Visible-light promoted de Mayo reaction by zirconium catalysis†
Wenzhao
Zhang
and
Sanzhong
Luo
 *
*
Center of Basic Molecular Science, Department of Chemistry, Tsinghua University, Beijing 100084, China. E-mail: luosz@tsinghua.edu.cn
First published on 26th October 2022
Abstract
Zirconium catalysis with ZrCl4 was developed to facilitate a de Mayo reaction under visible light conditions without any external photosensitizer and additive.
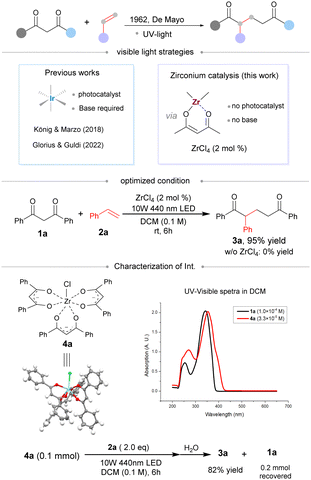
In 1962, de Mayo reported the photo-coupling of 1,3-dicarbonyls and alkenes under UV light irradiation. This reaction becomes a versatile route toward the access of 1,5-ketocarbonyl compounds (Scheme 1, top).1–3 However, a typical de Mayo reaction requires ultra violet light for effective conversion. The development of a visible light-process is not achieved until the renaissance of photochemical catalysis in organic synthesis.4,5 In 2018, Marzo and König reported a visible light-promoted de Mayo reaction catalyzed by an external iridium-based photosensitizer.6 A similar visible light process was also developed for the synthesis of medium-sized rings by Guldi and Glorius.7 In both cases, a basic additive was essential in order to facilitate the enol formation. Herein, we report a visible light-promoted de Mayo reaction by zirconium catalysis without any external photosensitizer or basic additive.
Zirconium complexes have been widely applied in organic synthesis as Lewis acid catalysts due to their low cost and toxicity, high stability and a high-coordination number.8 One interesting feature is their tendency to form stabilized zirconium enolates.8–10 For example, ZrCl4 easily forms enolate complex 4a with diketone 1a. The complex 4a is easily crystalized and shows strong absorption at 430 nm, which is red shifted compared with that of parent 1a (Scheme 1, below).11 Moreover, absorption of 4a would further red-shift to 445 nm at a reaction concentration of 2.0 × 10−3 mol L−1 (see Fig. S2, ESI†), showing significant absorption in the range of visible light. Though widely explored in chemical synthesis,8,9 zirconium enolate has not been examined in photochemical reactions. We examined the de Mayo reaction of Zr enolate 4a with styrene under visible light irradiation. To our delight, the reaction proceeded smoothly to give the desired adduct 3a in 82% yield. A ZrCl4-catalyzed version was hence further pursued.12
In our initial experiments, 1,3-diphenylpropanedione (1a) and styrene (2a) were employed as model substrates for the visible light de Mayo reaction. Under optimized conditions, 2 mol% loading of ZrCl4 is sufficient for complete conversion under 440 nm visible light. In contrast, no reaction was observed in the absence of ZrCl4 (Scheme 1, middle). The reaction did not proceed at all in the presence of only a base (Table 1, entries 1–3). Other Lewis acids have also been investigated; HfCl4 and ZrCl4 were identified as the two viable catalysts (Table 1, entries 4–8) and ZrCl4 was selected for further development as it is much more cheap. Other zirconium salts such as ZrF4 or Zr(OTf)4 were ineffective for the reaction (Table 1, entries 7 and 8). The change of solvent improved the yield greatly, and the loading of 2a could be further decreased with similar reactivity (Table 1, entries 9 and 10). Increasing the light power led to an enhanced reaction rate and the reaction was completed in 6 hours with 2 mol% loading of the catalyst (Table 1, entries 11 and 12).
| Entry | Catalyst | Ratio (1a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2a) 2a) |
Solvent | Yield (%) |
|---|---|---|---|---|
| a Reactions were performed at room temperature in 1.0 mL of solvent with 1a (0.1 mmol), 2a (0.5 mmol), and cat (10 mol%) under a 3 W blue LED in nitrogen for 36 h. Yield of the isolated product. b 10 W 440 nm LED, 3 h. c 2 mol% ZrCl4 loading, 10 W 440 nm LED, for 6 h. | ||||
| 1 | K2CO3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
MeCN | NR |
| 2 | H3PO4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
MeCN | NR |
| 3 | Quinuclidine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
MeCN | NR |
| 4 | AlCl3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
MeCN | NR |
| 5 | HfCl4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
MeCN | 62 |
| 6 | ZrCl4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
MeCN | 63 |
| 7 | ZrF4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
MeCN | NR |
| 8 | Zr(OTf)4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
MeCN | NR |
| 9 | ZrCl4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 5 |
DCM | 91 |
| 10 | ZrCl4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DCM | 89 |
| 11b | ZrCl4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DCM | 98 |
| 12c | ZrCl4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
DCM | 95 |
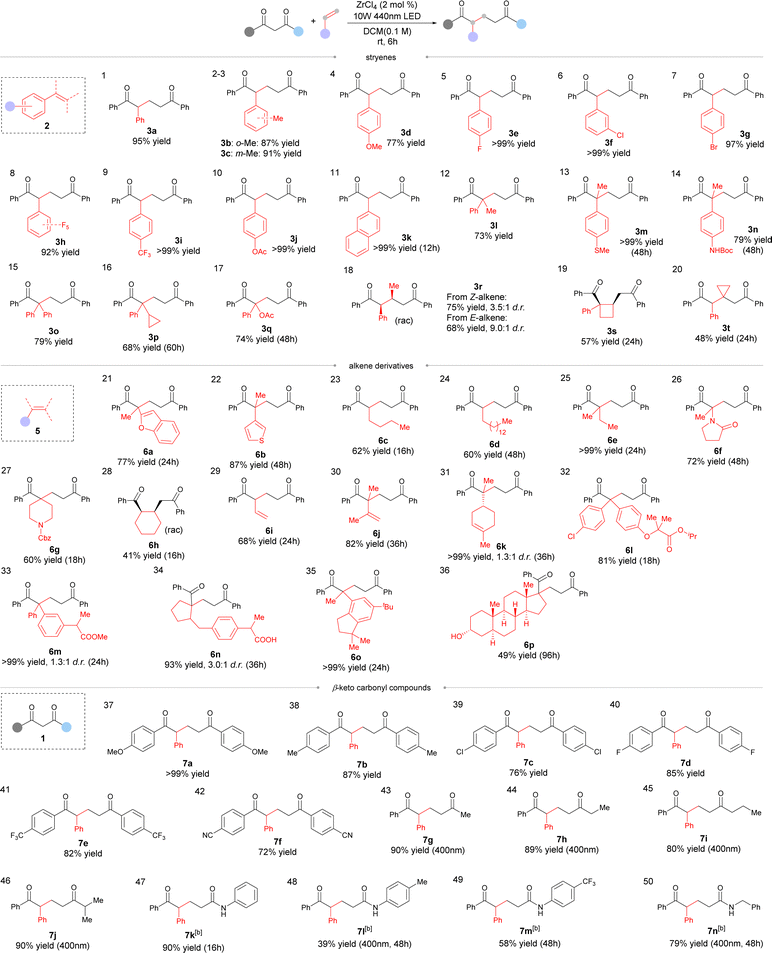
With the optimized conditions in hand, we examined the scope of styrene derivatives (Table 2). Substituents on styrenes such as methyl (Table 2, entries 2 and 3, 3b and 3c), MeO- (entry 4, 3d), halogen (entries 5–8, 3e–3h), trifluoromethyl (entry 9, 3i) or AcO (entry 10, 3j) were well tolerated to afford the desired products in good yields. 2-Vinylnaphthalene was also compatible, delivering 3k in a quantitative yield (Table 2, entry 11). 1,1-Disubstituted aryl alkenes worked well under the present conditions (Table 2, entries 12–17). 2-Aryl-1-propenes bearing a MeS- or BocNH- moiety were equally applicable (Table 2, entries 13 and 14, 3l and 3n). Diaryl alkene 2o provided a good yield of 79% (Table 2, entry 15), and cyclopropyl- or acetoxyl-substituted alkene was well tolerated, too (entries 16 and 17, 3p and 3q).
Aromatic internal alkenes were next examined. Both E- and Z-isomers of β-methylstyrene worked but with varying diastereoselectivity. In both cases, the reaction afforded syn-selective adducts as the major diastereoisomers (Table 2, entry 18, 3r). Notably, trisubstituted alkenes such as 2s and 2t also reacted well in this process, delivering moderate yields within 24 hours (Table 2, entries 19 and 20). The reactions with other aromatic alkenes such as benzofuran-substituted alkene 5a and thiophene derivative 5b proceeded smoothly to give the desired products with 77% and 87% isolated yields, respectively (Table 2, entries 21 and 22, 6a and 6b).
Aliphatic alkenes were also found to be applicable, showing moderate to good reactivity (Table 2, entries 23–28, 6c–6h). The reaction occurred with enamide to give the expected adduct 6f in 72% yield (Table 2, entry 26). N-Cbz-protected piperidine was also compatible, providing 6g in 60% yield (Table 2, entry 27). Cyclohexene worked to give a moderate 41% yield (Table 2, entry 28, 6h). The reaction also tolerated dienes such as butadiene and 2,3-dimethyl butadiene and a selective reaction of one of the two identical double bonds was generally observed (Table 2, entries 29 and 30, 6i and 6j). When (R)-cinene 5k was applied, a regioselective reaction at the terminal double bond was observed to give de Mayo adduct 6k in a quantitative yield with 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (Table 2, entry 31).
1 dr (Table 2, entry 31).
The scope of diketones was also examined. Both EDGs- and EWG-substituted aromatic diketones performed well in the reaction, giving good to excellent yields of the corresponding products (Table 2, entries 37–42, 7a–7f). Aliphatic ketones were also workable with high yields of the desired adducts (Table 2, entries 43–46, 7g–7j). In these cases, a shorter-wavelength light of 400 nm was applied for effective conversion. Apart from diketones, β-ketoamides were compatible in the reaction with light irradiation at 400 nm in the presence of 10 mol% of ZrCl4 (Table 2, entries 47–50, 7k–7n).
To demonstrate the synthetic potential of this methodology, alkene derivatives of natural products and pharmaceuticals were investigated in the reaction. Fenofibrate derivative 5l performed well in this process, delivering 6l in 81% yield after 18 hours (Table 2, entry 32). Ibuprofen and celestolide derivatives were also compatible, and the desired products were isolated in excellent yields (Table 2, entries 33 and 34, 6m and 6n). To our delight, androsterone derivative 5p was also reactive to give an alkene insertion adduct 6p in 49% yield after 4 days (Table 2, entry 35).
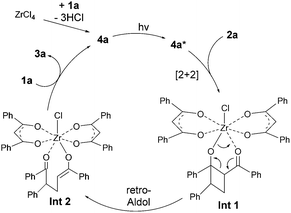
Mechanistically, the reaction is proposed to proceed via a photo-excited Zr enolate intermediate 4a* (Scheme 2). Subsequent photo-[2+2] and retro-aldol reactions lead to the desired adduct. The observed regioselectivity with asymmetric β-ketocarbonyls can be understood by considering the extent of conjugation in the enolate intermediate (e.g.4a/4a*) and the relative stability of the diradical intermediate involved in the photo-[2+2] process (see Scheme S1, ESI† for details).6,7
In concluon, we developed a ZrCl4 catalyzed de Mayo reaction under visible light conditions without any external photosensitizer or additive. The visible light zirconium catalysis proceeds via a photoactive Zr enolate intermediate, and 2% loading of ZrCl4 suffices to promote the reaction for a wide variety of alkenes and 1,3-dicarbonyls.
This work is supported by the Natural Science Foundation of China (91956000, 22031006 and 21861132003), Tsinghua University Initiative Scientific Research Program and Haihe Laboratory of Sustainable Chemical Transformations.
Conflicts of interest
There are no conflicts to declare.Notes and references
-
(a) P. de Mayo, P. H. Takashita and A. B. M. A. Satter, Proc. Chem. Soc., 1962, 119 CAS
; (b) P. de Mayo and P. H. Takashita, Can. J. Chem., 1963, 41, 440–449 CrossRef CAS
; (c) B. D. Challand, H. Hikino, G. Kornis, G. Lange and P. de Mayo, J. Org. Chem., 1969, 34, 794–806 CrossRef CAS
.
- For synthetic applications of de Mayo reaction, see:
(a) B. W. Disanayaka and A. C. Weedon, J. Org. Chem., 1987, 52, 2905–2910 CrossRef CAS
; (b) T. Bach and J. P. Hehn, Angew. Chem., Int. Ed., 2011, 50, 1000–1045 CrossRef CAS
; (c) Z.-J. Xu, Y. Zong, Y.-N. Qiao, J.-Z. Zhang, X. Liu, M.-Z. Zhu, Y. Xu, H. Zheng, L. Fang, X.-N. Wang and H.-X. Lou, Angew. Chem., Int. Ed., 2020, 59, 19919–19923 CrossRef CAS PubMed
; (d) J.-H. Gu, W.-J. Wang, J.-Z. Chen, J.-S. Liu, N.-P. Li, M.-J. Cheng, L.-J. Hu, C.-C. Li, W.-C. Ye and L. Wang, Org. Lett., 2020, 22, 1796–1800 CrossRef CAS PubMed
; (e) S. K. Kandappa, L. K. Valloli, S. Jockusch and J. Sivaguru, J. Am. Chem. Soc., 2021, 143, 3677–3681 CrossRef CAS PubMed
; (f) D. Tymann, D. C. Tymann, U. Bednarzick, L. Iovkova-Berends, J. Rehbein and M. Hiersemann, Angew. Chem., Int. Ed., 2018, 57, 15553–15557 CrossRef CAS PubMed
; (g) N. Salaverri, R. Mas-Ballesté, L. Marzo and J. Alemán, Commun. Chem., 2020, 3, 132 CrossRef CAS
.
- For synthetic applications of 1,5-diketones, see:
(a) T. Miyakoshi and H. Konno, Org. Biomol. Chem., 2019, 17, 2896–2905 RSC
; (b) G. dos Passos Gomes, I. A. Yaremenko, P. S. Radulov, R. A. Novikov, V. V. Chernyshev, A. A. Korlyukov, G. I. Nikishin, I. V. Alabugin and A. O. Terent’ev, Angew. Chem., Int. Ed., 2017, 56, 4955–4959 CrossRef CAS PubMed
; (c) A. G. Griesbeck, O. Hinze, H. Görner, U. Huchel, C. Kropf, U. Sundermeier and T. Gerke, Photochem. Photobiol. Sci., 2012, 11, 587–592 CrossRef CAS PubMed
.
- For reviews of photocatalysis, see:
(a) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322–5363 CrossRef CAS PubMed
; (b) N. A. Romero and D. A. Nicewicz, Chem. Rev., 2016, 116, 10075–10166 CrossRef CAS PubMed
; (c) L. Marzo, S. K. Pagire, O. Reiser and B. König, Angew. Chem., Int. Ed., 2018, 57, 10034–10072 CrossRef CAS PubMed
; (d) Q.-Q. Zhou, Y.-Q. Zou, L.-Q. Lu and W.-J. Xiao, Angew. Chem., Int. Ed., 2019, 58, 1586–1604 CrossRef CAS PubMed
; (e) T. P. Yoon, M. A. Ischay and J. Du, Nat. Chem., 2010, 2, 527–532 CrossRef CAS PubMed
; (f) M. H. Shaw, J. Twilton and D. W. C. MacMillan, J. Org. Chem., 2016, 81, 6898–6926 CrossRef CAS PubMed
; (g) J. Xuan and W.-J. Xiao, Angew. Chem., Int. Ed., 2012, 51, 6828–6838 CrossRef CAS
; (h) M. D. Kärkäs, J. A. Porco and C. R. J. Stephenson, Chem. Rev., 2016, 116, 9683–9747 CrossRef PubMed
; (i) D. Ravelli, S. Protti and M. Fagnoni, Chem. Rev., 2016, 116, 9850–9913 CrossRef CAS PubMed
; (j) Q. Liu and L.-Z. Wu, Natl. Sci. Rev, 2017, 4, 359–380 CrossRef CAS
; (k) Ci Jiang, W. Chen, W.-H. Zheng and H. Lu, Org. Biomol. Chem., 2019, 17, 8673–8689 RSC
; (l) C. Wang and Z. Lu, Org. Chem. Front., 2015, 2, 179–190 RSC
; (m) R. Brimioulle, D. Lenhart, M. M. Maturi and T. Bach, Angew. Chem., Int. Ed., 2015, 54, 3872–3890 CrossRef CAS
.
- For reviews of photosensitizer-free photoreactions, see:
(a) Y. Wei, Q.-Q. Zhou, F. Tan, L.-Q. Lu and W.-J. Xiao, Synthesis, 2019, 3021–3054 CAS
; (b) Y. Lang, C.-J. Li and H. Zeng, Org. Chem. Front., 2021, 8, 3594–3613 RSC
; (c) B. Baruaha and M. L. Deb, Org. Biomol. Chem., 2021, 19, 1191–1229 RSC
; (d) G. E. M. Crisenza, D. Mazzarella and P. Melchiorre, J. Am. Chem. Soc., 2020, 142(12), 5461–5476 CrossRef CAS PubMed
.
- R. Martinez-Haya, L. Marzo and B. König, Chem. Commun., 2018, 54, 11602–11605 RSC
.
- T. O. Paulisch, L. A. Mai, F. Strieth-Kalthoff, M. J. James, C. Henkel, D. M. Guldi and F. Glorius, Angew. Chem., Int. Ed., 2022, e202112695 CAS
.
- For reviews of zirconium catalysis, see:
(a) S. Kobayashi and K. Manabe, Pure Appl. Chem., 2000, 72, 1373–1380 CrossRef CAS
; (b) Z.-H. Zhang and T.-S. Li, Curr. Org. Chem., 2009, 13, 1–30 CrossRef CAS
; (c) L.-P. Mo and Z.-H. Zhang, Curr. Org. Chem., 2011, 15, 3800–3823 CrossRef CAS
; (d) G. Smitha, S. Chandrasekhar and C. S. Reddy, Synthesis, 2008, 829–855 CAS
; (e) K. Nikoofar and Z. Khademi, Res. Chem. Intermed., 2016, 42, 3929–3977 CrossRef CAS
.
- For zirconium catalyzed 1,3-dikeotne transformations, see:
(a) G. Smitha, S. Patnaik and C. S. Reddy, Synthesis, 2005, 711–713 CAS
; (b) E. Kolvari, M. A. Zolfigol, N. Koukabi, M. Gilandust and A.-V. Kordi, J. Iran. Chem. Soc., 2013, 10, 1183–1191 CrossRef CAS
; (c) N. Ronaghi, D. M. Fialho, C. W. Jones and S. France, J. Org. Chem., 2020, 85, 15337–15346 CrossRef CAS PubMed
.
- K. V. Zherikova and N. B. Morozova, J. Struct. Chem., 2012, 53, 761–767 CrossRef CAS
.
- C. Janiak and T. G. Scharmann, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1998, C54, 210–214 CrossRef CAS
.
- For selected Lewis acid catalyzed photoreactions, see:
(a) T. P. Yoon, Acc. Chem. Res., 2016, 49, 2307–2315 CrossRef CAS PubMed
; (b) D. P. Schwinger and T. Bach, Acc. Chem. Res., 2020, 53, 1933–1943 CrossRef CAS PubMed
; (c) X. Huang and E. Meggers, Acc. Chem. Res., 2019, 52(3), 833–847 CrossRef CAS PubMed
; (d) S. Mondal, F. Dumur, D. Gigmes, M. P. Sibi, M. P. Bertrand and M. Nechab, Chem. Rev., 2022, 122, 5842–5976 CrossRef CAS PubMed
; (e) Q. Xia, J. Dong, H. Song and Q. Wang, Chem. – Eur. J., 2019, 25, 2949–2961 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc05029c |
| This journal is © The Royal Society of Chemistry 2022 |