Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A reduced electrophilicity for simple Lewis acids A involved in non-covalent interactions with Lewis bases B†
Ibon
Alkorta
 a and
Anthony
Legon
a and
Anthony
Legon
 b
b
aInstituto de Química Médica (IQM-CSIC), Juan de la Cierva, 3, E-28006 Madrid, Spain. E-mail: ibon@iqm.csic.es; Tel: +34 915622900
bSchool of Chemistry, University of Bristol, Cantock's Close, Bristol BS8 1TS, UK. E-mail: a.c.legon@bristol.ac.uk; Tel: +44 (0)117 331 7708
First published on 1st March 2022
Abstract
Dissociation energies De for B⋯A = B + A can be written De = c′NBEA, where NB and EA are the nucleophilicities and electrophilicities of the Lewis base B and the Lewis acid A, respectively. A reduced electrophilicity is defined ΞA = EA/σmax, where σmax is the maximum electrostatic surface potential on iso-surface of A, the atom directly involved in the non-covalent interaction. This definition is tested for halogen-bonded complexes B⋯YX, with Lewis bases B = N2, CO, C2H2, C2H4, H2S, HCN H2O or NH3. De plotted against NB for several series B⋯YX yields straight lines of gradient EA. When De/σmax is the ordinate, the straight lines conflate to a single line, gradient ΞIX = EIX/σmax. Hydrogen-bonded complexes B⋯HX (X = F, Cl, Br, I), coinage-metal complexes B⋯MX (M = Cu, Ag, Au; X = F, Cl, Br, I), and alkali-metal bonded complexes B⋯MX (M = Li, Na: X = F, H, CH3) behave similarly. ΞA is an intrinsic property of the atom immediately involved in the non-covalent bond.
1. Introduction
The use of reduced quantities in chemistry/physics to identify or focus attention on relationships among properties of otherwise disparate substances/topics is not uncommon. For example, in connection with the well-known Law of Corresponding States, if the reduced densities ρ/ρc of liquids (where ρc is the density at the critical temperature Tc), are plotted against T/Tc, a single curve results onto which a number of liquid substances fit very well. Reduced potential energy functions that apply to a range of related diatomic molecules are a second example and provide useful generalisations that provoke questions about what is common to molecules that can be so described. Quantities such as reduced mass and reduced energies feature as simplifying and unifying tools in the theory of rotational spectroscopy. In this article, we shall show that, for a wide range of non-covalent interactions B⋯A of Lewis bases B with Lewis acids A (namely, interactions through hydrogen, halogen, coinage-metal and alkali-metal bonds), we can define a reduced electrophilicity of A. We shall show that the reduced electrophilicity is an interesting general property of complexes B⋯YX in which the non-covalent interaction is one of several different types. It is independent of the atom X in the molecule YX involved in the non-covalent interaction. Thus, the reduced electrophilicity is a property that describes the propensity of Y to form the appropriate non-covalent bond, independently of X.The terms nucleophile and electrophile were introduced by Ingold1 in 1933. In his book “Structure and Mechanism in Organic Chemistry”,2 Ingold gave the following definitions: “Reagents which act by donating their electrons to, or sharing them with, a foreign atomic nucleus will be called nucleophilic reagents or sometimes nucleophiles” and “Reagents which act by acquiring electrons, or a share in electrons, which previously belonged exclusively to a foreign molecule will be called electrophilic reagents, or sometimes electrophiles.’ Since 1933, there has been an extensive literature on the subject of nucleophilicity and electrophilicity. For example, using both words ‘nucleophilicity and electrophilicity’ together as a search topic in the Web Of Science returns 694 publications with an average citation of 30 per article. The great majority of the work is concerned with scales of nucleophilicity and electrophilicity based on rates of reaction, mainly involving organic molecules, as reviewed, for example, by Mayr and Patz3 in an article entitled: ‘Scales of Nucleophilicity and Electrophilicity: A System for Ordering Polar Organic and Organometallic Reactions.’ The definitions employed here, on the other hand, are based on the energy De of the (usually weak) non-covalent interaction of a pair of (initially isolated) molecules (a Lewis base B and a Lewis acid A) when they come together to form the isolated complex B⋯A without chemical reaction. Although these definitions are also consistent with those of Ingold quoted earlier, the subjects of the present article might be more correctly called, gas-phase nucleophilicities and electrophilicities of molecules involved in non-covalent interactions.
Some years ago,4 a method was proposed for reproducing the intermolecular stretching force constants kσ of simple hydrogen-bonded complexes B⋯HX by assigning dimensionless nucleophilicities NB and electrophilicities EHX to the Lewis base B and to the Lewis acids HX, respectively, according to eqn (1):
| kσ = cNBEHX, | (1) |
| De = Cσkσ, | (2) |
| De = c′NBEA | (3) |
Further investigations of complexes B⋯MX, (M = Cu, Ag or Au, X = F or Cl) involving the coinage-metal bond14 and of alkali-metal bonded complexes B⋯LiX and B⋯NaX (X = F, H or CH3)15 have revealed that the ab initio calculated values of De for the complexes involving these two types of non-covalent interaction can also be represented by eqn (3). However, when eqn (3) was fitted to De values by using the least-squares method described in ref. 13 although the fits were good, somewhat different NB values from those obtained in ref. 13 resulted, perhaps not surprisingly in view of the much more polar character of the LiX, NaX and CuX, AgX and AuX diatomic molecules than of HX, XY, etc. For the B⋯MX coinage metal series14 it was discovered that graphs of Deversus NB were good straight lines through the origin and led to the following order of electrophilicities EAuF > EAuCl > ECuF > ECuCl > EAgF ≈ EAgCl. It was also noted that, to good approximation, plots of De/σmaxversus NB, where σmax is the maximum positive value of the molecular electrostatic surface potential (MESP) on the 0.001 e bohr−3 electron density iso-surface, fall on the same straight line for both MF and MCl for a given M. This suggests that a reduced electrophilicity might be defined as (EMX/σmax).
In the present article, we examine whether this definition of reduced electrophilicity is generally valid. To do so we present ab initio calculations of the equilibrium dissociation energy De accompanying the process B⋯IX = B + IX for the series of halogen-bonded complexes B⋯IX in which the Lewis base B is N2, CO, C2H2, C2H4, H2S, HCN H2O or NH3 and IX is variously IF, ICl, IBr or I2. The corresponding series B⋯BrX (X = Br, Cl or F) and B⋯ClX (X = Cl or F) are also investigated. The calculations of De were conducted at the CCSD(T)(F12c)/cc-pVDZ-F12 level of theory. A least-squares fit of the De values to eqn (3) was carried out using the set of known NB values13 for the Lewis bases to determine the EYX (Y = I, Br, Cl) for the Lewis acids involved. Not only can eqn (3) be tested for this extended series, but also we shall investigate whether the graph of De/σmaxversus NB is a single straight line for the full set of iodine-bonded complexes B⋯IX (X = F, Cl, Br and I), and also for several other series. Put another way, we ask the question: Does EIX/σmax provide a reduced electrophilicity for the set of Lewis acids IF, ICl, IBr and I2, i.e. one independent of the atom attached to I? Some previously published De values for the series of hydrogen-bonded complexes B⋯HX (X = F, Cl, Br and I)10,11 and the series of alkali-metal-bonded complexes B⋯NaX and B⋯LiX (X = F, H and CH3)15 will be treated in the same way to test whether the concept of reduced electrophilicity is generally applicable.
2. Theoretical methods
The geometry optimizations of the isolated monomers and complexes were carried out using the CCSD(T)(F12c) explicitly correlated, coupled-cluster method.16,17 The cc-pVDZ-F12 basis sets, which are optimised for use with the CCSD(T)-F12 method,18 were used for all of the first and second row atoms. Pseudo-potential (PP) basis sets were employed for the atoms Br and I in combination with the ECP-10-MDF19 and ECP-28-MDF20 effective core potentials to account for scalar relativistic effects. The frozen-core approximation was used in all the calculations, which employed the MOLPRO package.21,22 Dissociation energies, so computed at the CCSD(T)(F12c)/cc-pVDZ-F12 level, were corrected for basis set superposition error (BSSE)23 by means of the counterpoise keyword in MOLPRO. De values for 132 new complexes and 12 monomers were calculated specifically for this article. The remaining De values employed here are from earlier publications: the data for coinage-metals complexes are from ref. 14, those for the alkali-metal complexes are from ref. 15 and some of the B⋯HX (X = F, Cl, Br, I) and B⋯XY (X and Y are halogen atoms) are from ref. 10 and 11.Molecular electrostatic surface potentials (MESP) were calculated by using the MP2/aug-cc-pVTZ wavefunction in the GAUSSIAN program24 and represented at the 0.001 e bohr−3 electron density iso-surface with the Jmol program25 (an open-source Java viewer for chemical structures in 3D. available at http://www.jmol.org/). The extreme values (maxima and minima) on the iso-surface were calculated with the multiwfn program.26
The results for 132 new complexes and 12 monomers are presented in this article for the first time and are recorded in the ESI.† Those for complexes involving the Li and Na non-covalent bonds were published previously in ref. 15) and those involving Cu, Ag and Au with X = F and Cl were first published in ref. 14 but results for CuBr and CuI are new. All values of De for the coinage-metal complexes were calculated at the CCSD(T)/aug-cc-pVTZ, aug-cc-pVTZ-PP levels as appropriate and the binding energy De was obtained by using the CBS extrapolation method.27,28 In excess of 300 complexes are discussed.
3. Results
3.1 The iodine-bonded series B⋯IX (X = F, Cl, Br, I)
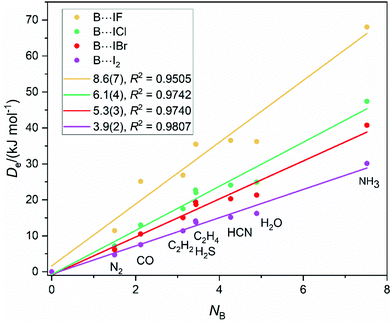
Fig. 1 shows a graph in which the equilibrium dissociation energies De of the iodine-bonded complexes B⋯IX (X = F, Cl, Br and I) are plotted against the nucleophilicity NB of the series of Lewis bases B = N2, CO, C2H2, C2H4, H2S, HCN H2O or NH3, the latter quantities as determined in ref. 13. De is the energy required to move the components of B⋯IX from their equilibrium distance in the complex to become infinitely separated molecules B and IX relaxed to their hypothetical equilibrium states. The Lewis base PH3 has been omitted from the list because it was shown previously that complexes such as H3P⋯ICl29 and H3P⋯ClF30 are of a different type from the remainder and have significant electric charge redistribution on complex formation. The linear-regression fits to the points (with the origin taken as a point under the assumption that the nucleophilicity NB = 0 when De = 0) are shown as solid straight lines. The R2 values shown in Fig. 1 indicate that the fits are satisfactory, with that for B⋯IF the worst at 0.95. Clearly, eqn (3) is obeyed by each of the B⋯IX series. The gradient of each straight line corresponds to the electrophilicity of the Lewis acid according to eqn (3) given that c′ = 1.00 kJ mol−1 was used in the determination of the NB values by the least squares fit to 250De values in ref. 13. The conclusion is therefore that EIF = 8.6(7) > EICl = 6.1(4) > EIBr = 5.3(3) > EI2 = 3.9(2), which order is consistent with electron withdrawing power of the X atom in the order F > Cl > Br > I.The concept of reduced electrophilicity discussed in the Introduction can now be tested for the B⋯IX series. Dividing eqn (3) by the value of σmax (the maximum positive value of the MESP on the 0.001 e bohr−3 electron density iso-surface near to the I atom along the axis of the IX diatomic molecule), we have
| De/σmax = c′NB(EA/σmax) = NBΞA, | (4) |
| Lewis acid YX | X = I | X = Br | X = Cl | X = F | X = CN | X = CP | X = CCH | X = H | X = CH3 |
|---|---|---|---|---|---|---|---|---|---|
| a Calculated at the MP2/aug-cc-pVTZ level of theory. | |||||||||
| IX | 125.7 | 158.4 | 180.1 | 241.3 | 201.3 | — | 142.0 | — | — |
| BrX | — | 118.8 | 141.3 | 205.6 | — | — | — | — | — |
| ClX | — | — | 107.0 | 172.1 | — | — | — | — | — |
| HX | 119.4 | 160.1 | 190.2 | 287.9 | 216.9 | 126.3 | 134.8 | ||
| CuX | 342.8 | 366.1 | 379.8 | 405.1 | — | — | — | — | — |
| AgX | 296.0 | 305.1 | 308.0 | 294.5 | — | — | — | — | — |
| AuX | 270.1 | 314.6 | 346.7 | 404.8 | — | — | — | — | — |
| LiX | — | — | — | 764.2 | — | — | — | 809.5 | 793.1 |
| NaX | — | — | — | 566.8 | — | — | — | 510.0 | 471.0 |
Fig. 2 confirms that there is indeed a reduced electrophilicity ΞA having the value 0.034(2) for the iodine atom in the four diatomic molecules IF, ICl, IBr and I2. The scatter and R2 are rather larger for IF, but the gradients of the four straight lines are identical within their quoted errors. In fact, some points are obscured by superposition of others. It is of interest to examine, in the same way, the situation when the Lewis acids are the pseudo-halogens ICN and ICCH. The graph of Deversus NB is shown in Fig. 3 while that when the ordinate is De/σmax is in Fig. 4. The latter diagram shows that the gradients in Fig. 4 are equal within the fitting error and hence both ICN and ICCH can be described by a reduced electrophilicity Ξ = 0.0229(4). We note, however, that this value is different from that for the IX (X = F, Cl, Br, I) given in Fig. 2.
3.2 The bromine-bonded series B⋯BrX (X = F, Cl, Br)
Fig. 5 shows the graphs of Deversus NB for the three series B⋯BrX (X = F, Cl, Br). The same pattern as observed for the B⋯IX series is evident, with the largest scatter from its regression line again when X = F. Values of De are not given in ref. 10 and 11 for N2⋯Br2 and HCN⋯Br2 and these were calculated for the present work. Evidently, eqn (3) is obeyed by each series, as indicated by R2 values close to unity. The electrophilicities of the BrX are in the order X = F > Cl ≥ Br.The corresponding graphs of De/σmaxversus NB for the series B⋯BrF, B⋯BrCl and B⋯Br2 are set out in Fig. 6. Note again that the gradients, which yield the reduced electrophilicity ΞA for the molecules BrX, are almost equal within the errors of the fits and suggest that, as for the B⋯IX series, the definition of reduced electrophilicity used here is reasonable.
3.3 The chlorine-bonded series B⋯ClX (X = F, Cl)
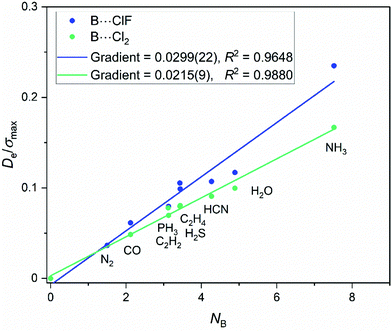
There are only two members of this series. Fig. 7 shows the graph of Deversus NB for both the B⋯ClF and B⋯Cl2 series. Eqn (3) appears to be obeyed by both. The De/σmaxversus NB plots shown in Fig. 8 have gradients = 0.030(2) and 0.022(1) for the B⋯ClF and B⋯Cl2 series, respectively. Hence, we cannot unambiguously assign a reduced electrophilicity to the ClF and Cl2 molecules for their interactions with the Lewis bases B, as was the case for the B⋯BrX and B⋯IX series. Nevertheless these values are much closer together than the gradients of 5.2(4) and 2.3(1) kJ mol−1 of the fitted points in the graphs of Deversus NB for the B⋯ClF and B⋯Cl2 series, respectively.3.4 Is there a reduced electrophilicity ΞA appropriate to the hydrogen halides HX?
The evidence presented in Sections 3.1–3.3 indicates that the quantity ΞA defined by means of eqn (3) and (4) might be used as a reduced electrophilicity for the Lewis acids A involved in series of halogen-bonded complexes. A reduced electrophilicity so defined is a measure of the propensity of, e.g. IX to form B⋯IX halogen bonds independently of the halogen atom X attached to it. It is of interest to investigate whether this concept has generality. In this section, hydrogen-bonded complexes B⋯HX having X = F, Cl, Br and I will be discussed first and then X = CN, CP and CCH will be considered.Dissociation energies De for the series B⋯HX (now including B = PH3), calculated at the explicitly-correlated level CCSD(T)(F12c)/cc-pVDZ-F12 and corrected for basis set superposition error (BSSE), have been published elsewhere.10,11 Using again NB values determined in ref. 13, Fig. 9 was constructed. It shows clearly that the B⋯HX series obey eqn (3) with well-determined gradients and R2 values, the latter close to 1. The gradients provide measures of the electrophilicities EHX of the hydrogen halides, which have the values 7.0(4), 4.5(2), 3.9(2) and 2.8(1) for X = F, Cl, Br and I, respectively, an order consistent with chemical intuition (the greater the slope the stronger the hydrogen bonds). Fig. 10 shows the corresponding set of graphs in which De/σmax is now the ordinate. The gradients determined by linear regression fits are closely bunched and have the mean value of 0.0239(7), which is, according to eqn (4), the reduced electrophilicity ΞHX of the hydrogen halides. The narrow spread of values indicates that the reduced electrophilicity of the hydrogen halides appears to be a well-defined quantity.
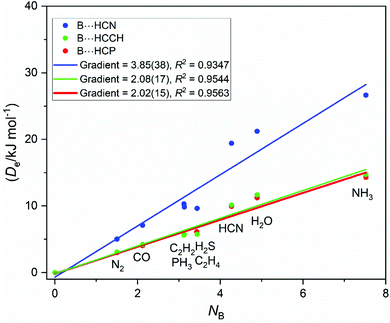
Given the results for the hydrogen halides, it is of interest to consider hydrogen acids of the type HCX in which H is now directly attached to a carbon atom. De values for B⋯HCN, B⋯HCP and B⋯HCCH complexed with the same set of Lewis bases B were calculated at the CCSD(T)(F12c)/cc-pVTZ-F12 level of theory and corrected for BSSE. The graphs of De plotted against NB for these three series are in Fig. 11. Note that the points for B⋯HCCH and B⋯HCP are close to coincident. Convergence in the geometry optimisation for H2S⋯HCN and H2S⋯HCCH was not achieved and so points for these are missing.
Although the scatter of the points is rather large for the HCN series, eqn (3) is obeyed by all three Lewis acids HCN, HCP and HCCH. Moreover, as can be seen in Fig. 12, the plots of De/σmax against NB for all three series lead to the same gradient within the fitting error. Thus, the reduced electrophilicity appears to be a property that can be assigned to these three Lewis acids also. We note, however, that ΞHCX = EHCX/σmax is different from the value obtained for the hydrogen halides.
3.5 Reduced electrophilicity and the coinage-metal halides MX (M = Cu, Ag, Au; X = F, Cl, Br, I)
Complexes of the type B⋯MX, where M = Cu, Ag, Au, X = F, Cl were discussed in ref. 14, where the possibility of a reduced electrophilicity was first raised. The Lewis bases B are again the nine simple molecules B = N2, CO, C2H2, C2H4, H2S, PH3, HCN, H2O, and NH3. For the present article, calculations have been extended to include X = Br and I. The graphs of Deversus NB for the series B⋯AuX (X = F, Cl, Br and I) are displayed in Fig. 13, while the plots with De/σmax as the ordinate are in Fig. 14. The corresponding pairs of diagrams for M = Cu are available as Fig. S1 and S2 of the ESI,† while for M = Ag Fig. S3 and S4 (ESI†) are appropriate. The magnitude and order of the De values for the B⋯MX complexes (and therefore the values of the NB of the Lewis bases when involved in such complexes, as published in ref. 14) are both quite different from those used13 for the hydrogen- or halogen-bonded series. Nevertheless, it is clear from Fig. 13 and Fig. S1, S3 (ESI†) that eqn (3) is valid for coinage-metal bonds as well as hydrogen- and halogen bonds. Moreover, the order of the electrophilicities of the aurous halides is EAuF > EAuCl > EAuBr > EAuI. The order for the argentous and cuprous halides is the same.Fig. 14 illustrates how division of the De value by σmax reduces the four straight lines in Fig. 13 to a single line. Thus, the gradients of the four lines in Fig. 14 are identical within the fitting error, with the mean value 0.0442(18). This is the dimensionless reduced electrophilicity ΞAuX of the AuX diatomic molecules. Fig. S2 and S4 (ESI†) indicate that ΞCuX = 0.0283(18) and ΞAgX = 0.0287(22).
The coinage-metal diatomic molecules are much more polar than the Lewis acids considered in earlier sections. For example, the electric dipole moments of CuCl and AgCl are 5.74 D31 and 6.076(6) D,32 respectively, while those of BrCl, ICl and HBr are 0.519(4) D,33 1.24(2) D34 and 0.8280(6) D,35 respectively. CuCl and AgCl (and presumably AuCl) also have large ionicities ic. The ionicity of a diatomic molecule is a measure of the fractional contribution of the structure M+Cl− to the valence-bond description of the molecule. It can be calculated36 from the nuclear quadrupole coupling constant χz(X) of the halogen atom X in the appropriate diatomic molecule by using eqn (5), in which eQqn,1,0 is the quadrupole coupling constant arising from a single electron in a npz orbital.
| ic = 1 + {χz(X)/(eQqn,1,0)} | (5) |
3.6 Reduced electrophilicity of the alkali-metal compounds LiX and NaX (X = F, H, CH3)
Application of eqn (5) to alkali-metal halides such as NaCl and LiCl shows values of ic that are close to 1, indicating that these molecules are essentially ion pairs in the gas phase.36 The question that then arises is: Does the concept of reduced electrophilicity apply as well to alkali-metal bonded complexes such as B⋯NaX and B⋯LiX as it does to hydrogen-bonded, halogen-bonded and coinage-metal-bonded complexes? De and NB values for B⋯MF, B⋯MH, B⋯MCH3 were recently published15 for M = Na and Li, with Lewis bases B = CO, HCN, H2O, NH3, H2S and PH3. A set of NB, ELiX and ENaX were derived by the least-squares method from 34 values of De for complexes that could be identified as isostructural with their hydrogen- and halogen-bonded counterparts. As for the coinage-metal bond series, the NB scale had some significant differences from that used for the hydrogen- and halogen-bonded series. The variation of De with NB for the alkali-metal bonded complexes (first published in ref. 15) is set out again in Fig. 15 (redrawn). Each series leads to an excellent straight line, which indicates eqn (3) is appropriate here also. The electrophilicities have the order ELiH > ELiF ≈ ELiCH3 > ENaF > ENaH > ENaCH3, as previously established.15The corresponding graphs of De/σmaxversus NB are set out in Fig. 16. The gradients of the three straight lines for the B⋯LiX complexes in Fig. 16 are very nearly equal. However, a separate linear regression fit of all the B⋯LiX points alone gives ΞLiX = ELiX/σmax = 0.01114(2) for the reduced electrophilicity of the LiX molecules, while for the B⋯NaX complexes alone the value is 0.01007(10). It appears that the reduced electrophilicities of the LiX and NaX molecules are very similar, but not equal.
4. Conclusions and discussion
The main conclusion of this work is that it is possible to define a reduced electrophilicity ΞA for a Lewis acid A when involved in non-covalent complexes B⋯A formed with Lewis bases B. This is found to be true for a range of non-covalent interaction types. A summary of the values of ΞA determined here is available in Table 2.| Lewis acid A | Reduced electrophilicity ΞA |
|---|---|
| a The reduced electrophilicity of a given Lewis acid A is defined as EA/σmax and is the gradient of the straight line obtained by plotting De/σmax against NB for a series of complexes B⋯A, where the NB and EA are the nucleophilicity and electrophilicity of the Lewis bases B and the Lewis acid A, respectively. σmax is the maximum positive value of the MESP of A on the 0.001 e bohr−3 iso-surface along its symmetry axis and near to the atom that is directly involved in the non-covalent interaction of A with B. | |
| IX (X = F, Cl, Br, I) | 0.033(2) |
| IX (X = CN, CCH) | 0.0229(4) |
| BrX (X = F, Cl, Br) | 0.033(3) |
| ClX (X = F, Cl) | ∼0.026(4) |
| HX (X = F, Cl, Br, I) | 0.0239(7) |
| HX (X = CN, CP, CCH) | 0.0164(13) |
| CuX (X = F, Cl Br, I) | 0.0283(18) |
| AgX (X = F, Cl, Br, I) | 0.0287(22) |
| AuX (X = F, Cl, Br, I) | 0.0442(18) |
| LiX (X = H, F, CH3) | 0.01114(2) |
| NaX (X = H, F, CH3) | 0.01007(10) |
Graphs of the equilibrium dissociation energy De for complexes B⋯IX plotted against the nucleophilicity NB of the Lewis bases B = N2, CO, C2H2, C2H4, H2S, HCN, H2O, and NH3 for the four series X = I, Br, Cl, F have been used to show that eqn (3), De = c′NBEA, is obeyed by each of these series of iodine-bonded complexes. It is thereby established that the order of the gas-phase non-covalent electrophilicities EIX of these dihalogen molecules is EIF > EICl > EIBr > EI2. Moreover, when De is divided by σmax, (i.e. the value of the molecular electrostatic potential on the 0.001 e bohr−3 electron density iso-surface on the molecular axis near to I), the plot of De/σmaxversus NB reveals that the four distinct straight lines of different gradient in the Deversus NB plots have become conflated to give a single straight line to a good level of approximation. The gradient of the single straight line is referred to as the reduced electrophilicity ΞIX = De/σmax of the IX molecules (X = I, Br, Cl and F). The same procedure followed for the series B⋯BrX and (less certainly) the series B⋯ClX allows similar conclusions and leads to the order of the reduced electrophilicities ΞIX = ΞBrX > ΞClX. The series B⋯ICX (X = N, CH) also behaved in the same manner as the B⋯IX series, but the conflation of the two straight lines when De/σmax is plotted against NB yields an ΞICX value smaller than that of the B⋯IX series.
It has also been possible to show that a similar conflation of four straight lines to a single straight line results when De/σmax is plotted against NB for the hydrogen-bonded series B⋯HX (X = F, Cl, Br, I) and hence provides a reduced electrophilicity ΞHX for the hydrogen halides. When HX is replaced by HCX, where X = CN. CP and CCH, a value of ΞHCX is again established but, as for the corresponding IX and ICX series, it is found that ΞHX > ΞHCX.
When the Lewis acid is more polar, as for AuX, AgX and CuX in the three series of coinage-metal bonded complexes B⋯MX (M = Cu, Ag, Au; X = F, Cl, Br, I), it is still possible to establish well-determined reduced electrophilicities for the Lewis acids in the order ΞAuX > ΞAgX ≈ ΞCuX. Even when the Lewis acid is close to being an ion pair, as in LiX and NaX (X = F, H, CH3), the approach is again successful and leads to well-determined reduced electrophilicities in the order ΞNaX ≈ ΞLiX.
It is important to discuss the significance of the newly-defined quantity referred to as reduced electrophilicity. Eqn (3) in Section 3.1 has been established to hold for a wide range of non-covalent interactions involved in axially symmetric complexes B⋯YX, where Y is the atom of the Lewis acid directly involved in that interaction. An important quantity in this context is the most positive electrostatic potential σmax at the 0.001 e bohr−3 iso-surface on the symmetry axis in the vicinity of the atom Y of the Lewis acid (0.001 e bohr−3 is the conventional choice of electron density for such surfaces). It was shown in Section 3.1 that division of eqn (3) by σmax to give eqn (4) and then a plot of De/σmaxversus NB leads in all cases considered here to a straight line through the origin. According to eqn (4), the gradient of this line is EYX/σmax, that is the electrophilicity of the molecule YX per unit maximum positive electrostatic potential on the symmetry axis at Y. We have shown (by consideration of four different types of non-covalent interaction and a range of axially symmetric Lewis acids YX) that the quantity EYX/σmax is an intrinsic property of the atom Y involved in the non-covalent interaction with the nucleophilic region of the Lewis base B. Thus, it is the same for all diatomic Lewis acids when the same atom Y is that involved in the non-covalent interaction with B. This result is made clear, for example, by Fig. 10, which involves the four hydrogen halides. When YX is a triatomic (or higher) molecule, as in Fig. 12 (which deals with the axially symmetric polyatomic Lewis acids HCN, HCP and HCCH), EYX/σmax is again an intrinsic property of the atom Y = H for the group, but has a slightly different value from that of the hydrogen halides (Fig. 10). Therefore it seems justified to give the quantity EYX/σmax the name reduced electrophilicity and the special symbol ΞYX. This new reduced quantity clearly points to systematic family relationships among groups of Lewis acids involving different types of non-covalent interaction. Further investigation might reveal further aspects the relationship.
Thus, we conclude that the reduced electrophilicity of a Lewis acid YX is an intrinsic property of the atom Y and its axial electrostatic surface potential directly involved in the non-covalent interaction. It appears to be independent of the nature of the remainder of YX, certainly so when YX is a diatomic molecule and nearly so for more complex YX. It therefore provides a measure of the propensity of atom Y to participate in a wide range of hydrogen bonds (if Y = H), halogen bonds (if Y is a halogen atom), coinage-metal bonds (if Y is a Group 11 atom), alkali-metal bonds (if Y is an alkali-metal atom).
The vast majority of data on nucleophilicities and electrophilicities involves quantities that are derived from the rate constants of chemical reaction in solution between more complex molecules than those discussed here (see ref. 3, for example). The electrophilicities and their reduced values presented in the Results section refer to the limiting, gas-phase interactions between pairs of molecules otherwise isolated and therefore comparison of the two scales is unlikely to be useful. Two papers have been published,38,39 however, that make a theoretical interpretation of the gas-phase N and E values set out initially in ref. 4.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
ACL thanks the University of Bristol for the award of a University Senior Research Fellowship. IA thanks the Ministerio de Ciencia e Innovación of Spain (PGC2018-094644-B-C22 and 5931125495-125495-4-21) and Comunidad de Madrid (P2018/EMT-4329 AIRTEC-CM) for financial support.References
- C. K. Ingold, J. Chem. Soc., 1933, 1120–1127 RSC
.
-
C. K. Ingold, Structure and Mechanism in Organic Chemistry, Cornell University Press, Ithaca, New York, 1953, pp. 200–201 Search PubMed
.
- H. Mayr and M. Patz, Angew. Chem., Int. Ed. Engl., 1994, 33, 938–957 CrossRef
.
- A. C. Legon and D. J. Millen, J. Am. Chem. Soc., 1987, 109, 356–358 CrossRef CAS
.
- A. C. Legon and D. J. Millen, J. Chem. Soc., Chem. Commun., 1987, 986–987 RSC
.
- A. C. Legon, J. Chem. Soc., Chem. Commun., 1998, 2585–2586 RSC
.
- A. C. Legon, Angew. Chem., Int. Ed., 1999, 38, 2686–2714 CrossRef PubMed
.
-
A. C. Legon, in Halogen Bonding: Fundamentals and Applications, ed. P. Metrangolo and G. ResnatiStructure and Bonding, Springer-Verlag, Berlin, 2008, ch. 2, vol. 126, pp. 17–64 Search PubMed
.
- D. J. Millen, Can. J. Chem., 1985, 63, 1477–1479 CrossRef CAS
.
- A. C. Legon, Phys. Chem. Chem. Phys., 2014, 16, 12415–12421 RSC
.
- A. C. Legon, Phys. Chem. Chem. Phys., 2014, 16, 25199 RSC
.
- I. Alkorta and A. C. Legon, New J. Chem., 2018, 42, 10548–10554 RSC
.
- I. Alkorta and A. C. Legon, Molecules, 2017, 22, 1786–1799 CrossRef PubMed
.
- I. Alkorta, N. R. Walker and A. C. Legon, Inorganics, 2021, 9, 13 CrossRef CAS
.
- I. Alkorta, J. G. Hill and A. C. Legon, Phys. Chem. Chem. Phys., 2020, 22, 16421–16430 RSC
.
- C. Hättig, D. P. Tew and A. Köhn, J. Chem. Phys., 2010, 132, 231102 CrossRef PubMed
.
- C. Hättig, W. Klopper, A. Köhn and D. P. Tew, Chem. Rev., 2012, 112, 4–74 CrossRef PubMed
.
- J. G. Hill and K. A. Peterson, J. Chem. Phys., 2014, 141, 094106 CrossRef PubMed
. See http://bit.ly/ccBasis for a repository of basis functions, including cc-pVDZ-F12 for Br and I, constructed by J. G. Hill.
- M. Doig, U. Wedig, H. Stoll and H. Preuss, J. Chem. Phys., 1987, 86, 866–872 CrossRef
.
- I. S. Lin, H. Stoll and P. Schwertfeger, J. Chem. Phys., 2006, 124, 034107 CrossRef PubMed
.
- H.-J. Werner, P. J. Knowles, G. Knizia, F. R. Manby and M. Schütz, ‘Molpro: a general-purpose quantum chemistry program package’, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 242–253 CAS
.
-
H.-J. Werner, P. J. Knowles, G. Knizia, F. R. Manby and M. Schütz, MOLPRO 2019.2, a package of ab initio programs, 2019 Search PubMed
.
- S. F. Boys and F. Bernardi, Mol. Phys., 1970, 19, 553–566 CrossRef CAS
.
-
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson and H. Nakatsuji, et al., Gaussian 16; Revision, A.03, Gaussian, Inc., Wallingford, CT, USA, 2016 Search PubMed
.
- Jmol: An Open-Source Java Viewer for Chemical Structures in 3D. Available online at: http://www.jmol.org/.
- T. Lu and F. Chen,
Multiwfn: A multifunctional wavefunction analyzer, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed
.
- D. Feller, J. Chem. Phys., 1993, 98, 7059–7071 CrossRef CAS
.
- A. Halkier, T. Helgaker, P. Jorgensen, W. Klopper and J. Olsen, Chem. Phys. Lett., 1999, 302, 437–446 CrossRef CAS
.
- A. C. Legon, Chem. Phys. Lett., 1999, 314, 472–480 CrossRef CAS
.
- R. A. Shaw, J. G. Hill and A. C. Legon, J. Phys. Chem. A, 2016, 120, 8461–8468 CrossRef CAS PubMed
.
- S. Hau and P. F. Bernath, J. Phys. Chem. A, 2015, 119, 1435–1438 CrossRef PubMed
.
- K. P. R. Nair and J. Hoeft, J. Phys. B: At. Mol. Phys., 1984, 17, 735–738 CrossRef CAS
.
- K. P. R. Nair, J. Hoeft and E. Tiemann, Chem. Phys. Lett., 1978, 58, 153–156 CrossRef CAS
.
- E. Herbst and W. Steinmetz, J. Chem. Phys., 1978, 56, 5342–5347 CrossRef
.
- F. A. van Dijk and A. Dymanus, Chem. Phys. Lett., 1970, 5, 387–389 CrossRef CAS
.
-
W. Gordy and R. L. Cook, Microwave Molecular Spectra, John Wiley and sons, New York, 2nd edn, 1984, ch. 14, p. 752 Search PubMed
.
- A. C. Legon and N. R. Walker, Phys. Chem. Chem. Phys., 2018, 20, 19332–19338 RSC
.
- A. Ebrahimi, H. Roohi, M. Habibi and M. Hasannejad, Chem. Phys. Lett., 2006, 327, 368–372 CAS
.
- A. Cedillo, R. Contreras, M. Galván, A. Aizman, J. Andrés and V. S. Safont, J. Phys. Chem. A, 2007, 111, 2442–2447 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d2cp00779g |
| This journal is © the Owner Societies 2022 |