria on the membrane surface improving its fouling resistance.381 Studies have demonstrated that rGO flakes dispersed in a cellulose-based membrane efficiently converted the light energy to heat, killing Escherichia coli bacteria within 3 min.382
MD has been also proven as a beneficial treatment for wastewater from textile industries ensuring the recovery of large portions of water and the concentration of dyes according to the ZLD paradigm.383,384 In this scenario, the integration of PMD with photocatalytic membranes (synergic exploitation of light and a photocatalytic semiconductor for the generation of oxidizing/reducing species inducing the total degradation of organic and inorganic pollutants to innocuous substances) demonstrated a great potential for use in energy-efficient and intensified wastewater treatment processes.385 Graphene-based membranes have been widely studied because of their excellent photocatalytic properties explored in many important energy and environmental applications including photocatalytic pollutant degradation, H2 production, and CO2 reduction.386 However, the role of carbon-based nanomaterials has been so far underestimated if one considers that their photothermal behaviour can play an important role in the enhancement of photocatalytic performance.387 For instance, the photothermal effect of rGO has been exploited in cooperation with the TiO2 P25 catalyst to develop a nanocomposite with superior efficiency for the photocatalytic degradation of methylene blue.387 Likewise, photothermal-assisted 3D flower-like CuS superparticles exhibited a 213% enhanced catalytic activity for the photodegradation of methylene blue in water.388
The use of PMD could also alleviate the pressure on water-stressed oil-producing countries, enabling the remediation of their wastewater with an organic carbon rejection ranging between 73% and 96%.389 Nevertheless, the implementation of photothermal materials is of interest for breaking azeotropic mixtures: Ag NPs in the PDMS membrane significantly improved the performance in terms of flux and selectivity in ethanol recovery from aqueous solution upon light exposure.390
Overall, advancements in photothermal membranes have injected new vitality into the field of membranes for water treatment applications, giving the realistic perspective to achieve the blue dream of low-energy water purification.42
7. Conclusions and outlook
MD is an emerging technology potentially able to mitigate water–energy nexus pressure, producing freshwater from different sources such as seawater, brackish water, brine and contaminated water. Unlike RO, the aptitude of MD to efficiently treat water with higher salt concentration may positively affect the sustainability of the desalination industry. However, the operativity of MD at the industrial scale has been so far hampered due to serious limitations related to low productivity in terms of transmembrane flux and low thermal efficiency mainly due to temperature polarization.Recently, the synergic combination of solar energy and MD has improved the sustainability of the process. The emergence of engineered photothermal materials with a wide range of physicochemical properties (i.e., morphology, structure, and absorption) and light-to-heat conversion higher than 90% is opening new avenues for MD. Recent advancements in the synthesis and functionalization of photothermal NPs and, in those of metal and carbon-based NPs, encourage the progress towards the production scale-up and high-volume applications. Furthermore, as concerns photothermal materials, the advent of high-throughput computational screening over thousands of candidate materials could favor the optimization of solar thermal conversion though detailed comparative evaluation of their physicochemical and electronic properties (dielectric functions, optical anisotropies, plasmonic excitations matching solar radiation, etc.), with subsequent benefits for solar-driven MD.
The dispersion of the photothermal material into/onto the membrane demonstrated the superior performance of the interfacial solar evaporation by the in situ harvesting of the nano-heating spots at the membrane–feed water interface, leading to mitigation or withdrawal of temperature polarization. Although in an embryonic phase, the recent advent of PMD is recognized as a promising arrow in the quiver when facing the challenge of sustainable water desalination and purification processes using renewable energies, such as sunlight. However, even if the efficiency of sun-powered PMD at lab-scale is proven, long-term stability of materials is still questionable. The mitigation of fouling (less aggressive in MD/PMD with respect to conventional pressure-driven membrane operations) is typically achieved by applying a hydrophilic coating on top of a hydrophobic membrane to improve the organic fouling resistance; this approach also limits the potential leakage of NPs, assuring longevity to the membranes.
In this scenario, the fundamental understanding of the effect of the membrane structure and the operating conditions of the process on the energy management is crucial for the scalability of PMD.
It is worth mentioning that modularity is another strength point, which paves the way for the practical and flexible integration of PMD within downstream process schemes for the post-treatment and the valorisation of waste effluents, including desalination of brine. These prospects are exciting with a view to circular economy and green transition, although the economic potential and the environmental footprint of PMD require further assessment.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Sergio Santoro acknowledges the European Commission for the financial support activated under the PON “Research and Innovation” 2014–2020, Action I.2 “Researchers’ Mobility”, Notice D. D. 407 of 02.27.2018 – AIM “Attraction and International Mobility”-Line 2 “Researchers’ Attraction”.References
- T. Distefano and S. Kelly, Ecol. Econ., 2017, 142, 130–147 CrossRef.
- C. Tortajada and A. K. Biswas, Curr. Opin. Environ. Sustain., 2018, 34, 21–25 CrossRef.
- J. R. Ziolkowska, Water Supply, 2015, 16, 563–578 CrossRef.
- J. Willet, K. Wetser, J. Vreeburg and H. H. M. Rijnaarts, Water Resour. Ind., 2019, 21, 100110 CrossRef.
- E. Drioli, S. Santoro, S. Simone, G. Barbieri, A. Brunetti, F. Macedonio and A. Figoli, React. Funct. Polym., 2014, 79, 1–7 CrossRef CAS.
- B. G. Ridoutt and S. Pfister, Glob. Environ. Chang., 2010, 20, 113–120 CrossRef.
- S. Santoro, H. Estay, A. H. Avci, L. Pugliese, R. Ruby-Figueroa, A. Garcia, M. Aquino, S. Nasirov, S. Straface and E. Curcio, Clean. Eng. Technol., 2021, 2, 100091 CrossRef.
- A. Alkaisi, R. Mossad and A. Sharifian-Barforoush, Energy Procedia, 2017, 110, 268–274 CrossRef.
- A. Ali, R. A. Tufa, F. Macedonio, E. Curcio and E. Drioli, Renewable Sustainable Energy Rev., 2018, 81, 1–21 CrossRef CAS.
- D. L. Shaffer, L. H. Arias Chavez, M. Ben-Sasson, S. Romero-Vargas Castrillón, N. Y. Yip and M. Elimelech, Environ. Sci. Technol., 2013, 47, 9569–9583 CrossRef CAS PubMed.
- A. Gherghel, C. Teodosiu and S. De Gisi, J. Clean. Prod., 2019, 228, 244–263 CrossRef CAS.
- N. Misdan, W. J. Lau and A. F. Ismail, Desalination, 2012, 287, 228–237 CrossRef CAS.
- F. Calise, F. L. Cappiello, R. Vanoli and M. Vicidomini, Appl. Energy, 2019, 253, 113575 CrossRef.
- M. Badruzzaman, N. Voutchkov, L. Weinrich and J. G. Jacangelo, Desalination, 2019, 449, 78–91 CrossRef CAS.
- I. C. Karagiannis and P. G. Soldatos, Desalination, 2008, 223, 448–456 CrossRef CAS.
- N. Voutchkov, Desalination, 2018, 431, 2–14 CrossRef CAS.
- Desalination and Water Reuse by the Numbers, https://idadesal.org/.
- A. Deshmukh, C. Boo, V. Karanikola, S. Lin, A. P. Straub, T. Tong, D. M. Warsinger and M. Elimelech, Energy Environ. Sci., 2018, 11, 1177–1196 RSC.
- ResearchAndMarkets.com, Major Reverse Osmosis System Components for Water Treatment: The Global Market, 2020.
- K. P. Lee, T. C. Arnot and D. Mattia, J. Membr. Sci., 2011, 370, 1–22 CrossRef CAS.
- A. H. Avci, D. A. Messana, S. Santoro, R. A. Tufa, E. Curcio, G. Di Profio and E. Fontananova, Membranes, 2020, 10, 168 CrossRef CAS PubMed.
- S. Santoro, R. A. Tufa, A. H. Avci, E. Fontananova, G. Di Profio and E. Curcio, Energy, 2021, 120563 CrossRef CAS.
- C. Fritzmann, J. Löwenberg, T. Wintgens and T. Melin, Desalination, 2007, 216, 1–76 CrossRef CAS.
- C. Laspidou, K. Hadjibiros and S. Gialis, Water, 2010, 2, 75–84 CrossRef CAS.
- I. Abd-Elaty, A. E. L. Shahawy, S. Santoro, E. Curcio and S. Straface, Sci. Total Environ, 2021, 795, 148928 CrossRef CAS PubMed.
- S. Al-Obaidani, E. Curcio, F. Macedonio, G. Di Profio, H. Al-Hinai and E. Drioli, J. Membr. Sci., 2008, 323, 85–98 CrossRef CAS.
- M. A. E. R. Abu-Zeid, Y. Zhang, H. Dong, L. Zhang, H. L. Chen and L. Hou, Desalination, 2015, 356, 1–14 CrossRef CAS.
- A. Cerda, M. Quilaqueo, L. Barros, G. Seriche, M. Gim-Krumm, S. Santoro, A. H. Avci, J. Romero, E. Curcio and H. Estay, J. Water Process Eng., 2021, 41, 102063 CrossRef.
- E. Drioli, A. Ali and F. Macedonio, Desalination, 2015, 356, 56–84 CrossRef CAS.
- X. Ji, E. Curcio, S. Al Obaidani, G. Di Profio, E. Fontananova and E. Drioli, Sep. Purif. Technol., 2010, 71, 76–82 CrossRef CAS.
- I. Hitsov, T. Maere, K. De Sitter, C. Dotremont and I. Nopens, Sep. Purif. Technol., 2015, 142, 48–64 CrossRef CAS.
- S. Santoro, I. M. Vidorreta, V. Sebastian, A. Moro, I. M. Coelhoso, C. A. M. Portugal, J. C. Lima, G. Desiderio, G. Lombardo, E. Drioli, R. Mallada, J. G. Crespo, A. Criscuoli and A. Figoli, J. Membr. Sci., 2017, 536, 156–166 CrossRef CAS.
- S. Santoro, I. Vidorreta, I. Coelhoso, J. C. Lima, G. Desiderio, G. Lombardo, E. Drioli, R. Mallada, J. Crespo, A. Criscuoli and A. Figoli, Molecules, 2019, 24, 638 CrossRef CAS PubMed.
- J. Koschikowski, M. Wieghaus and M. Rommel, Water Sci. Technol. Water Supply, 2003, 156, 295–304 CAS.
- X. Wang, L. Zhang, H. Yang and H. Chen, Desalination, 2009, 247, 403–411 CrossRef CAS.
- R. B. Saffarini, E. K. Summers, H. A. Arafat and J. H. Lienhard V, Desalination, 2012, 286, 332–341 CrossRef CAS.
- M. A. A. Hejazi, O. A. Bamaga, M. H. Al-Beirutty, L. Gzara and H. Abulkhair, Sep. Purif. Technol., 2019, 220, 300–308 CrossRef CAS.
- A. E. Kabeel, M. Abdelgaied and E. M. S. El-Said, Renew. Energy, 2017, 106, 192–200 CrossRef.
- F. Banat, N. Jwaied, M. Rommel, J. Koschikowski and M. Wieghaus, Desalination, 2007, 217, 17–28 CrossRef CAS.
- F. Banat, R. Jumah and M. Garaibeh, Renew. Energy, 2002, 25, 293–305 CrossRef CAS.
- L. Zhu, M. Gao, C. K. N. Peh and G. W. Ho, Mater. Horiz., 2018, 5, 323–343 RSC.
- C. Zhang, H.-Q. Liang, Z.-K. Xu and Z. Wang, Adv. Sci., 2019, 6, 1900883 CrossRef CAS PubMed.
- P. Zhang, Q. Liao, H. Yao, Y. Huang, H. Cheng and L. Qu, Energy Storage Mater., 2019, 18, 429–446 CrossRef.
- L. Shi, X. Wang, Y. Hu, Y. He and Y. Yan, Appl. Therm. Eng., 2020, 179, 115691 CrossRef CAS.
- Q. Huang, X. Liang, C. Yan and Y. Liu, Appl. Energy, 2021, 283, 116361 CrossRef.
- Y. Lin, H. Xu, X. Shan, Y. Di, A. Zhao, Y. Hu and Z. Gan, J. Mater. Chem. A, 2019, 7, 19203–19227 RSC.
- H. Ghasemi, G. Ni, A. M. Marconnet, J. Loomis, S. Yerci, N. Miljkovic and G. Chen, Nat. Commun., 2014, 5, 4449 CrossRef CAS PubMed.
- A. Politano, in Current Trends and Future Developments on (Bio-) Membranes, ed. A. Basile, E. Curcio and Inamuddin, Elsevier, 2019, pp. 55–80 Search PubMed.
- N. S. Fuzil, N. H. Othman, N. H. Alias, F. Marpani, M. H. D. Othman, A. F. Ismail, W. J. Lau, K. Li, T. D. Kusworo, I. Ichinose and M. M. A. Shirazi, Desalination, 2021, 517, 115259 CrossRef CAS.
- S. Santoro, A. H. Avci, M. Aquino, L. Pugliese, S. Straface and E. Curcio, Towards the Global Rise of Zero Liquid Discharge for Wastewater Management: The Mining Industry Case in Chile, The Handbook of Environmental Chemistry, Springer Berlin Heidelberg, Berlin, Heidelberg, 2021, pp. 1–14 DOI:10.1007/6982021785.
- A. G. Razaqpur, Y. Wang, X. Liao, Y. Liao and R. Wang, Water Res., 2021, 201, 117299 CrossRef CAS PubMed.
- S. W. Sharshir, A. M. Algazzar, K. A. Elmaadawy, A. W. Kandeal, M. R. Elkadeem, T. Arunkumar, J. Zang and N. Yang, Desalination, 2020, 491, 114564 CrossRef CAS.
- E. Curcio, G. Di Profio, E. Fontananova and E. Drioli, in Advances in Membrane Technologies for Water Treatment: Materials, Processes and Applications, ed. A. Basile, A. Cassano and N. K. Rastogi, Woodhead Publishing, Cambridge, 2015, pp. 411–441 Search PubMed.
- E. Curcio, G. Di Profio and E. Drioli, in Comprehensive Membrane Science and Engineering, ed. E. F. Drioli, Enrico and L. Giorno, Elsevier, Amsterdam, 2010 Search PubMed.
- N. Thomas, M. O. Mavukkandy, S. Loutatidou and H. A. Arafat, Sep. Purif. Technol., 2017, 189, 108–127 CrossRef CAS.
- E. Curcio and G. Di Profio, The Handbook of Continuous Crystallization, The Royal Society of Chemistry, 2020, pp. 321–352 Search PubMed.
- M. Gryta, J. Membr. Sci., 2005, 246, 145–156 CrossRef CAS.
- S. M. Huang, Y. H. Chen, W. Z. Yuan, S. Zhao, Y. Hong, W. B. Ye and M. Yang, Sep. Purif. Technol., 2019, 220, 334–344 CrossRef CAS.
- A. M. Alklaibi and N. Lior, J. Membr. Sci., 2006, 282, 362–369 CrossRef CAS.
- P. Wang and T. S. Chung, J. Membr. Sci., 2015, 474, 39–56 CrossRef CAS.
- G. Zuo, R. Wang, R. Field and A. G. Fane, Desalination, 2011, 283, 237–244 CrossRef CAS.
- G. Lewandowicz, W. Białas, B. Marczewski and D. Szymanowska, J. Membr. Sci., 2011, 375, 212–219 CrossRef CAS.
- Z. Xie, T. Duong, M. Hoang, C. Nguyen and B. Bolto, Water Res., 2009, 43, 1693–1699 CrossRef CAS PubMed.
- A. Criscuoli, P. Bafaro and E. Drioli, Desalination, 2013, 323, 17–21 CrossRef CAS.
- J. A. Andrés-Mañas, A. Ruiz-Aguirre, F. G. Acién and G. Zaragoza, Desalination, 2020, 475, 114202 CrossRef.
- S. O. Olatunji and L. M. Camacho, Front. Energy Res., 2018, 6, 130 CrossRef.
- A. Mourgues, N. Hengl, M. P. Belleville, D. Paolucci-Jeanjean and J. Sanchez, J. Membr. Sci., 2010, 355, 112–125 CrossRef CAS.
- S. Shukla, J. P. Méricq, M. P. Belleville, N. Hengl, N. E. Benes, I. Vankelecom and J. Sanchez Marcano, J. Membr. Sci., 2018, 545, 150–157 CrossRef CAS.
- S. Shukla, N. E. Benes, I. Vankelecom, J. P. Méricq, M. P. Belleville, N. Hengl and J. S. Marcano, J. Membr. Sci., 2015, 493, 167–178 CrossRef CAS.
- S. K. Hubadillah, Z. S. Tai, M. H. D. Othman, Z. Harun, M. R. Jamalludin, M. A. Rahman, J. Jaafar and A. F. Ismail, Sep. Purif. Technol., 2019, 217, 71–84 CrossRef CAS.
- Z. Cui, E. Drioli and Y. M. Lee, Prog. Polym. Sci., 2014, 39, 164–198 CrossRef CAS.
- S. A. Ali and M. A. J. Mazumder, J. Hazard. Mater., 2018, 350, 169–179 CrossRef CAS PubMed.
- L.-T. Huang, P.-S. Hsu, C.-Y. Kuo, S.-C. Chen and J.-Y. Lai, Desalination, 2008, 233, 64–72 CrossRef CAS.
- S. Feng, Z. Zhong, Y. Wang, W. Xing and E. Drioli, J. Membr. Sci., 2018, 549, 332–349 CrossRef CAS.
- L. Eykens, K. De Sitter, C. Dotremont, L. Pinoy and B. Van der Bruggen, Sep. Purif. Technol., 2017, 182, 36–51 CrossRef CAS.
- A. Figoli, S. Santoro, F. Galiano and A. Basile, Pervaporation membranes: Preparation, characterization, and application, 2015 Search PubMed.
- C. M. Hansen, Hansen solubility parameters: a user's handbook, CRC Press, Boca Raton, 2007 Search PubMed.
- M. H. V. Mulder, T. Franken and C. A. Smolders, J. Membr. Sci., 1985, 22, 155–173 CrossRef CAS.
- N. T. Hassankiadeh, Z. Cui, J. H. Kim, D. W. Shin, S. Y. Lee, A. Sanguineti, V. Arcella, Y. M. Lee and E. Drioli, J. Membr. Sci., 2015, 479, 204–212 CrossRef CAS.
- F. Russo, C. Ursino, E. Avruscio, G. Desiderio, A. Perrone, S. Santoro, F. Galiano and A. Figoli, Membranes, 2020, 10, 36 CrossRef CAS PubMed.
- J. Chang, J. Zuo, L. Zhang, G. S. O’Brien and T.-S. Chung, J. Membr. Sci., 2017, 539, 295–304 CrossRef CAS.
- T. Marino, F. Russo and A. Figoli, Membranes, 2018, 8, 71 CrossRef PubMed.
- S. Ebnesajjad, in Fluoroplastics (Second Edition), ed. S. Ebnesajjad, William Andrew Publishing, Oxford, 2015, vol. 1, pp. 382–395 Search PubMed.
- F. Russo, C. Ursino, B. Sayinli, I. Koyuncu, F. Galiano and A. Figoli, Clean Technol., 2021, 3, 761–786 CrossRef.
- M. Khayet, Adv. Colloid Interface Sci., 2011, 164, 56–88 CrossRef CAS PubMed.
- F. Sadeghi, A. Ajji and P. J. Carreau, J. Membr. Sci., 2007, 292, 62–71 CrossRef CAS.
- G.-G. Wu, C. Ding, W.-B. Chen, Y. Zhang, W. Yang and M.-B. Yang, Polymer, 2019, 175, 177–185 CrossRef CAS.
- N. G. P. Chew, S. Zhao, C. H. Loh, N. Permogorov and R. Wang, J. Membr. Sci., 2017, 528, 126–134 CrossRef CAS.
- R. B. Saffarini, B. Mansoor, R. Thomas and H. A. Arafat, J. Membr. Sci., 2013, 429, 282–294 CrossRef CAS.
- H. Chang, B. Liu, Z. Zhang, R. Pawar, Z. Yan, J. C. Crittenden and R. D. Vidic, Environ. Sci. Technol., 2021, 55, 1395–1418 CrossRef CAS PubMed.
- L. Eykens, K. De Sitter, C. Dotremont, L. Pinoy and B. Van der Bruggen, Ind. Eng. Chem. Res., 2016, 55, 9333–9343 CrossRef CAS.
- P. A. B. de Sampaio, Desalination, 2022, 533, 115769 CrossRef CAS.
- L. Li and K. K. Sirkar, J. Membr. Sci., 2016, 513, 280–293 CrossRef CAS.
- P. Wang and T.-S. Chung, J. Membr. Sci., 2015, 474, 39–56 CrossRef CAS.
- S. Santoro, E. Drioli and A. Figoli, Coatings, 2016, 6, 40 CrossRef.
- K. Xu, Y. Cai, N. T. Hassankiadeh, Y. Cheng, X. Li, X. Wang, Z. Wang, E. Drioli and Z. Cui, Desalination, 2019, 456, 13–22 CrossRef CAS.
- D. Tong, X. Wang, M. Ali, C. Q. Lan, Y. Wang, E. Drioli, Z. Wang and Z. Cui, Sep. Purif. Technol., 2016, 157, 1–8 CrossRef CAS.
- M. H. D. A. Farahani and V. Vatanpour, Sep. Purif. Technol., 2018, 197, 372–381 CrossRef CAS.
- Y. Wu, Y. Xia, X. Jing, P. Cai, A. D. Igalavithana, C. Tang, D. C. W. Tsang and Y. S. Ok, J. Hazard. Mater., 2020, 382, 120976 CrossRef CAS PubMed.
- S. Santoro, A. J. Moro, C. A. M. Portugal, J. G. Crespo, I. M. Coelhoso and J. C. Lima, J. Membr. Sci., 2016, 514, 467–475 CrossRef CAS.
- S. Santoro, V. Sebastian, A. J. Moro, C. A. M. Portugal, J. C. Lima, I. M. Coelhoso, J. G. Crespo and R. Mallada, J. Colloid Interface Sci., 2017, 486, 144–152 CrossRef CAS PubMed.
- O. Iglesias, M. J. Rivero, A. M. Urtiaga and I. Ortiz, Chem. Eng. J., 2016, 305, 136–148 CrossRef CAS.
- J. Guo, D. Y. S. Yan, F. L.-Y. Lam, B. J. Deka, X. Lv, Y. H. Ng and A. K. An, Chem. Eng. J., 2019, 378, 122137 CrossRef CAS.
- G. Zaragoza, A. Ruiz-Aguirre and E. Guillén-Burrieza, Appl. Energy, 2014, 130, 491–499 CrossRef.
- M. Khayet, Desalination, 2013, 308, 89–101 CrossRef CAS.
- M. A. E.-R. Abu-Zeid, Y. Zhang, H. Dong, L. Zhang, H.-L. Chen and L. Hou, Desalination, 2015, 356, 1–14 CrossRef CAS.
- I. Hitsov, T. Maere, K. De Sitter, C. Dotremont and I. Nopens, Sep. Purif. Technol., 2015, 142, 48–64 CrossRef CAS.
- M. Khayet, C. Cojocaru and A. Baroudi, Desalination, 2012, 287, 159–166 CrossRef CAS.
- M. Qtaishat, T. Matsuura, B. Kruczek and M. Khayet, Desalination, 2008, 219, 272–292 CrossRef CAS.
- S. O. Olatunji and L. M. Camacho, Front. Energy Res., 2018, 6, 130 CrossRef.
- J. Deshpande, K. Nithyanandam and R. Pitchumani, J. Membr. Sci., 2017, 523, 301–316 CrossRef CAS.
- M. Essalhi and M. Khayet, Sep. Purif. Technol., 2014, 133, 176–186 CrossRef CAS.
- X. Wu, G. Y. Chen, G. Owens, D. Chu and H. Xu, Mater. Today Energy, 2019, 12, 277–296 CrossRef.
- X. Niu, X. Hu, S. Chu and Q. Gong, Adv. Opt. Mater., 2018, 6, 1701292 CrossRef.
- A. Ciattoni, A. Marini, C. Rizza, M. Scalora and F. Biancalana, Phys. Rev. A, 2013, 87, 53853 CrossRef.
- S. Campione, I. Brener and F. Marquier, Phys. Rev. B, 2015, 91, 121408 CrossRef.
- T. Low, A. Chaves, J. D. Caldwell, A. Kumar, N. X. Fang, P. Avouris, T. F. Heinz, F. Guinea, L. Martin-Moreno and F. Koppens, Nat. Mater., 2017, 16, 182–194 CrossRef CAS PubMed.
- E. Ozbay, Science, 2006, 311, 189–193 CrossRef CAS PubMed.
- Q. Bao and K. P. Loh, ACS Nano, 2012, 6, 3677–3694 CrossRef CAS PubMed.
- P. Avouris and M. Freitag, IEEE J. Sel. Top. Quantum Electron., 2014, 20, 72–83 Search PubMed.
- C. W. Berry, N. Wang, M. R. Hashemi, M. Unlu and M. Jarrahi, Nat. Commun., 2013, 4, 1622 CrossRef CAS PubMed.
- A. Politano, L. Viti and M. S. Vitiello, APL Mater., 2017, 5, 35504 CrossRef.
- Z. Liang, J. Sun, Y. Jiang, L. Jiang and X. Chen, Plasmonics, 2014, 9, 859–866 CrossRef.
- V. Cebrián, F. Martín-Saavedra, L. Gómez, M. Arruebo, J. Santamaria and N. Vilaboa, Nanomedicine, 2013, 9, 646–656 CrossRef PubMed.
- J. Z. Zhang, J. Phys. Chem. Lett., 2010, 1, 686–695 CrossRef CAS.
- W.-C. Law, K.-T. Yong, A. Baev and P. N. Prasad, ACS Nano, 2011, 5, 4858–4864 CrossRef CAS PubMed.
- M. R. K. Ali, H. R. Ali, C. R. Rankin and M. A. El-Sayed, Biomaterials, 2016, 102, 1–8 CrossRef CAS PubMed.
- R. Sundararaman, P. Narang, A. S. Jermyn, W. A. Goddard III and H. A. Atwater, Nat. Commun., 2014, 5, 5788 CrossRef CAS PubMed.
- A. M. Brown, R. Sundararaman, P. Narang, W. A. Goddard and H. A. Atwater, ACS Nano, 2016, 10, 957–966 CrossRef CAS PubMed.
- M. L. Brongersma, N. J. Halas and P. Nordlander, Nat. Nanotechnol., 2015, 10, 25–34 CrossRef CAS PubMed.
- J. Liang, H. Liu, J. Yu, L. Zhou and J. Zhu, Nanophotonics, 2019, 8, 771–786 Search PubMed.
- G. Baffou and R. Quidant, Laser Photon. Rev., 2013, 7, 171–187 CrossRef CAS.
- J. Gargiulo, R. Berté, Y. Li, S. A. Maier and E. Cortés, Acc. Chem. Res., 2019, 52, 2525–2535 CrossRef CAS PubMed.
- C. Zhan, X.-J. Chen, Y.-F. Huang, D.-Y. Wu and Z.-Q. Tian, Acc. Chem. Res., 2019, 52, 2784–2792 CrossRef CAS PubMed.
- H.-K. Choi, K. S. Lee, H.-H. Shin, J.-J. Koo, G. J. Yeon and Z. H. Kim, Acc. Chem. Res., 2019, 52, 3008–3017 CrossRef CAS PubMed.
- C. J. Murphy, H.-H. Chang, P. Falagan-Lotsch, M. T. Gole, D. M. Hofmann, K. N. L. Hoang, S. M. McClain, S. M. Meyer, J. G. Turner, M. Unnikrishnan, M. Wu, X. Zhang and Y. Zhang, Acc. Chem. Res., 2019, 52, 2124–2135 CrossRef CAS PubMed.
- H.-Y. Ahn, S. Yoo, N. H. Cho, R. M. Kim, H. Kim, J.-H. Huh, S. Lee and K. T. Nam, Acc. Chem. Res., 2019, 52, 2768–2783 CrossRef CAS PubMed.
- B. Khlebtsov, V. Zharov, A. Melnikov, V. Tuchin and N. Khlebtsov, Nanotechnology, 2006, 17, 5167–5179 CrossRef CAS.
- H. Chen, L. Shao, T. Ming, Z. Sun, C. Zhao, B. Yang and J. Wang, Small, 2010, 6, 2272–2280 CrossRef CAS PubMed.
- G. Baffou and R. Quidant, Laser Photonics Rev., 2013, 7, 171–187 CrossRef CAS.
- A. Politano, A. Cupolillo, G. Di Profio, H. A. Arafat, G. Chiarello and E. Curcio, J. Phys.: Condens. Matter, 2016, 28, 363003 CrossRef CAS PubMed.
- S. Agnihotri, S. Mukherji and S. Mukherji, RSC Adv., 2014, 4, 3974–3983 RSC.
- G. Baffou, R. Quidant and F. J. García De Abajo, ACS Nano, 2010, 4, 709–716 CrossRef CAS PubMed.
- A. O. Govorov, W. Zhang, T. Skeini, H. Richardson, J. Lee and N. A. Kotov, Nanoscale Res. Lett., 2006, 1, 84 CrossRef.
- H. H. Richardson, M. T. Carlson, P. J. Tandler, P. Hernandez and A. O. Govorov, Nano Lett., 2009, 9, 1139–1146 CrossRef CAS PubMed.
- G. Baffou, P. Berto, E. Bermúdez Ureña, R. Quidant, S. Monneret, J. Polleux and H. Rigneault, ACS Nano, 2013, 7, 6478–6488 CrossRef CAS PubMed.
- J. T. Jørgensen, K. Norregaard, P. Tian, P. M. Bendix, A. Kjaer and L. B. Oddershede, Sci. Rep., 2016, 6, 30076 CrossRef PubMed.
- D. Elmaghraoui, A. Politano and S. Jaziri, J. Chem. Phys., 2020, 152, 114102 CrossRef CAS PubMed.
- L. Durdevic, H. M. L. Robert, B. Wattellier, S. Monneret and G. Baffou, Sci. Rep., 2019, 9, 4644 CrossRef PubMed.
- G. Baffou, P. Bon, J. Savatier, J. Polleux, M. Zhu, M. Merlin, H. Rigneault and S. Monneret, ACS Nano, 2012, 6, 2452–2458 CrossRef CAS PubMed.
- Q. Jiang, B. Rogez, J.-B. Claude, G. Baffou and J. Wenger, ACS Photonics, 2019, 6, 1763–1773 CrossRef CAS.
- G. Baffou, M. P. Kreuzer, F. Kulzer and R. Quidant, Opt. Express, 2009, 17, 3291–3298 CrossRef CAS PubMed.
- G. Baffou, Thermoplasmonics: Heating Metal Nanoparticles Using Light, Cambridge University Press, 2017, pp. 101–142 Search PubMed.
- S. Santoro, A. J. Moro, C. Portugal, J. G. Crespo, J. C. Lima and I. M. Coelhoso, J. Food Eng., 2016, 189, 37–44 CrossRef CAS.
- G. Naik, J. Kim, N. Kinsey and A. Boltasseva, in Modern Plasmonics, ed. N. V. Richardson and S. Holloway, North-Holland, 2014, vol. 4, pp. 189–221 Search PubMed.
- A. Boltasseva and H. A. Atwater, Science, 2011, 331, 290–291 CrossRef CAS PubMed.
- M. Rocca, Surf. Sci. Rep., 1995, 22, 1–71 CrossRef CAS.
- A. Politano, V. Formoso and G. Chiarello, Plasmonics, 2008, 3, 165 CrossRef CAS.
- H. Ditlbacher, A. Hohenau, D. Wagner, U. Kreibig, M. Rogers, F. Hofer, F. R. Aussenegg and J. R. Krenn, Phys. Rev. Lett., 2005, 95, 257403 CrossRef PubMed.
- M. G. Blaber, M. D. Arnold and M. J. Ford, J. Phys.: Condens. Matter, 2010, 22, 143201 CrossRef CAS PubMed.
- G. H. Chan, J. Zhao, G. C. Schatz and R. P. Van Duyne, J. Phys. Chem. C, 2008, 112, 13958–13963 CrossRef CAS.
- L. Zhou, Y. Tan, J. Wang, W. Xu, Y. Yuan, W. Cai, S. Zhu and J. Zhu, Nat. Photonics, 2016, 10, 393–398 CrossRef CAS.
- P. R. West, S. Ishii, G. V. Naik, N. K. Emani, V. M. Shalaev and A. Boltasseva, Laser Photon. Rev., 2010, 4, 795–808 CrossRef CAS.
- M. I. Stockman, Opt. Express, 2011, 19, 22029–22106 CrossRef PubMed.
- J. M. Sanz, D. Ortiz, R. Alcaraz de la Osa, J. M. Saiz, F. González, A. S. Brown, M. Losurdo, H. O. Everitt and F. Moreno, J. Phys. Chem. C, 2013, 117, 19606–19615 CrossRef CAS.
- A. Lalisse, G. Tessier, J. Plain and G. Baffou, J. Phys. Chem. C, 2015, 119, 25518–25528 CrossRef CAS.
- P. B. Johnson and R. W. Christy, Phys. Rev. B, 1972, 6, 4370–4379 CrossRef CAS.
- E. D. Palik, Handbook of optical constants of solids, Academic Press, Orlando, 1985 Search PubMed.
- M. D. Mcmahon, R. Lopez, H. M. Meyer, L. C. Feldman and R. F. Haglund, Appl. Phys. B, 2005, 80, 915–921 CrossRef CAS.
- A. Loiseau, V. Asila, G. Boitel-Aullen, M. Lam, M. Salmain and S. Boujday, Biosensors, 2019, 9, 1–40 CrossRef PubMed.
- G. A. Sotiriou, G. D. Etterlin, A. Spyrogianni, F. Krumeich, J.-C. Leroux and S. E. Pratsinis, Chem. Commun., 2014, 50, 13559–13562 RSC.
- K. Kalimuthu, B. S. Cha, S. Kim and K. S. Park, Microchem. J., 2020, 152, 104296 CrossRef CAS.
- V. Amendola, R. Pilot, M. Frasconi, O. M. Maragò and M. A. Iatì, J. Phys.: Condens. Matter, 2017, 29, 203002 CrossRef PubMed.
- I. Fabijanić, V. Janicki, J. Ferré-Borrull, M. Bubaš, V. Blažek Bregović, L. F. Marsal and J. Sancho-Parramon, Coatings, 2019, 9, 382 CrossRef.
- M. D. Susman, Y. Feldman, A. Vaskevich and I. Rubinstein, Chem. Mater., 2012, 24, 2501–2508 CrossRef CAS.
- V. G. Kravets, R. Jalil, Y.-J. Kim, D. Ansell, D. E. Aznakayeva, B. Thackray, L. Britnell, B. D. Belle, F. Withers, I. P. Radko, Z. Han, S. I. Bozhevolnyi, K. S. Novoselov, A. K. Geim and A. N. Grigorenko, Sci. Rep., 2014, 4, 5517 CrossRef CAS PubMed.
- V. Amendola, R. Saija, O. M. Maragò and M. A. Iatì, Nanoscale, 2015, 7, 8782–8792 RSC.
- S. Sarina, H. Zhu, E. Jaatinen, Q. Xiao, H. Liu, J. Jia, C. Chen and J. Zhao, J. Am. Chem. Soc., 2013, 135, 5793–5801 CrossRef CAS PubMed.
- T. Lin, C. Yang, Z. Wang, H. Yin, X. Lü, F. Huang, J. Lin, X. Xie and M. Jiang, Energy Environ. Sci., 2014, 7, 967–972 RSC.
- M. Ye, J. Jia, Z. Wu, C. Qian, R. Chen, P. G. O’Brien, W. Sun, Y. Dong and G. A. Ozin, Adv. Energy Mater., 2017, 7, 1601811 CrossRef.
- J. Wang, Y. Li, L. Deng, N. Wei, Y. Weng, S. Dong, D. Qi, J. Qiu, X. Chen and T. Wu, Adv. Mater., 2017, 29, 1603730 CrossRef PubMed.
- M. Li, U. Guler, Y. Li, A. Rea, E. K. Tanyi, Y. Kim, M. A. Noginov, Y. Song, A. Boltasseva, V. M. Shalaev and N. A. Kotov, ACS Energy Lett., 2018, 3, 1578–1583 CrossRef CAS.
- T. N. Lambert, N. L. Andrews, H. Gerung, T. J. Boyle, J. M. Oliver, B. S. Wilson and S. M. Han, Small, 2007, 3, 691–699 CrossRef CAS PubMed.
- C. Lee, H. Kim, C. Hong, M. Kim, S. S. Hong, D. H. Lee and W. I. Lee, J. Mater. Chem., 2008, 18, 4790–4795 RSC.
- D. D. Vaughn and R. E. Schaak, Chem. Soc. Rev., 2013, 42, 2861–2879 RSC.
- I. Kriegel, F. Scotognella and L. Manna, Phys. Rep., 2017, 674, 1–52 CrossRef CAS.
- Y. Li, W. Lu, Q. Huang, C. Li and W. Chen, Nanomedicine, 2010, 5, 1161–1171 CrossRef CAS PubMed.
- S. Ghosh, T. Avellini, A. Petrelli, I. Kriegel, R. Gaspari, G. Almeida, G. Bertoni, A. Cavalli, F. Scotognella, T. Pellegrino and L. Manna, Chem. Mater., 2016, 28, 4848–4858 CrossRef CAS PubMed.
- C. Yan, Q. Tian and S. Yang, RSC Adv., 2017, 7, 37887–37897 RSC.
- X. Yang, Y. Yang, L. Fu, M. Zou, Z. Li, A. Cao and Q. Yuan, Adv. Funct. Mater., 2018, 28, 1704505 CrossRef.
- H. Li, Y. He, Y. Hu and X. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 9362–9368 CrossRef CAS PubMed.
- C. S. Yung, N. A. Tomlin, K. Heuerman, M. W. Keller, M. G. White, M. Stephens and J. H. Lehman, Carbon N. Y., 2018, 127, 195–201 CrossRef CAS.
- J. Lehman, A. Sanders, L. Hanssen, B. Wilthan, J. Zeng and C. Jensen, Nano Lett., 2010, 10, 3261–3266 CrossRef CAS PubMed.
- C. Chen, Y. Li, J. Song, Z. Yang, Y. Kuang, E. Hitz, C. Jia, A. Gong, F. Jiang, J. Y. Zhu, B. Yang, J. Xie and L. Hu, Adv. Mater., 2017, 29, 1701756 CrossRef PubMed.
- A. A. Balandin, Nat. Mater., 2011, 10, 569–581 CrossRef CAS PubMed.
- B. Han, Y. Zhang, Q. Chen and H. Sun, Adv. Funct. Mater., 2018, 28, 1802235 CrossRef.
- G. Fugallo, A. Cepellotti, L. Paulatto, M. Lazzeri, N. Marzari and F. Mauri, Nano Lett., 2014, 14, 6109–6114 CrossRef CAS PubMed.
- J. Chen, X. Wang and T. Chen, Nanoscale Res. Lett., 2014, 9, 86 CrossRef PubMed.
- X. Wang, Y. He, G. Cheng, L. Shi, X. Liu and J. Zhu, Energy Convers. Manage., 2016, 130, 176–183 CrossRef CAS.
- X. Yu, X. Cai, H. Cui, S.-W. Lee, X.-F. Yu and B. Liu, Nanoscale, 2017, 9, 17859–17864 RSC.
- H. Lin, X. Wang, L. Yu, Y. Chen and J. Shi, Nano Lett., 2017, 17, 384–391 CrossRef CAS PubMed.
- F. Shahzad, M. Alhabeb, C. B. Hatter, B. Anasori, S. Man Hong, C. M. Koo and Y. Gogotsi, Science, 2016, 353, 1137–1140 CrossRef CAS PubMed.
- X. Zhao, L.-M. Peng, C.-Y. Tang, J.-H. Pu, X.-J. Zha, K. Ke, R.-Y. Bao, M.-B. Yang and W. Yang, Mater. Horiz., 2020, 7, 855–865 RSC.
- J. K. El-Demellawi, S. Lopatin, J. Yin, O. F. Mohammed and H. N. Alshareef, ACS Nano, 2018, 12, 8485–8493 CrossRef CAS PubMed.
- D. Xu, Z. Li, L. Li and J. Wang, Adv. Funct. Mater., 2020, 30, 2000712 CrossRef CAS.
- M. Alhabeb, K. Maleski, B. Anasori, P. Lelyukh, L. Clark, S. Sin and Y. Gogotsi, Chem. Mater., 2017, 29, 7633–7644 CrossRef CAS.
- P. Lakhe, E. M. Prehn, T. Habib, J. L. Lutkenhaus, M. Radovic, M. S. Mannan and M. J. Green, Ind. Eng. Chem. Res., 2019, 58, 1570–1579 CrossRef CAS.
- J. Li, X. Li and B. der Bruggen, Environ. Sci.: Nano, 2020, 7, 1289–1304 RSC.
- B. Y. Li and Y. Zou, Adv. Mater., 2008, 20, 2952–2958 CrossRef.
- B.-P. Jiang, L. Zhang, Y. Zhu, X.-C. Shen, S.-C. Ji, X.-Y. Tan, L. Cheng and H. Liang, J. Mater. Chem. B, 2015, 3, 3767–3776 RSC.
- Q. Zou, J. Huang and X. Zhang, Small, 2018, 14, 1803101 CrossRef PubMed.
- S. Huang, Q. He, S. Xu and L. Wang, Anal. Chem., 2015, 87, 5451–5456 CrossRef CAS PubMed.
- M. Gizdavic-nikolaidis, J. Travas-sejdic, G. A. Bowmaker, R. P. Cooney and P. A. Kilmartin, Synth. Met., 2004, 140, 225–232 CrossRef CAS.
- H. Sun, F. Lv, L. Liu, Q. Gu and S. Wang, Adv. Ther., 2018, 1, 1800057 CrossRef.
- J. Yang, J. Choi, D. Bang, E. Kim, E.-K. Lim, H. Park, J.-S. Suh, K. Lee, K.-H. Yoo, E.-K. Kim, Y.-M. Huh and S. Haam, Angew. Chem., Int. Ed., 2011, 50, 441–444 CrossRef CAS PubMed.
- Z. Zha, X. Yue, Q. Ren and Z. Dai, Adv. Mater., 2013, 25, 777–782 CrossRef CAS PubMed.
- K. Turcheniuk, T. Dumych, R. Bilyy, V. Turcheniuk, J. Bouckaert, V. Vovk, V. Chopyak, V. Zaitsev, P. Mariot, N. Prevarskaya, R. Boukherroub and S. Szunerits, RSC Adv., 2016, 6, 1600–1610 RSC.
- H. Chen, M. C. Roco and J. Son, J. Nanopart. Res., 2013, 15, 1951 CrossRef.
- B. Henderson-Sellers, Q. J. R. Meteorol. Soc., 1984, 110, 1186–1190 CrossRef.
- X. Wang, G. Ou, N. Wang and H. Wu, ACS Appl. Mater. Interfaces, 2016, 8, 9194–9199 CrossRef CAS PubMed.
- Z. Hong, J. Pei, Y. Wang, B. Cao, M. Mao, H. Liu, H. Jiang, Q. An, X. Liu and X. Hu, Energy Convers. Manage., 2019, 199, 112019 CrossRef CAS.
- A. Iqbal, M. S. Mahmoud, E. T. Sayed, K. Elsaid, M. A. Abdelkareem, H. Alawadhi and A. G. Olabi, J. Environ. Manage., 2021, 277, 111415 CrossRef CAS PubMed.
- Z. Li, W. Cai, X. Wang, Y. Hu and Z. Gui, J. Colloid Interface Sci., 2021, 582, 496–505 CrossRef CAS PubMed.
- Y. Zeng, J. Yao, B. A. Horri, K. Wang, Y. Wu, D. Li and H. Wang, Energy Environ. Sci., 2011, 4, 4074–4078 RSC.
- X. Wang, Y. He, X. Liu, L. Shi and J. Zhu, Sol. Energy, 2017, 157, 35–46 CrossRef CAS.
- M. Gao, C. K. Peh, H. T. Phan, L. Zhu and G. W. Ho, Adv. Energy Mater., 2018, 8, 1800711 CrossRef.
- Z. Gan, Z. Chen, L. Liu, L. Zhang, W. Tu and Y. Liu, Sol. RRL, 2017, 1, 1600032 CrossRef.
- A. H. Elsheikh, S. W. Sharshir, M. Kamal, A. Ali and J. Shaibo, Sol. Energy, 2019, 177, 561–575 CrossRef CAS.
- G. Ni, S. H. Zandavi, S. M. Javid, S. V. Boriskina, T. A. Cooper and G. Chen, Energy Environ. Sci., 2018, 11, 1510–1519 RSC.
- R. Enright, N. Miljkovic, J. L. Alvarado, K. Kim and J. W. Rose, Nanoscale Microscale Thermophys. Eng., 2014, 18, 223–250 CrossRef.
- R. N. Wenzel, Ind. Eng. Chem., 1936, 28, 988–994 CrossRef CAS.
- A. B. D. Cassie and S. Baxter, Trans. Faraday Soc., 1944, 40, 546–551 RSC.
- A. Aili, Q. Ge and T. Zhang, J. Heat Transfer, 2017, 139, 112401–112411 CrossRef.
- E. Curcio, X. Ji, G. Di Profio, A. O. Sulaiman, E. Fontananova and E. Drioli, J. Membr. Sci., 2010, 346, 263–269 CrossRef CAS.
- S. Cao, P. Rathi, X. Wu, D. Ghim, Y.-S. Jun and S. Singamaneni, Adv. Mater., 2020, 2000922 Search PubMed.
- F. E. Ahmed, R. Hashaikeh and N. Hilal, Desalination, 2019, 453, 54–76 CrossRef CAS.
- A. Amri, Z. T. Jiang, T. Pryor, C.-Y. Yin and S. Djordjevic, Renewable Sustainable Energy Rev., 2014, 36, 316–328 CrossRef CAS.
- X. Wang, Y. He, X. Liu, G. Cheng and J. Zhu, Appl. Energy, 2017, 195, 414–425 CrossRef CAS.
- J. Chen, J. Feng, Z. Li, P. Xu, X. Wang, W. Yin, M. Wang, X. Ge and Y. Yin, Nano Lett., 2019, 19, 400–407 CrossRef CAS PubMed.
- L. Yi, S. Ci, S. Luo, P. Shao, Y. Hou and Z. Wen, Nano Energy, 2017, 41, 600–608 CrossRef CAS.
- X. Wang, Y. He, X. Liu and J. Zhu, Powder Technol., 2017, 321, 276–285 CrossRef CAS.
- Y. Wang, C. Wang, X. Song, S. K. Megarajan and H. Jiang, J. Mater. Chem. A, 2018, 6, 963–971 RSC.
- X. Han, W. Wang, K. Zuo, L. Chen, L. Yuan, J. Liang, Q. Li, P. M. Ajayan, Y. Zhao and J. Lou, Nano Energy, 2019, 60, 567–575 CrossRef CAS.
- Y. Shi, R. Li, Y. Jin, S. Zhuo, L. Shi, J. Chang, S. Hong, K.-C. Ng and P. Wang, Joule, 2018, 2, 1171–1186 CrossRef CAS.
- Y. Wang, C. Wang, X. Song, M. Huang, S. K. Megarajan, S. F. Shaukat and H. Jiang, J. Mater. Chem. A, 2018, 6, 9874–9881 RSC.
- S. Hong, Y. Shi, R. Li, C. Zhang, Y. Jin and P. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 28517–28524 CrossRef CAS PubMed.
- X. Gao, H. Ren, J. Zhou, R. Du, C. Yin, R. Liu, H. Peng, L. Tong, Z. Liu and J. Zhang, Chem. Mater., 2017, 29, 5777–5781 CrossRef CAS.
- H. Sun, X. Li, J. Chen, H. Zhu, H. Miao, Y. Li, X. Liu and G. Shi, Soft Matter, 2021, 17, 4730–4737 RSC.
- M. Keshavarz Hedayati and M. Elbahri, Materials, 2016, 9, 497 CrossRef PubMed.
- Refractive index of polymers by index, https://scipoly.com/technical-library/refractive-index-of-polymers-by-index.
- E. Curcio and E. Drioli, Sep. Purif. Rev., 2005, 34, 35–86 CrossRef CAS.
- X. Wu, Q. Jiang, D. Ghim, S. Singamaneni and Y.-S. Jun, J. Mater. Chem. A, 2018, 6, 18799–18807 RSC.
- Y. Z. Tan, E. H. Ang and J. W. Chew, J. Membr. Sci., 2019, 572, 171–183 CrossRef CAS.
- Renewable Resource Data Center, Reference solar spectral irradiance: ASTM G-173, 2009.
- W. Wien and O. Lummer, Ann. Phys., 1895, 292, 451–456 CrossRef.
- J. Y. Lu, S. H. Nam, K. Wilke, A. Raza, Y. E. Lee, A. AlGhaferi, N. X. Fang and T. Zhang, Adv. Opt. Mater., 2016, 4, 1255–1264 CrossRef CAS.
- K.-T. Lin, H. Lin, T. Yang and B. Jia, Nat. Commun., 2020, 11, 1389 CrossRef CAS PubMed.
- G. Ni, G. Li, S. V. Boriskina, H. Li, W. Yang, T. Zhang and G. Chen, Nat. Energy, 2016, 1, 16126 CrossRef CAS.
- X. Li, R. Lin, G. Ni, N. Xu, X. Hu, B. Zhu, G. Lv, J. Li, S. Zhu and J. Zhu, Natl. Sci. Rev., 2017, 5, 70–77 CrossRef.
- K. Pietrak and T. S. Winiewski, J. Power Technol, 2014, 95, 14–24 Search PubMed.
- Y. Fu, J. Hansson, Y. Liu, S. Chen, A. Zehri, M. K. Samani, N. Wang, Y. Ni, Y. Zhang, Z.-B. Zhang, Q. Wang, M. Li, H. Lu, M. Sledzinska, C. M. S. Torres, S. Volz, A. A. Balandin, X. Xu and J. Liu, 2D Mater., 2019, 7, 12001 CrossRef.
- M. Seyhan, C. L. Altan, B. Gurten and S. Bucak, AIP Adv., 2017, 7, 45101 CrossRef.
- G. Langer, J. Hartmann and M. Reichling, Rev. Sci. Instrum., 1997, 68, 1510–1513 CrossRef CAS.
- B. Kumanek and D. Janas, J. Mater. Sci., 2019, 54, 7397–7427 CrossRef CAS.
- R. Yan, J. R. Simpson, S. Bertolazzi, J. Brivio, M. Watson, X. Wu, A. Kis, T. Luo, A. R. Hight Walker and H. G. Xing, ACS Nano, 2014, 8, 986–993 CrossRef CAS PubMed.
- Y. Li, T. Zhang, Y. Qin, T. Day, G. Jeffrey Snyder, X. Shi and L. Chen, J. Appl. Phys., 2014, 116, 203705 CrossRef.
- B. A. Lunn, J. Unsworth, N. G. Booth and P. C. Innis, J. Mater. Sci., 1993, 28, 5092–5098 CrossRef CAS.
- G. Wang, Y. Fu, X. Ma, W. Pi, D. Liu and X. Wang, Carbon, 2017, 114, 117–124 CrossRef CAS.
- G. Wang, Y. Fu, A. Guo, T. Mei, J. Wang, J. Li and X. Wang, Chem. Mater., 2017, 29, 5629–5635 CrossRef CAS.
- F. Jiang, H. Liu, Y. Li, Y. Kuang, X. Xu, C. Chen, H. Huang, C. Jia, X. Zhao, E. Hitz, Y. Zhou, R. Yang, L. Cui and L. Hu, ACS Appl. Mater. Interfaces, 2018, 10, 1104–1112 CrossRef CAS PubMed.
- Q. Chen, Z. Pei, Y. Xu, Z. Li, Y. Yang, Y. Wei and Y. Ji, Chem. Sci., 2018, 9, 623–628 RSC.
- X. Wu, M. E. Robson, J. L. Phelps, J. S. Tan, B. Shao, G. Owens and H. Xu, Nano Energy, 2019, 56, 708–715 CrossRef CAS.
- X. Li, W. Xu, M. Tang, L. Zhou, B. Zhu, S. Zhu and J. Zhu, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, 13953–13958 CrossRef CAS PubMed.
- F. Domínguez-Muñoz, B. Anderson, J. M. Cejudo-López and A. Carrillo-Andrés, Energy Build., 2010, 42, 2159–2168 CrossRef.
- X. Lin, M. Yang, W. Hong, D. Yu and X. Chen, Front. Mater., 2018, 5, 74 CrossRef.
- N. N. Hamadneh, W. S. Khan and W. A. Khan, J. King Saud Univ. Sci., 2019, 31, 618–627 CrossRef.
- X. Hu, W. Xu, L. Zhou, Y. Tan, Y. Wang, S. Zhu and J. Zhu, Adv. Mater., 2017, 29, 1604031 CrossRef PubMed.
- A. Politano, P. Argurio, G. Di Profio, V. Sanna, A. Cupolillo, S. Chakraborty, H. A. Arafat and E. Curcio, Adv. Mater., 2017, 29, 1603504 CrossRef PubMed.
- Z. Hua, B. Li, L. Li, X. Yin, K. Chen and W. Wang, J. Phys. Chem. C, 2017, 121, 60–69 CrossRef CAS.
- A. H. Avci, S. Santoro, A. Politano, M. Propato, M. Micieli, M. Aquino, Z. Wenjuan and E. Curcio, Chem. Eng. Process. – Process Intensif., 2021, 164, 108382 CrossRef CAS.
- T. Li, Q. Fang, H. Lin and F. Liu, J. Mater. Chem. A, 2019, 7, 17505–17515 RSC.
- J. Zhao, Y. Yang, C. Yang, Y. Tian, Y. Han, J. Liu, X. Yin and W. Que, J. Mater. Chem. A, 2018, 6, 16196–16204 RSC.
- A. P. Bell, J. A. Fairfield, E. K. McCarthy, S. Mills, J. J. Boland, G. Baffou and D. McCloskey, ACS Nano, 2015, 9, 5551–5558 CrossRef CAS PubMed.
- P. B. Roder, B. E. Smith, E. J. Davis and P. J. Pauzauskie, J. Phys. Chem. C, 2014, 118, 1407–1416 CrossRef CAS.
- G. Baffou, R. Quidant and C. Girard, Appl. Phys. Lett., 2009, 94, 153109 CrossRef.
- T. Ma, C. Yang, W. Guo, H. Lin, F. Zhang, H. Liu, L. Zhao, Y. Zhang, Y. Wang, Y. Cui, J. Zhao and F. Qu, ACS Appl. Mater. Interfaces, 2020, 12, 27140–27149 CrossRef CAS PubMed.
- Y. Chang, Z. Wang, Y. Shi, X. Ma, L. Ma, Y. Zhang and J. Zhan, J. Mater. Chem. A, 2018, 6, 10939–10946 RSC.
- T. Gao, Y. Li, C. Chen, Z. Yang, Y. Kuang, C. Jia, J. Song, E. M. Hitz, B. Liu, H. Huang, J. Yu, B. Yang and L. Hu, Small Methods, 2019, 3, 1800176 CrossRef.
- X. Wang, Y. He and X. Liu, Energy Convers. Manage., 2018, 173, 158–166 CrossRef CAS.
- W. Xu, X. Hu, S. Zhuang, Y. Wang, X. Li, L. Zhou, S. Zhu and J. Zhu, Adv. Energy Mater., 2018, 8, 1702884 CrossRef.
- C. Wang, Y. Wang, X. Song, M. Huang and H. Jiang, Adv. Sustainable Syst., 2019, 3, 1800108 CrossRef.
- H.-H. Yu, L.-J. Yan, Y.-C. Shen, S.-Y. Chen, H.-N. Li, J. Yang and Z.-K. Xu, Research, 2020, 2020, 3241758 CrossRef CAS PubMed.
- I. A. Said, S. Wang and Q. Li, Ind. Eng. Chem. Res., 2019, 58, 18829–18835 CrossRef CAS.
- P. D. Dongare, A. Alabastri, S. Pedersen, K. R. Zodrow, N. J. Hogan, O. Neumann, J. Wu, T. Wang, A. Deshmukh, M. Elimelech, Q. Li, P. Nordlander and N. J. Halas, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 6936–6941 CrossRef CAS PubMed.
- W. Li, Y. Chen, L. Yao, X. Ren, Y. Li and L. Deng, Desalination, 2020, 478, 114288 CrossRef CAS.
- J. Huang, Y. Hu, Y. Bai, Y. He and J. Zhu, Desalination, 2020, 489, 114529 CrossRef CAS.
- Y. Z. Tan, H. Wang, L. Han, M. B. Tanis-Kanbur, M. V. Pranav and J. W. Chew, J. Membr. Sci., 2018, 565, 254–265 CrossRef CAS.
- M. U. Farid, J. A. Kharraz and A. K. An, ACS Appl. Mater. Interfaces, 2021, 13, 3805–3815 CrossRef CAS PubMed.
- Y. Peng, Y. Wang, W. Li and J. Jin, J. Mater. Chem. A, 2021, 9, 10678–10684 RSC.
- W. Li, L. Deng, H. Huang, J. Zhou, Y. Liao, L. Qiu, H. Yang and L. Yao, ACS Appl. Mater. Interfaces, 2021, 13, 26861–26869 CrossRef CAS PubMed.
- S. Cao, X. Wu, Y. Zhu, R. Gupta, A. Tan, Z. Wang, Y.-S. Jun and S. Singamaneni, J. Mater. Chem. A, 2020, 8, 5147–5156 RSC.
- Q. Huang, S. Gao, Y. Huang, M. Zhang and C. Xiao, J. Membr. Sci., 2019, 582, 203–210 CrossRef CAS.
- J. Liu, H. Guo, Z. Sun and B. Li, J. Membr. Sci., 2021, 634, 119434 CrossRef CAS.
- Y. Zhang, K. Li, L. Liu, K. Wang, J. Xiang, D. Hou and J. Wang, Chemosphere, 2020, 256, 127053 CrossRef CAS PubMed.
- D. Ghim, X. Wu, M. Suazo and Y.-S. Jun, Nano Energy, 2021, 80, 105444 CrossRef CAS.
- Y.-S. Jun, X. Wu, D. Ghim, Q. Jiang, S. Cao and S. Singamaneni, Acc. Chem. Res., 2019, 52, 1215–1225 CrossRef CAS PubMed.
- X. Wu, S. Cao, D. Ghim, Q. Jiang, S. Singamaneni and Y.-S. Jun, Nano Energy, 2021, 79, 105353 CrossRef CAS.
- J. Wu, K. R. Zodrow, P. B. Szemraj and Q. Li, J. Mater. Chem. A, 2017, 5, 23712–23719 RSC.
- M. Drobek, A. Figoli, S. Santoro, N. Navascués, J. Motuzas, S. Simone, C. Algieri, N. Gaeta, L. Querze, A. Trotta, G. Barbieri, R. Mallada, A. Julbe and E. Drioli, Microporous Mesoporous Mater., 2015, 207, 126–133 CrossRef CAS.
- M. R. Esfahani, S. A. Aktij, Z. Dabaghian, M. D. Firouzjaei, A. Rahimpour, J. Eke, I. C. Escobar, M. Abolhassani, L. F. Greenlee, A. R. Esfahani, A. Sadmani and N. Koutahzadeh, Sep. Purif. Technol., 2019, 213, 465–499 CrossRef CAS.
- A. Politano, G. Di Profio, E. Fontananova, V. Sanna, A. Cupolillo and E. Curcio, Desalination, 2019, 451, 192–199 CrossRef CAS.
- G. Guan, R. Wang, F. Wicaksana, X. Yang and A. G. Fane, Ind. Eng. Chem. Res., 2012, 51, 13405–13413 CrossRef CAS.
- Y. Liao, R. Wang, M. Tian, C. Qiu and A. G. Fane, J. Membr. Sci., 2013, 425–426, 30–39 CrossRef CAS.
- X. Wu, Q. Jiang, D. Ghim, S. Singamaneni and Y. S. Jun, J. Mater. Chem. A, 2018, 6, 18799–18807 RSC.
- X. Huang, Y.-H. Yu, O. L. de Llergo, S. M. Marquez and Z. Cheng, RSC Adv., 2017, 7, 9495–9499 RSC.
- M. Wu, S. Ding, L. Deng and X. Wang, Sep. Purif. Technol., 2022, 281, 119995 CrossRef CAS.
- S. K. Das, Chem. Eng. Sci., 2019, 203, 293–301 CrossRef CAS.
- I.-L. Ngo, S. Jeon and C. Byon, Int. J. Heat Mass Transf., 2016, 98, 219–226 CrossRef CAS.
- D. M. Warsinger, J. Swaminathan, E. Guillen-Burrieza, H. A. Arafat and J. H. Lienhard V, Desalination, 2015, 356, 294–313 CrossRef CAS.
- M. Gryta, Desalination, 2007, 216, 88–102 CrossRef CAS.
- H. W. Warsinger, C. Martin, D. Swaminathan, J. Chung, D. M. Jeong, S. Lienhard, J. H. Martin Warsinger and D. Warsinger, Int. Desalin. Assoc., 2015, 0–14 Search PubMed.
- M. Gryta, M. Tomaszewska, J. Grzechulska and A. W. Morawski, J. Membr. Sci., 2001, 181, 279–287 CrossRef CAS.
- M. Khayet, A. Velázquez and J. I. Mengual, J. Membr. Sci., 2004, 240, 123–128 CrossRef CAS.
- Y.-F. Yang, Y. Li, Q.-L. Li, L.-S. Wan and Z.-K. Xu, J. Membr. Sci., 2010, 362, 255–264 CrossRef CAS.
- M. Krivorot, A. Kushmaro, Y. Oren and J. Gilron, J. Membr. Sci., 2011, 376, 15–24 CrossRef CAS.
- L. D. Tijing, Y. C. Woo, J. S. Choi, S. Lee, S. H. Kim and H. K. Shon, J. Membr. Sci., 2015, 475, 215–244 CrossRef CAS.
- T. Young, Philos. Trans. R. Soc. Lond., 1805, 95, 65–87 CrossRef.
- C. J. Van Oss, M. K. Chaudhury and R. J. Good, Chem. Rev., 1988, 88, 927–941 CrossRef CAS.
- Z. Wang, Y. Chen, F. Zhang and S. Lin, Desalination, 2019, 450, 46–53 CrossRef CAS.
- M. R. Choudhury, N. Anwar, D. Jassby and M. S. Rahaman, Adv. Colloid Interface Sci., 2019, 269, 370–399 CrossRef CAS PubMed.
- E. Guillen-Burrieza, M. O. Mavukkandy, M. R. Bilad and H. A. Arafat, J. Membr. Sci., 2016, 515, 163–174 CrossRef CAS.
- S. O. L. and, W. J. Lau, P. S. Goh and A. F. Ismail, Membr. Water Treat., 2014, 5, 14–29 Search PubMed.
- J. Kavitha, M. Rajalakshmi, A. R. Phani and M. Padaki, J. Water Process Eng., 2019, 32, 100926 CrossRef.
- F. Qu, Z. Yan, H. Yu, G. Fan, H. Pang, H. Rong and J. He, Desalination, 2020, 486, 114493 CrossRef CAS.
- F. He, K. K. Sirkar and J. Gilron, J. Membr. Sci., 2009, 345, 53–58 CrossRef CAS.
- J. Beltrán-Heredia, J. Sánchez-Martín and M. Barrado-Moreno, Chem. Eng. J., 2012, 180, 128–136 CrossRef.
- M. A. Aboulhassan, S. Souabi, A. Yaacoubi and M. Baudu, Int. J. Environ. Sci. Technol., 2006, 3, 327–332 CrossRef CAS.
- T. Horseman, Y. Yin, K. S. S. Christie, Z. Wang, T. Tong and S. Lin, ACS ES&T Eng, 2021, 1, 117–140 Search PubMed.
- B. Bhushan and Y. C. Jung, Prog. Mater. Sci., 2011, 56, 1–108 CrossRef CAS.
- J.-G. Lee, E.-J. Lee, S. Jeong, J. Guo, A. K. An, H. Guo, J. Kim, T. Leiknes and N. Ghaffour, J. Membr. Sci., 2017, 526, 395–408 CrossRef CAS.
- X. Fan, Y. Liu, X. Quan, H. Zhao, S. Chen, G. Yi and L. Du, J. Membr. Sci., 2016, 514, 501–509 CrossRef CAS.
- Y. C. Woo, L. D. Tijing, W.-G. Shim, J.-S. Choi, S.-H. Kim, T. He, E. Drioli and H. K. Shon, J. Membr. Sci., 2016, 520, 99–110 CrossRef CAS.
- R. Moradi, J. Karimi-Sabet, M. Shariaty-Niassar and M. A. Koochaki, Polymers, 2015, 7, 1444–1463 CrossRef CAS.
- N. A. Zakaria, S. Q. Zaliman, C. P. Leo, A. L. Ahmad, B. S. Ooi and P. E. Poh, J. Environ. Chem. Eng., 2022, 10, 107346 CrossRef CAS.
- S. S. Ray, M. Gandhi, S.-S. Chen, H.-M. Chang, C. T. N. Dan and H. Q. Le, Environ. Sci.: Water Res. Technol., 2018, 4, 1612–1623 RSC.
- M. Baghbanzadeh, D. Rana, C. Q. Lan and T. Matsuura, Sep. Purif. Technol., 2016, 157, 60–71 CrossRef CAS.
- Z.-Q. Dong, X.-H. Ma, Z.-L. Xu and Z.-Y. Gu, RSC Adv., 2015, 5, 67962–67970 RSC.
- J. Li, L.-F. Ren, H. S. Zhou, J. Yang, J. Shao and Y. He, J. Membr. Sci., 2021, 620, 118924 CrossRef CAS.
- M. Wang, G. Liu, H. Yu, S.-H. Lee, L. Wang, J. Zheng, T. Wang, Y. Yun and J. K. Lee, ACS Appl. Mater. Interfaces, 2018, 10, 13452–13461 CrossRef CAS PubMed.
- R. Mardosaitė, A. Jurkeviči
![[u with combining overline]](https://www.rsc.org/images/entities/char_0075_0305.gif) tė and S. Račkauskas, Cryst. Growth Des., 2021, 21, 4765–4779 CrossRef.
tė and S. Račkauskas, Cryst. Growth Des., 2021, 21, 4765–4779 CrossRef. - R. Huang, Z. Liu, Y. C. Woo, W. Luo, S. R. Gray and M. Xie, Environ. Sci.: Water Res. Technol., 2020, 6, 1786–1793 RSC.
- Z. Xiao, R. Zheng, Y. Liu, H. He, X. Yuan, Y. Ji, D. Li, H. Yin, Y. Zhang, X.-M. Li and T. He, Water Res., 2019, 155, 152–161 CrossRef CAS PubMed.
- J. A. Kharraz and A. K. An, J. Membr. Sci., 2020, 595, 117596 CrossRef CAS.
- X. Liao, P. Dai, Y. Wang, X. Zhang, Y. Liao, X. You and A. G. Razaqpur, J. Membr. Sci., 2022, 650, 120423 CrossRef CAS.
- B. Gong, H. Yang, S. Wu, J. Yan, K. Cen, Z. Bo and K. K. Ostrikov, ACS Sustainable Chem. Eng., 2019, 7, 20151–20158 CrossRef CAS.
- W. Zhang, Y. Lu, J. Liu, X. Li, B. Li and S. Wang, J. Membr. Sci., 2020, 595, 117563 CrossRef CAS.
- S. Lin, S. Nejati, C. Boo, Y. Hu, C. O. Osuji and M. Elimelech, Environ. Sci. Technol. Lett., 2014, 1, 443–447 CrossRef CAS.
- B. J. Deka, J. Guo, N. K. Khanzada and A. K. An, Water Res., 2019, 165, 114982 CrossRef CAS PubMed.
- L.-H. Chen, A. Huang, Y.-R. Chen, C.-H. Chen, C.-C. Hsu, F.-Y. Tsai and K.-L. Tung, Desalination, 2018, 428, 255–263 CrossRef CAS.
- Y.-R. Chen, R. Xin, X. Huang, K. Zuo, K.-L. Tung and Q. Li, J. Membr. Sci., 2021, 620, 118913 CrossRef CAS.
- S. Zhang, J. Chen, J. Zheng, X. Chen, H. Xu, F. I. T. Petrescu, L. M. Ungureanu, Y. Li and G. Shi, Nanomaterials, 2022, 12, 859 CrossRef CAS PubMed.
- P. Vijaya Kumar, S. Mary Jelastin Kala and K. S. Prakash, Mater. Lett., 2019, 236, 19–22 CrossRef CAS.
- S. Cao, Q. Jiang, X. Wu, D. Ghim, H. Gholami Derami, P.-I. Chou, Y.-S. Jun and S. Singamaneni, J. Mater. Chem. A, 2019, 7, 24092–24123 RSC.
- S. Santoro, P. Timpano, A. H. Avci, P. Argurio, F. Chidichimo, M. De Biase, S. Straface and E. Curcio, Desalination, 2021, 520, 115378 CrossRef CAS.
- D. J. Johnson and N. Hilal, Desalination, 2021, 500, 114852 CrossRef CAS.
- Q. Ma, Z. Xu and R. Wang, Water Res., 2021, 198, 117154 CrossRef CAS PubMed.
- R. Fillet, V. Nicolas, V. Fierro and A. Celzard, Sol. Energy Mater. Sol. Cells, 2021, 219, 110814 CrossRef CAS.
- Y. Liu, K. Ai and L. Lu, Chem. Rev., 2014, 114, 5057–5115 CrossRef CAS PubMed.
- S. Sun, B. Sun, Y. Wang, M. Fordjour Antwi-Afari, H.-Y. Mi, Z. Guo, C. Liu and C. Shen, Sep. Purif. Technol., 2021, 278, 119621 CrossRef.
- Z. Wang, H.-C. Yang, F. He, S. Peng, Y. Li, L. Shao and S. B. Darling, Matter, 2019, 1, 115–155 CrossRef.
- I. R. Salmón and P. Luis, Chem. Eng. Process. – Process Intensif., 2018, 123, 258–271 CrossRef.
- E. Drioli, G. Di Profio and E. Curcio, Membrane-Assisted Crystallization Technology, Imperial College Press, 2015 Search PubMed.
- E. Curcio, X. Ji, A. M. Quazi, S. Barghi, G. Di Profio, E. Fontananova, T. Macleod and E. Drioli, J. Membr. Sci., 2010, 360, 493–498 CrossRef CAS.
- C. A. Quist-Jensen, A. Ali, S. Mondal, F. Macedonio and E. Drioli, J. Membr. Sci., 2016, 505, 167–173 CrossRef CAS.
- C. Finnerty, L. Zhang, D. L. Sedlak, K. L. Nelson and B. Mi, Environ. Sci. Technol., 2017, 51, 11701–11709 CrossRef CAS PubMed.
- X. Li, J. Li, J. Lu, N. Xu, C. Chen, X. Min, B. Zhu, H. Li, L. Zhou, S. Zhu, T. Zhang and J. Zhu, Joule, 2018, 2, 1331–1338 CrossRef CAS.
- M. Gao, C. K. Peh, L. Zhu, G. Yilmaz and G. W. Ho, Adv. Energy Mater., 2020, 10, 2000925 CrossRef CAS.
- A. H. Avci, C. Van Goethem, T. Rijnaarts, S. Santoro, M. Aquino, G. Di Profio, I. F. J. Vankelecom, W. M. De Vos, E. Fontananova and E. Curcio, Mol. Psychiatry, 2021, 26, 265 CrossRef CAS PubMed.
- J. H. Jhaveri and Z. V. P. Murthy, Desalination, 2016, 379, 137–154 CrossRef CAS.
- A. Nguyen, L. Zou and C. Priest, J. Membr. Sci., 2014, 454, 264–271 CrossRef CAS.
- J. R. Ray, S. Tadepalli, S. Z. Nergiz, K.-K. Liu, L. You, Y. Tang, S. Singamaneni and Y.-S. Jun, ACS Appl. Mater. Interfaces, 2015, 7, 11117–11126 CrossRef CAS PubMed.
- Q. Jiang, D. Ghim, S. Cao, S. Tadepalli, K.-K. Liu, H. Kwon, J. Luan, Y. Min, Y.-S. Jun and S. Singamaneni, Environ. Sci. Technol., 2019, 53, 412–421 CrossRef CAS PubMed.
- A. Criscuoli, J. Zhong, A. Figoli, M. C. Carnevale, R. Huang and E. Drioli, Water Res., 2008, 42, 5031–5037 CrossRef CAS PubMed.
- S. Mozia, A. W. Morawski, M. Toyoda and T. Tsumura, Desalination, 2010, 250, 666–672 CrossRef CAS.
- Y. Shi, J. Huang, G. Zeng, W. Cheng and J. Hu, J. Membr. Sci., 2019, 584, 364–392 CrossRef CAS.
- X. Li, J. Yu, S. Wageh, A. A. Al-Ghamdi and J. Xie, Small, 2016, 12, 6640–6696 CrossRef CAS PubMed.
- Z. Gan, X. Wu, M. Meng, X. Zhu, L. Yang and P. K. Chu, ACS Nano, 2014, 8, 9304–9310 CrossRef CAS PubMed.
- X. Wang, Y. He, Y. Hu, G. Jin, B. Jiang and Y. Huang, Sol. Energy, 2018, 170, 586–593 CrossRef CAS.
- I. A. Said, T. R. Chomiak, Z. He and Q. Li, Sep. Purif. Technol., 2020, 250, 117170 CrossRef CAS.
- Y. Li, T. Verbiest, R. Strobbe and I. F. J. Vankelecom, J. Mater. Chem. A, 2013, 1, 15031–15038 RSC.
| This journal is © The Royal Society of Chemistry 2022 |