Open Access Article
Open Access ArticleCore-electron contributions to the molecular magnetic response†
Mesías
Orozco-Ic
 *a,
Nickolas D.
Charistos
*a,
Nickolas D.
Charistos
 b,
Alvaro
Muñoz-Castro
b,
Alvaro
Muñoz-Castro
 c,
Rafael
Islas
d,
Dage
Sundholm
c,
Rafael
Islas
d,
Dage
Sundholm
 *a and
Gabriel
Merino
*a and
Gabriel
Merino
 *e
*e
aDepartment of Chemistry, Faculty of Science, University of Helsinki, P.O. Box 55, A. I. Virtasen aukio 1, FIN-00014 Helsinki, Finland. E-mail: mesias.orozcoic@helsinki.fi; dage.sundholm@helsinki.fi
bAristotle University of Thessaloniki, Department of Chemistry, Laboratory of Quantum and Computational Chemistry, Thessaloniki, 54 124, Greece
cGrupo de Química Inorgánica y Materiales Moleculares, Facultad de Ingeniería, Universidad Autonoma de Chile, El Llano Subercaseaux 2801, Santiago, Chile
dDepartamento de Ciencias Químicas, Facultad de Ciencias Exactas, Universidad Andres Bello, Av. República 275, 8370146, Santiago, Chile
eDepartamento de Física Aplicada, Centro de Investigación y de Estudios Avanzados, Unidad Mérida. Km 6 Antigua Carretera a Progreso. Apdo. Postal 73, Cordemex, 97310, Mérida, Yuc., Mexico. E-mail: gmerino@cinvestav.mx
First published on 19th April 2022
Abstract
Orbital contributions to the magnetic response depend on the method used to compute them. Here, we show that dissecting nuclear magnetic shielding tensors using natural localized molecular orbitals (NLMOs) leads to anomalous core contributions. The arbitrariness of the assignment might significantly affect the interpretation of the magnetic response of nonplanar molecules such as C60 or [14]helicene and the assessment of their aromatic character. We solve this problem by computing the core- and σ-components of the induced magnetic field (and NICS) and the magnetically induced current density by removing the valence electrons (RVE). We estimate the core contributions to the magnetic response by performing calculations on the corresponding highly charged molecules, such as C6H630+ for benzene, using gauge-including atomic orbitals and canonical molecular orbitals (CMOs). The orbital contributions to nuclear magnetic shielding tensors are usually estimated by employing a natural chemical shielding (NCS) analysis in NLMO or CMO bases. The RVE approach shows that the core contribution to the magnetic response is small and localized at the nuclei, contrary to what NCS calculations suggest. This may lead to a completely incorrect interpretation of the magnetic σ-orbital response of nonplanar structures, which may play a major role in the overall magnetic shielding of the system. The RVE approach is thus a simple and inexpensive way to determine the magnetic response of the core- and σ-electrons.
Introduction
One of the many ways to diagnose whether a molecule is aromatic or not is by analyzing its response to an external magnetic field.1,2 In the presence of this field, some cyclic molecules induce a current density forming two- or three-dimensional circuits.3,4 In planar aromatic molecules, the external magnetic field (Bext) is usually applied perpendicularly to the molecular plane sustaining a diatropic current density that concomitantly creates an induced magnetic field, Bind, in the opposite direction to the external field.5–7 However, in nonplanar systems, Bext cannot be applied perpendicularly to the rings, which implies that the x- and y-components of Bind also become relevant.8,9Recently, we found that the response of the core electrons to the external magnetic field (Bindcore) in helicenes produces a cumulative and curvature-sensitive shielding cone due to the overlapping of the magnetic responses of the rings.9 In C60, this response is even more pronounced, resulting in strong shielding within the cage.10,11 These facts motivated us to study in detail the characteristics of Bindcore in nonplanar structures.
Typically, nuclear magnetic shielding tensors are calculated with the popular gauge-including atomic orbital (GIAO) scheme,12,13 using a methodology based on analytical gradient theory that employs explicit field-dependent basis functions, which is implemented in most electronic structure programs. Furthermore, a dissection of the shielding tensor into individual orbital contributions can be obtained by transforming canonical molecular orbitals (CMOs) into the natural localized molecular orbitals (NLMOs) by a unitary transformation using the natural chemical shielding (NCS) analysis.14 Alternatively, a direct dissection of the shielding tensor to CMO contributions, within the GIAO scheme, can be provided by numerical integration.15 The shielding tensor can then be constructed and interpreted in terms of CMO contributions. However, Steiner and Fowler proved that dissections such as the NCS scheme provide unphysical core- and σ-contributions to current densities and magnetic shieldings due to the occupied-occupied mixing of the orbitals.16 For nonplanar molecules, this type of dissection can also lead to a mixing of the σ- and π-contributions to the shielding tensor. The ipsocentric method,17,18 which works on a continuous set of gauge-origin transformations,19,20 removes these unphysical occupied–occupied transitions in the orbital contributions of the current density because it locates the origin at the individual evaluation points when calculating the current density. Consequently, the corresponding ipsocentric orbital contributions to the shielding tensor can be realized from the Biot–Savart integral expression.16,21 A comprehensive analysis of the core-electron contributions to the magnetic response has not been explored, except in benzene.16,19 These calculations performed with the ipsocentric approach suggest that the core-electron contribution is minimal, contrary to what is predicted by the NCS analysis.
Unlike GIAO-based magnetic property calculations, the ipsocentric approach has not been widely adopted by the chemical community, so addressing a possible solution to the problem of orbital contributions in GIAOs is quite attractive and convincing. Moreover, modern analyses of the magnetic response consist of calculating these properties in the molecular environment leading to a detailed quantitative and qualitative description of the magnetic behavior and not only at one point in space. Since the core-electron orbitals are much lower in energy than the valence orbitals (at least in organic molecules), the core contribution can also be estimated by computing the magnetic response, whose valence electrons have been removed, using GIAO and the analytical gradient approach. This strategy can also provide a corrected σ-electron contribution.
Here, we first analyze benzene to estimate the core- and σ-contributions in the prototypical planar aromatic ring. Next, we investigate the role of curvature in the magnetic response of the core electrons in corannulene, C60, and [14]helicene. The discussion of the magnetic response is focused on the induced magnetic field5–7 (Bind) and the magnetically induced current densities (Jind),22–24 computed in the molecular surroundings. Removal of valence electrons (RVE) provides different aspects of the core- and σ-electron effects of the magnetic response compared to previous studies,9–11 whereas the core-electron contributions do not affect the topology of the current density and ring-current strengths because the core contributions of the induced current density are localized close to the nuclei.
Computational details
The geometries were fully optimized at the PBE0-D3/def2-TZVP level.25–27 Magnetic shielding tensors were determined at the PBE0/def2-SVP level25,26 using the GIAO method.12,13 These computations were performed with Gaussian 09.28 While the Aromagnetic code29 was used in the Bind calculations, the Jind analysis was performed using the gauge-including magnetically induced current (GIMIC)22–24 program. The Aromagnetic and GIMIC programs are interfaced to Gaussian. We assumed a Bext direction oriented parallel to the highest symmetry axis (z-axis) of the molecule; the z-component of Bind (Bindz) is then the largest one. Bindz is a scalar field equal to the zz-component of the nucleus-independent chemical shift30 (NICSzz) for planar molecules. GIMIC integrates the Jind flux through planes that intersect bonds or atoms yielding ring-current strengths (Jind).22–24 The current-density flux in different parts of the plane can be determined by calculating the derivative of Jind (dJind/dr) with respect to one of the plane coordinates (r). The r coordinate can be chosen to begin with r = 0 at the horizontal axis in, for example, the center of a molecular ring.22–24 The integration planes usually extend 5 Å in three directions and end outside the molecule, where the current density practically vanishes.The magnetic shielding tensor was dissected into orbital contributions using the NCS analysis14 as implemented in the NBO631 code. Bindcore and Jindcorewere also computed using the RVE method, that is, for highly charged molecules whose valence electrons have been removed, but without relaxing the molecular structure. The RVE approach takes advantage of the fact that a weak perturbation due to a weak homogeneous external magnetic field does not modify the electronic structure of the core electrons. Therefore, calculating the induced current density or magnetic shielding of a system without its valence electrons is equivalent to calculating the magnetic response of the core electrons. The RVE approach is valid as long as the energy gap between the lowest occupied σ orbital and the highest occupied core-electron orbital (ΔLσ-Hcore) is large (> 250 eV). The ΔLσ-Hcore values are included in Table S1 of the ESI.† The Bindcore and Jindcore contributions of benzene, corannulene, C60, and [14]helicene have been estimated by calculating the magnetic responses of C6H630+, C20H1090+, C60240+, and C58H32264+, respectively, at the fixed structures from the neutral counterparts. We also calculated the NICS(0) values to compare and validate the RVE approach with the isotropic values reported by Fowler and Steiner. This also proves the applicability of the RVE approach to NICS, which is one of the most widely used aromaticity indices.
Steiner and Fowler noted that the total shielding is the same for any dissection scheme.16 For planar molecules, the same is true for the π-contribution and, consequently, for the contribution resulting from the sum of the core-electron and the σ-electron components (core + σ). The challenge is to separate the (core + σ)-contribution into its core- and σ-parts. Here, the σ-contribution is obtained by subtracting the core-electron component provided by RVE from the (core + σ)-contribution of the original system. Furthermore, to confirm the magnitude of the contributions coming from occupied–occupied transitions in the core-electron component, a detailed analysis of the shielding tensor was performed with the nuclear magnetic resonance (NMR) module15,32,33 of ADF,34 employing the PBE0 functional and the Slater type triple-ζ with two polarization function (TZ2P) basis set.
Results and discussion
Benzene
Aromaticity in organic molecules, such as benzene, is explained in terms of the delocalization of the π-electrons because they are primarily responsible for the ring current and the corresponding magnetic shielding contribution.6,18,35 In contrast, while σ-electrons induce a local magnetic response near the molecular plane, the core electrons should be located at the nuclei. Calculations of orbital contributions of NICS(0) and Bindz(0) in benzene, using the Biot–Savart expression, show that the core contribution is tiny in both cases.16,21 This contrasts with the NCS results, whose core contribution to Bindz(0), Bindz,core(0), of −10.52 ppm is almost 30% of the π-one to the overall Bindz(0) (see Table 1). These results could be interpreted to mean that core electrons also contribute to the benzene ring current, which is at odds with the accepted notion of aromaticity where π-electrons are the ones inducing that response (or σ-electrons in the case of all-metallic systems).| NICS(0) | B indz (0) | |
|---|---|---|
| core (NCS) | −3.69 | −10.52 |
| core (RVE) | −0.14 | 0.36 |
| σ (NCS) | 19.60 | 33.68 |
| σ (RVE) | 16.03 | 22.81 |
| core + σ | 15.90 | 23.17 |
| π | −25.0 | −36.41 |
| Total | −9.10 | −13.24 |
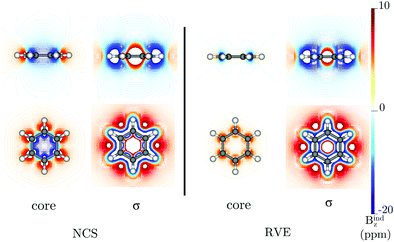
RVE yields a core contribution (coreRVE) to NICScore(0) and Bindz,core(0) of −0.14 and 0.36 ppm, respectively, (see Table 1) in C6H630+, which are similar to the values of −0.13 and 0.46 ppm reported by Steiner and Fowler and much smaller than those obtained by the NCS (coreNCS) approach. The authors also showed that the contributions from the core orbitals are small in the center of the ring, but no visualization of the magnetic response of the core electrons was reported. However, since single-point shielding calculations (such as NICS(0) or Bindz(0)) do not provide a complete image of the overall magnetic behavior, we compute Bindz,core on a grid. Fig. 1 shows that while coreNCS yields a shielding cone with magnetic shieldings between −10 and −20 ppm around the ring, RVE provides a negligible core contribution at the ring center. Consequently, the σ-contributions obtained via NCS (σNCS) and RVE (σRVE) show differences mainly in the molecular plane. The size of the deshielding cone inside the ring also differs.
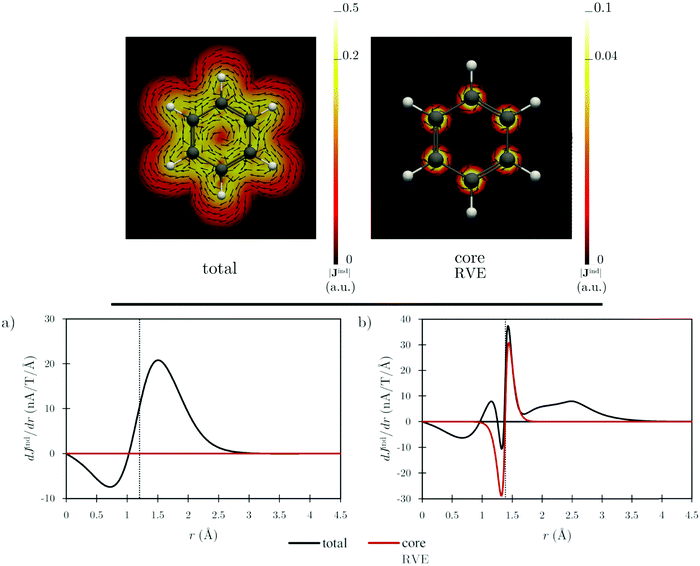
The diatropic current density induced by the core orbitals, using RVE, is localized at the carbon atoms, implying that the core contribution to the ring-current strength in a plane intersecting the C–C bond is negligible, as shown by the dJind/dr profile in Fig. 2a. Integration of the total Jind flowing through that plane yields a ring-current strength of 12 nA T−1.22–24 The dJind/dr profile of the current density flowing through a plane intersecting the carbon and hydrogen atoms yields positive and negative peaks in the vicinity of the carbon atoms for both the total response and the core contribution, respectively. Integrating one of the peaks of the red curve in Fig. 2b yields a value of 4.38 nA T−1, which is the strength of the current of the carbon core. The negative peak at the carbon core is much smaller when current-density contributions from all-orbitals are considered, resulting in a current with a magnitude of about 45% (2 nA T−1) of the coreRVE contribution.36
Corannulene
The orientation of the rings with respect to the external magnetic field influences the strength of the magnetic response of the π-electrons, especially in nonplanar organic molecules.8,9,37 This could lead to a scenario in which the separation of σ and π contributions is complicated. In the case of nonplanar organic molecules, such as the systems discussed here, whose hybridization does not deviate considerably from sp2, it is possible to separate the different orbital contributions.38,39 This is also the principle of approximations such as the pseudo-π model,40 which provide results quite similar to those of the π-electron response computed within the ipsocentric approach, allowing its applicability in both planar and nonplanar molecules.35,38,40–45 Thus, since the π-block is “easy” to identify, the (core + σ)-contribution, which is the result of subtracting the π-block contribution from the total magnetic response, is therefore also feasible to determine. Under these considerations, the π-type contributions of spherical, cylindrical, or quasi-planar molecules are ascertainable. Moreover, subtracting the coreRVE response from the (core + σ)-contribution yields the σ-corrected component as well.In the case of corannulene, there is a bowl-to-bowl inversion process through a planar transition state. Given the shallow curvature, both sides (convex and concave) of corannulene generate a similar magnetic response.46 Interestingly, the ring current of the molecular structure of the planar transition state and the bowl minimum has the same tropicity, while its strength is slightly affected by the deformation from planarity.47 Previous analyses identified a diatropic current in the peripheral carbon π-circuit and a paratropic ring current in the central five-membered ring (5-MR).47,48Table 2 summarizes the Bindz(0) and NICS(0) values in the center of the 5-MR and 6-MRs. For the 6-MRs, Bindz(0) is close to zero, which has been interpreted as a nonaromatic character of these rings, whereas for the 5-MRs, Bindz (0) is positive due to the paratropic ring current of the hub. The coreRVE and σRVE values differ significantly from the coreNCS and σNCS ones. The RVE values of the Bindz,core(0) are smaller than 0.42 ppm, whereas the coreNCS values are about 30 times larger with the opposite sign. The difference is even more striking when comparing the Bindz isolines (see Fig. 3). In NCS formulation, the core electrons seem to induce a long-range shielding zone in both planar and nonplanar forms, whereas in the RVE approach, a weak magnetic response of the core is obtained, localized in the vicinity of the carbon nuclei. Concomitantly, the σ-shielding appears more pronounced in the bowl-shaped configuration considering the RVE approach.
| Point group | core (NCS) | core (RVE) | σ (NCS) | σ (RVE) | core + σ | π | Total | |
|---|---|---|---|---|---|---|---|---|
| D 5h | 5-MR | −11.06 (−2.74) | 0.29 (−0.41) | 45.42 (21.80) | 34.07 (19.44) | 34.36 (19.03) | 30.46 (−6.00) | 64.82 (13.03) |
| 6-MR | −12.20 (−3.97) | 0.42 (−0.19) | 38.31 (20.09) | 25.70 (16.32) | 26.11 (16.13) | −23.77 (−21.21) | 2.34 (−5.08) | |
| C 5v | 5-MR | −15.14 (−5.18) | 0.38 (−0.28) | 28.94 (10.78) | 13.42 (5.88) | 13.80 (5.60) | 43.45 (4.45) | 57.25 (10.05) |
| 6-MR | −12.05 (−5.16) | 0.29 (−0.17) | 29.12 (14.98) | 16.77 (10.02) | 17.07 (9.85) | −18.30 (−17.21) | −1.23 (−7.36) |
 | ||
| Fig. 3 The isolines of the core- and σ-contributions to Bindz calculated for the corannulene structures using the NCS scheme (left) and with the RVE approach (right). | ||
The RVE calculations reveal that Jindcore is highly localized at the nuclei implying that the atomic currents of the carbon atoms do not contribute to the total ring current. Bending the corannulene structure does not significantly affect the core-electron contribution to the ring current, which also remains localized at each carbon atom given by their 1s2 core orbitals (see Fig. 4). The dJind/dr profiles, in the plane intersecting the C–C bonds of the 5-MR and a 6-MR (Fig. S1 in the ESI†), show that the transitions between maxima and minima are smooth and somewhat smaller for the bowl-shaped structure because the diatropic and paratropic contributions to the current density are weaker than those of the planar transition state. Like benzene, the core-electron contribution to dJind/dr vanishes when the current strength is computed in a plane intersecting the C–C bond (Fig. S1a in ESI†). The strength of the atomic current of the bowl-shaped structure is 4.35 nA T−1, which is almost the same strength as for the planar form of 4.32 nA T−1 (Table 3).
| Structure | Hub | Rim |
|---|---|---|
| Planar | −17.94 | 15.38 |
| Bowl-shaped | −12.97 | 14.74 |
C60 fullerene
C60 exhibits a π-type current inside and outside the cage flowing in the paratropic and diatropic directions, respectively.35,49 When the core- and σ-contributions are calculated using NCS, large alternating core shielding and σ-deshielding contributions are obtained.10,11 NCS provides core- and σ-contributions to Bindz(0) of −29.76 and 6.40 ppm, respectively, whereas the coreRVE values are −0.25 and −23.11 ppm. Comparison of coreNCS and coreRVE values reveal diametral differences inside and outside the cage (Fig. 5). The coreRVE contribution is negligible inside the carbon cage. Therefore, the orbital contributions to Bindz obtained with the NCS scheme are incorrect, leading to an incorrect interpretation of the σ-component. Therefore, instead of what had been pointed out before,10,11 the inside of the fullerene is fully shielded because of the σ-electrons. | ||
| Fig. 5 The isolines of the core and σ-contributions to Bindz calculated in a plane parallel (top) and perpendicular (bottom) to the C3 axis of C60 with the NCS (left) and RVE (right) approaches. | ||
Likewise, the Jind calculations also show that the currents in Fig. 6 are localized to the vicinity of the nuclei. From the Bindz calculations, it can be concluded that the truly σ-contribution to Jind has some diatropic circulation inside the cage.
[14]helicene
The shielding of [14]helicene is strongly affected by its curved structure due to the overlapping and superposition of the shielding cones.9 The π-component induces a strong deshielding region along the helical axis that covers some rings, which significantly affects the interpretation of the local aromaticity when NICS(0) or any of its variants are used. The deshielding region is mainly caused by the π-current density flowing along the periphery of the helical structure.35,50 NCS yields large core contributions to Bindz covering the entire structure,9 whereas RVE leads to a completely different interpretation with a core contribution located at the carbon atoms (see Fig. 7). Therefore, in large nonplanar molecules, such as helicenes, the σ-electrons contribute significantly to the shielding cone. This does not mean that the σ-electrons sustain the ring currents in helicenes. In contrast, σ-electrons respond with local shieldings at the C–C bonds, which accumulate and produce the pronounced global shielding cone in these curved structures. It has been shown that π-electrons promote the ring currents in helicenes but form a helical circulation along the edge of the molecular frame that produces a deshielding cone along the helical axis.9,35The role of occupied-to-occupied contributions
The orbital contributions to the magnetic response depend on the gauge-origin. The paramagnetic component of the orbital current density, which formally can be written as a sum over the states in the first-order orbitals, gives rise to unphysical occupied-to-occupied transitions when the gauge-origin does not coincide with the point at which the current density is calculated.17,18Finally, to investigate the origin of the substantial diamagnetic response of core orbitals predicted by a scheme working with GIAOs, we have also used the NMR module of ADF.15,32,33 In this case, orbital contributions to the shielding tensor  can be divided into diamagnetic and paramagnetic terms. The paramagnetic term of the core-electron orbitals can be further analyzed for contributions from occupied-to-occupied and occupied-to-virtual orbitals excitations, and contributions from gauge transformations, according to the GIAO formulation:
can be divided into diamagnetic and paramagnetic terms. The paramagnetic term of the core-electron orbitals can be further analyzed for contributions from occupied-to-occupied and occupied-to-virtual orbitals excitations, and contributions from gauge transformations, according to the GIAO formulation:
 | (1) |
This orbital analysis (Table S2 in the ESI†) is in good agreement with the NCS procedure. The different terms of Eqn 1 for Bindz,core(0) and NICScore(0) of benzene, corannulene and C60 are summarized in Table 4. They show that the strongly shielded response of core orbitals originate exclusively from the occupied-to-occupied term involving core-to-valence excitations, whereas the other components of the shielding tensor have minimal paramagnetic contributions of ∼1 ppm (∼0.2 ppm) to Bindz (NICS), in full agreement with Fowler and Steiner's affirmations.16
| Molecule | Core | |||
|---|---|---|---|---|
| Total | occ–occ | dia + gauge + occ–vir | ||
| Benzene | −12.37 (−4.28) | −13.29 (−4.53) | 0.92 (0.24) | |
| Corannulene D5h | 5-MR | −22.91 (−7.95) | −24.03 (−8.02) | 1.12 (0.07) |
| 6-MR | −15.90 (−5.50) | −16.97 (−5.71) | 1.07 (0.21) | |
| Corannulene C5v | 5-MR | −22.16 (−8.65) | −23.37 (−8.79) | 1.21 (0.14) |
| 6-MR | −16.27 (−6.56) | −17.17 (−6.78) | 0.90 (0.22) | |
| C60 | −28.62 (−28.64) | −29.16 (−29.17) | 0.55 (0.53) | |
| [14]helicene | A | −19.03 (−7.24) | −19.95 (−7.53) | 0.92 (0.29) |
| B | −21.95 (−8.91) | −22.85 (−9.14) | 0.90 (0.22) | |
| C | −22.63 (−9.87) | −23.56 (−10.12) | 0.93 (0.25) | |
| D | −22.06 (−10.55) | −22.97 (−10.78) | 0.91 (0.24) | |
| E | −22.13 (−11.79) | −23.05 (−12.04) | 0.92 (0.25) | |
| F | −23.13 (−14.08) | −24.07 (−14.34) | 0.94 (0.27) | |
| G | −24.52 (−15.98) | −25.48 (−16.28) | 0.97 (0.30) | |
Conclusions
Orbital dissection schemes involving CMO (or NLMO), which works on GIAOs such as the NCS analysis, result in spurious unphysical core-electron contributions due to occupied-to-occupied orbital transitions that lead to a mixing of nuclear magnetic shielding contributions from the core and σ orbitals,16 especially in nonplanar molecules. We have tackled this problem by estimating the core contribution from magnetic shielding calculations using GIAOs on highly charged molecules by removing the valence electrons (RVE). The RVE approach offers a clear representation of their contribution to the overall magnetic behavior, which can be extended to structures involving different elements from the periodic table. RVE yield the magnetic response of the core orbitals, whereas the σ-contribution was estimated by subtracting the core-electron component from the (core + σ)-contribution. RVE shows that the core-electron contributions are localized to the vicinity of the nuclei, which agrees with previous calculations using orbital contributions to the current density and the Biot–Savart integral expression but in disagreement with conclusions drawn from calculations using the NCS approach. The RVE approach can be applied in the cases where the energy gap between the lowest occupied σ orbital and the highest occupied core-electron orbital is large (>250 eV). In organic systems, this condition is usually satisfied, but in inorganic systems, especially in molecules with heavy atoms, it may not be satisfied, and there may be a significant contribution of core electrons to the total response.51–53 In nonplanar molecules the determination of the orbital contributions is not always reliable because the molecular curvature can lead to combined σ and π characters in their orbitals. However, those highly symmetrical systems have π-type orbitals that can be easily identified, so the RVE approach can be employed to obtain the core-electron and σ-corrected contributions as well.RVE calculations on the planar (benzene) and nonplanar molecules (corannulene, C60, and [14]helicene) indicate that the core electrons do not significantly contribute to the magnetic response, despite the curvature of the π-surface. The core-electron contribution to the magnetic shielding cone and the ring-current strength is very small for the case of 1s2 core orbitals. RVE on C60 and [14]helicene reveals that the σ orbitals play an important role in their magnetic shielding, whereas the contribution from the core orbitals is negligible. These conclusions do not agree with those drawn from the NCS calculations. So, the RVE approach will be helpful when determining the correct orbital contributions to the magnetic response of molecules with multiple aromatic characters or of molecules containing heavier elements where the core- and σ-electron contributions are also relevant.
Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The work in Mexico was supported by Conacyt (Proyecto Sinergia 1561802) and in Finland by the Academy of Finland (Project 314821). We also thank Magnus Ehrnrooth foundation and the Swedish Cultural Foundation in Finland for financial support.References
- P. v. R. Schleyer, Introduction: Aromaticity, Chem. Rev., 2001, 101, 1115–1118 CrossRef CAS PubMed.
- G. Merino and M. Solà, Celebrating the 150th Anniversary of the Kekulé Benzene Structure, Phys. Chem. Chem. Phys., 2016, 18, 11587–11588 RSC.
- P. Lazzeretti, Ring Currents, Prog. Nucl. Magn. Reson. Spectrosc., 2000, 36, 1–88 CrossRef CAS.
- J. A. N. F. Gomes and R. B. Mallion, Aromaticity and Ring Currents, Chem. Rev., 2001, 101, 1349–1384 CrossRef CAS PubMed.
- G. Merino, T. Heine and G. Seifert, The Induced Magnetic Field in Cyclic Molecules, Chem. – Eur. J., 2004, 10, 4367–4371 CrossRef CAS PubMed.
- T. Heine, R. Islas and G. Merino, σ and π Contributions to the Induced Magnetic Field: Indicators for the Mobility of Electrons in Molecules, J. Comput. Chem., 2007, 28, 302–309 CrossRef CAS PubMed.
- R. Islas, T. Heine and G. Merino, The Induced Magnetic Field, Acc. Chem. Res., 2012, 45, 215–228 CrossRef CAS PubMed.
- F. A. L. Anet and D. J. O’Leary, The Shielding Tensor. Part I: Understanding Its Symmetry Properties, Concepts Magn. Reson., Part A, 1991, 3, 193–214 CrossRef CAS.
- M. Orozco-Ic, J. Barroso, N. D. Charistos, A. Muñoz-Castro and G. Merino, Consequences of the Curvature on the Induced Magnetic Field: Case of Helicenes, Chem. – Eur. J., 2020, 26, 326–330 CrossRef CAS PubMed.
- N. D. Charistos and A. Muñoz-Castro, Induced Magnetic Field of Fullerenes: Role of σ- and π- Contributions to Spherical Aromatic, Nonaromatic, and Antiaromatic Character in C60q (q = 10, 0, −6, −12), and Related Alkali-Metal Decorated Building Blocks, Li12C60 and Na6C60, J. Phys. Chem. C, 2018, 122, 9688–9698 CrossRef CAS.
- Z. Chen, J. I. Wu, C. Corminboeuf, J. Bohmann, X. Lu, A. Hirsch and P. v. R. Schleyer, Is C60 Buckminsterfullerene Aromatic?, Phys. Chem. Chem. Phys., 2012, 14, 14886–14891 RSC.
- R. Ditchfield, Self-Consistent Perturbation Theory of Diamagnetism, Mol. Phys., 1974, 27, 789–807 CrossRef CAS.
- K. Wolinski, J. F. Hinton and P. Pulay, Efficient Implementation of the Gauge-Independent Atomic Orbital Method for NMR Chemical Shift Calculations, J. Am. Chem. Soc., 1990, 112, 8251–8260 CrossRef CAS.
- J. A. Bohmann, F. Weinhold and T. C. Farrar, Natural Chemical Shielding Analysis of Nuclear Magnetic Resonance Shielding Tensors from Gauge-Including Atomic Orbital Calculations, J. Chem. Phys., 1997, 107, 1173–1184 CrossRef CAS.
- G. Schreckenbach and T. Ziegler, Calculation of NMR Shielding Tensors Using Gauge-Including Atomic Orbitals and Modern Density Functional Theory, J. Phys. Chem., 1995, 99, 606–611 CrossRef CAS.
- E. Steiner and P. W. Fowler, On the Orbital Analysis of Magnetic Properties, Phys. Chem. Chem. Phys., 2004, 6, 261–272 RSC.
- E. Steiner and P. W. Fowler, Patterns of Ring Currents in Conjugated Molecules: A Few-Electron Model Based on Orbital Contributions, J. Phys. Chem. A, 2001, 105, 9553–9562 CrossRef CAS.
- E. Steiner, P. W. Fowler and R. W. A. Havenith, Current Densities of Localized and Delocalized Electrons in Molecules, J. Phys. Chem. A, 2002, 106, 7048–7056 CrossRef CAS.
- T. A. Keith and R. F. W. Bader, Calculation of Magnetic Response Properties using a Continuous Set of Gauge Transformations, Chem. Phys. Lett., 1993, 210, 223–231 CrossRef CAS.
- T. A. Keith and R. F. W. Bader, Topological Analysis of Magnetically Induced Molecular Current Distributions, J. Chem. Phys., 1993, 99, 3669–3682 CrossRef CAS.
- G. Acke, S. Van Damme, R. W. A. Havenith and P. Bultinck, Interpreting the Behavior of the NICSzz by Resolving in Orbitals, Sign, and Positions, J. Comput. Chem., 2018, 39, 511–519 CrossRef CAS PubMed.
- J. Jusélius, D. Sundholm and J. Gauss, Calculation of Current Densities Using Gauge-Including Atomic Orbitals, J. Chem. Phys., 2004, 121, 3952–3963 CrossRef PubMed.
- H. Fliegl, S. Taubert, O. Lehtonen and D. Sundholm, The Gauge Including Magnetically Induced Current Method, Phys. Chem. Chem. Phys., 2011, 13, 20500–20518 RSC.
- D. Sundholm, H. Fliegl and R. J. F. Berger, Calculations of Magnetically Induced Current Densities: Theory and Applications, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2016, 639–678 CAS.
- C. Adamo, M. Cossi and V. Barone, An Accurate Density Functional Method for the Study of Magnetic Properties: The PBE0 Model, J. Mol. Struct. THEOCHEM, 1999, 493, 145–157 CrossRef CAS.
- F. Weigend and R. Ahlrichs, Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman, D. J. Fox, Gaussian 16, revision D.01, Gaussian Inc., 2016 Search PubMed.
- M. Orozco-Ic, J. L. Cabellos and G. Merino, Aromagnetic, 2016, Cinvestav-Merida, Mexico.
- Z. Chen, C. S. Wannere, C. Corminboeuf, R. Puchta and P. v. R. Schleyer, Nucleus-Independent Chemical Shifts (NICS) as an Aromaticity Criterion, Chem. Rev., 2005, 105, 3842–3888 CrossRef CAS PubMed.
- F. Weinhold, C. R. Landis and E. D. Glendening, What Is NBO Analysis and How Is It Useful?, Int. Rev. Phys. Chem., 2016, 35, 399–440 Search PubMed.
- G. Schreckenbach, On the Relation between a Common Gauge Origin Formulation and the GIAO Formulation of the NMR Shielding Tensor, Theor. Chem. Acc., 2002, 108, 246–253 Search PubMed.
- C. M. Widdifield and R. W. Schurko, Understanding Chemical Shielding Tensors Using Group Theory, MO Analysis, and Modern Density-Functional Theory, Concepts Magn. Reson., Part A, 2009, 34A(2), 91–123 CrossRef CAS.
- G. te Velde, F. M. Bickelhaupt, E. J. Baerends, C. Fonseca Guerra, S. J. A. van Gisbergen, J. G. Snijders and T. Ziegler, Chemistry with ADF, J. Comput. Chem., 2001, 22, 931–967 CrossRef CAS.
- M. Orozco-Ic, M. Dimitrova, J. Barroso, D. Sundholm and G. Merino, Magnetically Induced Ring-Current Strengths of Planar and Nonplanar Molecules: New Insights from the Pseudo-π Model, J. Phys. Chem. A, 2021, 125, 5753–5764 CrossRef CAS PubMed.
- M. Dimitrova and D. Sundholm, Current density, current-density pathways, and molecular aromaticity, in Aromaticity: Modern Computational Methods and Applications, Elsevier, 2021, pp. 155–194 Search PubMed.
- A. G. Papadopoulos, N. D. Charistos and A. Muñoz-Castro, Magnetic Response of Aromatic Rings Under Rotation: Aromatic Shielding Cone of Benzene Upon Different Orientations of the Magnetic Field, ChemPhysChem, 2017, 18, 1499–1502 CrossRef CAS PubMed.
- A. Soncini, R. G. Viglione, R. Zanasi, P. W. Fowler and L. W. Jenneskens, Efficient mapping of ring currents in fullerenes and other curved carbon networks, C. R. Chim., 2006, 9, 1085–1093 CrossRef CAS.
- G. Monaco, F. F. Summa and R. Zanasi, Program Package for the Calculation of Origin-Independent Electron Current Density and Derived Magnetic Properties in Molecular Systems, J. Chem. Inf. Model., 2021, 61, 270–283 CrossRef CAS PubMed.
- P. W. Fowler and E. Steiner, Pseudo-π Currents: Rapid and Accurate Visualisation of Ring Currents in Conjugated Hydrocarbons, Chem. Phys. Lett., 2002, 364, 259–266 CrossRef CAS.
- N. D. Charistos, A. Muñoz-Castro and M. P. Sigalas, The Pseudo-π Model of the Induced Magnetic Field: Fast and Accurate Visualization of Shielding and Deshielding Cones in Planar Conjugated Hydrocarbons and Spherical Fullerenes, Phys. Chem. Chem. Phys., 2019, 21, 6150–6159 RSC.
- M. Antić, S. Đorđević, B. Furtula and S. Radenković, Magnetically Induced Current Density in Nonplanar Fully Benzenoid Hydrocarbons, J. Phys. Chem. A, 2020, 124, 371–378 CrossRef PubMed.
- P. W. Fowler, W. Myrvold, C. Gibson, J. Clarke and W. H. Bird, Ring-Current Maps for Benzenoids: Comparisons, Contradictions, and a Versatile Combinatorial Model, J. Phys. Chem. A, 2020, 124, 4517–4533 CrossRef CAS PubMed.
- M. Orozco-Ic and G. Merino, The Magnetic Response of Starphenes, Chemistry, 2021, 3, 1381–1391 CrossRef CAS.
- M. Orozco-Ic, R. R. Valiev and D. Sundholm, Non-intersecting ring currents in [12]infinitene, Phys. Chem. Chem. Phys., 2022, 24, 6404–6409 RSC.
- A. Reisi-Vanani and A. A. Rezaei, Evaluation of the Aromaticity of Non-Planar and Bowl-Shaped Molecules by NICS Criterion, J. Mol. Graphics Modell., 2015, 61, 85–88 CrossRef CAS PubMed.
- E. Steiner, P. W. Fowler and L. W. Jenneskens, Counter-Rotating Ring Currents in Coronene and Corannulene, Angew. Chem., Int. Ed., 2001, 40, 362–366 CrossRef CAS.
- A. Acocella, R. W. A. Havenith, E. Steiner, P. W. Fowler and L. W. Jenneskens, A Simple Model for Counter-Rotating Ring Currents in [n]circulenes, Chem. Phys. Lett., 2002, 363, 64–72 CrossRef CAS.
- M. P. Johansson, J. Jusélius and D. Sundholm, Sphere Currents of Buckminsterfullerene, Angew. Chem., Int. Ed., 2005, 44, 1843–1846 CrossRef CAS PubMed.
- E. Cherni, B. Champagne, S. Ayadi and V. Liégeois, Magnetically-Induced Current Density Investigation in Carbohelicenes and Azahelicenes, Phys. Chem. Chem. Phys., 2019, 21, 14678–14691 RSC.
- Z. Badri, S. Pathak, H. Fliegl, P. Rashidi-Ranjbar, R. Bast, R. Marek, C. Foroutan-Nejad and K. Ruud, All-Metal Aromaticity: Revisiting the Ring Current Model among Transition Metal Clusters, J. Chem. Theory Comput., 2013, 9, 4789–4796 CrossRef CAS PubMed.
- C. Foroutan-Nejad, J. Vícha and A. Ghosh, Relativity or Aromaticity? A First-Principles Perspective of Chemical Shifts in Osmabenzene and Osmapentalene Derivatives, Phys. Chem. Chem. Phys., 2020, 22, 10863–10869 RSC.
- G. Periyasamy, N. A. Burton, I. H. Hillier and J. M. H. Thomas, Electron Delocalization in the Metallabenzenes: A Computational Analysis of Ring Currents, J. Phys. Chem. A, 2008, 112(26), 5960–5972 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1 with dJind/dr plots of corannulene is reported. Table S1 shows the ΔLσ-Hcore values for all systems and Table S2 shows the orbital components of the Bindz(0) and NICS(0) values computed with ADF's NMR module. See DOI: https://doi.org/10.1039/d1cp05713h |
| This journal is © the Owner Societies 2022 |