Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Are you still using organic dyes? Colorimetric formaldehyde analysis for true photocatalytic-activity evaluation†
Fitri Rizki
Amalia
a,
Mai
Takashima‡
 *ab and
Bunsho
Ohtani§
*ab and
Bunsho
Ohtani§
 ab
ab
aGraduate School of Environmental Science, Hokkaido University, Sapporo 060-0810, Japan. E-mail: Takashima.m@cat.hokudai.ac.jp
bInstitute for Catalysis, Hokkaido University, Sapporo 001-0021, Japan
First published on 12th September 2022
Abstract
We report easily available and highly reliable colorimetric formaldehyde analysis using only a spectrophotometer/colorimeter, which enables the evaluation of the “true” photocatalytic activity induced by photoabsorption of a “photocatalyst”, compared with gas-chromatographic analyses of gaseous products, even though the reliability of colorimetric organic-dye analysis is much worse under visible-light irradiation.
Although numerous papers have reported “photocatalytic activities” of various kinds of materials, there seems to be no clear definition of “photocatalytic activity” as a specific property or ability of each photocatalyst.1 In almost all the reports, what was shown as photocatalytic activity is an absolute or relative rate of reaction occurring (or amount of a product obtained) in the presence of a possible photocatalyst under photoirradiation, even if non-photocatalytic reaction(s) is (are) possibly included. In a strict kinetic sense,2 reaction rate must be discussed, assuming (and confirming) a reaction stoichiometry. In this case, the rate of a given reaction can be evaluated by measuring only one of the substrates or products included in its stoichiometry. Furthermore, by clarifying the reaction stoichiometry, it is possible to claim that a photocatalyst is not changed during the reaction, i.e., working “catalytically”. The problem is that the above-mentioned points have negligibly been considered in most of the papers on photocatalysis, especially organic decomposition. This may give or have already given unrecognized confusions in the field of photocatalysis.
One of the examples of failed evaluations of photocatalytic activity is dye-decoloration studies under visible-range (VR) irradiation, since the dye-decoloration may occur through a dye-sensitization mechanism even if the used “photocatalyst” does not induce photocatalysis at all under VR irradiation3–5 and its stoichiometry has not been clarified within the authors’ knowledge. Then, why is it that this unreliable method has been often employed? One possible reason is its simplicity, only measuring the absorbance of the centrifuged (or filtered) reaction solution (even without dilution) and no use of analytical instruments such as gas or liquid chromatographs, which are much more expensive compared with spectrophotometers (or colorimeters) in not well-equipped laboratories. This is not a problem in a scientific sense. A fatal problem is that it is easy and convenient to obtain relatively high “fake” photocatalytic activity since a highly diluted (but giving detectable absorbance) dye solution can be easily decolored by a dye-sensitization mechanism (the photoexcited electron of a dye molecule is injected to a solid material and reduces oxygen adsorbed on the surface of the solid, which leads to dye degradation),6 and not by the true photocatalysis, under VR irradiation, even with a much smaller molar amount of dye than that of the “photocatalyst” in the reaction mixture.7 As a result, more than 3000 papers (more than 30,000 papers in total) on dye-decoloration studies have been published per year in the recent five years (Web of Science search with keywords “photocataly* AND ((methylene blue) OR rhodamine)” and the real number of “dye-decoloration” papers must be much higher).
One of the possible solutions for this problem is to find a reaction using a colorless,8 not VR-light absorbing, substrate and a colorimetric method only using a spectrophotometer or a colorimeter. Here, the authors propose a method to obtain the “true” photocatalytic activity using formaldehyde (HCHO) as a key compound (there were a few published papers on photocatalytic HCHO destruction9 and HCHO liberation,10–12 but no comparison was made with the other reaction systems or with the other analytical methods) being colorimetrically analyzed by derivatization with Hantzsch reaction13 to liberate yellow diacetyldihydrolutidine (DDL) having intense VR photoabsorption (ε = ca. 8 × 103 mol−1 L cm−1 in aqueous solution). Two photocatalytic-reaction systems are examined. One is methanol dehydrogenation to yield HCHO and hydrogen (H2) under deaerated conditions and the other is the oxidation of HCHO under aerobic conditions, where, in a usual manner, H2 and carbon dioxide (CO2), respectively, have been analyzed by gas chromatography (GC) as one of the easiest ways for well-equipped laboratories.
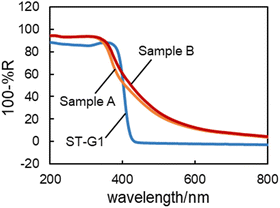
Here the results of two reactions and rhodamine B (RhB) decoloration under high-intensity (ca. 100 mW) green or UV LED irradiation are examined using three kinds of commercial titanium(IV) oxide (titania) powders: white, i.e., not VR-absorbing, ST-G1 (Showa Denko Ceramics), and two VR-absorbing titanias (Sample A and Sample B from a private company in Japan) as representative titania photocatalyst samples. Fig. 1 shows the summary of photoinduced reactions measured by colorimetric-HCHO analysis, RhB decoloration and GC (for detailed reaction and analysis conditions, see the ESI†).
 | ||
| Fig. 1 Yields of products or consumption of substrates of photoinduced reactions by three kinds of samples under green and UV-LED irradiation. | ||
For RhB decoloration, green and UV-LED irradiation induced the reaction in all the suspensions, though green-LED irradiation required ca. twelve-times longer irradiation to obtain a similar amount (negligible change in RhB concentration was observed in the absence of titania under both irradiation conditions). It seems strange that non-VR-absorbing white ST-G1 showed appreciable “activity” even under green-LED irradiation, suggesting that self-sensitized dye decoloration proceeded. Actually, the action spectra, i.e., irradiation-wavelength dependence, of RhB decoloration by Sample A and Sample B (Fig. S1, ESI†) clearly show that VR absorption by (possibly surface adsorbed) RhB enhanced the decoloration under VR irradiation as was observed for methylene-blue decoloration.14 Indeed, it can be said that the higher decoloration rate of RhB by the VR-absorbing Sample A and Sample B compared with that by ST-G1 is attributable to their “true” photocatalytic activity induced by the VR absorption (see Fig. 2). However, it should be noted that this is just speculation, and this RhB-decoloration study cannot prove it. In the HCHO consumption analysis under aerated conditions, similar to RhB decoloration, the VR-absorbing samples showed appreciable HCHO consumption. In contrast, ST-G1 showed negligible activity, suggesting the “true” VR-induced photocatalytic activities of Sample A and Sample B. The result of action-spectrum analysis supported this photocatalytic activity under VR irradiation, as shown in Fig. S2 (ESI†); the action spectrum for the HCHO consumption resembled the diffuse reflectance spectrum of Sample A.
On the other hand, those VR-absorbing samples induced negligible H2 and HCHO liberation from deaerated aqueous methanol under green-LED irradiation; Sample B gave a little HCHO liberation, but almost no H2 was evolved. It is well known that the loading of noble-metal cocatalysts such as platinum is the most significant requisite for efficient H2 evolution,15,16 but not changing the action spectrum.17 Consequently, in this experiment, samples were platinized before the LED irradiation. Since platinized VR-absorbing samples induced the dehydrogenation under UV irradiation with lower but appreciable product yields compared with that by ST-G1, the loaded platinum cocatalyst really works, at least partly. Consequently, these VR absorbing samples may have lower conduction band-bottom (CBB) energy, i.e., lower reduction ability, to drive H2 liberation by the modification to give VR absorption, while they have sufficiently lower valence band-top energy, i.e., sufficient oxidation ability to drive organic decomposition. It has been pointed out that a photocatalyst that cannot drive H2 evolution has CBB lower than the standard hydrogen potential (SHE; 0 V), and this means electrons at the CBB cannot reduce oxygen (O2) in the one-electron reduction mode.18 This suggests that these VR-sensitive titania photocatalysts drive oxidative organic decomposition, such as the present HCHO oxidative decomposition under aerobic conditions through two-electron reduction of O2, though further consideration of this mechanism may be out of the scope of this paper and will be discussed elsewhere (for detailed discussion, see ESI†).
Thus, it is suggested that the results of product-liberation and substrate-consumption analyses using HCHO as the substrate/product reflect the “true” photocatalytic activity induced by photoexcitation of possible photocatalysts, and the colorimetric HCHO analysis is significantly much more reliable than organic dye-decoloration analysis. Next, the superiority of the HCHO analysis compared with the conventional analysis is discussed as follows.
As is shown in Fig. 1, UV irradiation onto aqueous methanol suspension liberates H2 and HCHO with its possible stoichiometry:19
| CH3OH → HCHO + H2. | (1) |
Fig. 3 shows the relation between the liberated H2 and HCHO amounts by commercial titania photocatalysts under UV-VR irradiation; almost equimolar production (eqn (1)) was observed when their amount was less than ca. 300 μmol corresponding to ca. 0.5% conversion of methanol. Therefore, it can be said that as long as the product amount is less than this level of methanol conversion, the HCHO amount can be used for the rate, i.e., “true” photocatalytic activity.
 | ||
| Fig. 3 Plot of H2 evolution as a function of HCHO liberation in methanol dehydrogenation on representative titania. | ||
On the other hand, it is not easy to confirm the stoichiometry of oxidative decomposition of HCHO because of the difficulty of quantitative analysis of the reduction product of O2. Therefore, carbon material balance was examined in this study using commercial titania samples under UV-VR irradiation (for details, see ESI†). Fig. 4 shows the comparison of molar amount of HCHO consumption and possible product liberation. Except for Sample 3, almost fair carbon material balance was observed (one of the possible reasons for the not complete match is due to the interference of methyl formate in formic acid analysis and another possible reason is volatilization and surface adsorption of HCHO causing it to be underestimated). The significant point is that the product distribution was very different depending on the photocatalysts, probably due to the properties of HCHO and HCOOH, which may have unique interaction with the different characteristics of each titania photocatalyst,20,21 even though the HCHO conversion was in the same order. Therefore, for this oxidative HCHO decomposition, the stoichiometry cannot be uniquely determined. In other words, estimation of the rate of HCHO decomposition by measuring the HCHO consumption seems more appropriate compared with measuring CO2 liberation. As shown in Fig. 1, Sample A produced a negligible amount of CO2 under green-LED irradiation, while appreciable HCHO consumption was observed, presumably due to low selectivity for CO2 production by Sample A. As long as HCHO, which can be colorimetrically measured, is used as a substrate, product analysis, i.e., CO2 analysis, is less appropriate to evaluate the overall photocatalytic activity.
In conclusion, colorimetric analysis of HCHO as a substrate or a product of photocatalytic reactions enables the evaluation of “true” photocatalytic activity under VR or UV irradiation using only a spectrophotometer or colorimeter as an instrument with comparable or even higher sensitivity and reliability compared with the usual chromatographic analyses. This may open up a new stage for photocatalytic-activity discussion and, at the same time, reexamination of previously reported photocatalytic activities evaluated by dye decoloration.
Conceptualization: B. O.; methodology: M. T. and B. O.; investigation: F. R. A.; resources: B. O. and M. T.; supervision: M. T. and B. O.; visualization: F. R. A.; writing – original draft: F. R. A.; and writing – review and editing: F. R. A.; B. O.; and M. T. All the authors have read and agreed to the published version of the manuscript.
This work was financially supported by the Ministry of Education, Culture, Sports, Science and Technology (MEXT) Japan, Special Grant Program of Hokkaido University-Japan, Hokkaido University DX Fellowship grant no. JPMJSP2119-Japan and Nagoya University Research Fund.
Conflicts of interest
There are no conflicts to declare.Notes and references
- B. Ohtani, J. Photochem. Photobiol., C, 2010, 11, 157–178 CrossRef CAS.
- P. Muller, Pure Appl. Chem., 1994, 66, 1077–1084 CrossRef.
- Z. Liu, H. Zheng, H. Yang, L. Hao, L. Wen, T. Xu and S. Wu, RSC Adv., 2006, 6, 54215–54225 RSC.
- B. Ohtani, Chem. Lett., 2008, 37, 216–229 CrossRef.
- B. Ohtani, Catalyst, 2016, 6, 192 CrossRef.
- T. Watanabe, T. Takizawa and K. Honda, J. Phys. Chem., 1977, 81, 1845–1851 CrossRef CAS.
- S. Bae, S. Kim, S. Lee and W. Choi, Catal. Today, 2014, 224, 21–28 CrossRef CAS.
- C. Chen, M. Li, Y. Jia, R. Chong, L. Xu and X. Liu, J. Colloid Interface Sci., 2020, 564, 442–453 CrossRef CAS PubMed.
- E.-M. Shin, R. Senthurchelvan, J. Munoz, S. Basak, K. Rajeshwar, G. Benglas-Smith and B. C. Howell III, J. Electrochem. Soc., 1996, 143, 1562–1570 CrossRef CAS.
- S. Pradhan, D. Ghosh and S. Chen, ACS Appl. Mater. Interfaces, 2009, 1, 2060–2065 CrossRef CAS.
- A. Y. Ahmed, T. A. Kandiel, T. Oekermann and D. Bahnemann, J. Phys. Chem. Lett., 2011, 2, 2461–2465 CrossRef CAS.
- Z. Li, A. Ivanenko, X. Meng and Z. Zhang, J. Hazard. Mater., 2019, 380, 120822 CrossRef CAS PubMed.
- T. Nash, Biochem. J., 1953, 55, 416–421 CrossRef CAS PubMed.
- X. Yan, T. Ohno, K. Nishijima, R. Abe and B. Ohtani, Chem. Phys. Lett., 2006, 429, 606–610 CrossRef CAS.
- B. Ohtani, K. Iwai, S.-I. Nishimoto and S. Sato, J. Phys. Chem. B, 1997, 101, 3349–3359 CrossRef CAS.
- B. Ohtani, O. O. P. Mahaney, F. Amano, N. Murakami and R. Abe, J. Adv. Oxid. Technol., 2010, 13, 247–261 CAS.
- T. Torimoto, Y. Aburakawa, Y. Kawahara, S. Ikeda and B. Ohtani, Chem. Phys. Lett., 2004, 392, 220–224 CrossRef CAS.
- H. Hori, M. Takashima, M. Takase and B. Ohtani, Chem. Lett., 2017, 46, 1376–1378 CrossRef CAS.
- S.-i Nishimoto, B. Ohtani and T. Kagiya, J. Chem. Soc., Faraday Trans. 1, 1985, 81, 2467–2474 RSC.
- M. J. Wang, S. L. Shen, L. Li, Z. H. Tang and J. H. Yang, J. Mater. Sci., 2017, 52, 5155–5164 CrossRef CAS.
- W. T. Chen, A. Chan, D. Sun-Waterhouse, J. Llorca, H. Idriss and G. I. N. Waterhouse, J. Catal., 2018, 367, 27–42 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc04291f |
| ‡ Present address: Graduate School of Engineering, Nagoya University, Furo-cho, Chikusa-ku, Nagoya 464-8603, Japan. |
| § Present address: Nonprofitable Organization touche NPO, 1-6-414, North 4, West 14, Chuo-ku, Sapporo, 060-0004, Japan. |
| This journal is © The Royal Society of Chemistry 2022 |