Supramolecular aggregation in sterically encumbered monoarylphosphates and their H-bonded adducts: multigram synthesis of elusive 2,6-di-tert-butylphenyl phosphate†
Abstract
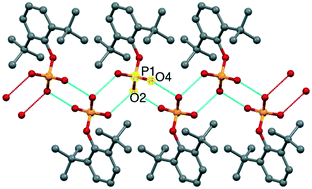
Sterically encumbered 2,6-di-tert-butylphenyl phosphate (dtbppH2) (1) has been synthesized in two steps from the reaction of either the lithium or sodium salt of 2,6-di-tert-butylphenol with POCl3, followed by controlled hydrolysis of the resultant (ArO)P(O)Cl2. Phosphate ester 1 has been characterized by analytical, spectroscopic, and single crystal X-ray diffraction studies. Crystallization of 1 in dimethylformamide (DMF) solution results in solvent decomposition to produce dimethyl amine, which then interacts with 1 to form the adduct [(Me2NH2)(dtbppH)]2·H2O (2). The presence of two acidic protons in 1 prompted us to investigate its supramolecular aggregation behaviour in the presence of aliphatic and aromatic amines such as 1,4-diazabicyclo[2.2.2]octane (DABCO) and 4,4′-bipyridine (BPY), producing the adducts [(DABCO-H)(dtbppH)] (3) and [(BPY-H2)(dtbppH)2(dtbppH2)]·MeOH (4), respectively. One-dimensional polymeric aggregation was observed for adduct 3 mediated by the presence of alternating 1,4-diazabicyclo[2.2.2]octan-1-ium cations and phosphate monoanions. Polymer 4 displays an interesting 1-D zigzag polymeric structure that contains alternating phosphate trimers and 4,4′-bipyridinium dications; the phosphate trimer itself is made up of a benzoic acid type dimer and a monomer which are linked together by P–O–H–O–P hydrogen bonds. In order to unravel the effect of steric bulkiness on the supramolecular aggregation, [(BPY-H)(dippH)] (5) and [(pip-H2)(dipp)] (6), the adducts formed by relatively less-bulkier 2,6-diisopropylphenyl phosphate (dippH2) with piperazine (pip) and 4,4′-bipyridine have been investigated.


 Please wait while we load your content...
Please wait while we load your content...