Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and 83Kr NMR spectroscopy of Kr@C60†
Gabriela
Hoffman
,
George R.
Bacanu
 ,
Elizabeth S.
Marsden
,
Elizabeth S.
Marsden
 ,
Mark C.
Walkey
,
Mark C.
Walkey
 ,
Mohamed
Sabba
,
Mohamed
Sabba
 ,
Sally
Bloodworth
,
Sally
Bloodworth
 ,
Graham J.
Tizzard
,
Graham J.
Tizzard
 ,
Malcolm H.
Levitt
,
Malcolm H.
Levitt
 and
Richard J.
Whitby
and
Richard J.
Whitby
 *
*
Chemistry, Faculty of Engineering and Physical Sciences, University of Southampton, Southampton, SO17 1BJ, UK. E-mail: rjw1@soton.ac.uk
First published on 12th September 2022
Abstract
Synthesis of Kr@C60 is achieved by quantitative high-pressure encapsulation of the noble gas into an open-fullerene, and subsequent cage closure. Krypton is the largest noble gas entrapped in C60 using ‘molecular surgery’ and Kr@C60 is prepared with >99.4% incorporation of the endohedral atom, in ca. 4% yield from C60. Encapsulation in C60 causes a shift of the 83Kr resonance by −39.5 ppm with respect to free 83Kr in solution. The 83Kr spin-lattice relaxation time T1 is approximately 36 times longer for Kr encapsulated in C60 than for free Kr in solution. This is the first characterisation of a stable Kr compound by 83Kr NMR.
Endohedral fullerenes (endofullerenes) are compounds in which atoms or small molecules are encapsulated inside fullerenes, providing a unique opportunity for study of the confined species in the isolated cavity.1a–d The noble gas endofullerenes of C60 and C70 (denoted e.g. Ng@C60 for the former case) are a specific group that have been the subject of sustained research activity, as compounds of great interest for study of the interactions between the encapsulated species and the encapsulating cage,2,3 the quantised energy level structure of the endohedral noble gas atom,2 and the effect of the noble gas upon the properties and reactivity of the fullerene cage.4a,b Many theoretical studies have addressed these areas,5a–h and our interest in the practical synthesis of noble gas endofullerenes is motivated by both the opportunity for direct study of these materials, and the value of resulting data as a test of theoretical models.
Early methods for preparation of Ng@C60 compounds relied upon direct encapsulation by exposure of C60 to the gas under high temperature and pressure, and led to approximately 0.1% incorporation of a single endohedral atom of He, Ne, Ar or Kr, and just 0.03% of Xe.6 Under similar conditions, an improved level of direct encapsulation into C60 ground with KCN was achieved, of 1% He and approx. 0.3% Ar, Kr or Xe.7a–c Enriched samples have been obtained using recycling HPLC, of ca. 0.1–1.0 mg Kr@C60 with 90–99% purity,8,9 and ca. 0.3 mg Xe@C60 with 50% purity.7b Resulting 13C NMR, UV-visible absorption, infrared, Raman, X-ray absorption and 129Xe NMR studies have confirmed a weak interaction between the noble gas atom and interior cage surface,7b,8 and observed the endohedral atom to influence cage vibrational and rotational properties.5d,9
With the development of the ‘molecular surgery’ method of endofullerene synthesis, high incorporation to facilitate spectroscopic studies on a macroscopic (multi-milligram) scale has become possible, the synthesis of H2@C60 and 4He@C60 being early examples.10,11 Murata's open-fullerenes 1 and 3 (Fig. 1)12,13 are key intermediates for ‘filling’ in the syntheses of HF@C60, H2@C60 and H2O@C60 (1), Ar@C60 and CH4@C60 (3),13–17 and we recently developed a one-pot filling and partial closure of a phosphorous ylid derivative 2 that enabled efficient synthesis of noble gas endofullerenes 3He@C60, 4He@C60 and Ne@C60.18 Incorporation of approx. 50–60% of the noble gas was accomplished, and enrichment of Ne@C60 to >99.5% encapsulation of the noble gas atom was achieved by recycling preparative HPLC.
 | ||
| Fig. 1 Open-cage fullerenes 1–3 are key precursors to ‘filling’ by a single atom or molecule in reported syntheses of H2O@C60, HF@C60, H2@C60 (1),11,16,17 HD@C60, D2@C60, 3He@C60, 4He@C60, Ne@C60 (2),18 CH4@C60, Ar@C60 (3).14,15 | ||
With the aim of elucidating the energy level structure of a confined noble gas atom and its interaction with the interior cage surface in detail, our syntheses of 3He@C60 and 4He@C60 have so far enabled characterisation of internuclear interactions in the form of the “non-bonded” J-coupling (0JHeC) and experimental interaction potential – each evaluated against theoretical models.2,3 We now describe preparation of pure Kr@C60 on a scale of tens of milligrams suitable for detailed study, including by 13C and 83Kr NMR discussed herein. We also report upon current limitations to the application of molecular surgery methods for synthesis of Xe@C60.
Synthesis of Kr@C60 was carried out according to the methods we have reported for preparation of CH4@C6015 and Ar@C60,14 and is described in Scheme 1. The bis(hemiketal) hydrate of open fullerene 1 was prepared from C60 using our recent optimisation18 of the cage-opening steps earlier described by Murata and co-workers,13,19 before dehydration to give 1 and insertion of sulfur to furnish 3.12 DFT calculations (see ESI†) indicated  = 87 kJ mol−1 and ΔHbind = −57 kJ mol−1 for encapsulation of krypton by 3, similar to the values for CH4. Accordingly, heating powdered 3 under >1500 atm of krypton gas for 14 h gave Kr@3 with >99% filling estimated from the 1H NMR and ESI+ mass spectra.
= 87 kJ mol−1 and ΔHbind = −57 kJ mol−1 for encapsulation of krypton by 3, similar to the values for CH4. Accordingly, heating powdered 3 under >1500 atm of krypton gas for 14 h gave Kr@3 with >99% filling estimated from the 1H NMR and ESI+ mass spectra.
Encapsulation of xenon by 3 was calculated to have  = 152 kJ mol−1 and ΔHbind = −56 kJ mol−1, and attempted preparation of Xe@3 by heating 3 at 212 °C under 1850 atm of xenon gas for 17 h gave <1% xenon incorporation, from the ESI+ mass spectrum. Higher temperature or a longer reaction time led to substantial decomposition, so xenon ‘filling’ of 3 does not constitute a viable route for the synthesis of Xe@C60 for which a larger cage opening is needed.
= 152 kJ mol−1 and ΔHbind = −56 kJ mol−1, and attempted preparation of Xe@3 by heating 3 at 212 °C under 1850 atm of xenon gas for 17 h gave <1% xenon incorporation, from the ESI+ mass spectrum. Higher temperature or a longer reaction time led to substantial decomposition, so xenon ‘filling’ of 3 does not constitute a viable route for the synthesis of Xe@C60 for which a larger cage opening is needed.
The rate of first-order thermal dissociation of Kr@3 was measured between 433 and 453 K. Arrhenius and Eyring plots are shown in the ESI.† All parameters for loss of krypton from the fullerene (Ea exit = 138.5 ± 5.6 kJ mol−1, ΔH‡ = 134.8 ± 5.6 kJ mol−1, ΔS‡ = −40.1 ± 14.6 J K−1 mol−1, (log)A = 11.3 and ΔG‡ = 152.4 ± 0.1 kJ mol−1 at 165 °C, closely matched those for loss of CH4 from 3.20
Oxidation of Kr@3 gave sulfoxide Kr@4 cleanly, and photochemical desulfinylation of Kr@4 led to the ring-contracted product Kr@1, isolated as its hydrate Kr@5 with >99% encapsulation. Separation of Kr@C60 and H2O@C60 is possible using recycling preparative HPLC so it was unnecessary to conduct exhaustive drying of Kr@5 or to use conditions for the following step that avoid re-encapsulation of traces of water (cf. our Ar@C60 synthesis).14 The final ring-closure steps for conversion of Kr@5 to Kr@C60 were therefore conducted under the conditions we originally reported for the synthesis of H2O@C60;17 involving dehydration to Kr@1, intramolecular Wittig reaction of the phosphonium ylid Kr@2 to give Kr@6, then similar Wittig closure of a phosphite ylid upon heating Kr@6 with (iPrO)3P. Reaction with N-phenylmaleimide in a final step that involves sequential [4 + 2], retro[4 + 2] and [2 + 2 + 2] cycloaddition completed the cage closure. Removal of H2O@C60 and enrichment of the krypton encapsulation, was achieved by recycling preparative HPLC.8,21a,b Overall, Kr@C60 was recovered with >99.4% incorporation of the noble gas, and in 3.6–4.1% yield from C60 over repeated batch syntheses. A crystal structure of the nickel(II) octaethylporphyrin/benzene solvate of Kr@C60 was obtained, in which the noble gas atom is centred in the cage (see ESI†) as in the previously reported structure of ca. 9% filled Kr@C60 {NiII(OEP)} 2C6H6.21a
Krypton is the largest noble gas so far encapsulated in C60 by the ‘molecular surgery’ methods described here, enabling synthesis on a suitable scale for detailed NMR characterisation. The 13C NMR resonance of Kr@C60 in 1,2-dichlorobenzene-d4 has a chemical shift of δc = 143.20 ppm at 298 K, deshielded by +0.390 ± 0.001 ppm relative to empty C60 (Fig. 2a). Yamamoto et al. reported a consistent value of Δδ = +0.39 ppm in benzene-d6,8 and it has been previously noted that deshielding of the cage 13C NMR resonance in the noble gas@C60 series, with respect to C60, becomes greater with the increasing van der Waals radius of the trapped atom.1a,18
We observe a pair of side peaks to the main 13C NMR resonance (Fig. 2b), due to minor isotopomers of Kr@C60 that each contain two adjacent 13C nuclei separated by either a hexagon–pentagon (HP) or shorter hexagon–hexagon (HH) bond, present in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio respectively. One-bond secondary isotope shifts of 1ΔHP = 12.45 ± 0.01 ppb and 1ΔHH = 19.77 ± 0.02 ppb, shielded relative to the main Kr@C60 peak, are smaller than those measured for empty C60 (1ΔHP = 12.56 ± 0.01 ppb and 1ΔHH = 19.98 ± 0.02 ppb)22 and are the smallest secondary isotope shifts yet measured for atomic or molecular endofullerenes.3,14,18,22
1 ratio respectively. One-bond secondary isotope shifts of 1ΔHP = 12.45 ± 0.01 ppb and 1ΔHH = 19.77 ± 0.02 ppb, shielded relative to the main Kr@C60 peak, are smaller than those measured for empty C60 (1ΔHP = 12.56 ± 0.01 ppb and 1ΔHH = 19.98 ± 0.02 ppb)22 and are the smallest secondary isotope shifts yet measured for atomic or molecular endofullerenes.3,14,18,22
The only stable krypton isotope with a nuclear spin is 83Kr (I = 9/2, 11.58% natural abundance) and 83Kr NMR has been used to study the surface and void adsorption properties of porous nanomaterials.23a,b Hyperpolarised 83Kr gas is used for MRI imaging of the lungs, despite the relatively short spin-lattice relaxation time of the quadrupolar 83Kr spin.24a,b To our knowledge, Kr@C60 offers the first opportunity for 83Kr NMR spectroscopy of a stable compound of krypton. The 83Kr NMR spectrum of Kr@C60 in 1,2-dichlorobenzene-d4 solution with dissolved krypton gas at 298 K is shown in Fig. 3. The δ = 0 origin of the 83Kr NMR chemical shift scale corresponds to low-pressure Kr gas on the unified IUPAC referencing scale,25 but using an updated Ξ parameter for 83Kr as determined by Makulski (see ESI†).26
The 83Kr chemical shift for 83Kr@C60 in 1,2-dichlorobenzene-d4 solution is δKr = 64.3 ± 0.1 ppm, shifted by −39.5 ppm with respect to the resonance of free 83Kr in solution. For comparison 3He@C60 and 129Xe@C60 are reported at −6.04 and −16.5 ppm respectively from the dissolved gasses in this solvent.27 In benzene-d6 solution 83Kr@C60 is at δKr = 64.3 ± 0.3 ppm, shifted by −32.7 ppm from the dissolved gas (see ESI†). The reported shifts of 129Xe@C60 in benzene are 179.2 ppm from Xe gas, and −8.89 ppm relative to dissolved gas.7b The shift of 3He@C60 is −6.3 ± 0.15 ppm in 1-methylnaphthalene or CS2 with respect to either dissolved or free gas.28
Simple (non-relativistic) calculations for 3He@C60, 83Kr@C60 and 129Xe@C60 predict cage induced shifts of −7.0, 29.9 and 71.7 ppm, consistent with the relative order, if not absolute values, observed.29 Recent calculations on 129Xe@C60 are in good agreement with the experimental shift.30 Whilst 3He observes the shielding effect of the cage on the field inside, for 83Kr and 129Xe the shift is dominated by an increasing direct interaction between the atomic orbitals and the π-electron orbitals of the cage.
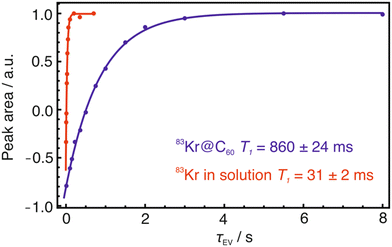
The protective effect of the cage is revealed by measurement of 83Kr linewidths and relaxation times. The 83Kr peak of 83Kr@C60 has a linewidth of 2.7 ± 0.1 Hz at half-height, which is much smaller than the linewidth of 10.8 ± 0.1 Hz for free 83Kr in solution (Fig. 3b and c). Similarly, the 83Kr spin-lattice relaxation time constant of 83Kr@C60 (T1 = 860 ± 24 ms) is much longer than that of free 83Kr in 1,2-dichlorobenzene-d4 solution (T1 = 31 ± 2 ms), and is longer than that reported for 83Kr dissolved in any other solvent at room temperature (Fig. 4).31 Presumably the high symmetry and rigidity of C60 greatly reduces the magnitude of fluctuating electric field gradients at the location of the Kr nucleus, which are responsible for quadrupolar relaxation.
In summary, Kr@C60 is prepared in a yield of approx. 4% from C60, with >99% krypton incorporation, using methods which overcome the severe limitation of only 0.1–0.3% direct krypton incorporation that results in very low mass recovery in the previously reported synthesis. An intermediate open-cage fullerene, 3, encapsulates krypton under high pressure but was shown to have a cage opening too small for the entry of xenon gas. The larger scale synthesis of Kr@C60 has enabled measurement of fine structure in the solution-phase 13C NMR spectrum, and characterisation by 83Kr NMR spectroscopy – the first example for a krypton compound (i.e., one in which the noble gas cannot escape without breaking covalent bonds). Endohedral 83Kr has a chemical shift of 64.3 ppm in 1,2-dichlorobenzene-d4, with respect to 83Kr gas. This is less deshielded than 83Kr in solution, presumably because the cage protects Kr from direct interactions with the solvent molecules. The 83Kr spin-lattice relaxation for 83Kr@C60 is approximately 36 times slower than for free 83Kr in solution, indicating that the cage shields the endohedral atom from fluctuating electric field gradients.
This research was supported by EPSRC grants EP/P009980/1, EP/P030491/1 and EP/T004320/1. The authors acknowledge the use of the IRIDIS High Performance Computing Facility at the University of Southampton.
Conflicts of interest
The authors declare no conflict of interest.Notes and references
- (a) R. G. Lawler, Nanostruct. Sci. Technol., 2017, 229 CrossRef; (b) M. H. Levitt, Philos. Trans. R. Soc., A, 2013, 371, 20120429 CrossRef; (c) M. H. Levitt and A. J. Horsewill, Philos. Trans. R. Soc., A, 2013, 371, 20130124 CrossRef; (d) A. A. Popov, S. F. Yang and L. Dunsch, Chem. Rev., 2013, 113, 5989 CrossRef CAS PubMed.
- G. R. Bacanu, T. Jafari, M. Aouane, J. Rantaharju, M. Walkey, G. Hoffman, A. Shugai, U. Nagel, M. Jiménez-Ruiz, A. J. Horsewill, S. Rols, T. Rõõm, R. J. Whitby and M. H. Levitt, J. Chem. Phys., 2021, 155, 144302 CrossRef CAS.
- G. R. Bacanu, J. Rantaharju, G. Hoffman, M. C. Walkey, S. Bloodworth, M. Concistrè, R. J. Whitby and M. H. Levitt, J. Am. Chem. Soc., 2020, 142, 16926 CrossRef CAS.
- (a) S. Jalife, J. Arcudia, S. Pan and G. Merino, Chem. Sci., 2020, 11, 6642 RSC; (b) M. Saunders, R. J. Cross, H. A. Jiménez-Vázquez, R. Shimshi and A. Khong, Science, 1996, 271, 1693 CrossRef CAS.
- (a) V. V. Albert, J. R. Sabin and F. E. Harris, Int. J. Quantum Chem., 2007, 107, 3061 CrossRef CAS; (b) M. Y. Amusia and L. V. Chernysheva, Fullerenes, Nanotubes, Carbon Nanostruct., 2020, 28, 179 CrossRef CAS; (c) S. K. Chaudhuri, R. K. Chaudhuri, P. K. Mukherjee and S. A. Chattopadhyay, J. Chem. Phys., 2017, 147, 034111 CrossRef PubMed; (d) F. Cimpoesu, S. Ito, H. Shimotani, H. Takagi and N. Dragoe, Phys. Chem. Chem. Phys., 2011, 13, 9609 RSC; (e) A. Jaworski and N. Hedin, Phys. Chem. Chem. Phys., 2021, 23, 21554 RSC; (f) T. Kupka, eMagRes, 2016, 5, 959 CAS; (g) L. Pizzagalli, Phys. Chem. Chem. Phys., 2022, 24, 9449 RSC; (h) H. Yan, S. Yu, X. Wang, Y. He, W. Huang and M. Yang, Chem. Phys. Lett., 2008, 456, 223 CrossRef CAS.
- M. Saunders, H. A. Jiménez-Vázquez and R. J. Cross, J. Am. Chem. Soc., 1994, 116, 2193 CrossRef CAS.
- (a) R. J. Cross, A. Khong and M. Saunders, J. Org. Chem., 2003, 68, 8281 CrossRef CAS; (b) M. S. Syamala, R. J. Cross and M. Saunders, J. Am. Chem. Soc., 2002, 124, 6216 CrossRef CAS; (c) A. Takeda, Y. Yokoyama, S. Ito, T. Miyazaki, H. Shimotani, K. Yakigaya, T. Kakiuchi, H. Sawa, H. Takagi, K. Kitazawa and N. Dragoe, Chem. Commun., 2006, 912 RSC.
- K. Yamamoto, M. Saunders, A. Khong, R. J. Cross, M. Grayson, M. L. Gross, A. F. Benedetto and R. B. Weisman, J. Am. Chem. Soc., 1999, 121, 1591 CrossRef CAS.
- S. Ito, A. Takeda, T. Miyazaki, Y. Yokoyama, M. Saunders, R. J. Cross, H. Takagi, P. Berthet and N. Dragoe, J. Phys. Chem. B, 2004, 108, 3191 CrossRef CAS.
- Y. Morinaka, F. Tanabe, M. Murata, Y. Murata and K. Komatsu, Chem. Commun., 2010, 46, 4532 RSC.
- K. Komatsu, M. Murata and Y. Murata, Science, 2005, 307, 238 CrossRef CAS.
- T. Futagoishi, M. Murata, A. Wakamiya, T. Sasamori and Y. Murata, Org. Lett., 2013, 15, 2750 CrossRef CAS PubMed.
- K. Kurotobi and Y. Murata, Science, 2011, 333, 613 CrossRef CAS PubMed.
- S. Bloodworth, G. Hoffman, M. C. Walkey, G. R. Bacanu, J. M. Herniman, M. H. Levitt and R. J. Whitby, Chem. Commun., 2020, 56, 10521 RSC.
- S. Bloodworth, G. Sitinova, S. Alom, S. Vidal, G. R. Bacanu, S. J. Elliott, M. E. Light, J. M. Herniman, G. J. Langley, M. H. Levitt and R. J. Whitby, Angew. Chem., Int. Ed., 2019, 58, 5038 CrossRef CAS.
- A. Krachmalnicoff, R. Bounds, S. Mamone, S. Alom, M. Concistrè, B. Meier, K. Kouřil, M. E. Light, M. R. Johnson, S. Rols, A. J. Horsewill, A. Shugai, U. Nagel, T. Rõõm, M. Carravetta, M. H. Levitt and R. J. Whitby, Nat. Chem., 2016, 8, 953 CrossRef CAS PubMed.
- A. Krachmalnicoff, M. H. Levitt and R. J. Whitby, Chem. Commun., 2014, 50, 13037 RSC.
- G. Hoffman, M. C. Walkey, J. Gräsvik, G. R. Bacanu, S. Alom, S. Bloodworth, M. E. Light, M. H. Levitt and R. J. Whitby, Angew. Chem., Int. Ed., 2021, 60, 8960 CrossRef CAS PubMed.
- Y. Hashikawa, M. Murata, A. Wakamiya and Y. Murata, J. Am. Chem. Soc., 2017, 139, 16350 CrossRef CAS PubMed.
- S. Bloodworth, J. Gräsvik, S. Alom, K. Kouřil, S. J. Elliott, N. J. Wells, A. J. Horsewill, S. Mamone, M. Jiménez-Ruiz, S. Rols, U. Nagel, T. Rõõm, M. H. Levitt and R. J. Whitby, Chem. Phys. Chem., 2018, 19, 266 CrossRef CAS PubMed.
- (a) H. M. Lee, M. M. Olmstead, T. Suetsuna, H. Shimotani, N. Dragoe, R. J. Cross, K. Kitazawa and A. L. Balch, Chem. Commun., 2002, 1352 RSC; (b) T. Suetsuna, N. Dragoe, H. Shimotani, A. Takeda, S. Ito, R. J. Cross, M. Saunders, H. Takagi and K. Kitazawa, Fullerenes, Nanotubes, Carbon Nanostruct., 2002, 10, 15 CrossRef CAS.
- G. R. Bacanu, G. Hoffman, M. Amponsah, M. Concistrè, R. J. Whitby and M. H. Levitt, Phys. Chem. Chem. Phys., 2020, 22, 11850 RSC.
- (a) Z. I. Cleveland and T. Meersmann, Magn. Reson. Chem., 2007, 45, S12–S23 CrossRef CAS PubMed; (b) C. F. Horton-Garcia, G. E. Pavlovskaya and T. Meersmann, J. Am. Chem. Soc., 2005, 127, 1958 CrossRef CAS PubMed.
- (a) Z. I. Cleveland, G. E. Pavlovskaya, N. D. Elkins, K. F. Stupic, J. E. Repine and T. Meersmann, J. Magn. Reson., 2008, 195, 232 CrossRef CAS PubMed; (b) J. S. Six, T. Hughes-Riley, D. M. L. Lilburn, A. C. Dorkes, K. F. Stupic, D. E. Shaw, P. G. Morris, I. P. Hall, G. E. Pavlovskaya and T. Meersmann, Magn. Reson. Imaging, 2014, 32, 48 CrossRef PubMed.
- R. K. Harris, E. D. Becker, S. M. Cabral de Menezes, P. Granger, R. E. Hoffman and K. W. Zilm, Pure Appl. Chem., 2008, 80, 59 CrossRef CAS.
- W. Makulski, Magn. Reson. Chem., 2014, 52, 430 CrossRef CAS PubMed.
- M. Frunzi, R. J. Cross and M. Saunders, J. Am. Chem. Soc., 2007, 129, 13343 CrossRef CAS PubMed.
- M. Saunders, H. A. Jiménez-Vázquez, R. J. Cross, S. Mroczkowski, D. I. Freedberg and F. A. L. Anet, Nature, 1994, 367, 256 CrossRef CAS.
- M. Bühl, S. Patchkovskii and W. Thiel, Chem. Phys. Lett., 1997, 275, 14 CrossRef.
- S. Standara, P. Kulhánek, R. Marek and M. Straka, J. Comput. Chem., 2013, 34, 1890 CrossRef CAS.
- R. K. Mazitov, K. M. Enikeev and A. V. Ilyasov, Z. Phys. Chem., 1987, 155, 55 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2141931. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc03398d |
| This journal is © The Royal Society of Chemistry 2022 |