Supramolecular architectures featuring Se⋯N secondary-bonding interactions in crystals of selenium-rich molecules: a comparison with their congeners†
Edward R. T.
Tiekink
 *
*
Research Centre for Crystalline Materials, School of Medical and Life Sciences, Sunway University, 47500 Bandar Sunway, Selangor Darul Ehsan, Malaysia. E-mail: edwardt@sunway.edu.my
First published on 23rd November 2022
Abstract
Supramolecular aggregates featuring Se⋯N contacts have been identified in 88 crystals containing two, three or four selenium atoms, corresponding to 9% of crystals where Se⋯N contacts have the potential to form. Molecules with two selenium atoms make up the majority of examples (58) followed by molecules with three (12) and four (18) selenium atoms. A wide range of supramolecular aggregation patterns has been identified in the 88 crystals featuring Se⋯N contacts with one- (42%) and two- (31%) dimensional aggregation forming the majority compared with zero- (17%) and three- (10%) dimensional aggregation making up the rest. The overwhelming majority of Se⋯N contacts correspond to chalcogen-bonding interactions based on σ-hole interactions. Correlations in intermolecular separations were evident for isostructural crystals which showed that a comparable Te⋯N interaction was stronger than the equivalent Se⋯N interaction, and the Se⋯N interaction was always stronger than the equivalent S⋯N interaction. When compared to crystals of mono-nuclear selenium compounds, a very similar adoption rate was observed in their crystals but with a bias towards smaller aggregation patterns, i.e. 50, 44, 5 and 1% for zero-, one-, two- and three- dimensional, respectively. This observation is clearly consistent with the greater opportunity for multi-nuclear selenium molecules to form more interactions. However, as exemplified by the 18 tetra-nuclear molecules, not all selenium atoms participated in a Se⋯N contact as two, 10, two and four selenium atoms, out of a possible four, formed a Se⋯N contact in their crystals.
Introduction
The 21st amino acid, selenocysteine, a natural bearer of selenium, is found in at least 25 selenoproteins.1,2 Prominent amongst these are the thioredoxin reductases and glutathione peroxidases, the primary function of which is to protect the body against oxidative damage.3,4 Thyroid hormones can also be activated by deiodinase enzymes for which selenocysteine is present in the active site.5 As an extension to their natural biological functions, synthetic selenium-containing compounds have long attracted the interest of medicinal chemists owing to their potential applications as anti-bacterial, anti-cancer, anti-inflammatory, etc. agents.6–9 Among these, cytoprotective Ebselen™ (2-phenyl-1,2-benzoselenazol-3(2H)-one) has entered the pharmacopoeia as an anti-oxidant/anti-inflammatory drug, being a mimic of glutathione peroxidase.10,11 Prompted by these considerations, considerable effort has been made to ascertain the mechanisms of action and modes of supramolecular association with biologically-relevant substrates.12–21 Over and above biological roles/applications, selenium compounds attract interest in terms of the sensing of anions and molecules,22–24 and, increasingly, catalysis,25–28 materials,29–31 covalent organic frameworks (COFs)32,33 and as fluorescence probes34 – all again demanding investigations of the supramolecular chemistry of selenium. Complementing these specialist reviews are more general overviews of the broader roles and implications of chalcogen-bonding.35–39In keeping with the specific categorization of different intermolecular interactions,40 rather than the generic “secondary-bonding”,41 end-on, highly directional interactions involving chalcogen atoms were termed “chalcogen-bonding” in 2018 by Wang et al.,42 with a formal definition appearing soon after in 2019.43 However, the explanation behind chalcogen-bonding and related interactions, notably halogen-bonding, came earlier through the development of the σ-hole concept.44–49 In chalcogen-bonding, at the extension of a R–Ch bond, an electron-deficient σ-hole develops with the potential to interact with a nucleophile, Ch = any chalcogen. Not surprisingly, theoretical aspects and the energetics of chalcogen-bonding have attracted the interest of computational chemists in recent years.50–57
Recent, comparative studies on the model homo-dimer comprising two five-membered chalcogen-substituted adiazolyl rings connected by two Ch⋯N interactions, i.e. via a four-membered {⋯Ch–N}2 synthon, for Ch = O, S, Se and Te, are especially worth highlighting.56 Within the planar, geometry-optimised dimers, the Ch⋯N interactions were found to be cooperative with energies in the order 4 < 7 < 17 kcal mol−1 for Ch = S, Se and Te, respectively; a negligible interaction energy was calculated for the oxygen congener. These trends are in accord with earlier studies.58,59 Further, these results correlate with the depth of the σ-hole which is correlated with the Vmax values on the respective calculated molecular electrostatic potential maps (MEP's) being 12.9, 19.2 and 31.5 kcal mol−1 for Ch = S, Se and Te, respectively.56 These results are consistent with significant electrostatic contributions (ca. 58%) calculated for related Ch⋯N chalcogen bonds in a similar dimeric aggregate featuring a four-membered {⋯Ch–N}2 synthon.54 The latter study also showed that orbital contributions to the Ch⋯N chalcogen bonds were most important for tellurium cf. sulphur, while the reverse was true for the dispersion term.54 Given that radicals are also included in the present survey, it is salient to mention that allied calculations on similar five-membered ring systems (with each bearing a pair of adjacent Ch atoms) also feature {⋯Ch–N}2 synthons that show almost the same relative contributions to the bonding associated with the Ch⋯N chalcogen bonds.55
In continuation of a long-held interest in ascertaining the nature of supramolecular aggregation patterns featuring secondary bonding interactions of selenium and tellurium, and the propensity of these to form in molecular crystals,60–67 the present bibliographic survey covers molecules with two, three or four selenium atoms forming secondary-bonding interactions with nitrogen, as a complement to a recent survey detailing analogous interactions in crystals involving mono-nuclear selenium compounds.66 Thus, crystals of bi-, tri- and tetra-nuclear selenium compounds are evaluated for arrangements featuring Se⋯N contacts with the results compared with their mono-nuclear analogues; higher nuclearity selenium compounds with Se⋯N interactions were not found. Further, those crystals having Se⋯N contacts are compared with their tellurium, sulphur and oxygen congeners in order to determine the practical effects of changing the nature of the chalcogen atom in terms of forming Ch⋯N interactions.
Methods
The CSD68 (version 5.42, two updates) was searched for Se⋯N contacts employing ConQuest (version 2.0.4)69 for all molecules with more than one selenium atom and employing a distance protocol, i.e. any Se⋯N separation had to be equal to or less than the sum of the van der Waals radii, assumed to be 3.45 Å.70,71 Non-ionic, non-polymeric structures of organic molecules with R ≤ 0.075 and having three-dimensional coordinates not suffering from errors were extra conditions applied to the search protocols. This resulted in 121 “hits”, which included a rather large number of duplicates, mainly owing to the intense interest in the radical chemistry of selenium compounds. Manual sorting was conducted with DIAMOND72 to remove examples where the Se⋯N interactions were acting in concert with other obvious interactions (three examples) leaving, in all, 88 examples for further detailed analysis and description. Geometric data, images, full bibliographic details and descriptions of supramolecular aggregation, including additional intra- and inter-molecular contacts not having an impact upon the Se⋯N interactions, are included in ESI† Tables S1 (di-nuclear selenium species), S2 (di-nuclear selenium species co-crystallised with additional species), S3 (tri-nuclear selenium species) and S4 (tetra-nuclear species); equivalent data for oxygen, sulphur and tellurium congeners of 1–88 are available in ESI† Table S5. All diagrams are original and were generated with DIAMOND.72Supramolecular architectures
The discussion is arranged in the following fashion. Di-nuclear selenium aggregates are discussed before tri- and tetra-nuclear species; for the significantly more numerous di-nuclear selenium species, those co-crystallised with other molecules are split from those that do not. Within each category, aggregates involving the participation of one selenium atom are discussed before those involving two selenium atoms, etc. Generally, within each of the above categories, zero-dimensional aggregation patterns are discussed before one-dimensional patterns, etc. Wherever possible, groups of common supramolecular motifs are grouped together with less common ones, “miscellaneous” aggregates are described at the end of each category/sub-category. The focus herein is upon supramolecular aggregation patterns found in the crystals of 1–88: the interested reader is referred to the original publication for details of the specific application motivating their study.Supramolecular architectures featuring di-nuclear selenium molecules involving the participation of one selenium atom only
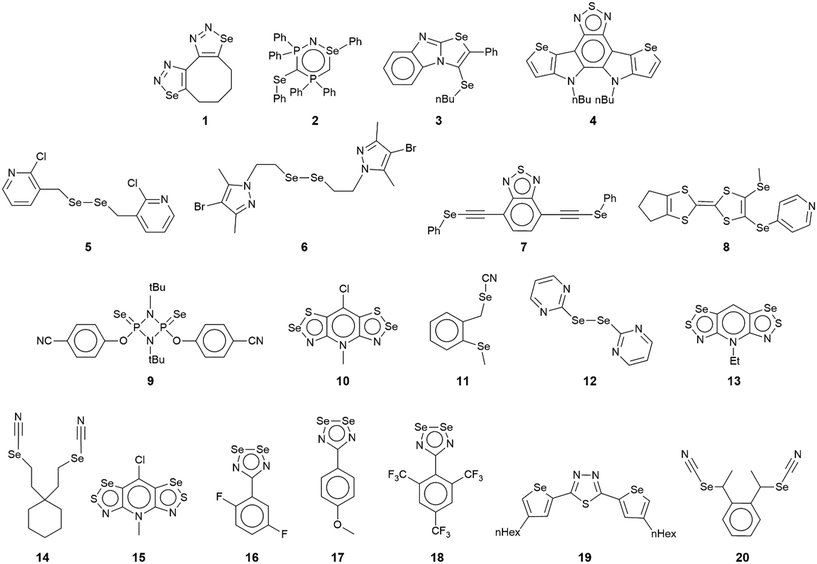
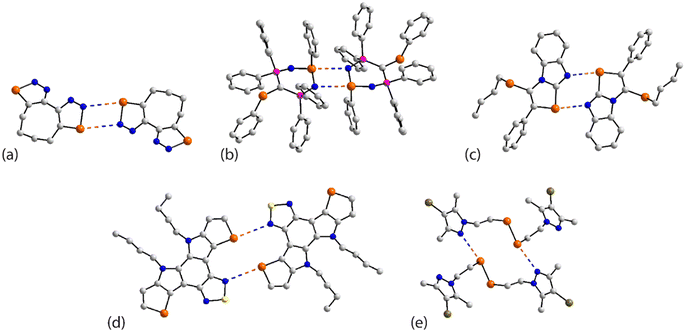
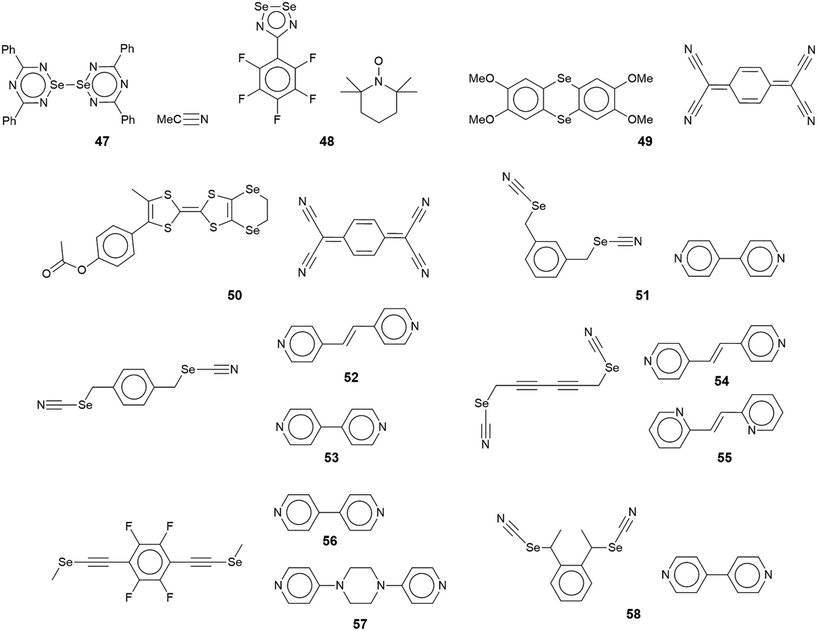
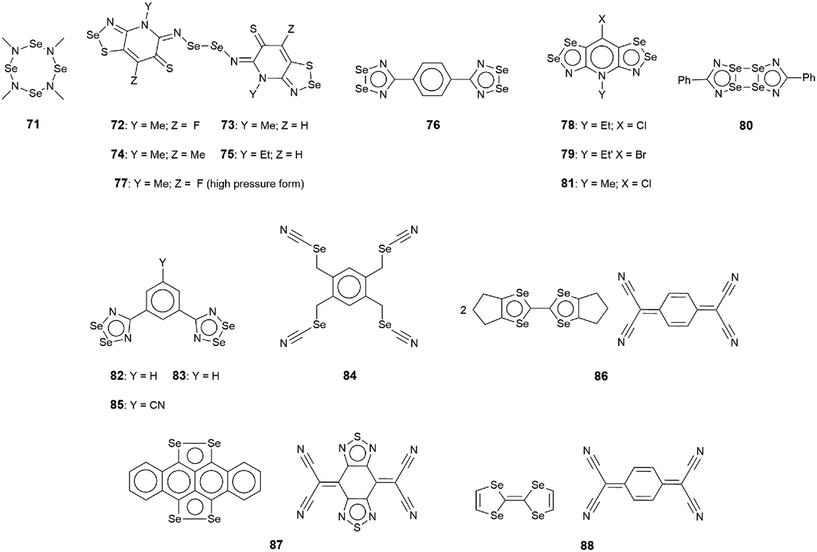
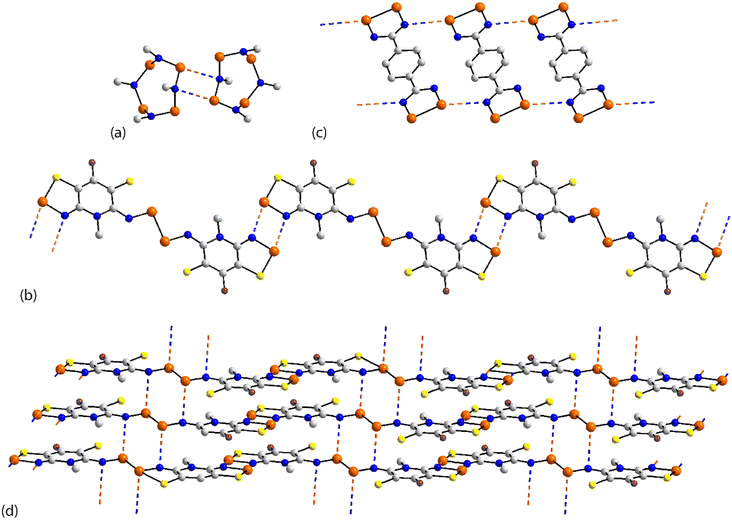
The chemical diagrams for the 20 molecules discussed in this category are shown in Fig. 1, with key geometric data listed in Table 1. Dimeric aggregates featuring a pair of Se⋯N contacts are noted in the crystals of 1–6.73–78 A four-membered, centrosymmetric {⋯Se–N}2 synthon is found in each of 1,73Fig. 2(a), and 2,74Fig. 2(b). The synthon in 1 is often repeated throughout this survey (see below) and featured prominently in the review of mono-nuclear selenium compounds.66 The analogous synthon in 2 also involves a ring selenium atom, rather than the exocyclic selenium. Similarly, the ring-selenium in 3 (ref. 75) also forms the contact leading to a six-membered {⋯SeCN}2 synthon, Fig. 2(c). In 4,76 for which each selenium atom resides within a five-membered ring, only one of these associates with a symmetry related molecule, over a 2-fold axis of symmetry, to form a 10-membered {⋯SeC3N}2 synthon, Fig. 2(d). The next two molecules feature Se–Se bonds. In 5,77 three independent molecules comprise the crystallographic asymmetric-unit. One of these self-associated about a centre of inversion to form a dimer featuring a 10-membered {⋯Se2C2N}2 synthon; this corresponds to the shorter Se⋯N separation for this entry in Table 1. A second, non-symmetric dimer is formed between the remaining two molecules; one of the Se⋯N contacts in this dimer is marginally longer than 3.45 Å, the van der Waals cut-off separation employed in this analysis.70,71 In 6,78 a centrosymmetric, 12-membered {⋯Se2C2N2}2 synthon is found, Fig. 2(e).| No. | Se⋯N; Nca | W–Se⋯N | X–Se⋯N | Y–N⋯Se | Z–N⋯Se | REFCODE | Ref. |
|---|---|---|---|---|---|---|---|
| a Nc = normalised contact, i.e. the ratio of d(Se⋯N) to ∑vdW radii (3.45 Å). | |||||||
| 1 | 3.381(9); 0.98 | C, 146.4(3) | N, 75.8(3) | Se, 104.2(3) | N, 134.8(6) | QAFBUY | 73 |
| 2 | 3.023(3); 0.88 | N, 171.39(9) | N, 76.30(9) | Se, 103.70(10) | P, 128.77(14) | XIVPEX | 74 |
| C, 81.95(12) | |||||||
| 3 | 2.9798(18); 0.86 | N, 167.79(7) | N, 82.72(7) | C, 113.72(13) | C, 142.40(14) | TAXZEZ | 75 |
| 4 | 3.433(3); 1.00 | C, 171.64(12) | C, 92.96(12) | S, 113.59(11) | C, 138.6(2) | VEHVIQ | 76 |
| 5 | 3.378(7); 0.98 | C, 163.4(3) | Se, 83.53(14) | (Cl)C, 100.4(5) | C, 99.5(6) | DIHHEI | 77 |
| 3.413(8); 0.99 | C, 154.8(3) | Se, 86.11(14) | (Cl)C, 108.2(6) | C, 91.4(6) | |||
| 3.451(7); 1.00 | C, 161.5(3) | Se, 79.61(13) | (Cl)C, 103.8(6) | C, 100.7(5) | |||
| 6 | 3.141(6); 0.91 | Se, 168.61(12) | C, 75.8(3) | N, 127.3(5) | C, 127.4(4) | GIWXAO | 78 |
| 7 | 3.345(3); 0.97 | (Ph)C, 162.90(9) | C, 77.96(11) | (S)C, 105.30(12) | C, 96.03(16) | BAFKOM | 79 |
| 8 | 3.360(7); 0.97 | C, 168.5(3) | C, 74.3(2) | C, 119.3(6) | C, 121.1(5) | NIBWOM | 80 |
| 9 | 3.365(3); 0.98 | P, 153.87(5) | C, 83.6(2) | BULQIK | 81 | ||
| 10 | 3.001(5); 0.87 | N, 162.61(19) | S, 74.82(12) | Se, 81.28(18) | C, 162.6(4) | SUQQUR | 82 |
| 11 | 3.125(4); 0.91 | C, 166.75(12) | (N)C, 72.72(12) | C, 135.0(3) | YUNSIK | 83 | |
| 12 | 3.349(14); 0.97 | C, 163.7(5) | Se, 90.0(2) | C, 120(1) | C, 126(1) | SEDZEI | 84 |
| 13 | 3.405(3); 0.99 | S, 174.46(6) | C, 87.84(10) | S, 95.35(12) | C, 122.75(19) | HIGQOE | 85 |
| 14 | 3.199(2); 0.93 | (N)C, 145.69(8) | C, 76.77(8) | C, 126.28(17) | GOHMEW | 86 | |
| 15 | 3.234(3); 0.94 | C, 176.51(11) | S, 89.78(5) | S, 94.13(12) | C, 121.2(2) | SUQRAY | 82 |
| 16 | 3.15(2); 0.91 | N, 169.2(7) | Se, 101.7(4) | Se, 93.7(8) | C, 155(2) | WERQEP | 87 |
| 3.33(2); 0.97 | N, 163.0(9) | Se, 106.0(4) | Se, 96.0(8) | C, 145(2) | |||
| 17 | 3.415(13); 0.99 | Se, 157.8(2) | N, 89.3(5) | Se, 90.4(5) | C, 144(1) | WIMNEL10 | 88 |
| 18 | 2.840(9); 0.82 | Se, 161.05(16) | N, 72.6(3) | Se, 107.5(3) | C, 136.9(6) | UBOJON | 89 |
| 3.254(8); 0.94 | N, 134.0(3) | Se, 129.93(13) | Se, 137.0(3) | C, 105.5(5) | |||
| 3.288(9); 0.95 | Se, 135.29(14) | N, 130.2(3) | Se, 133.0(3) | C, 108.1(6) | |||
| 19 | 3.048(6); 0.88 | C, 164.2(3) | C, 78.9(3) | N, 91.3(4) | C, 115.7(5) | SIKWIT | 90 |
| 3.369(6); 0.98 | C, 157.0(2) | C, 82.6(3) | N, 64.8(3) | C, 122.8(4) | |||
| 20 | 3.283(7); 0.95 | (N)C, 162.6(2) | C, 75.12(17) | C, 149.3(6) | MAHHEM | 91 | |
| 3.393(6); 0.98 | C, 167.36(17) | (N)C, 73.1(2) | C, 144.7(5) | ||||
One-dimensional, supramolecular chains form the largest group in this category, the adoption of distinct topologies notwithstanding. Each of 7,79Fig. 3(a), 8 (ref. 80) and 9,81Fig. 3(b), employs a single selenium atom to form a linear chain. In 8, the Se⋯N contact occurs between the methylselanyl and pyridyl-nitrogen atoms, whereas in 9, a rare contact is formed between selenide-selenium and nitrile-nitrogen atoms.
 | ||
| Fig. 3 Supramolecular aggregation patterns featuring Se⋯N contacts in the crystals of (a) 7, (b) 9, (c) 10 and (d) 14. Additional colour codes: chloride, cyan; oxygen, red. | ||
The largest class of one-dimensional, supramolecular polymers are those with zigzag topologies as noted for 10,82Fig. 3(c), 11,8312 (ref. 84) and 13;85 molecules 10 and 13 are radicals. Glide symmetry is responsible for the adopted topology in each case. The contacts in 10 involve ring atoms, while in 11 a diorganoselenium centre bound to a nitrile ligand associates with symmetry related nitrile-nitrogen atoms. In 12, which features a central Se–Se bond and four potential pyridyl-nitrogen donors, only a single interaction is formed within the chain. The molecule of 13 is a pseudo positional isomer of 10, at least in the sense that the selenium and sulphur atoms in each ring have been interchanged; the interactions to form the chain also involve ring-atoms.
The three remaining chains have helical (21) topologies. These are found for 14,86Fig. 3(d), 15 (ref. 82) and 16.87 The chain in 14 is closely related to that formed in 11; 15, a radical, is a strict positional isomer of 10. Each of the two independent molecules in 16 has a Se–Se bond within a five-membered ring; 16 is a radical. The two independent molecules form a dimeric aggregate and these assemble into a helical chain.
The remaining four molecules in this category may be considered “miscellaneous”. The first of these, namely 17 (ref. 88) has a molecular structure similar to that for the aforementioned 16, but there is only a single Se⋯N contact to form a non-symmetric, two-molecule species, Fig. 4(a). The aggregate in 18,89 a radical, comprises six molecules, three of which are crystallographically independent as the aggregate is located about a centre of inversion. As seen from Fig. 4(b), centrosymmetrically related molecules associate via a four-membered {⋯SeN}2 synthon; this involves the shorter of the three independent Se⋯N separations (Table 1). The terminal molecule forms an interaction as seen in 17, while the middle molecule functions as a bridge by forming two Se⋯N contacts, one being an acceptor and the other a donor, as noted above for 16.
 | ||
| Fig. 4 Supramolecular aggregation patterns featuring Se⋯N contacts in the crystals of (a) 17, (b) 18, (c) 19 and (d) 20. Additional colour code: fluoride, plum. | ||
An unusual mode of association is noted within a helical chain in the crystal of 19.90 Here, one selenium atom interacts with both thiadiazolyl–nitrogen atoms; the Se⋯N separations differ by ca. 0.32 Å. In the final crystal in this category, 20,91 the selenium atom again forms two Se⋯N contacts, as for 19, but this time with well separated nitrile-nitrogen atoms to form a two-dimensional array, Fig. 4(d); the array has a jagged topology. In this case, the Se⋯N separations differ by about 0.11 Å.
While a more detailed discussion on geometric parameters will be given below in the discussion of congeners, a few comments on those pertaining to 1–20 are worthwhile. First and foremost, the crucial angle data are indicative of σ-hole interactions. The σ-hole develops at the selenium atom connected to a range of atoms, primarily nitrogen and carbon, but also sulphur, phosphorus and selenium. While most of the angles subtended at the selenium atom involved in forming the Se⋯N contact are >150°, several are more acute but are likely to still be associated with σ-hole interactions. Thus, the narrowest angle of 134.0(3)° is found in the six-molecule aggregate in 18 where steric congestion is likely to have an impact upon the angles involved in the chalcogen bond. The range of Se⋯N separations is great, i.e. from a short 2.840(9) Å for the “centrosymmetric” contacts in 18, to a long 3.451(7) Å for one of the contacts in 5. With respect of the sum of the van der Waals radii, these experimental distances may be expressed in terms of normalised contacts (Nc), i.e. the ratio of d(Se⋯N) to the sum of the van der Waals radii (3.45 Å),70,71 of 0.82 to 1.00, respectively.
Supramolecular architectures featuring di-nuclear selenium molecules involving the participation of both selenium atoms – two Se⋯N contacts per molecule
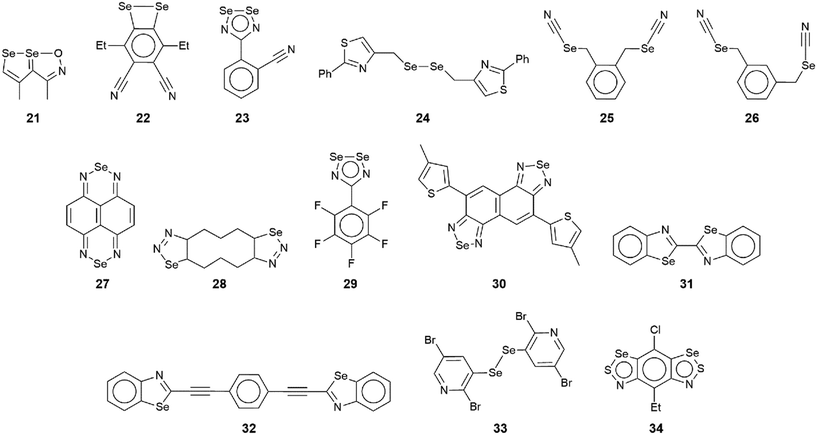
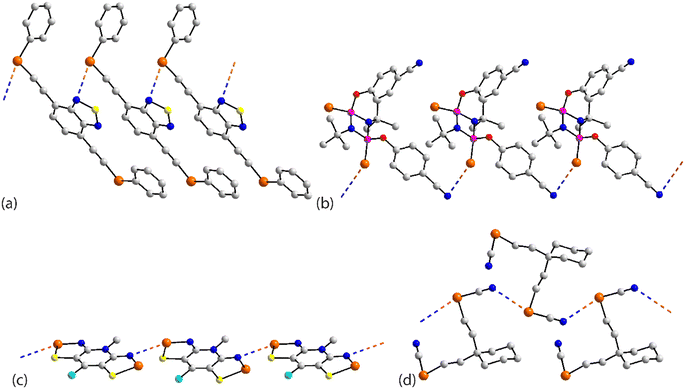
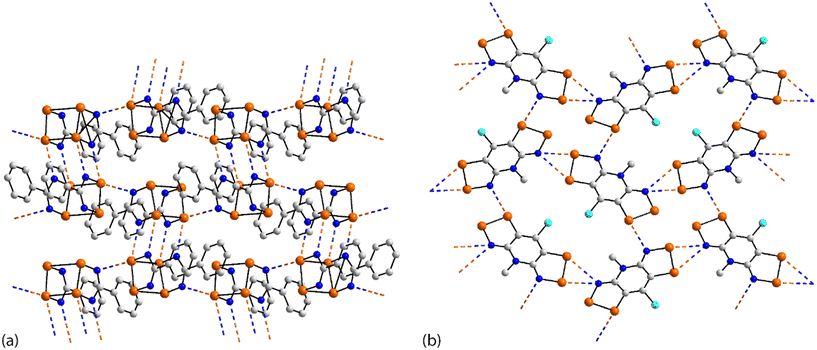
The chemical structures for 21–34, which fall into this sub-category, i.e. where each selenium atom forms a Se⋯N contact, are given in Fig. 5 and geometric parameters in Table 2. From the data included in Table 2, all Se⋯N contacts are indicative of chalcogen-bonding interactions. The first of the three aggregates, namely 21,9222 (ref. 93) and 23,94 have both selenium atoms interacting with the same nitrogen atom. A two-molecule aggregate is formed in 21, Fig. 6(a), where both selenium atoms of one molecule accept electron-density from the ring-nitrogen of another. This molecule is of additional interest in that it contains both selenium(II) and selenium(IV) atoms, being the only example in the present survey of a molecule with a selenium(IV) centre. In each of 22 and 23, the donor atom is a nitrile-nitrogen. The topology of the chain in 22 is linear whereas in 23, a radical species, there are two independent molecules which assemble into an undulating zigzag chain, Fig. 6(b). While there are differences in the magnitudes of the Se⋯N separations, these do not exceed 0.21 Å (for one of the molecules in 23, Table 2).| No. | Se⋯N; Nc | W–Se⋯N | X–Se⋯N | Y–N⋯Se | Z–N⋯Se | REFCODE | Ref. |
|---|---|---|---|---|---|---|---|
| a Standard uncertainties are not available. | |||||||
| 21 | 3.213(10); 0.93 | C, 157.6(3) | Se, 70.8(2) | O, 94.7(6) | C, 140.1(7) | FIKYIH | 92 |
| O, 117.9(3) | |||||||
| 3.337(11); 0.97 | C, 152.3(4) | Se, 65.4(2) | O, 112.6(7) | C, 129.9(7) | |||
| 22 | 3.049(5); 0.88 | C, 144.24(17) | Se, 69.07(10) | C, 172.2(5) | HOGBOW | 93 | |
| 3.124(6); 0.91 | C, 140.24(17) | Se, 65.73(9) | C, 128.3(4) | ||||
| 23 | 2.96(2); 0.86 | N, 165.2(6) | Se, 72.65(8) | C, 133(1) | PAHRUL | 94 | |
| 3.173(13); 0.92 | N, 151.1(5) | Se, 63.02(7) | C, 164(1) | ||||
| 2.97(2); 0.86 | N, 164.2(4) | Se, 72.2(4) | C, 136(2) | ||||
| 3.16(2); 0.92 | N, 150.9(5) | Se, 63.5(4) | C, 165(2) | ||||
| 24 | 3.342(3); 0.97 | Se, 171.91(6) | C, 77.85(14) | C, 113.40(2) | C, 119.97(3) | FEYBAP | 95 |
| 25 | 2.969(9); 0.86 | C, 172.6(3) | C, 77.8(3) | N, 174.0(8) | NARBIR | 96 | |
| 2.985(8); 0.87 | C, 173.0(3) | C, 75.1(3) | N, 173.3(7) | ||||
| 26 | 2.97(2); 0.86 | (N)C, 175.9(7) | C, 79.3(5) | C, 174(2) | FEDHUU | 97 | |
| 3.02(2); 0.88 | (N)C, 175.7(7) | C, 80.6(5) | C, 176(2) | ||||
| 3.01(2); 0.87 | (N)C, 172.9(7) | C, 78.5(5) | C, 172(2) | ||||
| 3.02(2); 0.88 | (N)C, 174.1(7) | C, 80.2(5) | C, 174(2) | ||||
| 27 | 2.90; 0.84 | N, 175.3 | N, 72.2 | Se, 107.8 | C, 131.3 | NAPSEZ10 | 98 |
| 28 | 3.094(15); 0.90 | C, 155.64(6) | N, 69.08(6) | N, 138.12(12) | Se, 110.92(6) | ZEJKUU | 99 |
| 29 | 3.092(6); 0.90 | Se, 165.43(11) | N, 80.8(2) | Se, 98.9(2) | C, 144.5(4) | UBOHOL | 89 |
| 3.097(6); 0.90 | Se, 168.72(11) | N, 80.5(2) | Se, 98.9(2) | C, 145.5(4) | |||
| 30 | 2.966(3); 0.86 | N, 163.66(10) | N, 71.25(9) | Se, 108.75(10) | C, 143.84(19) | EJIYED | 100 |
| 31 | 3.234(4); 0.94 | C, 176.95(15) | C, 95.92(15) | C, 109.0(2) | C, 133.7(3) | WULTOP | 101 |
| 32 | 3.381(5); 0.98 | C, 162.56(16) | C, 84.69(15) | C, 109.2(3) | C, 131.5(3) | RUVWEM | 102 |
| 33 | 3.414(9); 0.99 | C, 140.5(3) | Se, 117.24(14) | C, 99.8(6) | (Br)C, 98.1(5) | ABOWUK | 103 |
| 34 | 3.279(2); 0.95 | C, 164.51(9) | S, 102.17(4) | S, 81.28(8) | C, 123.38(16) | YODSUG | 104 |
 | ||
| Fig. 6 Supramolecular aggregation patterns featuring Se⋯N contacts in the crystals of (a) 21, (b) 23, (c) 24, (d) 25 and (e) 27. | ||
The molecule in 24 (ref. 95) is constructed about a central Se–Se bond, with each selenium interacting with a thiazolyl-nitrogen atom. In so doing, centrosymmetric, eight-membered {⋯SeC2N}2 synthons are formed, Fig. 6(c). Aggregates resembling supramolecular ladders are formed in the crystals of the di-nitrile substituted molecules in each of 25 (ref. 96) and 26.97 In 25, Fig. 6(d), the nitrile residues are orientated in the same direction to form a regular ladder whereas in 26, the nitrile residues are directed to opposite sides of the molecule in each of the two independent molecules so that the staves of the supramolecular ladder are skewed with respect to the rails. Supramolecular tapes are evident in the crystals of 27,9828,9929 (ref. 89) and 30;10029 is a radical. The common feature is the formation of four-membered {⋯SeN}2 synthons, as illustrated in Fig. 6(e) for 27; these are centrosymmetric in all tapes except for 29. By virtue of being constructed about a central, non-planar ten-membered ring, the tape in 28 deviates from planarity and may be considered as resembling a ribbon.
The four remaining aggregation patterns are based on two-dimensional arrays but each with a distinctive topology. The molecule in 31 (ref. 101) is centrosymmetric and the four heteroatoms of the two selenazoyl rings form Se⋯N contacts with four different molecules to form the assembly shown in Fig. 7(a); the layer is flat. The molecule of 32 (ref. 102) is also centrosymmetric but rather than being directly connected, the selenazoyl rings are separated by 1,4-phenylenediethyne spacers. The connections are as just described for 31 but lead to a more open arrangement as evidenced in Fig. 6(b) with the topology of the array being distinctly zigzag in nature when viewed side-on. The molecule in 33 (ref. 103) is disposed about a 2-fold axis which bisects the Se–Se bond. Each molecule participates in four Se⋯N(pyridyl) contacts to form the jagged array illustrated in Fig. 6(c). An aesthetically impressive open framework is formed in the crystal of the radical 34,104Fig. 6(d), where the molecule lies on a mirror plane relating the two selenathiazoyl rings. Each molecule associates with four others, about a site of symmetry ![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) , to form interconnected 20-membered {⋯SeC3Se⋯NCNCN}2 and 12-membered {⋯SeSN}4 synthons.
, to form interconnected 20-membered {⋯SeC3Se⋯NCNCN}2 and 12-membered {⋯SeSN}4 synthons.
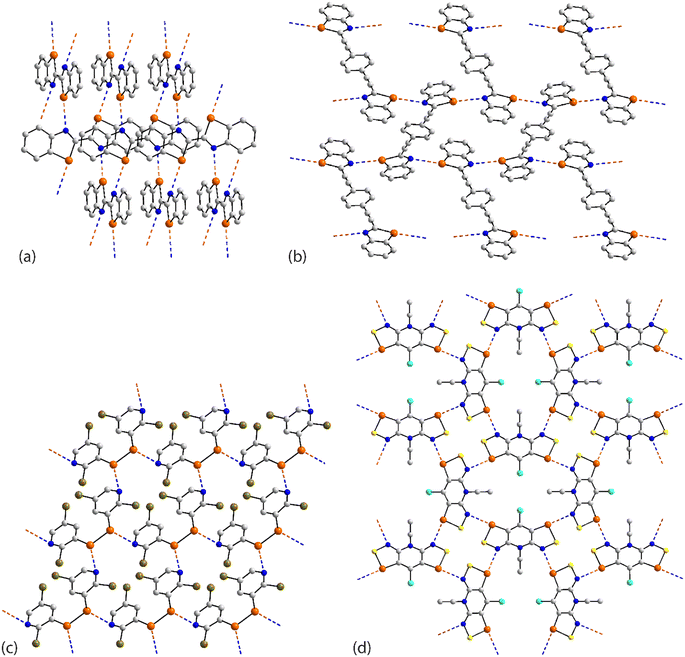 | ||
| Fig. 7 Supramolecular aggregation patterns featuring Se⋯N contacts in the crystals of (a) 31, (b) 32, (c) 33 and (d) 34. | ||
Supramolecular architectures featuring di-nuclear selenium molecules involving the participation of both selenium atoms – more than two Se⋯N contacts per molecule
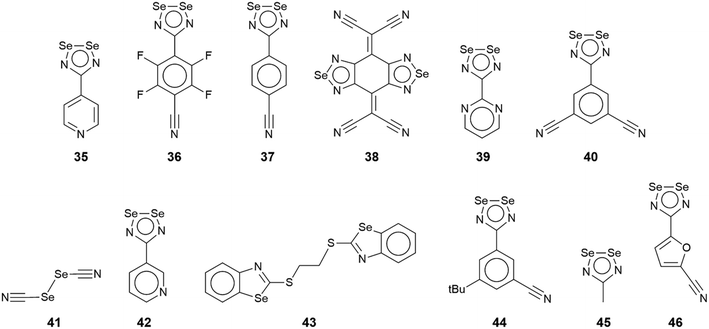
In this sub-category, comprising crystals 35–46 (Fig. 8), each selenium atom participates in the formation of Se⋯N contacts with, on average, more than one contact per selenium atom. Given this scenario, only two- and three-dimensional architectures are generated. Selected geometric data for 35–46 are collated in Table 3.| No. | Se⋯N; Nc | W–Se⋯N | X–Se⋯N | Y–N⋯Se | Z–N⋯Se | REFCODE | Ref. |
|---|---|---|---|---|---|---|---|
| a For parameters characterising the other independent molecules, see ESI† Table S1. | |||||||
| 35 | 2.962(3); 0.86 | N, 162.77(8) | Se, 72.73(6) | C, 106.93(19) | C, 136.0(2) | TINQIT | 105 |
| 3.172(3); 0.92 | N, 154.33(8) | Se, 63.09(6) | C, 94.43(19) | C, 149.0(2) | |||
| 3.169(3); 0.92 | Se, 154.38(5) | N, 75.71(9) | Se, 105.62(9) | C, 133.0(2) | |||
| 3.212(3); 0.93 | Se, 154.52(5) | N, 74.46(9) | Se, 104.07(9) | C, 135.7(2) | |||
| 36 | 3.085(12); 0.89 | N, 158.2(4) | Se, 69.5(3) | C, 163(1) | UBOJED | 89 | |
| 3.145(13); 0.91 | N, 155.2(4) | Se, 66.8(3) | C, 153(1) | ||||
| 3.188(14); 0.92 | Se, 163.5(2) | N, 82.3(4) | Se, 97.4(4) | C, 144(1) | |||
| 3.192(13); 0.93 | Se, 167.6(2) | N, 82.3(4) | Se, 97.1(4) | C, 146(1) | |||
| 37 | 3.096(18); 0.90 | N, 159.9(5) | Se, 68.1(4) | C, 160(2) | PAHREV | 94 | |
| 3.01(2); 0.87 | N, 158.7(5) | Se, 68.0(4) | C, 156(2) | ||||
| 3.050(11); 0.88 | Se, 157.8(3) | N, 76.9(4) | Se, 103.8(4) | C, 135.9(9) | |||
| 3.071(11); 0.89 | Se, 154.9(3) | N, 76.5(4) | Se, 102.7(4) | C, 137(1) | |||
| 38 | 2.940(4); 0.85 | N, 169.49(11) | N, 82.94(11) | C, 169.5(3) | GEFVOC10 | 106 | |
| 39 | 3.160(6); 0.92 | Se, 91.28(11) | N, 82.8(2) | Se, 98.1(2) | C, 86.8(4) | BARWUO | 107 |
| 3.190(7); 0.93 | Se, 89.37(12) | N, 81.5(2) | Se, 97.6(3) | C, 87.1(4) | |||
| 3.152(6); 0.91 | N, 167.1(2) | Se, 88.00(11) | Se, 95.0(2) | C, 148.3(4) | |||
| 2.949(6); 0.86 | N, 172.4(2) | Se, 97.14(12) | C, 97.3(4) | C, 147.3(5) | |||
| 40 | 3.310(16); 0.96 | N, 154.2(5) | Se, 64.2(3) | C, 96(1) | WAJGUJ | 108 | |
| 3.311(16); 0.96 | N, 160.8(5) | Se, 73.4(3) | C, 135(1) | ||||
| 2.978(13); 0.86 | Se, 159.7(3) | N, 78.1(4) | Se, 104.3(5) | C, 139(1) | |||
| 3.054(12); 0.89 | Se, 157.4(2) | N, 75.9(5) | Se, 101.6(5) | C, 137.9(9) | |||
| 3.396(17); 0.98 | Se, 146.9(3) | N, 98.4(5) | C, 138(1) | ||||
| 41 | 2.877(4); 0.83 | Se, 164.33(8) | C, 69.44(16) | C, 158.7(4) | XUNHOF | 109 | |
| 2.937(4); 0.85 | C, 172.89(16) | Se, 91.61(8) | C, 133.4(4) | ||||
| 3.157(4); 0.92 | Se, 165.35(8) | C, 71.11(15) | C, 127.7(3) | ||||
| 3.199(5); 0.93 | C, 178.39(17) | Se, 82.40(7) | C, 93.0(3) | ||||
| 42 | 3.065(3); 0.89 | N, 158.47(11) | Se, 68.12(6) | C, 95.2(2) | C, 143.4(2) | TINQAL | 105 |
| 3.074(3); 0.89 | N, 158.92(11) | Se, 67.71(6) | C, 105.9(2) | C, 137.4(2) | |||
| 3.411(3); 0.99 | Se, 161.09(6) | N, 100.38(11) | Se, 102.13(12) | C, 139.7(2) | |||
| 43 | 3.227(3); 0.94 | C, 161.03(13) | C, 89.41(14) | C, 97.3(2) | C, 129.4(3) | PUZWIQ | 110 |
| 3.375(4); 0.98 | C, 168.51(14) | C, 103.54(14) | C, 93.7(3) | C, 112.5(3) | |||
| 3.234(3); 0.94 | C, 171.09(14) | C, 100.16(14) | C, 96.0(2) | C, 110.6(2) | |||
| 44 | 2.997(3); 0.87 | N, 165.38(12) | Se, 75.55(7) | C, 135.5(3) | PECXED02 | 111 | |
| 3.303(3); 0.96 | N, 153.08(12) | Se, 61.49(7) | C, 96.3(3) | ||||
| 2.894(4); 0.84 | Se, 167.81(7) | N, 77.19(13) | Se, 104.05(14) | C, 141.0(3) | |||
| 2.933(4); 0.85 | Se, 165.86(7) | N, 76.08(13) | Se, 102.59(14) | C, 141.6(3) | |||
| 3.063(4); 0.89 | N, 156.26(14) | Se, 69.57(7) | Se, 88.47(13) | C, 153.7(3) | |||
| 3.142(3); 0.91 | N, 152.99(13) | Se, 65.98(6) | Se, 91.50(12) | C, 141.2(3) | |||
| 45 | 3.186(14); 0.92 | Se, 80.9(3) | N, 92.7(5) | Se, 99.4(5) | C, 84.3(9) | ZEXVON | 112 |
| 3.346(15); 0.97 | Se, 79.2(2) | N, 92.1(5) | Se, 96.6(5) | C, 81.2(9) | |||
| 3.186(14); 0.92 | Se, 80.9(3) | N, 92.7(5) | Se, 99.4(5) | C, 84.3(9) | |||
| 3.300(13); 0.96 | Se, 169.7(3) | N, 95.1(5) | Se, 83.0(5) | C, 156(1) | |||
| 2.971(14); 0.86 | N, 160.2(5) | Se, 69.3(3) | Se, 112.7(6) | C, 130(1) | |||
| 3.048(15); 0.88 | N, 157.1(6) | Se, 65.7(3) | Se, 108.3(6) | C, 121(1) | |||
| 46 | 2.974(10); 0.86 | N, 164.5(3) | Se, 74.2(2) | C, 145.7(7) | YIFHUQ | 113 | |
| 3.234(8); 0.94 | N, 153.7(3) | Se, 62.25(16) | C, 152.3(7) | ||||
| 3.459(9); 1.00 | Se, 158.80(16) | N, 83.8(3) | C, 111.8(7) | ||||
| 3.030(6); 0.88 | Se, 153.98(12) | N, 79.4(2) | Se, 106.1(3) | C, 127.8(5) | |||
| 3.202(6); 0.93 | Se, 141.79(12) | N, 74.7(2) | Se, 99.3(3) | C, 136.0(5) | |||
| 3.050(7); 0.88 | Se, 151.56(12) | N, 78.8(2) | Se, 108.0(3) | C, 125.3(5) | |||
| 3.278(7); 0.95 | Se, 135.57(12) | N, 72.0(2) | Se, 100.0(2) | C, 131.9(5) | |||
| 3.004(8); 0.87 | N, 164.5(3) | Se, 73.80(16) | C, 147.7(7) | ||||
| 3.241(8); 0.94 | N, 154.8(3) | Se, 62.8(15) | C, 150.8(7) | ||||
| 3.315(9); 0.96 | Se, 165.08(15) | N, 90.0(3) | C, 105.0(7) | ||||
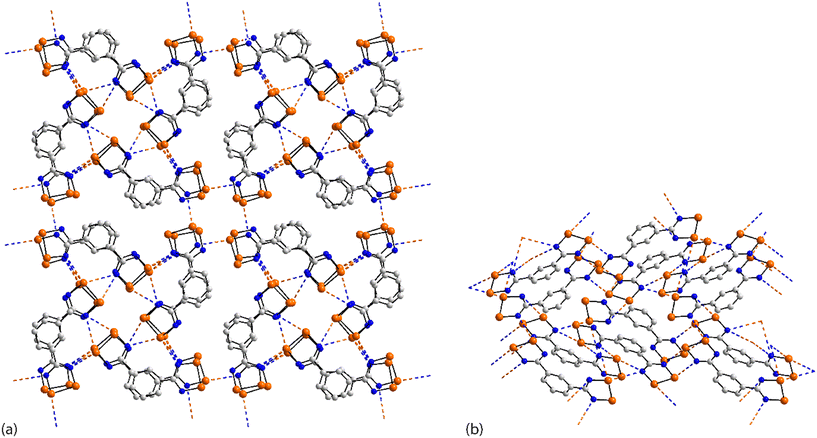
The rich diversity of structural motifs in this section is emphasised by the observation that only 35,10536 (ref. 89) and 37 (ref. 94) have closely related two-dimensional arrays with the remaining assemblies being without precedent; 35–37 are radical species. As illustrated in Fig. 9(a) for 35, the motif comprises tapes mediated by four-membered {⋯SeN}2 synthons connected by pyridyl-nitrogen interacting with two selenium atoms. The Se⋯N(pyridyl) separations are dissimilar (entries 1 & 2 for 35, Table 3) but the differences are not great. The {⋯SeN}2 synthon is non-symmetric (entries 3 & 4 for 35, Table 3) but, again, the differences in the Se⋯N separations are not chemically significant. In each of 36 and 37, the tapes are linked by bifurcated nitrile-nitrogen interactions across the edge of the respective five-membered ring. There are two independent molecules comprising the asymmetric-unit of each 36 and 37 with data for only one of each listed in Table 3. The tapes in 36 comprise alternating independent molecules connected laterally so may be described as being constructed from rows of alternating molecules. In this case, the Se⋯N(nitrile) separations (entries 1 & 2 for 36, Table 3) tend to be shorter than the Se⋯N(ring) ring contacts. In 37, the construction of the array is similar to that in 36 but no trends are evident in the magnitudes of the Se⋯N separations.
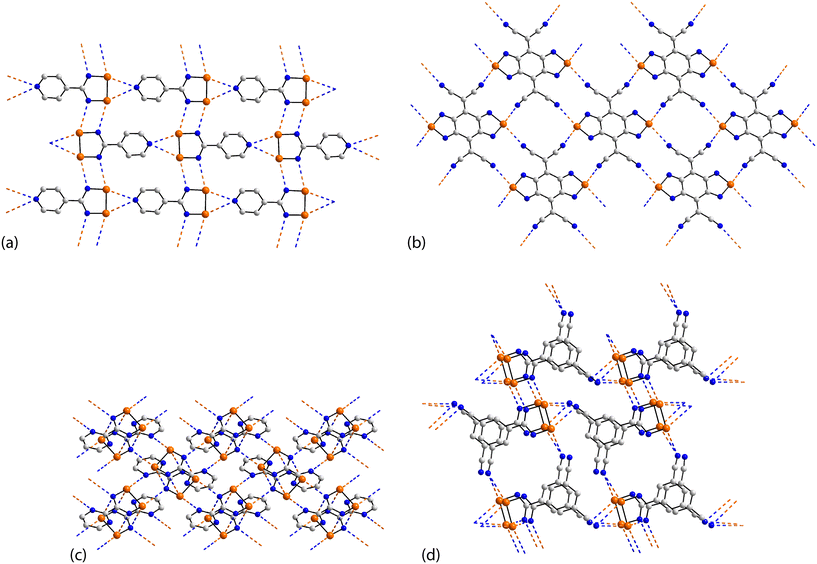 | ||
| Fig. 9 Supramolecular aggregation patterns featuring Se⋯N contacts in the crystals of (a) 35, (b) 38, (c) 39 and (d) 40. | ||
In common with 35–37, a flat, two-dimensional array is evident in the crystal of 38.106 The molecule has 2/m symmetry. Each Se atom forms two Se⋯N interactions, and each nitrile–nitrogen atom forms one. The result is a grid comprising edge-shared 12-membered {⋯Se⋯NC3N}2 and 14-membered {⋯SeNC4N}2 synthons, Fig. 9(b).
A more complicated pattern is apparent in the crystal of 39;107 this is a radical species. Centrosymmetrically related diselenadiazolyl residues are connected by four ring-Se⋯N(ring) contacts which do not conform to the definition of chalcogen-bonding as the angles subtended at the selenium and nitrogen atoms participating in these contacts are close to orthogonal (entries 1 & 2 for 39, Table 3). As such, this interaction is consistent with a relatively rare example of a π-hole interaction in this survey.114–118 The supramolecular rectangles are connected laterally by additional ring-Se⋯N(ring) contacts (entry 3 for 39, Table 3) and Se⋯N(pyrimidyl) contacts, leading to a two-dimensional array, Fig. 9(c). The layer is basically flat with the exception of the pyrimidyl rings which project out of and to either side of the plane.
Two molecules comprise the asymmetric-unit of radical 40 (ref. 108) which differ in their mode of association. The first molecule self-associates via Se⋯N(nitrile) interactions from a bifurcated nitrile atom (entries 1 & 2 for 40, Table 3). A non-symmetric synthon {⋯SeN}2 forms between the two independent molecules (entries 3 & 4 for 40, Table 3). The second contact for the second selenium is with a nitrile-nitrogen atom of the second independent molecule (entry 5 for 40, Table 3). The second molecule associates as just described but the interaction corresponding to entry 5 is now self-association, a crucial contact (see below). Thus, the difference between the independent molecules relates to the interactions involving the nitrile-nitrogen atoms: in the first independent molecule, one nitrile group forms two contacts while for the second molecule, each nitrile group forms two Se⋯N contacts; each selenium atom forms two Se⋯N contacts. The result is a double layer, Fig. 9(d) with the crucial contact between layers being the additional self-association between the second independent molecules (entry 5 for 40, Table 3).
In arguably the simplest molecule covered in this survey, each of the two crystallographically independent molecules in 41 (ref. 109) lacks symmetry. Each of the selenium atoms forms two Se⋯N(nitrile) interactions (entries 1 & 2 for 41, Table 3 pertain to the first independent molecule); each nitrile-nitrogen atom also participates in two Se⋯N(nitrile) contacts. The result is a three-dimensional architecture as represented in Fig. 10(a).
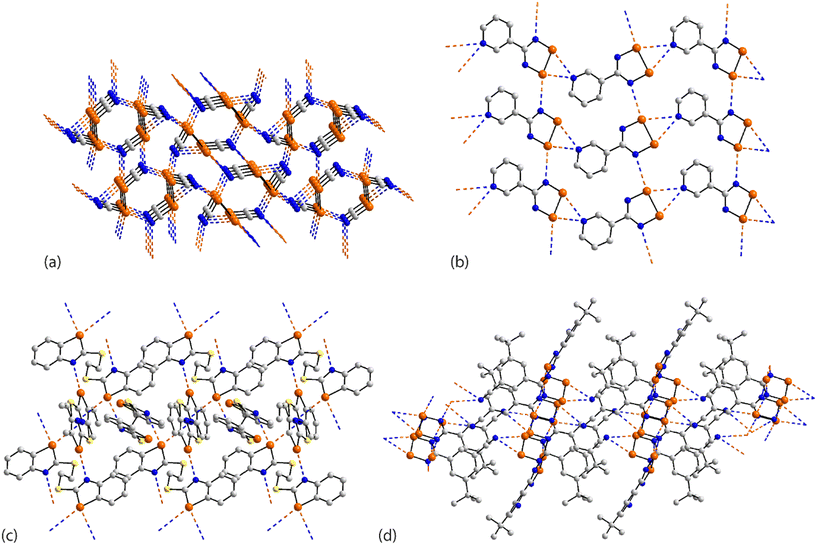 | ||
| Fig. 10 Supramolecular aggregation patterns featuring Se⋯N contacts in the crystals of (a) 41, (b) 42, (c) 43 and (d) 44. | ||
Whereas the selenium atoms in 35–41 participated in the same number of Se⋯N interactions each, in the remaining aggregates to be described in this sub-category, the selenium atoms form disparate numbers of Se⋯N interactions. This is exemplified first in the crystal of (radical) 42 (ref. 105) which comprises two crystallographically independent molecules but which form an identical pattern of Se⋯N contacts. The pyridyl-nitrogen atom bridges the Se–Se edge of a symmetry related molecule, forming experimentally equivalent Se⋯N separations (entries 1 & 2 for 42, Table 3). One of the selenium atoms forms a second contact, with a ring-nitrogen to form a chain with a Se⋯N separation (entry 3 for 42, Table 3) significantly longer than the first two contacts. The result is a two-dimensional array with an undulating topology, Fig. 10(b). The second independent molecule self-associates in an analogous fashion, and with a similar pattern of Se⋯N separations.
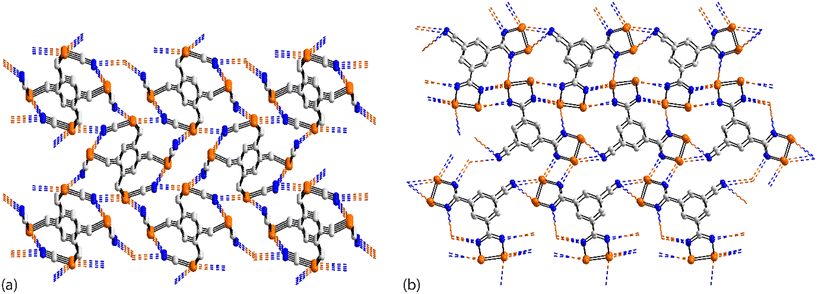
The asymmetric-unit of 43 (ref. 110) comprises three independent molecules, each disposed about a centre of inversion, and each having a different mode of association in terms of forming Se⋯N contacts in the crystal. In the first molecule, the selenium atom forms two Se⋯N contacts (entries 1 & 2 for 43, Table 3), in the second, one, and in the third, none. In terms of nitrogen donors, the first and third molecules provide these whereas the second molecule does not. The result is a two-dimensional array, as shown in Fig. 10(c).
There are also three independent molecules in 44 (ref. 111), a radical species, which are, again, distinguished by their pattern of Se⋯N contacts; there are 10 independent Se⋯N contacts in the crystal. Two independent molecules have one selenium atom forming two contacts and the other, one contact, whereas the third independent molecule sees both selenium atoms forming two contacts. For the first two molecules, the nitrile-nitrogen atom forms two contacts but for the third, this residue does not participate in Se⋯N contacts. Further, the ring nitrogen atoms in the first two molecules form 2 + 1 (i.e. for the nitrogen atom syn to the t-butyl group) or 1 + 2 Se⋯N contacts, while neither the ring-nitrogen atom for the third molecule participates in a Se⋯N contact. The data in Table 3 refer to the first independent molecule; see ESI† Table S1 for additional data. This molecule self-associates via bifurcated nitrile-nitrogen interactions with the Se–Se edge (entries 1 & 2 for 44, Table 3), and associates, via a non-symmetric {⋯SeN}2 synthon, with the second independent molecule (entries 3 & 4 for 44, Table 3). The ring-nitrogen atom syn to the t-butyl group bridges the Se–Se edge of the third independent molecule (entries 5 & 6 for 44, Table 3). A view of the resulting two-dimensional array is given in Fig. 10(d).
The asymmetric unit of 45 (ref. 112) comprises four independent molecules, which differ in their modes of association via Se⋯N contacts; the crystal features 12 separate Se⋯N contacts. The selenium atoms in, arbitrarily chosen, independent molecules one, two, three and four form 2 + 0, 3 + 1, 2 + 0 and 2 + 1 Se⋯N contacts, respectively, and the ring-nitrogen atoms participate in 2 + 2, 2 + 0, 2 + 2 and 1 + 1 Se⋯N contacts, respectively. The interactions involving the first molecule are described herein, as indicative of the others, being connected to the other three molecules comprising the asymmetric-unit. Two face-to-face orientated molecules, one and two, rotated by 90°, are arranged to allow for the construction of a rectangle through two π-hole contacts (entries 1 & 2 for 45, Table 3). One ring-nitrogen connects to selenium atoms of molecules two and three (entries 3 & 4 for 45, Table 3) while the second ring-nitrogen connects to the Se–Se edge of the fourth independent molecule (entries 5 & 6 for 45, Table 3); these later contacts form the shorter of the Se⋯N separations. The net result of the association via Se⋯N is a two-dimensional array, Fig. 11(a).
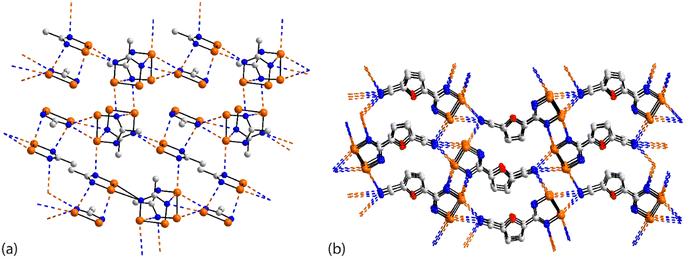 | ||
| Fig. 11 Supramolecular aggregation patterns featuring Se⋯N contacts in the crystals of (a) 45 and (b) 46. | ||
The last aggregate to be discussed in this sub-category is that formed in 46 for which eight molecules comprise the asymmetric-unit.113 Remarkably, all eight molecules form the same pattern of Se⋯N contacts whereby one selenium atom forms three Se⋯N contacts and the other, two. One ring-nitrogen atom forms two Se⋯N contacts but the second, none; the nitrile-nitrogen atom forms three contacts. The result of the 40 Se⋯N interactions, two of which are marginally longer than the sum of the van der Waals radii, is a three-dimensional architecture, Fig. 11(b). The Se–Se edge of the five-membered ring is bridged by a bifurcated nitrile-nitrogen atom derived from the fourth independent molecule (entries 1 & 2 for 46, Table 3). The selenium atom forming two contacts connects to a nitrile-nitrogen from the seventh independent molecule (entry 3 for 46, Table 3) while the Se–N edge with the other selenium atom participates in two, non-symmetric {⋯SeN}2 synthons with similar edges derived from the fifth and sixth independent molecules (entries 4–7 for 46, Table 3). Finally, the nitrile-nitrogen atom spans a Se–Se edge of the fourth independent molecule (entries 8 & 9 for 46, Table 3) and connects to a selenium atom of a second sixth independent molecule.
Supramolecular architectures featuring di-nuclear selenium molecules in multi-component crystals
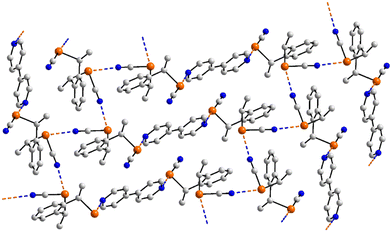
The chemical diagrams for the 12 multi-component crystals of selenium to be discussed herein are given in Fig. 12 with the key geometric parameters listed in Table 4. In the crystal of 47,119 two selenium-containing radicals assemble about a centre of inversion to form an incomplete rectangle through two non-symmetric Se⋯N contacts, Fig. 13(a). The two radical species in 48 (ref. 120) do not interact with each other but the two independent selenium-containing molecules do, to form a dimeric aggregate about a non-symmetric, four-membered {⋯SeN}2 synthon, Fig. 13(b). In 49,121Fig. 13(c), the co-formers in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-crystal assemble into a two-molecule aggregate. In the 2
1 co-crystal assemble into a two-molecule aggregate. In the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-crystal 50,122 two nitrile-nitrogen atoms derived from the centrosymmetric tetracyanoquinodimethane molecule connect to two selenium atoms to form a three-molecule aggregate, Fig. 13(d).
1 co-crystal 50,122 two nitrile-nitrogen atoms derived from the centrosymmetric tetracyanoquinodimethane molecule connect to two selenium atoms to form a three-molecule aggregate, Fig. 13(d).
| No. | Se⋯N; Nc | W–Se⋯N | X–Se⋯N | Y–N⋯Se | Z–N⋯Se | REFCODE | Ref. |
|---|---|---|---|---|---|---|---|
| a Standard uncertainties are not available. | |||||||
| 47 | 3.032(7); 0.88 | N, 163.6(3) | N, 76.9(3) | Se, 109.4(4) | C, 128.5(6) | DUVFEF10 | 119 |
| Se, 67.60(17) | |||||||
| 3.235(8); 0.94 | N, 144.0(3) | N, 71.3(3) | Se, 101.4(4) | C, 131.3(7) | |||
| Se, 118.10(18) | |||||||
| 48 | 2.914(6); 0.85 | Se, 166.66(11) | N, 76.45(19) | Se, 105.7(2) | C, 138.6(4) | SOBPIL | 120 |
| 3.024(6); 0.88 | Se, 164.15(11) | N, 73.48(19) | Se, 101.3(2) | C, 143.8(4) | |||
| 49 | 3.45; 1.00 | C, 140.6 | C, 86.0 | C, 95.4 | BOMDIQ | 121 | |
| 50 | 3.260(7); 0.95 | C, 171.3(2) | Se, 70.3(3) | C, 95.7(5) | ASIHUF | 122 | |
| 51 | 2.830(2); 0.82 | (N)C, 177.18(9) | C, 84.30(9) | C, 107.4(2) | C, 137.06(19) | FEDJAC | 97 |
| 52 | 2.865(2); 0.83 | (N)C, 167.84(9) | C, 83.00(8) | C, 105.56(18) | C, 138.63(19) | FUNGIH | 123 |
| 53 | 2.897(2); 0.84 | (N)C, 176.70(11) | C, 83.00(10) | C, 108.1(2) | C, 136.2(2) | FEDJEG | 97 |
| 54 | 2.7368(12); 0.79 | (N)C, 174.42(6) | C, 79.29(6) | C, 117.34(10) | C, 121.33(10) | QANSAD | 124 |
| 55 | 2.792(3); 0.81 | (N)C, 172.37(14) | C, 81.39(13) | C, 107.4(2) | C, 132.0(3) | QANSEH | 124 |
| 56 | 3.055(3); 0.89 | (C)C, 176.09(11) | C, 80.01(11) | C, 105.78(18) | C, 134.83(19) | IMOQUZ | 125 |
| 57 | 2.995(2); 0.87 | (C)C, 171.58(11) | C, 78.55(13) | C, 103.93(19) | C, 141.0(2) | IMORIO | 125 |
| 58 | 3.003(4); 0.87 | (N)C, 173.62(13) | C, 82.52(10) | C, 104.7(3) | C, 128.2(3) | MAHHOW | 91 |
| 3.198(3); 0.93 | C, 165.56(9) | (N)C, 71.16(11) | C, 131.2(2) | ||||
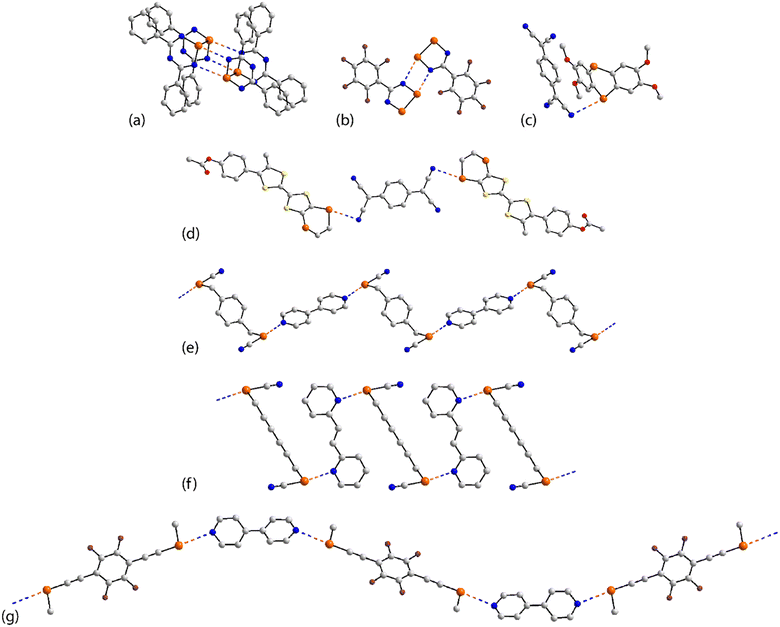 | ||
| Fig. 13 Supramolecular aggregation patterns featuring Se⋯N contacts in the crystals of (a) 47, (b) 48, (c) 49, (d) 50, (e) 53, (f) 55 and (g) 56. | ||
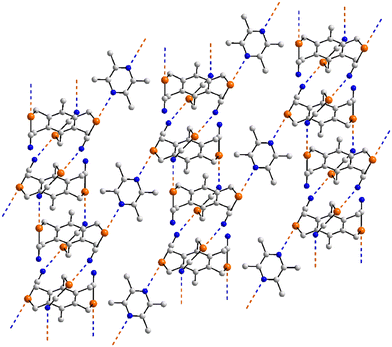
To a first approximation, the supramolecular chains formed in the crystals of 51,9752,12353,97Fig. 13(e), and 54 (ref. 124) have a flattened, step-ladder topology, with small twists. The bipyridyl-type ligands are disposed about a centre of inversion as are the selenium-containing molecules in 52–54; in 51, the selenium co-former has 2-fold symmetry. In 55,124 owing to the nitrogen-donors being in the 2-positions of the pyridyl rings, the chain has a more jagged topology, Fig. 13(f). A more open topology is evident in the crystal of 56,125Fig. 13(g), where the bi-nuclear selenium compound is disposed about a centre of inversion and the 4,4′-bipyridyl co-former about a 2-fold axis. The supramolecular chain in 57 (ref. 125) is similar; each co-former is disposed about a centre of inversion.
In the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-crystal 58,91 the 4,4′-bipyridyl conformer is located about a centre of inversion. One of the independent selenium atoms forms a Se⋯N interaction with a pyridyl-nitrogen atom (entry 1 for 58, Table 4) to form a three-molecule aggregate. These are connected into a two-dimensional array via Se⋯N(nitrile) contacts, Fig. 14, which form the longer separations; the second nitrile-nitrogen does not participate in a Se⋯N contact.
1 co-crystal 58,91 the 4,4′-bipyridyl conformer is located about a centre of inversion. One of the independent selenium atoms forms a Se⋯N interaction with a pyridyl-nitrogen atom (entry 1 for 58, Table 4) to form a three-molecule aggregate. These are connected into a two-dimensional array via Se⋯N(nitrile) contacts, Fig. 14, which form the longer separations; the second nitrile-nitrogen does not participate in a Se⋯N contact.
Supramolecular architectures featuring tri-nuclear selenium molecules
There are only 12 crystals in this category with the chemical diagrams of the interacting species shown in Fig. 15; selected geometric parameters are collected in Table 5.| No. | Se⋯N; Nc | W–Se⋯N | X–Se⋯N | Y–N⋯Se | Z–N⋯Se | REFCODE | Ref. |
|---|---|---|---|---|---|---|---|
| 59 | 3.166(4); 0.92 | N, 166.25(15) | Se, 100.82(8) | Se, 85.45(15) | Se, 97.34(16) | IVEDAO | 126 |
| C, 125.2(3) | |||||||
| 60 | 3.119(12); 0.90 | C, 165.7(4) | Se, 74.2(3) | C, 128(1) | SACMUF | 127 | |
| 3.345(16); 0.97 | Se, 142.3(2) | Se, 63.8(3) | C, 153(1) | ||||
| 61 | 3.011(5); 0.87 | (N)C, 169.31(19) | C, 79.09(18) | C, 170.1(4) | PEYQIW | 128 | |
| 3.023(5); 0.88 | (N)C, 168.48(19) | C, 79.52(19) | C, 168.6(4) | ||||
| 3.379(6); 0.98 | (C)C, 171.01(18) | (N)C, 76.3(2) | C, 106.7(4) | ||||
| 3.243(8); 0.94 | (N)C, 156.5(3) | C, 75.1(3) | C, 140.2(7) | ||||
| 62 | 3.378(6); 0.98 | P, 163.78(11) | P, 68.51(10) | C, 117.2(5) | REQQIN | 129 | |
| 3.310(6); 0.96 | P, 158.06(11) | P, 66.52(10) | C, 154.5(5) | ||||
| 3.345(5); 0.97 | P, 149.55(10) | P, 75.64(11) | C, 135.8(5) | ||||
| 63 | 3.064(3); 0.89 | Se, 169.55(6) | Se, 87.70(6) | C, 132.0(3) | XUNHUL | 109 | |
| 3.029(3); 0.88 | C, 173.60(13) | Se, 87.6(6) | C, 113.9(3) | ||||
| 3.215(3); 0.93 | Se, 162.10(5) | C, 72.05(11) | C, 122.1(2) | ||||
| 64 | 3.441(4); 1.00 | C, 84.66(11) | C, 117.39(11) | C, 73.89(19) | C, 89.5(2) | SAMHIZ | 130 |
| 65 | 2.989(8); 0.87 | (N)C, 175.3(2) | C, 80.41(17) | Se, 154.5(5) | XOBWOD | 131 | |
| 3.063(4); 0.89 | (N)C, 178.8(2) | C, 81.78(16) | Se, 141.0(4) | ||||
| 66 | 3.192(9); 0.93 | (N)C, 174.7(3) | C, 79.7(2) | Se, 142.3(7) | XOBWIX | 131 | |
| 3.315(6); 0.96 | (N)C, 177.1(3) | C, 80.3(2) | Se, 136.6(6) | ||||
| 67 | 2.964(3); 0.86 | (N)C, 176.10(11) | C, 80.34(10)° | C, 179.1(2) | PEYQOC | 128 | |
| 3.385(3); 0.98 | (C)C, 164.24(10) | C, 77.09(11) | C, 105.4(2) | ||||
| 2.972(3); 0.86 | (N)C, 173.82(11) | C, 77.21(11) | C, 176.7(3) | ||||
| 3.318(3); 0.96 | (C)C, 172.40(10) | C, 77.74(10) | C, 107.6(2) | ||||
| 2.960(4); 0.86 | (N)C, 175.42(13) | C, 78.40(13) | C, 178.9(3) | ||||
| 68 | 3.171(5); 0.92 | (N)C, 172.64(18) | C, 77.52(13) | C, 115.7(3) | C, 126.3(3) | XOBWET | 131 |
| 3.029(6); 0.88 | (N)C, 175.03(18) | C, 78.56(15) | C, 164.4(4) | ||||
| 3.096(5); 0.90 | (N)C, 178.83(18) | C, 81.48(15) | C, 137.1(4) | ||||
| 69 | 3.328(9); 0.97 | N, 157.2(4) | N, 71.8(4) | Se, 122.2(4) | C, 114.4(6) | RANPOP | 32 |
| 70 | 3.269(6); 0.95 | N, 151.6(2) | N, 69.1(2) | Se, 97.3(2) | C, 148.1(5) | RANPUV | 32 |
| 2.934(7); 0.85 | N, 167.9(2) | N, 78.3(2) | Se, 110.1(3) | C, 127.9(5) | |||
| 3.237(7); 0.94 | N, 172.9(2) | N, 79.0(2) | Se, 113.4(3) | C, 116.5(5) | |||
| 3.356(7); 0.97 | N, 148.1(3) | N, 115.7(3) | Se, 92.3(3) | C, 112.7(5) |
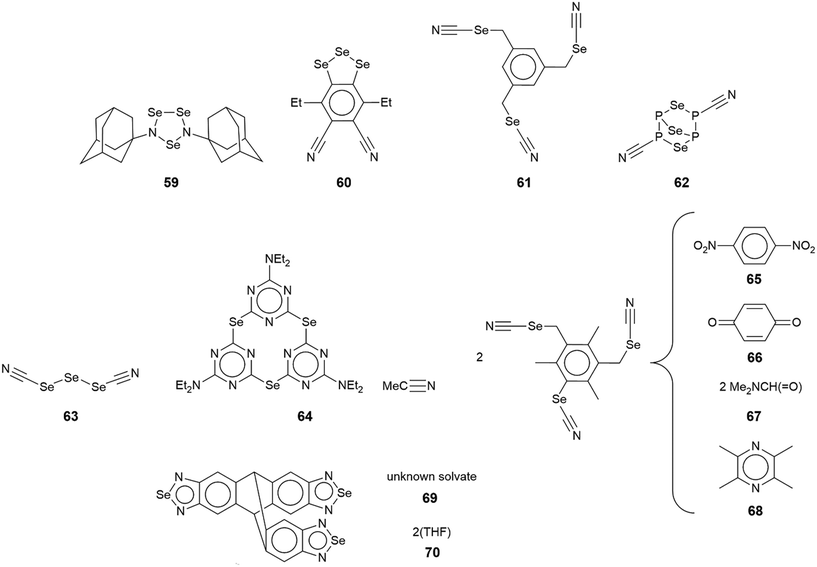
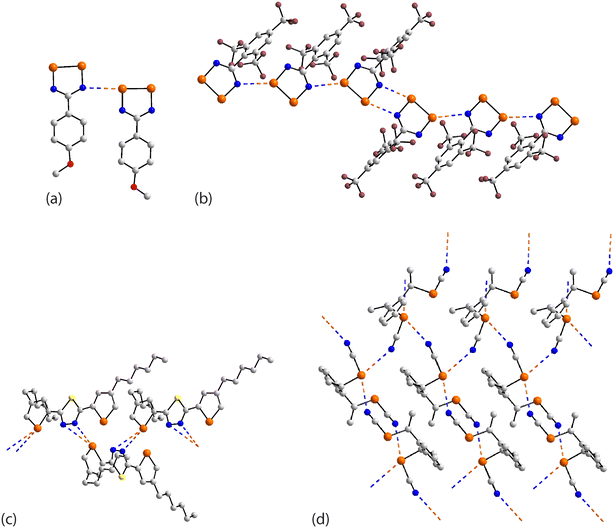
In the first example of this category, 59,126 only one of the three selenium atoms forms a Se⋯N interaction within a helical chain (21 screw), Fig. 16(a). In 60,127 a sequence of three connected selenium atoms within a five-membered triselenolyl ring is noted with two of these being bridged non-symmetrically by a bifurcated nitrile-nitrogen atom within a helical chain (21 screw), Fig. 16(b). The molecule in crystal 61 (ref. 128) features several times in this category. Each of the three selenium atoms of the parent molecule participates in Se⋯N contacts. Two of the selenium atoms cooperate to form a two-dimensional array of bridged ladders, Fig. 16(c), upper view. The rails of the ladder correspond to the shorter Se⋯N separations (entries 1 & 2 for 61, Table 5). One of the selenium atoms forms two Se⋯N contacts by generating a link (stave) to a neighbouring rail (entry 3 for 61, Table 5); a second, supporting link is made by the other selenium atom but at a longer separation (3.464(5) Å). The third CH2SeCN residue lies to the same side of the array and self-associates with a second layer (about the glide axis) to form a double-layer, Fig. 16(c), lower view; the Se⋯N separation (entry 4 for 61, Table 5) is intermediate between those forming the rails and rungs, respectively.
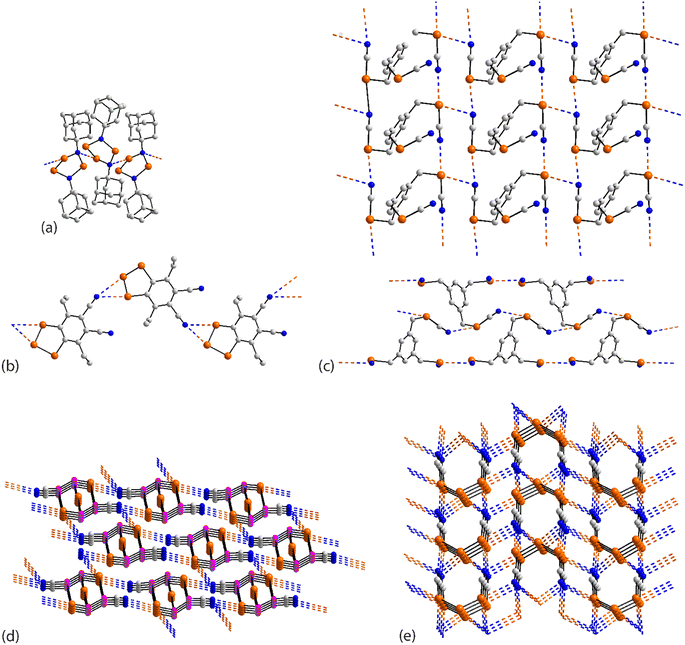 | ||
| Fig. 16 Supramolecular aggregation patterns featuring Se⋯N contacts in the crystals of (a) 59, (b) 60, (c) 61, (d) 62 and (e) 63. | ||
A phosphorus-rich species comprises crystal 62.129 Two of the selenium atoms form Se⋯N contacts, one forms two interactions and the other, one. The Se⋯N contacts are formed with nitrile-nitrogen atoms with one nitrile being bifurcated, bridging selenium atoms derived from two different molecules. The result is a three-dimensional architecture, Fig. 16(d). In 63,109 where the central selenium atom lies on a crystallographic mirror plane, each selenium forms two Se⋯N contacts; each nitrile-nitrogen atom is trifurcated. The Se⋯N separation involving the selenium atom on the mirror plane (entry 1 for 63, Table 5) is intermediate between those formed by the selenium atom in the general position. The aforementioned connections exist within a three-dimensional architecture, Fig. 16(e).
The remaining aggregates in this category are found within multi-component crystals. In the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 acetonitrile solvate 64,130 only one of the selenium atoms participates in a Se⋯N contact within a helical chain (21 screw), Fig. 17(a). The 2
1 acetonitrile solvate 64,130 only one of the selenium atoms participates in a Se⋯N contact within a helical chain (21 screw), Fig. 17(a). The 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-crystals of 65 and 66 (ref. 131) are isostructural, each feature the molecule described above in 61 and each has the respective co-former disposed about a centre of inversion. As viewed from Fig. 17(b), two of the selenium atoms engage in Se⋯N(nitrile) interactions within a linear, supramolecular chain; the selenium atom not forming Se⋯N interactions forms close contacts with the oxygen-donors of the co-formers. In the 1
1 co-crystals of 65 and 66 (ref. 131) are isostructural, each feature the molecule described above in 61 and each has the respective co-former disposed about a centre of inversion. As viewed from Fig. 17(b), two of the selenium atoms engage in Se⋯N(nitrile) interactions within a linear, supramolecular chain; the selenium atom not forming Se⋯N interactions forms close contacts with the oxygen-donors of the co-formers. In the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dimethylformamide solvate 67,128 two of the selenium atoms participate in two Se⋯N contacts to form supramolecular ladders with the Se⋯N separations associated with the rails (entries 1 & 3 for 67, Table 5) being significantly shorter than those associated with the staves (entries 2 & 4 for 67, Table 5). The ladders are linked by the organic residues with the remaining selenium atom participating in an isolated chain of Se⋯N(nitrile) interactions between two ladders, Fig. 17(c).
1 dimethylformamide solvate 67,128 two of the selenium atoms participate in two Se⋯N contacts to form supramolecular ladders with the Se⋯N separations associated with the rails (entries 1 & 3 for 67, Table 5) being significantly shorter than those associated with the staves (entries 2 & 4 for 67, Table 5). The ladders are linked by the organic residues with the remaining selenium atom participating in an isolated chain of Se⋯N(nitrile) interactions between two ladders, Fig. 17(c).
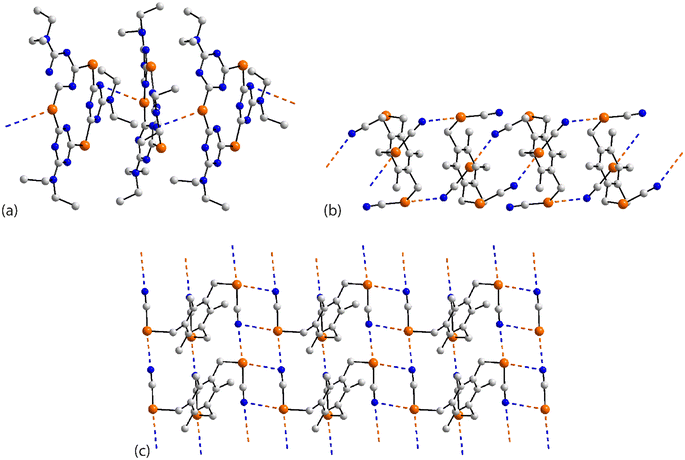 | ||
| Fig. 17 Supramolecular aggregation patterns featuring Se⋯N contacts in the crystals of (a) 64, (b) 65 and (c) 67. | ||
In the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-crystal 68,131 the co-former participates in Se⋯N contacts, unlike the situation in each of 65–67, with these inter-co-former interactions disrupting the normally observed ladder motif. As with the previous examples, the tri-nuclear compound has no symmetry but the co-former is situated about an inversion centre; each selenium atom forms a single Se⋯N contact. The tetramethylpyrazine molecule links two molecules via Se⋯N contacts (entry 1 for 68, Table 5). The other two independent contacts involve two nitrile-nitrogen atoms (entries 2 & 3 for 68, Table 5); the contacts involving the nitrile-nitrogen atoms are shorter than those involving pyrazine. The result is a jagged two-dimensional array, Fig. 18.
1 co-crystal 68,131 the co-former participates in Se⋯N contacts, unlike the situation in each of 65–67, with these inter-co-former interactions disrupting the normally observed ladder motif. As with the previous examples, the tri-nuclear compound has no symmetry but the co-former is situated about an inversion centre; each selenium atom forms a single Se⋯N contact. The tetramethylpyrazine molecule links two molecules via Se⋯N contacts (entry 1 for 68, Table 5). The other two independent contacts involve two nitrile-nitrogen atoms (entries 2 & 3 for 68, Table 5); the contacts involving the nitrile-nitrogen atoms are shorter than those involving pyrazine. The result is a jagged two-dimensional array, Fig. 18.
Two very recently described tri-nuclear selenium compounds are described as covalent organic frameworks (COFs).32 In the first of these, 69, isolated as an unknown solvate, the molecule has 2-fold symmetry with the selenium atom lying on the axis implying that it participates in two Se⋯N contacts with two symmetry related selendiazolyl ring-nitrogen atoms. This mode of association leads to a three-dimensional architecture, Fig. 19(a), as is found in the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 tetrahydrofuran solvate, 70,32 illustrated in Fig. 19(b). Here, there are two independent trinuclear selenium molecules in the asymmetric-unit which differ in terms of the Se⋯N contacts they form. One molecule employs one selenium atom of one residue (entry 1 for 70, Table 5) and a ring-nitrogen atom of another in forming Se⋯N interactions (entry 3 for 70, Table 5). The second molecule employs two selenium atoms, one of which forms two Se⋯N contacts (entries 2 & 3 for 70, Table 5), the other, one contact (entry 4 for 70, Table 5) and a ring-nitrogen atom from each residue. The most prominent interaction between the independent molecules is a four-membered {⋯SeN}2 synthon (entries 1 & 2 for 70, Table 5). The resulting architecture is porous allowing for the accommodation of the solvent tetrahydrofuran molecules.
3 tetrahydrofuran solvate, 70,32 illustrated in Fig. 19(b). Here, there are two independent trinuclear selenium molecules in the asymmetric-unit which differ in terms of the Se⋯N contacts they form. One molecule employs one selenium atom of one residue (entry 1 for 70, Table 5) and a ring-nitrogen atom of another in forming Se⋯N interactions (entry 3 for 70, Table 5). The second molecule employs two selenium atoms, one of which forms two Se⋯N contacts (entries 2 & 3 for 70, Table 5), the other, one contact (entry 4 for 70, Table 5) and a ring-nitrogen atom from each residue. The most prominent interaction between the independent molecules is a four-membered {⋯SeN}2 synthon (entries 1 & 2 for 70, Table 5). The resulting architecture is porous allowing for the accommodation of the solvent tetrahydrofuran molecules.
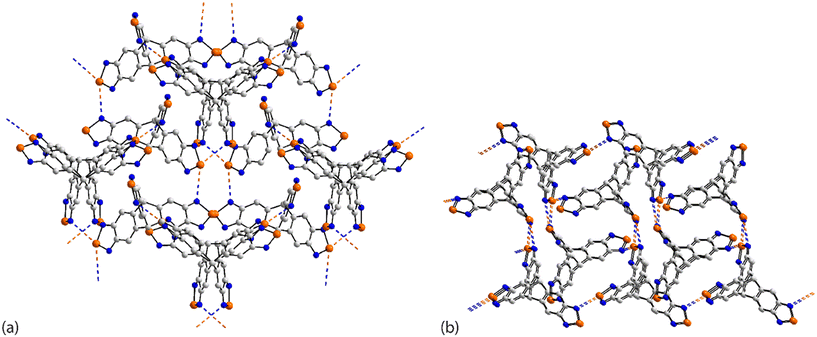 | ||
| Fig. 19 Supramolecular aggregation patterns featuring Se⋯N contacts in the crystals of (a) 69 and (b) 70. | ||
Supramolecular architectures featuring tetra-nuclear selenium molecules
According to the search of the CSD,68 the maximum number of selenium atoms in a molecule forming Se⋯N interactions in its crystal is four. Indeed, there are 18 examples of such molecules with their chemical diagrams shown in Fig. 20. Some of the supramolecular aggregation patterns are particularly complicated with full listings of geometric parameters given in ESI† Table S4; salient and representative data are presented in Table 6.| No. | Se⋯N; Nc | W–Se⋯N | X–Se⋯N | Y–N⋯Se | Z–N⋯Se | REFCODE | Ref. |
|---|---|---|---|---|---|---|---|
| a Standard uncertainties are not available. b For parameters characterising the other independent molecules, see ESI† Table S1. | |||||||
| 71 | 3.225(8); 0.94 | N, 174.0(3) | N, 76.8(3) | Se, 87.4(3) | Se, 103.2(3) | MUHDEA | 132 |
| C, 93.8(6) | |||||||
| 72 | 2.85; 0.83 | S, 169.2 | N, 78.3 | Se, 101.7 | C, 142.3 | BUQGUQ01 | 133 |
| 73 | 2.892(5); 0.84 | S, 171.07(11) | N, 78.97(18) | Se, 101.03(19) | C, 143.3(4) | NEBGUX | 134 |
| 74 | 2.98; 0.86 | S, 168.4 | N, 76.4 | Se, 103.6 | C, 140.7 | BUQHAX | 135 |
| 75 | 3.239(3); 0.94 | S, 165.55(5) | N, 78.25(9) | Se, 101.75(10) | C, 141.76(18) | XAKGUM | 136 |
| 76 | 3.43(3); 0.99 | Se, 155.6(5) | N, 91.0(9) | Se, 91(1) | C, 145(2) | VINJOR | 137 |
| 3.44(3); 1.00 | Se, 152.1(4) | N, 90(1) | Se, 92.5(9) | C, 142(2) | |||
| 77 | 2.45; 0.71 | S, 171.3 | N, 79.4 | Se, 100.6 | C, 143.6 | BUQGUQ03 | 133 |
| 3.03; 0.88 | Se, 97.3 | N, 93.1 | Se, 93.1 | C, 91.4 | |||
| 78 | 3.339(7); 0.97 | C, 165.5(3) | Se, 97.49(12) | Se, 82.4(2) | C, 123.1(5) | AFUDEL01 | 104 |
| 79 | 3.397(5); 0.99 | C, 165.3(2) | Se, 98.23(9) | Se, 81.67(17) | C, 123.6(4) | IZOXOL | 138 |
| 80 | 3.187(12); 0.92 | Se, 163.7(2) | Se, 70.5(2) | Se, 99.9(5) | C, 139.9(8) | JEFBIF | 139 |
| N, 83.7(4) | |||||||
| 3.301(12); 0.96 | Se, 157.5(2) | Se, 110.4(2) | Se, 96.2(5) | C, 145.0(8) | |||
| N, 80.1(4) | |||||||
| 3.091(13); 0.90 | N, 167.4(5) | Se, 88.5(2) | Se, 99.0(5) | C, 97.2(8) | |||
| Se, 99.6(2) | |||||||
| 81 | 3.115(3); 0.90 | N, 160.95(11) | Se, 71.62(5) | Se, 79.55(9) | C, 162.2(2) | SUQREC | 82 |
| 3.264(3); 0.95 | C, 149.72(11) | Se, 64.94(5) | Se, 102.77(10) | C, 133.3(2) | |||
| 3.026(3); 0.88 | C, 178.11(10) | Se, 91.49(6) | Se, 107.01(12) | C, 108.41(19) | |||
| 82 | 3.29(3); 0.95 | N, 157(1) | Se, 73.0(5) | Se, 80(1) | C, 148(2) | SOBSUX | 140 |
| 3.43(3); 0.99 | N, 154.0(9) | Se, 66.6(5) | Se, 120(1) | C, 124(2) | |||
| 3.25(3); 0.94 | Se, 152.8(5) | N, 101.0(9) | Se, 116(1) | C, 118(2) | |||
| 3.37(3); 0.98 | Se, 146.0(5) | N, 82.7(9) | Se, 103(1) | C, 134(2) | |||
| 3.37(3); 0.98 | Se, 148.5(5) | N, 102(1) | Se, 102(1) | C, 135(2) | |||
| 3.44(3); 1.00 | Se, 133.9(5) | N, 104(1) | Se, 107(1) | C, 110(2) | |||
| 3.11(3); 0.90 | N, 171(1) | Se, 95.0(5) | Se, 92(1) | C, 141(2) | |||
| 83 | 3.025(13); 0.88 | Se, 163.9(2) | N, 76.2(4) | Se, 103.4(5) | C, 142(1) | SOBSUX01 | 141 |
| 3.024(13); 0.88 | Se, 164.1(2) | N, 75.8(4) | Se, 104.5(5) | C, 138(1) | |||
| 3.432(15); 1.00 | N, 170.2(5) | Se, 92.5(2) | Se, 111.6(5) | C, 88(1) | |||
| 3.153(16); 0.91 | Se, 153.4(3) | N, 75.4(5) | Se, 104.6(5) | C, 135(1) | |||
| 3.353(16); 0.97 | N, 157.5(5) | Se, 93.5(3) | Se, 109.8(5) | C, 128(1) | |||
| 3.239(13); 0.94 | N, 155.3(5) | Se, 91.0(3) | Se, 115.5(6) | C, 124(1) | |||
| 3.311(14); 0.96 | Se, 145.6(3) | N, 74.2(5) | Se, 111.6(6) | C, 117(1) | |||
| 3.039(15); 0.88 | N, 171.0(6) | Se, 97.1(3) | Se, 103.0(5) | C, 95(1) | |||
| 84 | 3.085(5); 0.89 | (N)C, 164.0(2) | C, 79.3(2) | C, 155.6(4) | PEYRET | 128 | |
| 3.302(7); 0.96 | (C)C, 167.6(2) | C, 72.5(2) | C, 159.6(6) | ||||
| 3.370(6); 0.98 | (N)C, 170.0(3) | C, 79.41(17) | C, 124.8(4) | ||||
| 85 | 3.104(15); 0.90 | Se, 150.2(2) | N, 88.2(5) | Se, 99.2(5) | C, 132.3(9) | YAXWAV | 142 |
| 3.406(13); 0.99 | Se, 158.0(2) | N, 79.2(5) | Se, 89.0(5) | C, 148.5(9) | |||
| 3.300(13); 0.96 | N, 157.3(5) | Se, 75.5(2) | Se, 115.1(5) | C, 128.5(9) | |||
| 3.444(12); 1.00 | Se, 140.0(2) | N, 91.4(5) | Se, 112.9(6) | C, 120(1) | |||
| 2.877(12); 0.83 | Se, 163.5(3) | N, 77.7(5) | Se, 103.0(5) | C, 140.3(9) | |||
| 2.902(14); 0.87 | Se, 161.7(3) | N, 76.7(4) | Se, 102.7(5) | C, 139(1) | |||
| 3.055(14); 0.89 | N, 157.3(5) | Se, 69.3(3) | C, 152(1) | ||||
| 3.116(15); 0.90 | N, 155.2(5) | Se, 66.5(3) | C, 109(1) | ||||
| 3.108(12); 0.90 | Se, 151.1(2) | N, 92.0(5) | Se, 113.2(6) | C, 123.2(9) | |||
| 3.243(16); 0.94 | N, 162.0(6) | Se, 75.6(2) | Se, 117.1(6) | C, 126(1) | |||
| 86 | 3.29; 0.95 | C, 163.5 | C, 86.6 | C, 113.3 | BOWSUB | 143 | |
| 87 | 3.084(3); 0.89 | C, 160.51(12) | Se, 68.32(6) | C, 164.7(3) | PEFGUC01 | 144 | |
| 3.098(3); 0.90 | C, 158.57(13) | Se, 67.69(7) | C, 124.2(3) | ||||
| 88 | 3.165(3); 0.92 | C, 169.54(8) | C, 77.59(12) | N, 122.1(2) | ZUGRUO | 145 | |
| 3.177(3); 0.92 | C, 175.00(9) | C, 82.35(13) | N, 122.4(2) | ||||
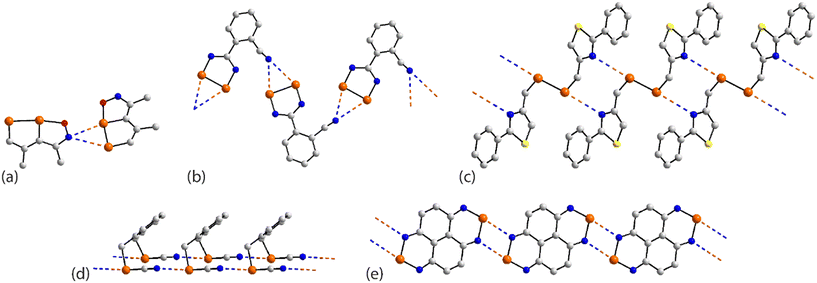
As seen from Fig. 21(a), the familiar {⋯SeCN}2 synthon features between centrosymmetrically related molecules in the crystal of 71.132 Here, only one selenium atom out of the four participates in a Se⋯N contact, being the only molecule in a single component crystal of a tetra-selenium species to do so. The centrosymmetric molecule in 72 (ref. 131) features a central Se–Se bond and two pendent thiaselenazolyl ring-selenium atoms and it is the latter that assemble about a centre of inversion and {⋯SeN}2 synthons to connect molecules into a zigzag chain, Fig. 21(b). The tapes noted in the crystals of 73,13474 (ref. 135) and 75 (ref. 136) resemble that formed in 72. A flat, supramolecular tape is evident in the crystal of 76,137Fig. 21(c), whereby two of selenium atoms of the centrosymmetric molecule participate in Se⋯N interactions. When 73 was subjected to pressure (1.86 GPa), the Se⋯N separation reduced from 2.892(5) Å, determined under ambient conditions,134 to 2.86 Å and reduced further to 2.70 Å at 2.76 GPa with the retention of the supramolecular chain; the high pressure data structures were based on powder data.146 When the pressure was increased even further (3.74 GPa; powder data), a phase change was noted whereby a distinct twist in the tape was detected and chains were linked into a layer via long Se⋯N separations (3.50 Å); this change led to a sudden band-gap closure and the formation of a metallic state.146 A more dramatic change was noted in allied experiments where 72 was subjected to greater pressure to give rise to 77.133 The ambient Se⋯N separation of 2.85 Å in 72 reduced to 2.45 Å at 8.40 GPa in 77 with this change being accompanied by the formation of new Se⋯N interactions (3.03 Å), involving the diselenol atoms, leading to centrosymmetric, six-membered {⋯Se2N}2 synthons which serve to connect the chains of Fig. 21(b) into a two-dimensional array, Fig. 21(d).
 | ||
| Fig. 21 Supramolecular aggregation patterns featuring Se⋯N contacts in the crystals of (a) 71, (b) 72, (c) 76 and (d) 77. | ||
The supramolecular assemblies in each of 78 (ref. 104) and 79 (ref. 138) resemble that formed by the binuclear molecule 34,104 illustrated in Fig. 7(d). This arises as only two of the available selenium atoms in 78 and in 79 participate in Se⋯N contacts.
The tetra-nuclear molecule in 80 (ref. 139) lacks symmetry and has a distinctive U-shape so the diselenadiazolyl rings face each other. Centrosymmetrically related molecules are connected by two non-symmetric {⋯SeN}2 synthons (entries 1 & 2 for 80, Table 6). The selenium atom participating in the shorter of these contacts forms a second Se⋯N interaction within a two-dimensional array, Fig. 22(a). The molecule in 81 (ref. 82) is closely related to 34,104Fig. 7(d), 78 (ref. 104) and 79 (ref. 138) but assembles with a different array of Se⋯N interactions. Here, the Se–Se bond of one diselenadiazolyl ring is bridged in a nearly symmetric fashion by a diselenadiazolyl-nitrogen atom (entries 1 & 2 for 81, Table 6). A selenium atom of the latter ring forms a single contact Se⋯N with the ring with the bridged Se–Se bond to form the array illustrated in Fig. 22(b).
 | ||
| Fig. 22 Supramolecular aggregation patterns featuring Se⋯N contacts in the crystals of (a) 80 and (b) 81. | ||
The next two supramolecular assemblies to be described are polymorphs and each presents a rather complicated array of Se⋯N contacts owing in no small part to the presence of multiple molecules in the asymmetric-unit. In 82 (ref. 140), two independent, non-symmetric molecules comprise the asymmetric-unit and each forms a distinct pattern of Se⋯N contacts. For the first independent molecule, the selenium atoms of one ring participate in Se⋯N contacts but those of the second ring do not. For the former, both are bridged by a diselenadiazolyl-nitrogen atom of a symmetry related molecule (entries 1 & 2 for 82, Table 6). One of these selenium atoms forms two additional contacts, one to a symmetry related molecule (entry 3 for 82, Table 6) and one to the second independent molecule (entry 4 for 82, Table 6). For this first independent molecule, one nitrogen atom of each ring forms Se⋯N contacts. For the ring where the selenium atoms form Se⋯N contacts, the contacts correspond to entries 1 & 2 for 82 in Table 2. For the second nitrogen atom, which also forms two contacts, one contact is made with the first independent molecule (entry 3 for 82, Table 6) and the other with the second independent molecule (entry 5 for 82, Table 6). One selenium atom of each ring of the second independent molecule participates in Se⋯N contacts. One of these forms two contacts corresponding to entries 5 & 6 for 82 in Table 6, i.e. linking both independent molecules; the nitrogen atoms of this ring do not form Se⋯N contacts. One selenium atom of the second diselenadiazolyl ring of the second independent molecule forms a single contact (entry 7 for 82, Table 6) to a symmetry related molecule. Both nitrogen atoms of this ring participate in Se⋯N contacts, one forms a single contact (entry 7 for 82, Table 6), while the other forms two contacts (entries 4 & 6 for 82, Table 6). The Se⋯N interactions extend in three-dimensions, Fig. 23(a).
 | ||
| Fig. 23 Supramolecular aggregation patterns featuring Se⋯N contacts in the polymorphic crystals of (a) 82 and (b) 83. | ||
The second polymorph, i.e. crystal 83,141 also presents two independent molecules, each with a different pattern of Se⋯N contacts. The most obvious difference between the polymorphs relates to the formation of {⋯SeN}2 synthons in this polymorph, features absent in the first described polymorph, 82. Each selenium atom of the diselenadiazolyl ring of the first independent molecule forms Se⋯N contacts. One of these participates in the formation of a non-symmetric {⋯SeN}2 synthon linking the independent molecules (entries 1 & 2 for 83, Table 6). The second selenium atom forms a significantly longer Se⋯N contact, forming another link between the independent molecules (entry 3 for 83, Table 6). Only one selenium atom of the second ring participates in Se⋯N contacts but this forms two such contacts, one being to a centrosymmetrically related molecule via a centrosymmetric {⋯SeN}2 synthon (entry 4 for 83, Table 6) and the other to a symmetry related molecule (entry 5 for 83, Table 6). The nitrogen atoms of the first ring form Se⋯N contacts, one, a single contact within the non-symmetric {⋯SeN}2 synthon (entry 2 for 83, Table 6). The second nitrogen atom forms two contacts, one to the selenium atom participating in the centrosymmetric {⋯SeN}2 synthon (entry 5 for 83, Table 6) and the other to a selenium atom of the second independent molecule (entry 6 for 83, Table 6) which also links to a nitrogen atom of the second ring (entry 7 for 83, Table 6), thereby providing a bridge between the ring-nitrogen atoms of the first independent molecule. This interaction corresponds to the second of two interactions formed by this nitrogen atom, the first being its participation in the centrosymmetric {⋯SeN}2 synthon (entry 5 for 83, Table 6). The second nitrogen atom of the second ring does not participate in a Se⋯N contact. Each selenium atom of the first diselenadiazolyl ring of the second independent molecule forms a Se⋯N contact. One of these is a participant in the non-symmetric {⋯SeN}2 synthon (entries 2 & 1 for 83, Table 6) while the other connects to a symmetry related molecule accounting for the final independent Se⋯N contact (entry 8 for 83, Table 6). Only one of the selenium atoms of the second ring forms Se⋯N contacts, connecting to two first independent molecules (entries 6 & 7 for 83, Table 6). For the first ring, one nitrogen atom forms a Se⋯N contact, participating in the non-symmetric synthon (entry 1 for 83, Table 6). By contrast, each nitrogen atom for the second ring forms a Se⋯N contact, one of these is to a symmetry related molecule (entry 8 for 83, Table 6) and the other nitrogen atom links to a symmetry related first independent molecule (entry 3 for 83, Table 6). As for the first polymorph, i.e.82, the Se⋯N interactions occur within a three-dimensional architecture, Fig. 23(b).
The molecule in 84 (ref. 128) is disposed about a centre of inversion with one pair of selenium atoms forming two Se⋯N(nitrile) interactions (entries 1 & 2 for 84, Table 6) and the second pair one Se⋯N(nitrile) interaction each. These occur within a three-dimensional array, Fig. 24(a). Two non-symmetric, independent molecules comprise the asymmetric-unit of 85 (ref. 142) but each forms the same pattern of Se⋯N contacts so details for the first independent molecule will only be given. Each Se–N edge of the first selenadiazolyl ring of the first independent molecule self-associates with symmetry related rings through their participation in the formation of non-symmetric {⋯SeN}2 synthons (entries 1 & 2 for 85, Table 6); these contacts lead to a supramolecular tape. One of these selenium atoms also forms two additional Se⋯N(selenadiazolyl) contacts, each with a different second independent molecule (entries 3 & 4 for 85, Table 6). The second ring of the first independent molecule presents a different pattern of Se⋯N contacts. One Se–N edge links a second independent molecule via a non-symmetric {⋯SeN}2 synthon (entries 5 & 6 for 85, Table 6) while, at the same time, the Se–Se bond is almost symmetrically bridged by a nitrile-nitrogen atom (entries 7 & 8 for 85, Table 6). Finally, a nitrogen atom of each selenadiazolyl ring is bridged in a non-symmetric fashion by a selenium atom derived from the second independent molecule (entries 9 & 10 for 85, Table 6). A representation of the three-dimensional assembly featuring Se⋯N contacts is given in Fig. 24(b).
 | ||
| Fig. 24 Supramolecular aggregation patterns featuring Se⋯N contacts in the crystals of (a) 84 and (b) 85. | ||
The three remaining assemblies to be described involve interactions between co-crystal co-formers. A three-molecule aggregate is observed in co-crystal 86.143 The central tetracyano-4-quinodimethane molecule is disposed about a centre of inversion and forms two Se⋯N contacts, Fig. 25(a). A linear supramolecular chain is featured in the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 co-crystal 87 (ref. 144), Fig. 25(b); each co-former is located about a centre of inversion. Here, each Se–Se edge is bridged in a symmetric fashion by Se⋯N(nitrile) contacts. Finally, in co-crystal 88 (ref. 145), each selenium atom links a different nitrile-nitrogen atom; each co-former is disposed about a centre of inversion. A flat, two-dimensional array accommodates the aforementioned Se⋯N(nitrile) contacts, Fig. 25(c).
1 co-crystal 87 (ref. 144), Fig. 25(b); each co-former is located about a centre of inversion. Here, each Se–Se edge is bridged in a symmetric fashion by Se⋯N(nitrile) contacts. Finally, in co-crystal 88 (ref. 145), each selenium atom links a different nitrile-nitrogen atom; each co-former is disposed about a centre of inversion. A flat, two-dimensional array accommodates the aforementioned Se⋯N(nitrile) contacts, Fig. 25(c).
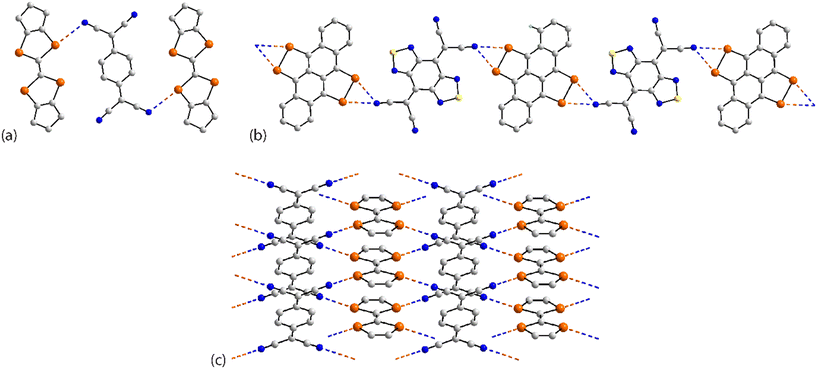 | ||
| Fig. 25 Supramolecular aggregation patterns featuring Se⋯N contacts in the crystals of (a) 86, (b) 87 and (c) 88. | ||
An evaluation of congeners of 1–88 for the presence of related Ch⋯N contacts
Having established the nature of supramolecular aggregation patterns featuring secondary Se⋯N contacts in crystals of multi-nuclear selenium compounds, the focus now turns to establishing the propensity of heavier, i.e. tellurium, and lighter, i.e. sulphur and oxygen, congeners to form analogous interactions in their crystals, as performed in analogous studies.66,67,147,148 Hence, the CSD68 was searched based on the composition of 1–88 with the molecular formula (or formulae in the case of multi-component crystals) used as the query structure but relaxed to allow the selenium atoms to be substituted by tellurium, sulphur and oxygen. Distance criteria were applied, as for the original search, based on the Te⋯N, S⋯N or O⋯N separation being less than or equal to the sum of the respective van der Waals radii,70,71 assumed to be 3.61, 3.35 and 3.07 Å, respectively. In this way, at least one congener was detected for 35 of the 88 crystals evaluated above. A summary of this analysis is presented in Table 7. The congeners are categorised such that isostructural congeners are highlighted with italic text with non-isostructural congeners emphasised in bold.| No. | Te-analogue | Se | S-analogue | O-analogue |
|---|---|---|---|---|
| 4 | VEHVIQ76 | VEHVOW 76 | ||
| 5 | DIHHAE 77 | DIHHEI77 | ||
| 9 | BULQIK81 | BULQUW 81 | ||
| 10 | SUQQUR84 | MOSTIX01 (ref. 149) | ||
| 12 | SEDZEI84 | PYMSUL11 (ref. 150) | ||
| PYMSUL12 (ref. 151) | ||||
| 13 | HIGQOE85 | ENABIE 152 | ||
| 14 | GOHMEW86 | GOHMAS 86 | ||
| 15 | SUQRAY82 | MOSTIX01 (ref. 149) | ||
| 16 | WERQEP87 | NIHBAH 153 | ||
| NIHBAH01 (ref. 87) | ||||
| 19 | SIKWIT90 | SIKWEP 90 | ||
| 27 | NAPSEZ10 (ref. 98) | NAPTDZ10 (ref. 154) | ||
| 30 | EJIYED100 | HAPKAM 155 | EJIYAZ 100 | |
| 31 | WULTUV 101 | WULTOP101 | FOCZON 156 | NOGBOA01 (ref. 156) |
| 32 | RUVWEM102 | RUVWOW 102 | RUVWIQ 102 | |
| 33 | FEGCIE 157 | ABOWU103 | ||
| 34 | YODSUG103 | MOSTOF 149 | ||
| 35 | TINQIT105 | TINQEP 105 | ||
| 36 | UBOJED89 | YOMXUT 158 | ||
| 37 | PAHREV94 | PAHRAR 94 | ||
| 38 | GEFVOC10 (ref. 102) | FARSOG01 (ref. 102) | ||
| 42 | TINQAL105 | ASODUJ01 (ref. 105) | ||
| 43 | PUZWIQ110 | MACMOT 159 | ||
| 45 | ZEXVON112 | JATNEX 160 | ||
| 48 | SOBPIL120 | SOBPEH 120 | ||
| 49 | BOMDIQ121 | MXTTCQ01 (ref. 121) | ||
| 56 | IMORAG 125 | IMOQUZ125 | ||
| 57 | IMOROU 125 | IMORIO125 | ||
| 60 | SACMOZ 127 | SACMUF127 | ||
| 71 | MUHDEA132 | TSTMIM 161 | ||
| 72 | BUQGUQ01 (ref. 131) | HOJQII03 (ref. 159) | ||
| 76 | VINJOR137 | VINJIL 137 | ||
| 78 | AFUDEL01 (ref. 104) | MOSTOD01 (ref. 104) | ||
| 81 | SUQREC82 | MOSTIX01 (ref. 149) | ||
| 85 | YAXWAV142 | YAXVUO 142 | ||
| 88 | VEVFIL 164 | ZUGRUO145 | TTFTCQ01 (ref. 164) |
The purpose of this survey is to ascertain the persistence of chalcogen-bonding interactions, Ch⋯N for the heavier and lighter congeners of the 35 multi-nuclear selenium species forming Se⋯N interactions in their crystals, keeping in mind that the propensity for Ch⋯N bond formation is expected to decrease in the order Ch = Te > Se > S > O. The unit-cell parameters, summaries of supramolecular association and bibliographic details for the identified congeners are given in ESI† Table S5.
As noted from Table 7, there are three oxygen congeners included in the listing, i.e. those of 30, 31 and 32, labelled 30O,10031O (ref. 156) and 32O,102 respectively. In none of the crystals of the oxygen-congeners were O⋯N interactions noted.
More tellurium-containing congeners are available in the literature, Table 7, i.e. seven examples. Isostructural relationships were noted for four of these, namely 33Te,15756Te,12557Te (ref. 125) and 60Te;127 the geometric parameters characterising these are discussed in more detail below. The three remaining crystals of tellurium congeners presented different supramolecular association patterns. In 5Te,77 Te⋯N separations longer than the sum of the van der Waals radii occur within a twisted ribbon as opposed to the dimeric aggregates formed by the three independent molecules of 5, Fig. 2(e). In 31Te,101 only one of the tellurium atoms forms a contact within the sum of the van der Waals radii within a zigzag chain in contrast to the two-dimensional array noted for 31, Fig. 7(a). In the last example among the tellurium congeners, 88Te,163 only one of the independent tellurium atoms of the centrosymmetric molecule forms a (close) Te⋯N contact to form a linear chain of alternating co-formers. The second tellurium atom forms a long Te⋯N contact (i.e. greater than the sum of the van der Waals radii) to form a two-dimensional array. This is in contrast to 88 where a distinctive two-dimensional array arises as both selenium atoms participate in close Se⋯N contacts, Fig. 25(c).
The largest group of congeners involve selenium and their sulphur analogues. There are 30 matches with two of these having a pair of sulphur-containing polymorphs, giving rise to 32 sulphur analogues. Of these, 16 are isostructural and most will be discussed in further detail below in terms of relevant geometric parameters. Of the 16 non-isostructural pairs, several patterns are apparent. Of the latter sub-set, nearly half of the crystals do not feature S⋯N contacts less than the sum of the van der Waals radii: 4S,769S,8112S (second polymorph),15132S,10243S,15948S (ref. 120) and 71S.161 The remaining non-isostructural crystals do feature close S⋯N contacts.
In 45S,160 a three-dimensional array features numerous S⋯N contacts but with different crystallographic symmetry to that noted in 45. A similar scenario persists in the following three congeners to be described. In 10S,149 an analogous pattern of S⋯N contacts is noted but within a helical chain as opposed to the zigzag chain of 10. In 16, Se⋯N contacts are noted within a helical (21) chain. There are two polymorphs of 16S.87,153 In the first of these,153 S⋯N contacts occur within a helical chain but propagated by 42 screw symmetry. In the second polymorph,87 two independent molecules comprise the asymmetric-unit and one of these assembles into a helical chain with 41 screw symmetry within which are S⋯N contacts.
In the following three crystals, S⋯N contacts are noted but the additional S⋯N contacts to increase the dimensionality of the supramolecular aggregation pattern are missing. Thus, in 35S,105 chains sustained by bifurcated S⋯N(nitrile) contacts persist as in 35 but the lateral {SeN⋯}2 contacts, to give the two-dimensional array shown in Fig. 9(a), do not. A similar situation pertains in 36S.158 In 42S,105 the chain with the bifurcated S⋯N(ring) contacts of 42, Fig. 10(b), persists but not the perpendicular connectivity. In 81S,149 a helical chain features S⋯N contacts but the cross-linking through additional S⋯N contacts, seen in 81, Fig. 22(b), does not persist.
Finally, and most exceptional among the congeners discussed thus far, in 30S,155 centrosymmetric molecules assemble into a two-dimensional array featuring two acceptor and two donor S⋯N contacts per molecule which contrasts the supramolecular tape in 30 featuring {SeN⋯}2 synthons.
The lack of isostructurality among the previous 16 sulphur-congeners can be related to the reduced propensity of S⋯N cf. Se⋯N chalcogen bond formation, an effect which is magnified in the molecular packing by the observation that Se⋯N chalcogen bonds increase the aromaticity of rings within their molecules which then enhances their likelihood of forming π⋯π interactions with consequent impact upon charge transport and magnetic properties.102,165,166
The attention now turns to seeking correlations between the Ch⋯N separation and/or Y–Ch⋯N with Ch = Se on the one hand, and Ch = Te or S on the other. Geometric data in support of this analysis are presented in Table 8. In keeping with the notion that a more valid comparison of geometric parameters is made when the data have been collected under similar experimental conditions,167,168 the data included in Table 8 for matched isostructural pairs have been collected at the same temperature based on the information incorporated in their CSD entries.
| Aggregate | Se⋯N | Nc | Y, Y–Se⋯N | Aggregate | Ch⋯N | Nca | Y–Ch⋯N |
|---|---|---|---|---|---|---|---|
| a ∑vdW = 3.61 and 3.35 Å for the tellurium and sulphur congeners, respectively. b Standard uncertainties are not available. | |||||||
| Tellurium congeners | |||||||
| 33 (ref. 103) | 3.414(9) | 0.99 | C, 140.5(3) | 33Te (ref. 157) | 3.421(11) | 0.95 | C, 144.1(4) |
| 56 (ref. 125) | 3.055(3) | 0.89 | C, 176.09(11) | 56Te (ref. 125) | 2.952(4) | 0.82 | C, 173.89(13) |
| 57 (ref. 125) | 2.995(2) | 0.87 | C, 171.58(11) | 57Te (ref. 125) | 2.853(3) | 0.79 | C, 171.85(12) |
| Sulphur congeners | |||||||
| 12 (ref. 84) | 3.349(14) | 0.97 | C, 163.7(5) | 12S (ref. 150) | 3.314(2) | 0.99 | C, 159.32(8) |
| 13 (ref. 85) | 3.405(3) | 0.99 | S, 174.46(6) | 13S (ref. 152) | 3.465(2) | 1.03 | S, 177.66(5) |
| 15 (ref. 82) | 3.234(3) | 0.94 | C, 176.51(11) | 15S (ref. 149) | 3.174(3) | 0.95 | C, 174.25(13) |
| 19 (ref. 90) | 3.048(6) | 0.88 | C, 164.2(3) | 19S (ref. 90) | 3.109(4) | 0.93 | C, 166.92(15) |
| 3.369(6) | 0.98 | C, 157.0(2) | 3.422(4) | 0.99 | C, 158.06(15) | ||
| 27 (ref. 98)b | 2.90 | 0.84 | N, 175.3 | 27S (ref. 154)b | 3.10 | 0.93 | N, 176.8 |
| 31 (ref. 101) | 3.234(4) | 0.94 | C, 176.95(15) | 99S (ref. 155) | 3.255(2) | 0.97 | C, 174.94(7) |
| 34 (ref. 104) | 3.279(2) | 0.95 | C, 164.51(9) | 34S (ref. 149) | 3.400(3) | 1.01 | C, 160.60(11) |
| 38 (ref. 106) | 2.940(4) | 0.88 | N, 169.49(11) | 38S (ref. 106) | 3.035(4) | 0.91 | N, 167.87(13) |
| 49 (ref. 121)b | 3.45 | 1.00 | C, 140.6 | 49S (ref. 121)b | 3.43 | 1.02 | C, 140.4 |
| 72 (ref. 133)b | 2.85 | 0.83 | S, 169.2 | 72S (ref. 162) | 3.092(3) | 0.92 | S, 172.83(7) |
| 76 (ref. 137) | 3.43(3) | 0.99 | Se, 155.6(5) | 76S (ref. 137) | 3.474(7) | 1.04 | S, 157.53(16) |
| 3.44(3) | 1.00 | Se, 152.1(4) | 3.519(7) | 1.05 | S, 154.56(16) | ||
| 78 (ref. 104) | 3.339(7) | 0.97 | C, 165.5(3) | 78S (ref. 104) | 3.349(16) | 1.00 | S, 161.02(7) |
| 88 (ref. 145) | 3.165(3) | 0.92 | C, 169.54(8) | 88S (ref. 164)b | 3.20 | 0.96 | C, 174.2 |
| 3.177(3) | 0.92 | C, 175.00(9) | 3.25 | 0.97 | C, 169.4 | ||
There are three pairs of isostructural selenium/tellurium congeners available for comparison based on the above criteria. While a direct comparison of the magnitudes of Ch⋯N indicates that the values involving tellurium are equal to or shorter than those involving selenium, a more informative parameter is Nc, as discussed above, with lower values indicating more significant contacts. For the three congeners in this sub-set, it appears that, relatively, the Ch⋯N chalcogen bonds are stronger for the tellurium congeners in accord with expectation. Except for the observation that the longer Ch⋯N contacts have more acute Y–Ch⋯N angles, there is no apparent correlation between Ch⋯N separations and Y–Ch⋯N angles.
There is a more extensive series of isostructural congeners between selenium and sulphur. Without exception, the Nc value for each Ch⋯N contact involving selenium cf. sulphur, indicates a more significant interaction in the selenium congener. Again, there is no apparent correlation between the Ch⋯N separation and Y–Ch⋯N angle, between congeners.
Overview
Intermolecular Se⋯N interactions have been found in 88 crystals of multi-nuclear selenium compounds; the maximum number of selenium atoms in any molecule covered in this survey was four. The overwhelming number of Se⋯N contacts corresponds to chalcogen-bonding interactions, based on σ-hole bonding, with a very small number of π-hole interactions. Of the 88 crystals, the vast majority correspond to bi-nuclear selenium molecules, i.e. 58 examples. This compares with 12 examples of tri-nuclear molecules and 18 of tetra-nuclear molecules. Of the bi-nuclear molecules, 13 zero-dimensional aggregates having Se⋯N contacts were observed compared with 27 one-, 16 two- and two three-dimensional aggregates. For the tri-nuclear molecules, there were no zero-dimensional aggregates, there being four, five and three examples of one-, two- and three-dimensional aggregates. A more even spread of aggregation patterns is noted for the tetra-nuclear species, i.e. two, six, six and four examples of zero-, one-, two- and three-dimensional aggregates having Se⋯N contacts. These distributions compare to those seen for mono-nuclear selenium molecules, for which half (i.e. 71/142) featured Se⋯N contacts within zero-dimensional aggregates, and nearly half (i.e. 63/142) within one-dimensional aggregation patterns; there were seven and one two- and three-dimensional patterns, respectively.66 Clearly, the presence of additional selenium atoms in a molecule enhances the opportunities for additional Se⋯N contacts to be formed leading to the greater likelihood of higher dimensional aggregation patterns.In the majority of aggregates described above, the selenium atom forms a single interaction with some examples of the selenium atom forming two contacts and a very small number with selenium forming three interactions. This was also true for the mono-nuclear selenium species.66 An analysis was also conducted to determine what percentage of the selenium atoms participated in Se⋯N contacts. For the bi-nuclear species, in 25/58 of the molecules in this sub-class only one selenium atom formed Se⋯N contacts with 33/58 having both selenium atoms forming Se⋯N contacts which correlates with the higher dimensionality of the supramolecular aggregates thus formed, as mentioned above. For the tri-nuclear molecules, in 3/12 molecules did only one selenium atom, out of a possible three, form a Se⋯N contact, in half of the examples, two selenium atoms formed contacts and only in 3/12 of the sub-set did all three selenium atoms form Se⋯N contacts. For the tetra-nuclear molecules, there were two examples where only one selenium, out of a possible four, formed a Se⋯N contact, 10/18 aggregates had two selenium atoms forming contacts, 2/8 involving three selenium atoms and in four examples did all four selenium atoms form Se⋯N contacts.
In terms of overall adoption rates, 9% of all possible crystals of multi-nuclear selenium compounds formed Se⋯N contacts. This value is identical to that computed for mono-nuclear selenium compounds.66 It should be noted that while these numbers are relatively low, certain classes of mono-nuclear selenium molecules, e.g. those having a selenadiazolyl ring, have significantly greater adoption rates, i.e. 75%,66 offering great opportunities for the design of specific supramolecular aggregates in their crystals.
It terms of the magnitude of the identified Se⋯N separations, it is well recognised that identifying systematic corrections of inherently weak intermolecular contacts is not possible.60,169 This is because the distances are highly dependent on the bonding/electronic environments about the interacting species: this idea pertains to σ-hole contacts as demonstrated in recent competition studies between chalcogen-bonding and halogen-bonding.170,171 Intermolecular distances are also susceptible to experimental conditions such as temperature, pressure, etc. The axiom notwithstanding, comparing geometric parameters determined under comparable experimental conditions can give clues as to trends.167,168 This can be the case when comparing geometric parameters determined for isostructural crystals measured under the same or very similar experimental conditions. With this proviso in mind, data presented in Table 8 indicate that for pairs of isostructural crystals, chalcogen bonds involving tellurium are stronger than those involving selenium and, in the same way, chalcogen bonds formed by selenium are stronger than comparable interactions in isostructural crystals where the interacting atom is sulphur.
There is clearly on-going interest in material applications for the heavier chalcogens, e.g. as synthetic precursors for nanoparticle generation,172 magnetic173 and conductive materials,174,175 in the biosynthesis of small molecules176 and in the medicinal chemistry/biological context.177 The aforementioned applications are complemented by the exciting recent report of the experimental imaging of the anisotropic distribution of electron density for a σ-hole for halogen atoms.178 The varied role of chalcogen-bonding in the chemical, materials, medicinal and biological sciences ensures continued experimental and computational chemistry investigations in each of these endeavours that are sure to provide new, fascinating results.
Conclusions
Supramolecular assembly based on Se⋯N interactions in crystals of di-, tri- and tetra-nuclear selenium molecules has been established in 9% of crystals where they can potentially form. A wide range in supramolecular aggregation patterns was identified in 88 crystals featuring Se⋯N contacts within zero-dimensional assemblies (17%), falling behind one- (42%), two- (31%) and three- (10%) dimensional assemblies. Correlations in intermolecular separations were evident for isostructural crystals where the X-ray intensity data had been measured under approximately the same experimental conditions. Thus, for pairs of isostructural selenium- and tellurium-containing crystals, the strength of the Te⋯N interaction was greater than the equivalent Se⋯N interaction. Conversely, for isostructural selenium- and sulphur-containing crystals, the Se⋯N interaction was always stronger than the equivalent S⋯N interaction. This being stated, comparisons of aggregation patterns of non-isostructural congeners showed that tellurium-containing molecules didn't always form as an extensive pattern of Te⋯N contacts as for selenium analogues, and conversely, sometimes, a more extensive pattern of interactions was noted for the sulphur congeners compared with their selenium counterparts. Clearly, molecules are going to assemble via a variety of intermolecular contacts in their crystals and while chalcogen-bonding interactions are of obvious importance, they are but one player in the overall supramolecular assembly options in crystals.Conflicts of interest
The author declares no conflict of interest.Acknowledgements
The author gratefully acknowledges Sunway University Sdn Bhd (Grant number GRTIN-RRO-56-2022) for support of this research.References
- T. C. Stadtman, Annu. Rev. Biochem., 1996, 65, 83–100 CrossRef CAS.
- C. Benstoem, A. Goetzenich, S. Kraemer, S. Borosch, W. Manzanares, G. Hardy and C. Stoppe, Nutrients, 2015, 7, 3094–3118 CrossRef CAS.
- J. Nordberg and E. S. J. Arnér, Free Radical Biol. Med., 2001, 31, 1287–1312 CrossRef CAS PubMed.
- C. Jacob, G. I. Giles, N. M. Giles and H. Sies, Angew. Chem., Int. Ed., 2003, 42, 4742–4758 CrossRef CAS PubMed.
- A. C. Bianco, D. Salvatore, B. Gereben, M. J. Berry and P. R. Larsen, Endocr. Rev., 2002, 23, 38–89 CrossRef CAS.
- E. R. T. Tiekink, Dalton Trans., 2012, 41, 6390–6395 RSC.
- H.-L. Seng and E. R. T. Tiekink, Appl. Organomet. Chem., 2012, 26, 655–662 CrossRef CAS.
- N. V. Barbosa, C. W. Nogueira, P. A. Nogara, A. F. de Bem, M. Aschner and J. B. T. Rocha, Metallomics, 2017, 9, 1703–1734 CrossRef CAS.
- Z. Chen, H. Lai, L. Hou and T. Chen, Chem. Commun., 2020, 56, 179–196 RSC.
- M. J. Parnham and H. Sies, Biochem. Pharmacol., 2013, 86, 1248–1253 CrossRef CAS.
- G. K. Azad and R. S. Tomar, Mol. Biol. Rep., 2014, 41, 4865–4879 CrossRef CAS.
- K. P. Bhabak and G. Mugesh, Acc. Chem. Res., 2010, 43, 1408–1419 CrossRef CAS PubMed.
- S. P. Thomas, K. Satheeshkumar, G. Mugesh and T. N. Guru Row, Chem, 2015, 21, 6793–6800 CrossRef CAS PubMed.
- K. Satheeshkumar and G. Mugesh, Chem. – Eur. J., 2011, 17, 4849–4857 CrossRef CAS.
- K. Arai, T. Matsunaga, H. Ueno, N. Akahoshi, Y. Sato, G. Chakrabarty, G. Mugesh and M. Iwaoka, Chem. – Eur. J., 2019, 25, 12751–12760 CrossRef CAS PubMed.
- S. Mondal, D. Manna, K. Raja and G. Mugesh, ChemBioChem, 2020, 21, 911–923 CrossRef CAS PubMed.
- S. Scheiner, CrystEngComm, 2021, 23, 6821–6837 RSC.
- O. Carugo, G. Resnati and P. Metrangolo, ACS Chem. Biol., 2021, 16, 1622–1627 CrossRef CAS PubMed.
- A. Singh, A. Kaushik, J. S. Dhau and R. Kumar, Coord. Chem. Rev., 2022, 450, 214254 CrossRef CAS.
- Elena Yu. Tupikina, Org. Biomol. Chem., 2022, 20, 5551–5557 RSC.
- S. Jena, J. Dutta, K. D. Tulsiyan, A. K. Sahu, S. S. Choudhury and H. S. Biswal, Chem. Soc. Rev., 2022, 51, 4261–4286 RSC.
- M. S. Taylor, Coord. Chem. Rev., 2020, 413, 213270 CrossRef CAS.
- R. Hein and P. D. Beer, Chem. Sci., 2022, 13, 7098–7125 RSC.
- A. Docker, C. H. Guthrie, H. Kuhn and P. D. Beer, Angew. Chem., Int. Ed., 2021, 60, 21973–21978 CrossRef CAS PubMed.
- T. Wirth, Tetrahedron, 1999, 55, 1–28 CrossRef CAS.
- K. T. Mahmudov, M. N. Kopylovich, M. F. C. Guedes da Silva and A. J. L. Pombeiro, Dalton Trans., 2017, 46, 10121–10138 RSC.
- J. Bamberger, F. Ostler and O. G. Mancheño, ChemCatChem, 2019, 11, 5198–5211 CrossRef CAS PubMed.
- X. Yuan and Y. Wang, Angew. Chem., Int. Ed., 2022, 61, e202203671 CAS.
- M. Fourmigué and A. Dhaka, Coord. Chem. Rev., 2020, 403, 213084 CrossRef.
- P. C. Ho, J. Z. Wang, F. Meloni and I. Vargas-Baca, Coord. Chem. Rev., 2020, 422, 213464 CrossRef CAS.
- B. Fan, F. Lin, X. Wu, Z. Zhu and A. K.-Y. Jen, Acc. Chem. Res., 2021, 54, 3906–3916 CrossRef CAS PubMed.
- W. Yang, R. Jiang, C. Liu, B. Yu, X. Cai and H. Wang, Cryst. Growth Des., 2021, 21, 6497–6503 CrossRef CAS.
- B. J. Eckstein, L. C. Brown, B. C. Noll, M. P. Moghadasnia, G. J. Balaich and C. M. McGuirk, J. Am. Chem. Soc., 2021, 143, 20207–20215 CrossRef CAS PubMed.
- R. Mamgain and F. V. Singh, ACS Org. Inorg. Au, 2022, 2, 262–288 CrossRef CAS.
- R. Gleiter, G. Haberhauer, D. B. Werz, F. Rominger and C. Bleiholder, Chem. Rev., 2018, 118, 2010–2041 CrossRef CAS.
- L. Vogel, P. Wonner and S. M. Huber, Angew. Chem., Int. Ed., 2019, 58, 1880–1891 CrossRef CAS.
- P. Scilabra, G. Terraneo and G. Resnati, Acc. Chem. Res., 2019, 52, 1313–1324 CrossRef CAS.
- N. Biot and D. Bonifazi, Coord. Chem. Rev., 2020, 413, 213243 CrossRef CAS.
- K. T. Mahmudov, A. V. Gurbanov, V. A. Aliyeva, M. F. C. Guedes da Silva, G. Resnati and A. J. L. Pombeiro, Coord. Chem. Rev., 2022, 464, 214556 CrossRef CAS.
- A. C. Legon, Phys. Chem. Chem. Phys., 2017, 19, 14884–14896 RSC.
- N. W. Alcock, Adv. Inorg. Chem. Radiochem., 1972, 15, 1–58 CrossRef CAS.
- H. Wang, J. Liu and W. Wang, Phys. Chem. Chem. Phys., 2018, 20, 5227–5234 RSC.
- C. B. Aakeroy, D. L. Bryce, G. R. Desiraju, A. Frontera, A. C. Legon, F. Nicotra, K. Rissanen, S. Scheiner, G. Terraneo, P. Metrangolo and G. Resnati, Pure Appl. Chem., 2019, 91, 1889–1892 CrossRef CAS.
- J. S. Murray, P. Lane, T. Clark and P. Politzer, J. Mol. Model., 2007, 13, 1033–1038 CrossRef CAS PubMed.
- P. Politzer, J. S. Murray and T. Clark, Phys. Chem. Chem. Phys., 2010, 12, 7748–7757 RSC.
- P. Politzer and J. S. Murray, Phys. Chem. Chem. Phys., 2013, 15, 11178–11189 RSC.
- P. Politzer and J. S. Murray, Crystals, 2017, 7, 212 CrossRef.
- J. S. Murray and P. Politzer, Crystals, 2020, 10, 76 CrossRef.
- P. Hobza and J. Řezáč, Chem. Rev., 2016, 116, 4911–4912 CrossRef CAS.
- M. H. Kolář and P. Hobza, Chem. Rev., 2016, 116, 5155–5187 CrossRef PubMed.
- D. J. Pascoe, K. B. Ling and S. L. Cockroft, J. Am. Chem. Soc., 2017, 139, 15160–15167 CrossRef CAS.
- J. D. Velásquez, G. Mahmoudi, E. Zangrando, A. V. Gurbanov, F. I. Zubkov, Y. Zorlu, A. Masoudias and J. Echeverría, CrystEngComm, 2019, 21, 6018–6025 RSC.
- B. Galmés, A. Juan-Bals, A. Frontera and G. Resnati, Chem. – Eur. J., 2020, 26, 4599–4606 CrossRef.
- M. Michalczyk, M. Malik, W. Zierkiewicz and S. Scheiner, J. Phys. Chem. A, 2021, 125, 657–668 CrossRef CAS PubMed.
- C. Wen, Y. Shi, Y. Lu, Z. Xu and H. Liu, J. Phys. Chem. A, 2021, 125, 8572–8580 CrossRef CAS PubMed.
- S. Scheiner, J. Phys. Chem. A, 2022, 126, 1194–1203 CrossRef CAS.
- S. Scheiner, J. Phys. Chem. A, 2022, 126, 4025–4035 CrossRef CAS PubMed.
- M. Risto, R. W. Reed, C. M. Robertson, R. Oilunkaniemi, R. S. Laitinen and R. T. Oakley, Chem. Commun., 2008, 3278–3280 RSC.
- M. Risto, A. Assoud, S. M. Winter, R. Oilunkaniemi, R. S. Laitinen and R. T. Oakley, Inorg. Chem., 2008, 47, 10100–10109 CrossRef CAS.
- E. R. T. Tiekink and J. Zukerman-Schpector, CrystEngComm, 2009, 11, 2701–2711 RSC.
- E. R. T. Tiekink and J. Zukerman-Schpector, Coord. Chem. Rev., 2010, 254, 46–76 CrossRef CAS.
- I. Caracelli, J. Zukerman-Schpector and E. R. T. Tiekink, Coord. Chem. Rev., 2012, 256, 412–438 CrossRef CAS.
- S. M. Lee, P. J. Heard and E. R. T. Tiekink, Coord. Chem. Rev., 2018, 375, 410–423 CrossRef CAS.
- E. R. T. Tiekink, Crystals, 2020, 10, 503 CrossRef CAS.
- E. R. T. Tiekink, Coord. Chem. Rev., 2021, 427, 213586 CrossRef CAS PubMed.
- E. R. T. Tiekink, Coord. Chem. Rev., 2021, 443, 214031 CrossRef CAS.
- E. R. T. Tiekink, Coord. Chem. Rev., 2022, 457, 214397 CrossRef CAS.
- C. R. Groom, I. J. Bruno, M. P. Lightfoot and S. C. Ward, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2016, 72, 171–179 CrossRef CAS.
- I. J. Bruno, J. C. Cole, P. R. Edgington, M. Kessler, C. F. Macrae, P. McCabe, J. Pearson and R. Taylor, Acta Crystallogr., Sect. B: Struct. Sci., 2002, 58, 389–397 CrossRef.
- A. Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS.
- A. Spek, Acta Crystallogr., Sect. E: Crystallogr. Commun., 2020, 76, 1–11 CrossRef CAS.
- K. Brandenburg, DIAMOND, Crystal Impact GbR, Bonn, Germany, 2006 Search PubMed.
- H. Detert and D. Schollmeyer, IUCrData, 2020, 5, x201585 CrossRef CAS PubMed.
- M. Nieger, D. Hanssgen and C. Mohr, Private Communication to the Cambridge Structural Database, Refcode XIVPEX, 2002 Search PubMed.
- J. A. Roehrs, R. P. Pistoia, D. F. Back and G. Zeni, Adv. Synth. Catal., 2012, 354, 1791–1796 CrossRef CAS.
- S. Ghosh, S. Das, N. R. Kumar, A. R. Agrawal and S. S. Zade, New J. Chem., 2017, 41, 11568–11575 RSC.
- K. K. Bhasin, N. Singh, S. Doomra, E. Arora, G. Ram, S. Singh, Y. Nagpal, S. K. Mehta and T. M. Klapötke, Bioinorg. Chem. Appl., 2007, 069263 CAS.
- S. Pundir, S. K. Mehta, S. M. Mobin and K. K. Bhasin, Indian J. Heterocycl. Chem., 2017, 27, 99–105 CAS.
- C. H. Silveira, M. G. Fronza, R. A. Balaguez, A. M. E. Larroza, L. Savegnago, D. F. Back, B. A. Iglesias and D. Alves, Dyes Pigm., 2021, 185, 108910 CrossRef CAS.
- N. Benbellat, Y. Le Gal, S. Golhen, A. Gouasmia and L. Ouahab, Synth. Met., 2012, 162, 1789–1797 CrossRef CAS.
- Y. X. Shi, R. Z. Liang, K. A. Martin, N. Weston, S. Gonzalez-Calera, R. Ganguly, Y. Li, Y. Lu, A. J. M. Ribeiro, M. J. Ramos, P. A. Fernandes and F. Garcia, Inorg. Chem., 2015, 54, 6423–6432 CrossRef CAS.
- A. A. Leitch, X. Yu, S. M. Winter, R. A. Secco, P. A. Dube and R. T. Oakley, J. Am. Chem. Soc., 2009, 131, 7112–7125 CrossRef CAS PubMed.
- A. Lari, C. Bleiholder, F. Rominger and R. Gleiter, Eur. J. Org. Chem., 2009, 2009, 2765–2774 CrossRef.
- A. S. Hodage, C. P. Prabhu, P. P. Phadnis, A. Wadawale, K. I. Priyadarsini and V. K. Jain, J. Organomet. Chem., 2012, 720, 19–25 CrossRef CAS.
- A. A. Leitch, J. L. Brusso, K. Cvrkalj, R. W. Reed, C. M. Robertson, P. A. Dube and R. T. Oakley, Chem. Commun., 2007, 3368–3370 RSC.
- D. B. Werz, F. R. Fischer, S. C. Kornmayer, F. Rominger and R. Gleiter, J. Org. Chem., 2008, 73, 8021–8029 CrossRef CAS PubMed.
- L. Beer, A. W. Cordes, D. J. T. Myles, R. T. Oakley and N. J. Taylor, CrystEngComm, 2000, 2, 109–114 RSC.
- M. Parvez, R. T. Boeré and K. H. Moock, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1995, 51, 2118–2121 CrossRef.
- R. L. Melen, R. J. Less, C. M. Pask and J. M. Rawson, Inorg. Chem., 2016, 55, 11747–11759 CrossRef CAS.
- H. Pang, P. J. Skabara, D. J. Crouch, W. Duffy, M. Heeney, I. McCulloch, S. J. Coles, P. N. Horton and M. B. Hursthouse, Macromolecules, 2007, 40, 6585–6593 CrossRef CAS.
- H.-T. Huynh, O. Jeannin, E. Aubert, E. Espinosa and M. Fourmigué, New J. Chem., 2021, 45, 76–84 RSC.
- C. Allen, J. C. A. Boeyens, A. G. Briggs, L. Denner, A. J. Markwell, D. H. Reid and B. G. Rose, J. Chem. Soc., Chem. Commun., 1987, 967–968 RSC.
- T. Kimura, T. Nakahodo, H. Fujihara and E. Suzuki, Inorg. Chem., 2014, 53, 4411–4417 CrossRef CAS PubMed.
- A. W. Cordes, R. C. Haddon, R. G. Hicks, R. T. Oakley and T. T. M. Palstra, Inorg. Chem., 1992, 31, 1802–1808 CrossRef CAS.
- R. A. Popa, E. Licarete, M. Banciu and A. Silvestru, Appl. Organomet. Chem., 2018, 32, e4252 CrossRef.
- S. L. W. McWhinnie, A. B. Brooks and I. Abrahams, Acta Crystallogr., Sect. C: Struct. Chem., 1998, 54, 126–128 CrossRef.
- H.-T. Huynh, O. Jeannin and M. Fourmigué, Chem. Commun., 2017, 53, 8467–8469 RSC.
- A. Gieren, V. Lamm, R. C. Haddon and M. L. Kaplan, J. Am. Chem. Soc., 1980, 102, 5070–5073 CrossRef CAS.
- G. A. Morales and F. R. Fronczek, J. Chem. Crystallogr., 1994, 24, 811–813 CrossRef CAS.
- K. Kawashima, I. Osaka and K. Takimiya, Chem. Mater., 2015, 27, 6558–6570 CrossRef CAS.
- N. Biot and D. Bonifazi, Chem. – Eur. J., 2020, 26, 2904–2913 CrossRef CAS PubMed.
- A. Kremer, C. Aurisicchio, F. De Leo, B. Ventura, J. Wouters, N. Armaroli, A. Barbieri and D. Bonifazi, Chem. – Eur. J., 2015, 21, 15377–15387 CrossRef CAS.
- K. K. Bhasin and V. Arora, Appl. Organomet. Chem., 2004, 18, 359–362 CrossRef CAS.
- C. M. Robertson, A. A. Leitch, K. Cvrkalj, R. W. Reed, D. J. T. Myles, P. A. Dube and R. T. Oakley, J. Am. Chem. Soc., 2008, 130, 8414–8425 CrossRef CAS PubMed.
- A. I. Taponen, J. W. L. Wong, K. Lekin, A. Assoud, C. M. Robertson, M. Lahtinen, R. Clérac, H. M. Tuononen, A. Mailman and R. T. Oakley, Inorg. Chem., 2018, 57, 13901–13911 CrossRef CAS PubMed.
- T. Suzuki, H. Fujii, Y. Yamashita, C. Kabuto, S. Tanaka, M. Harasawa, T. Mukai and T. Miyashi, J. Am. Chem. Soc., 1992, 114, 3034–3043 CrossRef CAS.
- J. Wu, D. J. MacDonald, R. Clérac, I.-R. Jeon, M. Jennings, A. J. Lough, J. Britten, C. Robertson, P. A. Dube and K. E. Preuss, Inorg. Chem., 2012, 51, 3827–3839 CrossRef CAS PubMed.
- W. M. Davis, R. G. Hicks, R. T. Oakley, B. Zhao and N. J. Taylor, Can. J. Chem., 1993, 71, 180–185 CrossRef CAS.
- C. J. Burchell, P. Kilian, A. M. Z. Slawin, J. D. Woollins, K. Tersago, C. Van Alsenoy and F. Blockhuys, Inorg. Chem., 2006, 45, 710–716 CrossRef CAS PubMed.
- Z. Časar, I. Leban, A. Majcen-Le Maréchal and D. Lorcy, J. Chem. Soc., Perkin Trans. 1, 2002, 1568–1573 RSC.
- R. T. Boere, Private Communication to the Cambridge Structural Database, Refcode PECXED02, 2020 Search PubMed.
- C. D. Bryan, A. W. Cordes, R. T. Oakley and R. E. vonH. Spence, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1995, 51, 2402–2404 CrossRef.
- J. F. Britten, O. P. Clements, A. W. Cordes, R. C. Haddon, R. T. Oakley and J. F. Richardson, Inorg. Chem., 2001, 40, 6820–6824 CrossRef CAS PubMed.
- H. Wang, W. Wang and W. J. Jin, Chem. Rev., 2016, 116, 5072–5104 CrossRef CAS PubMed.
- S. J. Grabowski, Phys. Chem. Chem. Phys., 2017, 19, 29742–29759 RSC.
- S. Scheiner, J. Phys. Chem. A, 2021, 125, 6514–6528 CrossRef CAS PubMed.
- R. Shukla, N. Claiser, M. Souhassou, C. Lecomte, S. J. Balkrishna, S. Kumar and D. Chopra, IUCrJ, 2018, 5, 647–653 CrossRef CAS.
- P. Politzer, J. S. Murray and T. Clark, Phys. Chem. Chem. Phys., 2021, 23, 16458–16468 RSC.
- R. T. Oakley, R. W. Reed, A. W. Cordes, S. L. Craig and J. B. Graham, J. Am. Chem. Soc., 1987, 109, 7745–7749 CrossRef CAS.
- M. A. Nascimento, E. Heyer, J. J. Clarke, H. J. Cowley, A. Alberola, N. Stephaniuk and J. M. Rawson, Angew. Chem., Int. Ed., 2019, 58, 1371–1375 CrossRef CAS PubMed.
- W. Hinrichs and G. Klar, J. Chem. Res., 1982, 336–337 CAS.
- L. Kaboub, J.-P. Legros, B. Donnadieu, A.-K. Gouasmia, L. Boudiba and J.-M. Fabre, J. Mater. Chem., 2004, 14, 351–362 RSC.
- V. Kumar, Y. Xu, C. Leroy and D. L. Bryce, Phys. Chem. Chem. Phys., 2020, 22, 3817–3824 RSC.
- A. Dhaka, I.-R. Jeon, O. Jeannin, E. Aubert, E. Espinosa and M. Fourmigué, Angew. Chem., Int. Ed., 2022, e202116650 CAS.
- A. Dhaka, O. Jeannin, I.-R. Jeon, E. Aubert, E. Espinosa and M. Fourmigué, Angew. Chem., Int. Ed., 2020, 59, 23583–23587 CrossRef CAS PubMed.
- T. Maaninen, H. M. Tuononen, G. Schatte, R. Suontamo, J. Valkonen, R. Laitinen and T. Chivers, Inorg. Chem., 2004, 43, 2097–2104 CrossRef CAS PubMed.
- T. Kimura, A. Yomogita, T. Matsutani, T. Suzuki, I. Tanaka, Y. Kawai, Y. Takaguchi, T. Wakahara and T. Akasaka, J. Org. Chem., 2004, 69, 4716–4723 CrossRef CAS PubMed.
- O. Jeannin, H.-T. Huynh, A. M. S. Riel and M. Fourmigué, New J. Chem., 2018, 42, 10502–10509 RSC.
- B. W. Tattershall, E. L. Sandham and W. Clegg, J. Chem. Soc., Dalton Trans., 1997, 81–88 RSC.
- J. Thomas, W. Van Rosson, K. Van Hecke, L. Van Meervelt, M. Smet, W. Maes and W. Dehaen, Chem. Commun., 2012, 48, 43–45 RSC.
- A. M. S. Riel, O. Jeannin, O. B. Berryman and M. Fourmigué, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2019, 75, 34–38 CrossRef CAS PubMed.
- A. J. Karhu, O. J. Pakkanen, J. M. Rautiainen, R. Oilunkaniemi, T. Chivers and R. S. Laitinen, Inorg. Chem., 2015, 54, 4990–4997 CrossRef CAS PubMed.
- K. Lekin, A. A. Leitch, J. S. Tse, X. Bao, R. A. Secco, S. Desgreniers, Y. Ohishi and R. T. Oakley, Cryst. Growth Des., 2012, 12, 4676–4684 CrossRef CAS.
- L. Beer, J. L. Brusso, R. C. Haddon, M. E. Itkis, H. Kleinke, A. A. Leitch, R. T. Oakley, R. W. Reed, J. F. Richardson, R. A. Secco and X. Yu, J. Am. Chem. Soc., 2005, 127, 18159–18170 CrossRef CAS PubMed.
- A. A. Leitch, X. Yu, C. M. Robertson, R. A. Secco, J. S. Tse and R. T. Oakley, Inorg. Chem., 2009, 48, 9874–9882 CrossRef CAS PubMed.
- L. Beer, J. L. Brusso, R. C. Haddon, M. E. Itkis, A. A. Leitch, R. T. Oakley, R. W. Reed and J. F. Richardson, Chem. Commun., 2005, 1543–1545 RSC.
- A. W. Cordes, R. C. Haddon, R. T. Oakley, L. F. Schneemeyer, J. V. Waszczak, K. M. Young and N. M. Zimmerman, J. Am. Chem. Soc., 1991, 113, 582–588 CrossRef CAS.
- A. A. Leitch, K. Lekin, S. M. Winter, L. E. Downie, H. Tsuruda, J. S. Tse, M. Mito, S. Desgreniers, P. A. Dube, S. Zhang, Q. Liu, C. Jin, Y. Ohishi and R. T. Oakley, J. Am. Chem. Soc., 2011, 133, 6051–6060 CrossRef CAS PubMed.
- P. Del Bel Belluz, A. W. Cordes, E. M. Kristof, P. V. Kristof, S. W. Liblong and R. T. Oakley, J. Am. Chem. Soc., 1989, 111, 9276–9278 CrossRef.
- M. P. Andrews, A. W. Cordes, D. C. Douglass, R. M. Fleming, S. H. Glarum, R. C. Haddon, P. Marsh, R. T. Oakley, T. T. M. Palstra, L. F. Schneemeyer, G. W. Trucks, R. Tycko, J. V. Waszczak, K. M. Young and N. M. Zimmerman, J. Am. Chem. Soc., 1991, 113, 3559–3568 CrossRef CAS.
- A. W. Cordes, R. C. Haddon, R. G. Hicks, R. T. Oakley, T. T. M. Palstra, L. F. Schneemeyer and J. V. Waszczak, J. Am. Chem. Soc., 1992, 114, 1729–1732 CrossRef CAS.
- A. W. Cordes, R. C. Haddon, R. G. Hicks, D. K. Kennepohl, R. T. Oakley, T. T. M. Palstra, L. F. Schneemeyer, S. R. Scott and J. V. Waszczak, Chem. Mater., 1993, 5, 820–825 CrossRef CAS.
- T. J. Emge, W. A. Bryden, D. O. Cowan and T. J. Kistenmacher, Mol. Cryst. Liq. Cryst., 1982, 90, 173–184 CrossRef CAS.
- K. Iwasaki, A. Ugawa, A. Kawamoto, Y. Yamashita, K. Yakushi, T. Suzuki and T. Miyashi, Bull. Chem. Soc. Jpn., 1992, 65, 3350–3357 CrossRef CAS.
- P. W. R. Corfield and S. J. La Placa, Acta Crystallogr., Sect. B: Struct. Sci., 1996, 52, 384–387 CrossRef.
- J. S. Tse, A. A. Leitch, X. Yu, X. Bao, S. Zhang, Q. Liu, C. Jin, R. A. Secco, S. Desgreniers, Y. Ohishi and R. T. Oakley, J. Am. Chem. Soc., 2010, 132, 4876–4886 CrossRef CAS PubMed.
- E. R. T. Tiekink, CrystEngComm, 2021, 23, 904–928 RSC.
- E. R. T. Tiekink, Crystals, 2021, 11, 433 CrossRef CAS.
- L. Beer, J. L. Brusso, A. W. Cordes, R. C. Haddon, M. E. Itkis, K. Kirschbaum, R. T. Oakley, D. S. MacGregor, A. A. Pinkerton and R. W. Reed, J. Am. Chem. Soc., 2002, 124, 9498–9509 CrossRef CAS PubMed.
- G. K. Batsala, V. Dokorou, N. Kourkoumelis, M. J. Manos, A. J. Tasiopoulos, T. Mavromoustakos, M. Simčič, S. Golič-Grdadoinik and S. K. Hadjikakou, Inorg. Chim. Acta, 2012, 382, 146–157 CrossRef CAS.
- G. J. Corban, C. D. Antoniadis, S. K. Hadjikakou, N. Kourkoumelis, V. Yu. Tyurin, A. Dolgano, E. R. Milaeva, M. Kubicki, P. V. Bernhardt, E. R. T. Tiekink, S. Skoulika and N. Hadjiliadis, Heteroat. Chem., 2012, 23, 498–511 CrossRef CAS.
- L. Beer, J. F. Britten, J. L. Brusso, A. W. Cordes, R. C. Haddon, M. E. Itkis, D. S. MacGregor, R. T. Oakley, R. W. Reed and C. M. Robertson, J. Am. Chem. Soc., 2003, 125, 14394–14403 CrossRef CAS PubMed.
- A. J. Banister, A. S. Batsanov, O. G. Dawe, P. L. Herbertson, J. A. K. Howard, S. Lynn, I. May, J. N. B. Smith, J. M. Rawson, T. E. Rogers, B. K. Tanner, G. Antorrena and F. Palacio, J. Chem. Soc., Dalton Trans., 1997, 2539–2542 RSC.
- A. Gieren, V. Lamm, R. C. Haddon and M. L. Kaplan, J. Am. Chem. Soc., 1979, 101, 7277–7281 CrossRef CAS.
- I. Osaka, M. Shimawaki, H. Mori, I. Doi, E. Miyazaki, T. Koganezawa and K. Takimiya, J. Am. Chem. Soc., 2012, 134, 3498–3507 CrossRef CAS PubMed.
- C. M. Fitchett, C. Richardson and P. J. Steel, Org. Biomol. Chem., 2005, 3, 498–502 RSC.
- K. K. Bhasin, V. Arora, T. M. Klapötke and M.-J. Crawford, Eur. J. Inorg. Chem., 2004, 4781–4788 CrossRef CAS.
- A. J. Banister, N. Bricklebank, W. Clegg, M. R. J. Elsegood, C. I. Gregory, I. Lavender, J. M. Rawson and B. K. Tanner, J. Chem. Soc., Chem. Commun., 1995, 679–680 RSC.
- Q. Liu, D. Shi, C. Ma, F. Pan, R. Qu, K. Yu and J. Xu, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2003, 59, o219–o220 CrossRef PubMed.
- A. J. Banister, M. I. Hansford, Z. V. Hauptman, S. T. Waitand and W. Clegg, J. Chem. Soc., Dalton Trans., 1989, 1705–1713 RSC.
- A. L. Macdonald and J. Trotter, Can. J. Chem., 1973, 51, 2504–2506 CrossRef CAS.
- K. Lekin, H. Phan, S. M. Winter, J. W. L. Wong, A. A. Leitch, D. Laniel, W. Yong, R. A. Secco, J. S. Tse, S. Desgreniers, P. A. Dube, M. Shatruk and R. T. Oakley, J. Am. Chem. Soc., 2014, 136, 8050–8062 CrossRef CAS PubMed.
- D. O. Cowan, M. D. Mays, T. J. Kistenmacher, T. O. Poehler, M. A. Beno, A. M. Kini, J. M. Williams, Y.-K. Kwok, K. D. Carlson, L. Xiao, J. J. Novoa and M.-H. Whangbo, Mol. Cryst. Liq. Cryst., 1990, 181, 43–58 CrossRef CAS.
- T. J. Kistenmacher, T. E. Phillips and D. O. Cowan, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1974, 30, 763–768 CrossRef CAS.
- D. Tzeli, I. D. Petsalakis, G. Theodorakopoulos, F.-U. Rahman, P. Ballester, J. Rebek Jr. and Y. Yu, ChemPhysChem, 2020, 21, 2187–2195 CrossRef CAS PubMed.
- K. Takimiya, K. Bulgarevich, K. Sahara, K. Kanazawa, H. Takenaka and K. Kawabata, Chin. J. Chem., 2022, 40, 2546–2558 CrossRef CAS.
- K. Kersten, R. Kaur and A. Matzger, IUCrJ, 2018, 5, 124–129 CrossRef CAS PubMed.
- P. Sacchi, M. Lusi, A. J. Cruz-Cabeza, E. Nauha and J. Bernstein, CrystEngComm, 2020, 22, 7170–7185 RSC.
- J. D. Dunitz and R. Taylor, Chem. – Eur. J., 1997, 3, 89–98 CrossRef CAS.
- T. Bunchuay, A. Docker, U. Eiamprasert, P. Surawatanawong, A. Brown and P. D. Beer, Angew. Chem., Int. Ed., 2020, 59, 12007–12012 CrossRef CAS PubMed.
- Y. Ishigaki, K. Shimomura, K. Asai, T. Shimajiri, T. Akutagawa, T. Fukushima and T. Susuki, Bull. Chem. Soc. Jpn., 2022, 95, 522–531 CrossRef CAS.
- S. Mishra, Chem. Commun., 2022, 58, 10136–10153 RSC.
- S. M. Winter, S. Hill and R. T. Oakley, J. Am. Chem. Soc., 2015, 137, 3720–3730 CrossRef CAS PubMed.
- N. A. Makhaeva, S. V. Amosova and V. A. Potapov, Molecules, 2022, 27, 5613 CrossRef CAS PubMed.
- D. Romito, E. Fresta, L. M. Cavinto, H. Kählig, H. Amenitsch, L. Caputo, Y. Chen, P. Samori, J.-C. Charlier, R. D. Costa and D. Bonifazi, Angew. Chem., Int. Ed., 2022, 61, e202202137 CrossRef CAS PubMed.
- C. M. Kayrouz, J. Huang, N. Hauser and M. R. Seyedsayamdost, Nature, 2022, 610, 199–204 CrossRef CAS PubMed.
- P. A. Tsuji and D. I. Hatfield, Int. J. Mol. Sci., 2022, 23, 808 CrossRef PubMed.
- B. Mallada, A. Gallardo, M. Lamanec, B. de la Torre, V. Špirko, P. Hobza and P. Jelinek, Science, 2021, 374, 863–867 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Tables S1–S4, comprising details of supramolecular aggregates in crystals 1–88 discussed herein, along with details of their chalcogen-containing congeners (Table S5). See DOI: https://doi.org/10.1039/d2ce01414a |
| This journal is © The Royal Society of Chemistry 2023 |