Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A computational study of direct CO2 hydrogenation to methanol on Pd surfaces†
Igor
Kowalec
 a,
Lara
Kabalan
a,
Lara
Kabalan
 a,
C. Richard A.
Catlow
a,
C. Richard A.
Catlow
 abc and
Andrew J.
Logsdail
abc and
Andrew J.
Logsdail
 *a
*a
aCardiff Catalysis Institute, School of Chemistry, Cardiff University, Cardiff, CF10 3AT, UK. E-mail: LogsdailA@cardiff.ac.uk
bUK Catalysis Hub, Research Complex at Harwell, RAL, Oxford, OX11 0FA, UK
cDepartment of Chemistry, University College London, London, WC1H 0AJ, UK
First published on 21st March 2022
Abstract
The reaction mechanism of direct CO2 hydrogenation to methanol is investigated in detail on Pd (111), (100) and (110) surfaces using density functional theory (DFT), supporting investigations into emergent Pd-based catalysts. Hydrogen adsorption and surface mobility are firstly considered, with high-coordination surface sites having the largest adsorption energy and being connected by diffusion channels with low energy barriers. Surface chemisorption of CO2, forming a partially charged CO2δ−, is weakly endothermic on a Pd (111) whilst slightly exothermic on Pd (100) and (110), with adsorption enthalpies of 0.09, −0.09 and −0.19 eV, respectively; the low stability of CO2δ− on the Pd (111) surface is attributed to negative charge accumulating on the surface Pd atoms that interact directly with the CO2δ− adsorbate. Detailed consideration for sequential hydrogenation of the CO2 shows that HCOOH hydrogenation to H2COOH would be the rate determining step in the conversion to methanol, for all surfaces, with activation barriers of 1.41, 1.51, and 0.84 eV on Pd (111), (100) and (110) facets, respectively. The Pd (110) surface exhibits overall lower activation energies than the most studied Pd (111) and (100) surfaces, and therefore should be considered in more detail in future Pd catalytic studies.
1. Introduction
Methanol synthesis by direct hydrogenation of CO2 has been recognised as a potential route towards sustainable fuels for transport and a circular fuel economy.1 The industrial synthesis of methanol involves syngas, which is a mixture of CO/CO2/H2 commonly produced from coal gasification. Whilst methanol synthesis from fossil fuels is efficient and profitable, environmental pressures are urging the chemical industry to transfer from a linearoil economy to net zero emissions by 2050.2 Bussche et al. built a steady-state kinetic model that revealed CO2, and not CO, is likely to be the main source of carbon in methanol synthesised from syngas;2–4 such knowledge encourages consideration of direct CO2 hydrogenation to methanol, using anthropogenic CO2 from the atmosphere. However, a better understanding of the interaction of CO2 with transition metal catalysts is required for the design of novel and effective catalytic systems. Many factors, such as the source of H2, affect the extent to which the process of direct methanol synthesis from CO2 can be “green”; however, the idea of using an atmospheric pollutant such as CO2 for fuel synthesis, and/or also generating feedstock for further synthesis of chemical compounds, such as formic acid, is broadly appealing.5A crucial step in the direct hydrogenation of CO2 to methanol is the initial CO2 activation. On a heterogeneous catalyst, the reverse water-gas shift (RWGS) reaction needs to be inhibited while maintaining a strong interaction between CO2 and the catalytic surface.5,6 Pd alone exhibits poor selectivity to methanol for direct CO2 hydrogenation, but the selectivity is greatly enhanced when it is alloyed with other transition metals, such as Zn.5,7–11 In order to understand fully the Pd-based alloy reactivity, it is necessary first to know the nature of the interactions between CO2 and Pd. The available experimental data for the interaction of CO2 with Pd facets are limited, but computation using density functional theory (DFT) is providing insight into the processes.12–15 Burghaus et al. reported that CO2 reactivity on clean Pd surfaces is weak, not favouring dissociation to CO unless an alkali metal species is coadsorbed.15 The weak interaction is considered to be predominantly a van der Waals physisorption, based on the theoretical and experimental observations at the Pd (111) surface.14,16,17 CO2 adsorption on Pd has been studied in the context of the RWGS reaction and utilisation of syngas, and desorption of CO2 from the Pd (111) surface is reported as requiring 0.26 eV of energy.14 Solymosi et al. reported that CO2 desorption from the Pd (100) surface also has a relatively low energy of 0.35 eV, which was, in contrast, associated with a chemisorption, involving a metal to empty CO2 π* orbital electron transfer.12 Evidence of CO2 chemisorption on Pd (110) in the presence of water was also reported by Brosseau et al.18 Therefore, the character of the CO2 interaction with Pd surfaces seems to depend on the surface structure. The differing adsorption energies can be correlated with surface energies, given physisorption was exclusively observed on the lowest energy (111) surface, and experimental evidence of chemisorption was observed for CO2 on the higher energy Pd (100) and (110) surfaces, though the latter is noted as being in the presence of water.12–14,18
Complementary to these observations, the rate of catalytic hydrogenation of CO2 on Pd increases greatly when the active species is paired with suitable metal oxide supports, such as TiO2 and ZnO, as they facilitate CO2 adsorption and activation.9,19,20 Ko et al. computed the adsorption of CO2 on transition metal surfaces, using the dispersion-corrected PBE-D2 density functional, and reported two types of CO2 adsorption on Pd (111): an exothermic physisorption (−0.33 eV) of undistorted CO2, parallel to the surface; and a less exothermic chemisorption (−0.18 eV) with CO2 in a bent geometry, and having a partial negative charge.21 In contrast, Zhang et al. recently calculated the CO2 chemisorption on Pd (111) to be endothermic (0.06 eV) using the PBE density functional, in agreement with Habas et al., who reported the adsorption energy of CO2 to be 0.22 eV above the dissociation limit, using DFT with the B3LYP density functional.22 Liu et al. have also shown that, when using the PBE density functional, the inclusion of the DFT-D2 correction dramatically changes the adsorption energy of chemisorbed species on the Pd (111) surface, from 0.30 eV to −0.18 eV.17 Although there is no consensus on the matter of the endo- or exothermicity of CO2 chemisorption on Pd surfaces, the reported values are generally small, which agrees with the experimental reports of a weak interaction between CO2 and Pd surfaces.
Direct CO2 hydrogenation to methanol is proposed to proceed via a surface formate intermediate (HCOO*, where * indicates an adsorbed species), with Medford et al. having shown that HCOO* could act as a poison for other reaction pathways due to its high thermodynamic stability on the catalyst surface.23 Variations of the mechanism proposed by Grabow, which progresses via formic acid (HCOOH) as shown in Fig. 1, have been presented, such as an initial Eley–Rideal type mechanism on Cu-based catalytic systems, where CO2 in the gas phase reacts with surface-bound hydrogen to yield formate.24 Recently, Huš et al. concluded that dioxymethylene (H2COO*) should be preferably considered over formic acid (HCOOH*) on Cu-based catalysts as the former is more strongly bound to the metallic surface and the activation energy towards hydroxymethoxy (H2COOH*) is lower.25
 | ||
| Fig. 1 Formate pathway of direct CO2 hydrogenation to methanol on metallic surfaces, as proposed by Grabow et al. (via HCOOH*, blue) and Huš et al. (via H2COO*, orange). * indicates a surface-bound species and δ-indicates that CO2 is partially charged (i.e. activated).25,26 | ||
Pd-based catalysts supported on ZnO are potent alternative catalysts for this reaction, with their reactivity attributed to the Pd-Zn binary metallic phases and their stabilisation of the HCOO* intermediate, similar to the Cu-based catalyst.9,20 Zhang et al. have reported DFT studies of an alternative CO2 formate mechanism that involves dissociation of HCOOH to HCO and OH, and subsequent hydrogenations of HCO to produce CH3OH.27 Furthermore, Brix et al. have recently considered the initial CO2 hydrogenation on Pd (111) to proceed via carboxylic acid (COOH), instead of formate, in a DFT study using the dispersion-corrected PBE-D3 density functional, and they observed a high energy barrier of 2.23 eV for CO2 hydrogenation to formate on Pd (111), in contrast to the barrier of 0.85 eV reported by Zhang et al.23,24
Whilst binary metallic alloy catalysts may offer more desirable selectivity, stability, and tunability than their monometallic counterparts, the lack of basic understanding of the behaviour of monometallic materials hinders the design of emergent multi-component materials. To achieve the required insight in the context of CO2 hydrogenation over Pd, we need to understand reactivity across all the prominent surface facets. Thus, we present here an in-depth investigation of CO2 interaction with low energy Pd (111), (110) and (100) surfaces using DFT calculations, followed by investigation of the direct CO2 hydrogenation to CH3OH, via the Grabow mechanism, on the Pd (111), (110) and (100) surfaces, in the context of rationalising CO2 reactivity on Pd-based catalysts.
2. Methodology
The Fritz Haber Institute ab initio molecular simulations (FHI-aims) software package has been used for full potential all-electron DFT calculations, with the Pythonic Atomic Simulation Environment (ASE) used for management of calculation geometries.28,29 The default convergence criteria within FHI-aims for self-consistent field (SCF) calculations were used, i.e. the changes between the current and previous SCF iterations in charge density, sum of eigenvalues, and total energy were below N × 1.67 × 10−5 e a0−3, 10−3 eV, and 10−6 eV, respectively, where N is the number of atoms in the model. Scalar relativistic treatment of kinetic energy for all elements was achieved by the atomic zero-order regular approximation (ZORA), and a Gaussian-type broadening with width of 0.01 eV was applied to the occupation of electronic states. The Perdew–Burke–Ernzerhof exchange correlation (XC) density functional has been used unless explicitly stated otherwise, paired with the Tkatchenko-Scheffler van der Waals dispersion correction (PBE + vdW). A default “light” basis set (version: 2010) has been used for geometry optimisations, providing structural accuracy;28,30,31 energy calculations were then performed with a “tight” basis set (version: 2010) on the optimised geometries, providing greater electronic accuracy and mitigation of basis set superposition error.28 For geometry optimisations, convergence was deemed complete when forces on all unconstrained atoms were less than 0.01 eV Å−1.Due to the closed-shell electronic configuration of Pd ([Kr] 4d10), spin-paired calculations were used in periodic calculations; gas-phase adsorbate structures were calculated both spin-paired and spin-unpaired, and the energy of the more stable configuration considered for reference in subsequent periodic calculations. The effect of the spin-paired approximation has been assessed towards the activation energies in relevant surface hydrogenation reactions in Section S3 of the ESI,† with a spin-paired treatment shown to introduce small error bars of ±0.05 eV.
2.1 Bulk models
For sampling the Brillouin zone of face-centred cubic (FCC) Pd in a primitive unit cell, a (9 × 9 × 9) Monkhorst–Pack k-grid provides converged accuracy, as detailed in Section S1 of the ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 The lattice constant (a0 = 3.91 Å), bulk modulus (B0 = 183.37 GPa), and cohesive energy (Ecoh = 4.00 eV) calculated for bulk FCC Pd match closely with the experimental observations of 3.88 Å, 180.40 GPa, and 3.89 eV, respectively.33,34
32 The lattice constant (a0 = 3.91 Å), bulk modulus (B0 = 183.37 GPa), and cohesive energy (Ecoh = 4.00 eV) calculated for bulk FCC Pd match closely with the experimental observations of 3.88 Å, 180.40 GPa, and 3.89 eV, respectively.33,34
2.2 Surface models
Using the optimised model of bulk FCC Pd, a surface supercell was created with dimensions of (3 × 3 × n), where n is the number of atomic layers in the z-direction perpendicular to the material surface. The x- and y-dimensions were chosen such that the adsorbates are significantly separated (7.5 Å), and a vacuum layer of 40 Å was added in the z-direction. The k-grid sampling was reduced appropriately for altered cell dimensions, with a k-grid of (3 × 3 × 1) applied. Due to the one-sided nature of the slab models considered, a dipole-correction was used in all calculations.The energy penalty for breaking chemical bonds at the surface of a material (Ecleave) is calculated as:
 | (1) |
To calculate the surface energy (Esurf), the energy of stabilisation provided by geometry relaxation (Erelax) needs to be obtained from the difference in total DFT energy of the optimised slab (ERelaxedSlab) and EUnrelaxedSlab:
 | (2) |
In summary, accurate results have been achieved herein with a 3 × 3 × 5 supercell surface model, with the bottom two layers constrained to their bulk positions and the three top surface layers unconstrained. The surface energies (Esurf) can subsequently be calculated as follows:
| Esurf = Ecleave + Erelax. | (3) |
| Ref. | XC | E surf/(J m−2) | ||
|---|---|---|---|---|
| Pd (111) | Pd (100) | Pd (110) | ||
| This work | PBE + vdW | 1.72 | 1.91 | 1.99 |
| Methfessel et al.35 | LDA | 1.64 | 1.86 | 1.97 |
| Vitos et al.36 | GGA | 1.92 | 2.33 | 2.23 |
| Patra et al.37 | LDA | 1.88 | 2.43 | 2.25 |
| PBE | 1.36 | 1.79 | 1.61 | |
| PBEsol | 1.63 | 2.15 | 1.93 | |
| SCAN | 1.54 | 2.03 | 1.83 | |
| SCAN + rVV10 | 1.77 | 2.29 | 2.05 | |
| Singh-Miller et al.38 | PBE | 1.31 | 1.49 | 1.55 |
| Da Silva et al.39 | LDA | 1.87 | — | — |
| PBE | 1.33 | — | — | |
| Skriver et al.40 | LDA | 1.88 | — | — |
| Tyson et al.41 | Experiment | 2.00 | — | — |
| Boer et al.42 | Experiment | 2.01 | — | — |
2.3 Surface adsorption
For catalytic surface reactions, the adsorption energy (Eads) measures the interaction between a surface and reactant, and is deduced from comparison of the energies of the optimised gas-phase adsorbate (EA), optimised surface (ES) and the combined system (EA–S).| Eads = EA–S − (EA + ES). | (4) |
| EBSSE = [EA(A–S) − EA(A)] + [ES(A–S) − ES(S)]. | (5) |
A more negative EBSSE indicates a greater overbinding error; however, by subtracting EBSSE from Eads, the counterpoise corrected adsorption energy can be established (ECPads) as:
| ECPads = Eads − EBSSE. | (6) |
With the “light” basis set, EBSSE is −0.08 eV for CO2 on Pd (111), but EBSSE was reduced to −0.02 eV with the “tight” basis set. Considering the low BSSE with the “tight” basis, which is used subsequently throughout this work, the EBSSE contribution to Eads was deemed negligible and was not subsequently calculated for species other than CO2.
2.4 Transition state structures
For kinetic studies, we have used a machine learning nudged elastic band (MLNEB) method to identify saddle points and minimum energy paths (MEPs).44,45 A spring constant of 0.05 eV Å−1 has been used throughout; the convergence criterion of forces on all unconstrained atoms of below 0.05 eV Å−1, with energy uncertainty below 0.03 eV, was deemed sufficiently accurate for CO2 adsorption. Comparison with a more stringent force criterion of 0.01 eV Å−1 altered the activation energy for CO2 adsorption on FCC (111) surface by 5 meV only (Section S2, ESI†).2.5 Vibrations and Thermodynamics
Vibration frequency calculations have been performed for structures in the reaction pathway using the ASE Vibrations module and the Frederiksen method.46 To reduce the computational cost, the number of vibrating Pd atoms in the structures was tested as a function of distance from the adsorbate, from the nearest Pd neighbours outwards. The vibrational energy converged at first Pd neighbours of the adsorbate, and this approximation has been applied throughout. The vibration frequencies were also calculated for transition state structures, and the saddle points were confirmed by the presence of an imaginary frequency vibration. Due to the use of a finite difference approach, occasional additional imaginary frequencies did occur due to numerical noise, but were always below 10i meV and thus did not impact on the results. Free energies of gas components were calculated using an ideal gas-phase approximation, whereas the energies of periodic surface structures with adsorbates were calculated using a harmonic approximation. Once the zero-point energy (ZPE) was established, the contribution of ZPE was added to Eads yielding enthalpy of adsorption, Hads.3. Results and discussions
3.1 Hydrogen adsorption
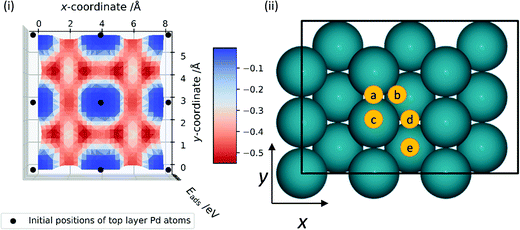
Prior to investigating the reaction steps in CO2 hydrogenation, an understanding of hydrogen behaviour on Pd is crucial as the interaction of H with CO2 is integral to the reaction profile. Thus, a survey was conducted of Eads for H, Eads(H), on the Pd surfaces; the H atom was positioned at various locations on the surface and optimised, with constraints in the xy-plane. Eads(H) on the Pd (111), (100), and (110) surfaces was calculated with respect to the gas-phase diatomic hydrogen molecule and is plotted across the xy-plane in Fig. 3(i), 4(i), and 5(i), respectively. The ZPE-correction was not applied in these scans and all energies presented in this section are based on differences in total electronic energy. The most stable adsorption site for H on the Pd (111) surface is the HCP hollow position, which is site B in Fig. 3(ii), with Eads(H) of −0.67 eV; similar stability over high coordination sites is observed on the (100) surface, where the hollow site (Fig. 4(ii), site C) has lower energy with Eads(H) of −0.54 eV, and the “FCC” site (Fig. 5(ii), site B) is of lower energy in the case of the (110) surface, with Eads(H) of −0.56 eV. The least stable adsorption site for H on all surfaces is atop, with Eads(H) of −0.12, −0.08 and 0.00 eV on the (111), (100) and (110) Pd surfaces, respectively. The typical reactant feed for CO2 hydrogenation is between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 1
3 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 molar ratio of CO2 and H2, and thus dissociated hydrogen would be readily available on the catalyst surface.9,20 High hydrogen mobility can be deduced from Fig. 3(i), 4(i) and 5(i), as differences in favourable Eads are low along specific channels, highlighted in red. The adsorption energies for H on the Pd (111) surface (−0.67 eV) and the (110) surface (−0.56 eV) compare reasonably with the experimental work of Conrad et al., who report initial heats of adsorption for ½H2 of 0.45 and 0.53 eV for Pd (111) and (110) surfaces, respectively.47 There is also good agreement with previous theoretical research, as Herron et al. calculated atomic hydrogen adsorption energies on Pd (111) using PW91 and reported them to be −0.59, −0.56, and 0.00 eV on FCC hollow, HCP hollow, and atop positions, respectively.48 Similarly, Fonseca et al. used PBE in their DFT study of hydrogen adsorption on Pd (111) and observed −0.66, −0.61, and −0.50 eV adsorption energies of hydrogen atom on FCC hollow, HCP hollow, and bridge position, respectively.49
9 molar ratio of CO2 and H2, and thus dissociated hydrogen would be readily available on the catalyst surface.9,20 High hydrogen mobility can be deduced from Fig. 3(i), 4(i) and 5(i), as differences in favourable Eads are low along specific channels, highlighted in red. The adsorption energies for H on the Pd (111) surface (−0.67 eV) and the (110) surface (−0.56 eV) compare reasonably with the experimental work of Conrad et al., who report initial heats of adsorption for ½H2 of 0.45 and 0.53 eV for Pd (111) and (110) surfaces, respectively.47 There is also good agreement with previous theoretical research, as Herron et al. calculated atomic hydrogen adsorption energies on Pd (111) using PW91 and reported them to be −0.59, −0.56, and 0.00 eV on FCC hollow, HCP hollow, and atop positions, respectively.48 Similarly, Fonseca et al. used PBE in their DFT study of hydrogen adsorption on Pd (111) and observed −0.66, −0.61, and −0.50 eV adsorption energies of hydrogen atom on FCC hollow, HCP hollow, and bridge position, respectively.49
3.2 CO2 adsorption
The adsorption energies and structures for CO2 on the Pd (111), (100) and (110) surfaces are reported in Table 2. The undistorted CO2 is most stable with a C–Pd bond distance, d(C–Pd), of 3.454 Å, which agrees with the physisorbed species observed by Habas et al.22Hads(CO2) is strongest on the close-packed (111) surface, and is found to relate linearly with the number of Pd atoms that neighbour the surface adsorption site; when Hads(CO2) is plotted as a function of surface atom coordination number, which are 9, 8, and 7 for the Pd (111), (100), and (110) surfaces, respectively, a linear fit returns R2 = 0.998.| Species | Pd surface | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 111 | 100 | 110 | |||||||
| H ads | d (C–Pd1) | ∠O–C–O | H ads | d (C–Pd1) | ∠O–C–O | H ads | d (C–Pd1) | ∠O–C–O | |
| CO2 | −0.21 | 3.45 | 179.5 | −0.18 | 3.28 | 179.1 | −0.16 | 3.26 | 179.2 |
| CO2TS | 0.12 | 2.37 | 154.8 | 0.00 | 2.45 | 160.6 | No energy barrier | ||
| CO2δ− | 0.09 | 2.10 | 140.3 | −0.09 | 2.06 | 140.6 | −0.19 | 2.06 | 140.2 |
The stronger physisorption, rather than chemisorption, observed for CO2 on the Pd (111) surface (Eads(CO2) = −0.21 eV) was reported previously by Ko et al.22 (−0.33 eV); they also identify a chemisorbed state CO2δ− with Eads = −0.16 eV,17 which compares with our observation of Hads(CO2δ−) = 0.09 eV. Similarly, Huš et al. observed that on Cu catalysts, CO2 binds to the metal surface in a bent geometry, where one of the oxygens binds to a secondary metal atom and the carbon binds to a metal atom underneath.25 Higham et al. observed an endothermic CO2 chemisorption on Cu (100) and (110) surfaces, similarly to our result on Pd (111).50 Energy differences between our results and those of Ko et al. are probably due to the choice of van der Waals correction;21 never-the-less, the observed trends are very similar, and the stability of the physisorbed CO2 implies that there is an energy barrier on the Pd (111) surface for the activation of CO2.
H ads(CO2δ−) is endothermic (0.09 eV) on the Pd (111) surface, matching the work of Zhang et al.,27 and exothermic (−0.09 and −0.19 eV) on the Pd (100) and (110) surfaces, respectively.27 Reduction of the size of the model surface, such that 1/4 monolayer (ML) coverage of CO2 is achieved on Pd (111), (100) and (110) surfaces, results in Hads(CO2δ−) of 0.12 eV, −0.03 eV and −0.16 eV, respectively. The higher (less favourable) Hads(CO2δ−) values for 1/4 ML coverage, when compared to the 1/9 ML coverage presented in Table 2, are intuitively linked to unfavourable interactions between neighbouring adsorbates. Hads(CO2δ−) is noted as increasingly negative (i.e. strengthens) with increasing Esurf for the Pd facets, and the energy difference between surface-bound CO2 and CO2δ− also decreases; these observations agree with experimental data that show an absence of chemisorption on the Pd (111) surface, and both physisorption and chemisorption on the Pd (100) surface.12–14 Despite differences in Hads(CO2δ−) on the surfaces examined, the adsorbed geometries of CO2 and CO2δ− are consistent across all surfaces (Table 2); only a small difference in angles (0.4°) is calculated for either the physisorbed or chemisorbed geometries when compared across the three facets. The impact of steric interactions for adsorbed CO2 can be quantified via the distortion energy, i.e., the gas-phase energy of the adsorbed bent CO2 geometry relative to the preferred linear CO2 configuration, which is 1.35, 1.33 and 1.33 eV for Pd (111), Pd (110), and (100) facets, respectively. Given that the overall adsorption energies are exothermic on Pd (100) and (110) surface facets, it can be concluded that the binding energy between surface Pd atoms and the CO2 must be significant to negate the distortion energy arising from the unfavourable bent CO2 geometry.
Mulliken charge analysis of the CO2 and CO2δ− species adsorbed on the Pd (111), (110), and (100) facets provides insight into the electronic charge of the surface species, and the data acquired are reported in Table 3. The notation used for describing charges on atoms of interest is shown in Fig. 6: O1 and O2 are oxygen atoms on CO2 molecule; the two closest Pd atoms interacting with CO2 are labelled Pd1 and Pd2, where Pd1 is closest to O1 and Pd2 is closest to O2; and Pdsurf, Pdsublayer, and Pdslab refer to the first, second and all layers of Pd atoms in the model, respectively.
| Pristine surfaces | CO2 | CO2δ− | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pd (111) | Pd (110) | Pd (100) | Gas | Pd (111) | Pd (110) | Pd (100) | Pd (111) | Pd (110) | Pd (100) | |
| q c | — | — | — | +0.48 | +0.47 | +0.45 | +0.44 | +0.39 | +0.38 | +0.38 |
| q O 1 | — | — | — | −0.24 | −0.22 | −0.22 | −0.22 | −0.19 | −0.26 | −0.23 |
| q O 2 | — | — | — | −0.24 | −0.23 | −0.22 | −0.22 | −0.23 | −0.24 | −0.25 |
| q Pd 1 | — | — | — | — | −0.02 | −0.05 | −0.05 | −0.32 | −0.10 | −0.15 |
| q Pd 2 | — | — | — | — | −0.01 | −0.02 | 0.00 | −0.30 | +0.04 | −0.07 |
| q Pdsublayer | +0.03 | +0.02 | +0.02 | — | +0.02 | +0.05 | 0.00 | −0.02 | +0.03 | 0.00 |
| q Pdsurf | −0.03 | −0.03 | −0.02 | — | 0.00 | −0.01 | 0.00 | −0.07 | 0.00 | −0.02 |
| q Pdslab | 0.00 | 0.00 | 0.00 | — | −0.02 | −0.01 | 0.00 | +0.04 | +0.11 | +0.10 |
For CO2 physisorption on the Pd (111) surface (Fig. 7a), the charge of the carbon (qC) is +0.47 e, very similar to the gas phase CO2 (qC = +0.48 e), and only small changes are observed on the surface Pd. For CO2δ− on the Pd (111) surface (Fig. 7b), negatively charged Pd atoms bond to an oxygen and carbon (qPd1 = −0.30 e, qPd2 = −0.32 e). The distance d(C–Pd2) is 2.85 Å, and there is a direct electronic interaction between Pd2 and the carbon atom of CO2. The average charge on the second layer of Pd atoms, qPdsublayer, decreases from +0.03 to +0.02 e upon physisorption and decreases further to −0.02 e upon chemisorption. The average charge on the first surface atomic layer of Pd, qPdsurf, is −0.03 e, 0.00 e and −0.07 e for pristine Pd (111) surface, Pd (111) slab with CO2, and Pd (111) slab with CO2δ−, respectively, suggesting that the electron density has been pulled to the first two layers of Pd, and to the CO2δ− adsorbate via Pd1 and Pd2. qC has decreased from +0.47 e to +0.39 e, indicating some metal (Pd1) to empty CO2 π* orbital electron transfer.12 The negatively charged oxygen close to the negative qPd1 and qPd2 will result in electrostatic repulsion, and thus are likely to contribute in the decreased stability of CO2δ− on the Pd (111) surface.22,51
 | ||
| Fig. 7 A red-white-blue (negative-neutral-positive charges) color-coded visualisation of the net Mulliken charge on atoms for (a) CO2 physisorbed and (b) CO2δ− chemisorbed on the Pd (111) surface. | ||
In contrast, for CO2δ− on Pd (110) the qC reduction is by 0.07 e upon chemisorption, with higher electron density on the oxygens and much less charge redistribution on the surface Pd atoms compared to the Pd (111) surface (qPd1 of −0.10 e and −0.30 e, respectively), which all contribute to the overall stability (i.e. lower Hads). For CO2δ− on a Pd (100) facet, the charges calculated are intermediary between the results on the Pd (111) and (110) surfaces, and Hads also falls between the values observed for Pd (111) and (110) surfaces.
The overall charge transfer from the metal to CO2δ− is −0.04, −0.10 and −0.11 e on Pd (111), (100), and (110) surfaces, respectively, which is small but correlates directly with adsorption strength. In the previous literature, Bader charge analysis has been considered for CO2 chemisorption on Pd (111) surfaces, and the transfer to CO2δ− reported as −0.28 and −0.43 e by Tang et al. and Habas et al., respectively;22,52 the direction of charge transfer is consistent with our own observations, with the quantitative difference attributed to methodological differences, i.e. Mulliken charge analysis has a stronger basis set dependency than Bader analysis.53,54 Importantly, we show qualitatively that the charge transfer to CO2δ− increases over Pd (111), (100), and (110) surfaces, indicating that Pd (100) and (110) surfaces are more suitable for CO2 activation than the most stable Pd (111) surface.
3.3 Interactions of intermediates with Pd surfaces.
Reaction intermediates from the Grabow mechanism, as introduced in Section 1.2, have been optimised on the pristine Pd (111), (100) and (110) surfaces, in each case starting from an atop position, which ensured that adsorbates were starting at a proximity allowing metal-adsorbate bond formation during the geometry optimisation process. For example, in the case of CO2, the chemisorbed species could easily be missed starting from the gas phase due to an energy barrier for the chemisorption of CO2 on Pd (111) and (100) surfaces. The calculated values of Hads are presented in Fig. 8 and tabulated in Section S4 of the ESI.† | ||
| Fig. 8 H ads of the intermediates in the direct CO2 hydrogenation to methanol, as studied on the low-index Pd surfaces, presented in order of increasing Esurf: (111), (100) and (110),26 in blue, orange and grey, respectively. Error bars of ±0.05 eV are provided to account for the spin-paired approximation applied to the adsorbed species, as described in Section 2.3. | ||
For the intermediates considered, the average difference between the highest and lowest Hads across the three surfaces is 0.22 eV; the smallest difference is for the CO2 molecule (0.05 eV), and the largest for H2CO, H2COOH, and CO2δ− (0.36, 0.33, and 0.29 eV, respectively). Plotting the surface energy (Esurf) of the low-index Pd surfaces against the adsorption enthalpy (Hads) of these intermediates on the corresponding surfaces (Fig. 9) illustrates where surface properties associate with these observations. In particular, Hads of CO2, CO2δ−, H2COOH, and H2CO present clear linear correlations with the stability of the surface facets, giving R2 of 0.988, 0.997, 0.987, and 1.000, respectively. HCOO, HCOOH, H3CO, and CH3OH give a poor linear fit, which indicates that other factors, such as steric effects, should be considered for rationalising the strength of these adsorbate interactions with the Pd surfaces. For example, due to additional space on the long-bridge site on the Pd (110) surface, the HCOOH can be accommodated in a different orientation from that on the Pd (111) and (100) surfaces (i.e. C–H atoms facing down, rather than up), which makes the resulting structures more difficult to compare directly.
3.4 Transition states and reaction profile
To gain further insight into reaction mechanisms, activation energies were calculated. Here, for each reaction step that involved a hydrogenation, it was necessary to set the transition state (TS) starting geometry such that a hydrogen atom was positioned near to the intermediate; optimisation of these starting models with proximal hydrogen in some instances led to instability of the intermediate adsorption structures, which, however, did not cause problems when directly applied to the TS calculations. Once a transition state structure was confirmed, the ZPE-corrected activation energy (Hact) was calculated for each reaction step as:| Hact = HTS (species) − Hads (species). | (7) |
| H act(CO2δ−) | 2.5H2 + H* + CO2*chem → TS1 + 2.5H2 | H act(H2COOH) | 1.5H2 + H2COOH* → TS4 + 1.5H2 |
| H act(HCOO) | 2H2 + HCOO* + H* → TS2 + 2H2 | H act(H2CO) | 0.5H2 + H2CO* + H* + H2O → TS5 + 0.5H2 + H2O |
| H act(HCOOH) | 1.5H2 + HCOOH* + H* → TS3 + 1.5H2 | H act(H3CO) | CH3O* + H* + H2O → TS6 + H2O |
The activation energy for CO2δ− hydrogenation, Hact(CO2δ−), is 1.13 eV, 1.10 eV and 0.81 eV on the Pd (111), (100) and (110) surfaces, respectively. The observation that Hact(CO2δ−) is lowest on the Pd (110) surface can be attributed to the additional space underneath the CO2δ− on the preferred long-bridge site, which facilitates the hydrogen atom binding to the carbon. The Hact(CO2δ−) on the Pd (111) surface (1.13 eV) matches the work of Zhang et al. (0.85 eV), though differs somewhat from the results of Brix et al. (2.23 eV); we believe that this difference stems from the use of a physisorbed CO2 geometry in their calculations, with a chemisorbed structure considered in our work and the calculations by Zhang et al.27,55
H act(HCOOH) is observed to follow the trend (100) > (111) > (110), i.e. different from the Esurf trend. The Hact(HCOOH) of 1.41 eV evaluated for the most commonly studied Pd (111) surface is larger than the 1.13 eV reported by Brix et al. Given that the adsorption energy of HCOOH on the Pd (111) surface is calculated as −0.58 eV, and desorption is considered as the reverse process, the high Hact(HCOOH) observed (1.41 eV) for the Pd (111) surface suggests that HCOOH is more likely to desorb than react further. The high activation barrier for HCOOH hydrogenation agrees with work by Huš et al. on Cu-based catalysts; however, formic acid is not amongst the product stream observed when using Pd catalysts experimentally, with CH3OH, CO and trace to significant amounts of CH4 reported.5,9,20,25,56 Thus, another intermediate, such as H2COO, might be of importance in leading to the experimental products, as was determined for Cu-based catalysts.25 In our work, the Hact(HCOOH) on the Pd (110) surface is about 40% lower than on Pd (111) and almost 45% lower than on the Pd (100). The reduction of Hact(HCOOH) might stem from lower stability of the HCOOH, and reduced stability of the hydrogen atom on Pd (110), which translates into a more accessible transition state.
The activation enthalpy for dissociation of H2COOH species, Hact(H2COOH), is highest on the Pd (100) surface, where the H2COOH intermediate is stabilised. Brix et al. reported a high Hact(H2COOH) of 2.01 eV on Pd (111), while we calculate Hact(H2COOH) to be only 0.40 eV; the significant discrepancy of 1.61 eV arises from a considerable difference in the transition state geometry, i.e., our transition state involves breaking of a single C–O bond, whereas both C–O bonds were broken in the transition state found by Brix et al. For hydrogenation of formaldehyde, Hact(H2CO) is similarly low (0.67–0.74 eV) on the three surfaces; however, on the (111) surface it is higher than Hads of H2CO (−0.58 eV), whilst on Pd (100) and (110) surfaces, H2CO is stabilised more (−0.83 and −0.94 eV) than on Pd (111). The stronger Hads on (100) and (110) surfaces means that H2CO desorption is less likely, and reactivity favoured, whilst desorption would be a competitive process on the (111) surface. Desorption of H2CO during CO2 hydrogenation to methanol on Pd catalysts is a major concern in experiment,5 and thus the Pd (100) and (110) surfaces may be preferable in catalyst design.
In most hydrogenation steps examined on the three Pd surfaces, the reaction pathway favoured migration of the hydrogen atom towards the least stable on-top site before bonding to the intermediate. Therefore, the relative stability of the hydrogen adsorption sites, as shown in Section 3.1, has a major impact on the Hact for most hydrogenation reactions on the Pd (111), (100) and (110) surfaces. Reducing the difference in stability for hydrogen atoms on the possible surface sites might be an important factor in the design of catalysts for CO2 hydrogenation to methanol, as it could lead to reduction of Hact for species reacting on a Pd-based catalyst.
A reaction profile based on the energy of initial, TS and final geometries, relative to the energy of isolated Pd (111), (100) and (110) surfaces and gas-phase reactants, is plotted in Fig. 11, with each individual step balanced stoichiometrically by energies of gas-phase molecules.
 | ||
| Fig. 11 The ZPE-corrected energy profile of CO2 hydrogenation to methanol, via the formate pathway, on Pd (111), (100), and (110) surfaces, plotted in blue, orange, and grey, respectively, relative to the energy of pristine surface and gas phase reactants.26 Energies of intermediate structures and transition state geometries have been stoichiometrically balanced with energies of gas phase reactants; * indicates surface bound species. | ||
Based on total electronic energy for all the surfaces, which is presented in the ESI,† the reaction energy for the conversion of CO2 to methanol is exothermic (−1.05 eV) relative to gas phase reactants, which agrees with reaction energy (−1.17 eV) derived from atomization energies.5,57,58 The gas-phase reaction enthalpy presented above (−0.26 eV) is underestimated by 0.26 eV with respect to experimental values reported in literature,5 and the magnitude of the error in gas-phase energies of molecules is typical for gradient corrected functionals, such as PBE.59 The highest Hact in the CO2 hydrogenation reaction across the Pd (100), (111) and (110) surfaces is Hact(HCOOH), with values of 1.51, 1.41, and 0.84 eV, respectively, which is therefore a likely rate determining step (RDS) for the reaction. However, in an experimental study by Aas et al., the decomposition of HCOOH on Pd (110) was shown to require 0.42 eV, which is only 50% of the Hact(HCOOH) on Pd (110), and therefore much more likely.60 An important feature of the reaction energy profile is that TS1 remains endothermic on all three surfaces, with respect to the gas phase reactants, which would inevitably influence the rate of the reaction. All transition states on Pd (110) remain either below net zero energy of the reaction or significantly lower than Pd (111) and Pd (100) when above, which indicates that Pd (110) is the most active among the surfaces investigated here. As highlighted in Section 3.1, the hydrogen atoms are stabilised strongly on the Pd (111), (100), and (110) surfaces; the binding energies of intermediates with a neighbouring hydrogen atom do not vary significantly from the sum of binding energies of the adsorbates calculated separately, which suggests that the presence of hydrogen neither stabilises nor destabilises the intermediates at the low 1/9 ML coverage of hydrogen considered.26 However, presence of a hydrogen atom at the nearest neighbouring site to chemisorbed CO2 was observed to result in CO2 desorption during geometry optimisation, to form a linear physisorbed species, which may indicate a lower stability of chemisorbed CO2 with increasing hydrogen ML coverage. Experimentally, the presence of H2 appears to induce a larger CO2 intake both at increased temperature and/or pressure, but this phenomenon has been linked to CO2 dissociation.61
3.5 Gibbs free energy analysis
Gibbs free energy changes (ΔG, eV) between the reaction steps a–v in Table 5 across Pd (111), (110) and (100) were calculated and are shown in Fig. 12 and 13. The pressure (p) used was 1 atm (1013125 Pa), which was applied to gas components. The temperatures (T) considered were 0 K (i.e., enthalpy), 300 K for ambient conditions, and 500 K as typical experimental conditions for CO2 hydrogenation over Pd catalysts.20 Graphs of the effect of temperature are shown for Pd (111) in Fig. 12, with other surfaces presented in Section S7 in the ESI.† A comparison of results for 500 K presented in Fig. 13.| (a) 3H2 + CO2*phys → TS0 + 3H2 | (b) 3H2 + TS0 → CO2*chem + 3H2 |
| (c) 3H2 + CO2*chem → CO2*chem+ H* +2.5H2 | (d) 2.5H2 + H* + CO2*chem → TS1 + 2.5H2 |
| (e) 2.5H2 + TS1 → HCOO* + 2.5H2 | (f) 2.5H2 + HCOO* → HCOO* + H* + 2H2 |
| (g) 2H2 + HCOO* + H* → TS2 + H2 | (h) 2H2 + TS2 → HCOOH* +2H2 |
| (i) 2H2 + HCOOH* → HCOOH* + H* + 1.5H2 | (j) 1.5H2 + HCOOH* + H* → TS3 + 1.5H2 |
| (k) 1.5H2 + TS3 → H2COOH* + 1.5H2 | (l) 1.5H2 + H2COOH* → H2COOH*rotated + 1.5H2 |
| (m) 1.5H2 + H2COOH*rotated → TS4 + 1.5H2 | (n) 1.5H2 + TS4 → H2CO* + OH* + 1.5H2 |
| (o) 1.5H2 + H2CO* + OH* → H2CO* + H2O* +H2 | (p) H2 + H2CO* + H2O* → H2CO* + H2 + H2O |
| (q) H2 +H2CO* + H2O → H2CO* + H* + 0.5 H2 + H2O | (r) 0.5H2 + H2CO* + H* + H2O → TS5 + 0.5H2 + H2O |
| (s) 0.5H2 + TS5 + H2O → CH3O* + 0.5H2 + H2O | (t) CH3O* + 0.5H2 + H2O → CH3O* + H* + H2O |
| (u) CH3O* + H* + H2O → TS6 + H2O | (v) H2O + TS6 → CH3OH* + H2O |
 | ||
| Fig. 12 The Gibbs free energy changes between reaction steps in CO2 hydrogenation reaction via formate on Pd (111) at p of 1 atm and T of 0 K, 300 K and 500 K; reaction steps a–v are explained in Table 5. | ||
 | ||
| Fig. 13 The Gibbs free energy changes between reaction steps in CO2 hydrogenation reaction via formate on Pd (111), (110), and (100) at p of 1 atm and T of 500 K; reaction steps a–v are explained in Table 5. | ||
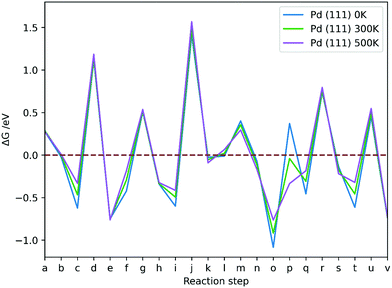
As T increases (Fig. 12), formation of species on Pd (111) from respective TS structure shows more negative (favourable) ΔG for formation of H2COOH (k), H2CO* (n) and H3CO* (s), and less negative for CO2δ− (b) and HCOOH* (h), while HCOO* (e) and CH3OH* (v) are not significantly affected. On Pd (100), formation of species from respective TS structure shows similar trends to Pd (111) with an increase of T, but formation of H2CO* (n) and CH3OH* (v) is increasingly more favourable also. On Pd (110), elevated T facilitates formation of CO2δ− (b), HCOO* (e), H2COOH (k) and CH3OH* (v), but formation of HCOOH* (h) shows a ΔG increase, while H2CO (n) and H3CO* (s) are not significantly affected. Overall, the changes are subtle and are most prominent for processes involving H adsorption, which becomes less favourable as T increases, and for H2O desorption, which is more favourable as T increases.
Large positive ΔG is observed for all reaction steps involving breaking of Pd–H bonds and attaching of the hydrogen to the adsorbates, i.e. formation of TS1 (d), TS2 (g), TS3 (j), TS5 (r) and TS6 (u), which indicates that the very strong Pd–H interaction at 1/9 ML hydrogen coverage impedes the reaction on Pd (111), (100) and (110) surfaces even at the first hydrogenation step to formate.
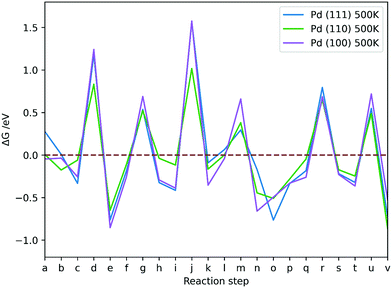
The Pd (110) surface has the lowest ΔG values for TS formations, except for dissociation of H2COOH (m), which is more favourable on the Pd (111) surface. The ΔG associated with TS formation are not strongly affected by T, which suggests that the flat low-index surfaces of Pd are not the likely source of methanol formation in supported metallic Pd catalysts. The conclusion is in agreement with experiment, showing that pure unsupported Pd does not produce methanol at 463 K and atmospheric pressure.9 Moreover, changing T was shown to have a very limited effect on formation of intermediates in CO2 hydrogenation on Pd catalysts. Adsorbing hydrogen on Pd surfaces is less favourable at higher T, but formation of TS structures remains unaffected, thus rendering the process less feasible at high T; however, elevated T is necessary to activate CO2 on Pd (100), showing that low T CO2 activation is key for CO2 hydrogenation to be kinetically viable.
4. Summary and conclusions
Direct hydrogenation of CO2 to methanol on transition metal catalysts is a promising approach for green energy storage, and new and more efficient catalysts need to be designed to make the technology viable; here, we have investigated the CO2 hydrogenation reaction via the formate pathway on Pd (111), (100), and (110) surfaces to support this endeavour.Firstly, we investigated the stability of H on the Pd surfaces, showing that high coordination sites have the largest adsorption energy, and these high stability sites are interlinked via channels with low diffusion barriers; we also show for CO2 adsorption that the preference of physical or chemical adsorption is dependent on the stability of the Pd surface facet. For the CO2 hydrogenation reaction, the transition state for CO2δ− hydrogenation (TS1), to form formate, is endothermic, which will influence the overall rate of the reaction. Hact(HCOOH) is the highest energy step in the reaction profile on the Pd (111), (100), and (110) surfaces (TS3), and it can be considered as the likely rate determining step of this reaction on the surfaces examined. Based on thermodynamical analysis, the formation of TS1 has a high barrier that is not significantly influenced by reaction conditions, which indicates that flat surfaces of metallic Pd are unlikely to be the source of formate, and subsequently, methanol in product streams of reactions using supported monometallic Pd catalysts. Increased temperature was found to facilitate CO2 chemisorption on Pd (100) and (110), but has an adverse effect on multiple other reaction steps, including the free energy of hydrogen adsorption. Novel Pd-based polymetallic nanoparticle catalysts for direct CO2 hydrogenation to methanol could be designed to lower the barrier to initial CO2 hydrogenation, TS1, and lower the barrier for formic acid hydrogenation (TS3) or facilitate a mechanism that proceeds via an alternative intermediate, such as H2COO. Importantly, a Pd-based CO2 hydrogenation catalyst should have lower Pd-H binding strength to facilitate the reaction.
Overall, we show that the most stable geometry of CO2 adsorbed on Pd surface varies across (111), (100) and (110) facets and future studies should not be limited to consideration of the lowest energy (111) surface facet. Inclusion of zero-point energy has shown the Pd (100) surface to be unsuitable for CO2 hydrogenation, highlighting that consideration of enthalpy is important for accuracy in computational catalysis. Low-index Pd surfaces are therefore unlikely to be the source of methanol formation on supported monometallic Pd catalysts, which indicates the potential importance of low-coordination metallic sites and metal-support interfacial sites. The H2COO intermediate could be alternatively considered as part of the formate pathway, and future work will entail modelling of the reaction with this intermediate also considered on multi-component Pd-based catalytic systems, which have been shown to manifest great selectivity to CH3OH in direct CO2 hydrogenation.20,62–64
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors are grateful for funding by the EPSRC Centre-to Centre Project (Grant reference: EP/S030468/1). IK acknowledges the Cardiff School of Chemistry for a PhD studentship award. We are grateful to Matthias Scheffler, Yuanyuan Zhou, Herzain Rivera, Graham Hutchings, Mike Bowker and David Willock for useful discussions. AJL acknowledges funding by the UKRI Future Leaders Fellowship program (MR/T018372/1). The authors acknowledge computational resources and support from: the Supercomputing Wales project, which is part-funded by the European Regional Development Fund (ERDF) via the Welsh Government; the UK National Supercomputing Services ARCHER and ARCHER2, accessed via membership of the Materials Chemistry Consortium, which is funded by Engineering and Physical Sciences Research Council (EP/L000202/1, EP/R029431/1, EP/T022213/1); and the Isambard UK National Tier-2 HPC Service operated by GW4 and the UK Met Office, and funded by EPSRC (EP/P020224/1).References
- G. Hutchings, M. Davidson, P. Atkins, P. Collier, N. Jackson, A. Morton, M. Muskett, M. Rosseinsky, P. Styring, P. Thornley and C. Williams, Sustainable synthetic carbon based fuels for transport: Policy Briefing, The Royal Society, 2019 Search PubMed.
- S. Roy, A. Cherevotan and S. C. Peter, ACS Energy Lett., 2018, 3, 1938–1966 CrossRef CAS.
- K. M. V. Bussche and G. F. Froment, J. Catal., 1996, 161, 1–10 CrossRef.
- M. Bowker, R. A. Hadden, H. Houghton, J. N. K. Hyland and K. C. Waugh, J. Catal., 1988, 109, 263–273 CrossRef CAS.
- M. Bowker, ChemCatChem, 2019, 11, 4238–4246 CrossRef CAS PubMed.
- J. Ko, B.-K. Kim and J. W. Han, J. Phys. Chem. C, 2016, 120, 3438–3447 CrossRef CAS.
- J. Xu, X. Su, X. Liu, X. Pan, G. Pei, Y. Huang, X. Wang, T. Zhang and H. Geng, Appl. Catal. Gen., 2016, 514, 51–59 CrossRef CAS.
- I. Melián-Cabrera, M. L. Granados and J. L. G. Fierro, J. Catal., 2002, 210, 285–294 CrossRef.
- N. Iwasa, H. Suzuki, M. Terashita, M. Arai and N. Takezawa, Catal. Lett., 2004, 96, 75–78 CrossRef CAS.
- N. Iwasa, T. Mayanagi, N. Ogawa, K. Sakata and N. Takezawa, Catal. Lett., 1998, 54, 119–123 CrossRef CAS.
- R. Manrique, R. Jiménez, J. Rodríguez-Pereira, V. G. Baldovino-Medrano and A. Karelovic, Int. J. Hydrogen Energy, 2019, 44, 16526–16536 CrossRef CAS.
- F. Solymosi and A. Berkó, J. Catal., 1986, 101, 458–472 CrossRef CAS.
- F. Solymosi, J. Mol. Catal., 1991, 65, 337–358 CrossRef CAS.
- T. Matsushima and H. Asada, J. Chem. Phys., 1986, 85, 1658–1668 CrossRef CAS.
- U. Burghaus, Prog. Surf. Sci., 2014, 89, 161–217 CrossRef CAS.
- C.-L. Kao, A. Carlsson and R. J. Madix, Surf. Sci., 2002, 497, 356–372 CrossRef CAS.
- X. Liu, L. Sun and W.-Q. Deng, J. Phys. Chem. C, 2018, 122, 8306–8314 CrossRef CAS.
- R. Brosseau, T. H. Ellis and H. Wang, Chem. Phys. Lett., 1991, 177, 118–122 CrossRef CAS.
- A. Erdöhelyi, M. Pásztor and F. Solymosi, J. Catal., 1986, 98, 166–177 CrossRef.
- H. Bahruji, M. Bowker, G. Hutchings, N. Dimitratos, P. Wells, E. Gibson, W. Jones, C. Brookes, D. Morgan and G. Lalev, J. Catal., 2016, 343, 133–146 CrossRef CAS.
- J. Ko, B.-K. Kim and J. W. Han, J. Phys. Chem. C, 2016, 120, 3438–3447 CrossRef CAS.
- M.-P. Habas, F. Mele, M. Sodupe and F. Illas, Surf. Sci., 1999, 431, 208–219 CrossRef CAS.
- A. J. Medford, J. Sehested, J. Rossmeisl, I. Chorkendorff, F. Studt, J. K. Nørskov and P. G. Moses, J. Catal., 2014, 309, 397–407 CrossRef CAS.
- S. Kattel, P. J. Ramírez, J. G. Chen, J. A. Rodriguez and P. Liu, Science, 2017, 355, 1296–1299 CrossRef CAS PubMed.
- M. Huš, D. Kopač, N. S. Štefančič, D. L. Jurković, V. D. B. C. Dasireddy and B. Likozar, Catal. Sci. Technol., 2017, 7, 5900–5913 RSC.
- L. C. Grabow and M. Mavrikakis, ACS Catal., 2011, 1, 365–384 CrossRef CAS.
- M. Zhang, Y. Wu, M. Dou and Y. Yu, Catal. Lett., 2018, 148, 2935–2944 CrossRef CAS.
- V. Blum, R. Gehrke, F. Hanke, P. Havu, V. Havu, X. Ren, K. Reuter and M. Scheffler, Comput. Phys. Commun., 2009, 180, 2175–2196 CrossRef CAS.
- A. H. Larsen, J. J. Mortensen, J. Blomqvist, I. E. Castelli, R. Christensen, M. Dulak, J. Friis, M. N. Groves, B. Hammer, C. Hargus, E. D. Hermes, P. C. Jennings, P. B. Jensen, J. Kermode, J. R. Kitchin, E. L. Kolsbjerg, J. Kubal, K. Kaasbjerg, S. Lysgaard, J. B. Maronsson, T. Maxson, T. Olsen, L. Pastewka, A. Peterson, C. Rostgaard, J. Schiøtz, O. Schütt, M. Strange, K. S. Thygesen, T. Vegge, L. Vilhelmsen, M. Walter, Z. Zeng and K. W. Jacobsen, J. Phys.: Condens. Matter, 2017, 29, 273002 CrossRef PubMed.
- M. Ernzerhof and G. E. Scuseria, J. Chem. Phys., 1999, 110, 5029–5036 CrossRef CAS.
- A. Tkatchenko and M. Scheffler, Phys. Rev. Lett., 2009, 102, 073005 CrossRef PubMed.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Condens. Matter Mater. Phys., 1976, 13, 5188–5192 CrossRef.
- J. W. Arblaster, Platinum Met. Rev., 2012, 56, 181–189 CrossRef CAS.
- P. Janthon, S. (Andy) Luo, S. M. Kozlov, F. Viñes, J. Limtrakul, D. G. Truhlar and F. Illas, J. Chem. Theory Comput., 2014, 10, 3832–3839 CrossRef CAS PubMed.
- M. Methfessel, D. Hennig and M. Scheffler, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 4816–4829 CrossRef CAS PubMed.
- L. Vitos, A. V. Ruban, H. L. Skriver and J. Kollár, Surf. Sci., 1998, 411, 186–202 CrossRef CAS.
- A. Patra, J. E. Bates, J. Sun and J. P. Perdew, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E9188–E9196 CrossRef CAS PubMed.
- N. E. Singh-Miller and N. Marzari, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 235407 CrossRef.
- J. L. F. Da Silva, C. Stampfl and M. Scheffler, Surf. Sci., 2006, 600, 703–715 CrossRef CAS.
- H. L. Skriver and N. M. Rosengaard, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 46, 7157–7168 CrossRef CAS PubMed.
- W. R. Tyson and W. A. Miller, Surf. Sci., 1977, 62, 267–276 CrossRef CAS.
- F. R. de Boer, W. C. M. Mattens, R. Boom, A. R. Miedema and A. K. Niessen.
- S. S. Xantheas, J. Chem. Phys., 1996, 104, 8821–8824 CrossRef CAS.
- M. H. Hansen, J. A. G. Torres, P. C. Jennings, Z. Wang, J. R. Boes, O. G. Mamun and T. Bligaard, ArXiv190400904 Phys.
- G. Henkelman, B. P. Uberuaga and H. Jónsson, J. Chem. Phys., 2000, 113, 9901–9904 CrossRef CAS.
- T. Frederiksen, M. Paulsson, M. Brandbyge and A.-P. Jauho, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 205413 CrossRef.
- H. Conrad, G. Ertl and E. E. Latta, Surf. Sci., 1974, 41, 435–446 CrossRef.
- J. A. Herron, S. Tonelli and M. Mavrikakis, Surf. Sci., 2012, 606, 1670–1679 CrossRef CAS.
- S. Fonseca, G. Maia and L. M. C. Pinto, Electrochem. Commun., 2018, 93, 100–103 CrossRef CAS.
- M. D. Higham, M. G. Quesne and C. R. A. Catlow, Dalton Trans., 2020, 49, 8478–8497 RSC.
- P. Atkins and J. de Paula, Physical Chemistry, Oxford University Press, 10th edn, 2014 Search PubMed.
- Q. Tang, F. Shi, K. Li, W. Ji, J. Leszczynski, A. G. Russell, E. G. Eddings, Z. Shen and M. Fan, Fuel, 2020, 280, 118446 CrossRef CAS.
- J. S. Gómez-Jeria, J. Chil. Chem. Soc., 2009, 54, 482–485 Search PubMed.
- J. Dobado, H. Martinez, J. Molina and M. Sundberg, Quantum Systems in Chemistry and Physics, 2000, vol. 2, pp. 337–353 Search PubMed.
- F. Brix, V. Desbuis, L. Piccolo and É. Gaudry, J. Phys. Chem. Lett., 2020, 11, 7672–7678 CrossRef CAS PubMed.
- T. Fujitani, M. Saito, Y. Kanai, T. Watanabe, J. Nakamura and T. Uchijima, Appl. Catal. Gen., 1995, 125, L199–L202 CrossRef CAS.
- R. Haunschild and W. Klopper, J. Chem. Phys., 2012, 136, 164102 CrossRef PubMed.
- J. Wellendorff, K. T. Lundgaard, K. W. Jacobsen and T. Bligaard, J. Chem. Phys., 2014, 140, 144107 CrossRef PubMed.
- A. Posada-Borbón and H. Grönbeck, ACS Catal., 2021, 11, 9996–10006 CrossRef.
- N. Aas, Y. Li and M. Bowker, J. Phys.: Condens. Matter, 1991, 3, S281 CrossRef CAS.
- F. Solymosi, A. Erdöhelyi and M. Lancz, J. Catal., 1985, 95, 567–577 CrossRef CAS.
- J. Díez-Ramírez, J. L. Valverde, P. Sánchez and F. Dorado, Catal. Lett., 2016, 146, 373–382 CrossRef.
- J. Díez-Ramírez, J. A. Díaz, P. Sánchez and F. Dorado, J. CO2 Util., 2017, 22, 71–80 CrossRef.
- H. Bahruji, M. Bowker, W. Jones, J. Hayward, J. Ruiz Esquius, D. J. Morgan and G. J. Hutchings, Faraday Discuss., 2017, 197, 309–324 RSC (i) (ii).
Footnote |
| † Electronic supplementary information (ESI) available: The accompanying supporting information contains details of the calculation methods, and detailed energetics for all steps in the reaction profiles. All structures associated with the presented work are available from the NOMAD repository at DOI: 10.17172/NOMAD/2021.05.24-1 (all data) and 10.17172/NOMAD/2021.05.25-1 (optimised structures). All underpinning energetic data is available at DOI: 10.17035/d.2022.0164034480. See DOI: 10.1039/d2cp01019d |
| This journal is © the Owner Societies 2022 |