Open Access Article
Open Access ArticleAscorbate-assisted nitric oxide release from photocontrollable nitrosonium ion releasers for potent ex vivo photovasodilation†
Naoya
Ieda
*a,
Yuka
Yoshikawa
a,
Natsumi
Tomita
a,
Kei
Ohkubo
 b,
Yuji
Hotta
a,
Mitsuyasu
Kawaguchi
a,
Kazunori
Kimura
a and
Hidehiko
Nakagawa
b,
Yuji
Hotta
a,
Mitsuyasu
Kawaguchi
a,
Kazunori
Kimura
a and
Hidehiko
Nakagawa
 *a
*a
aGraduate School of Pharmaceutical Sciences, Nagoya City University, 3-1, Tanabe-dori, Mizuho-ku, Nagoya, Aichi, 467-8603, Japan. E-mail: ieda@phar.nagoya-cu.ac.jp; deco@phar.nagoya-cu.ac.jp
bInstitute for Open and Transdisciplinary Research Initiatives & Institute for Advanced Co-Creation Studies, Osaka University, 1-6 Yamada-oka, Suita, Osaka 565-0871, Japan
First published on 7th July 2022
Abstract
We found that N-nitrosoaminoanisole derivatives tethered to dyes work as photocontrollable nitrosonium cation releasers and are converted to potent nitric oxide releasers in the presence of sodium ascorbate. The N-nitrosoaminoanisole derivative 2 worked as a more potent photovasodilating reagent ex vivo than previously reported nitric oxide releasers.
In mammals, reduced blood flow may lead to circulatory system disease and organ failure,1 and drugs that improve blood flow by relaxing blood vessels are therefore promising candidates for the treatment of various cardiovascular diseases and stroke. Nitric oxide (NO) is an endogenous signaling molecule that causes a potent but transient relaxation of vessels,2 so chemicals that enable spatiotemporal control of NO concentration are of great interest. In particular, many studies have focused on photocontrollable NO releasers,3 but so far, there have been few reports of successful optical control of blood vessel dilation.4 Our group has developed a series of visible-light-controlled NO releasers, composed of a light-harvesting antenna moiety and an N-nitrosoaminophenol moiety for NO release. As shown in Fig. 1a, photoinduced electron transfer (PeT) to the antenna moiety from the N-nitrosoaminophenol moiety generates a phenoxyl radical intermediate that releases NO to form a stable quinone. Our design has achieved high cross section (εΦNO: 365) as a red light controllable NO releaser (λ: 660 nm) and enabled us to regulate vasodilation of ex vivo rat aorta, as well as the intracavernous pressure of rats in vivo (Table S1, ESI†).5 Interestingly, during structural optimization of a green-light-controllable NO releaser, we found that the hydroxyl group of NO-Rosa5 (1) is necessary for NO release, and furthermore, that the O-methylated compound, NO-Rosa6 (2), released nitrosonium ion (NO+) instead of NO (Fig. 1c).5c A plausible mechanism of NO+ release from 2 is shown in Fig. 1b. After PeT, the radical cation releases NO+via a mesolytic cleavage mechanism.6 In the present work, we compared the NO- and NO+-releasing abilities of 1 and 2, as well as a red-light-controllable NO releaser, NORD-1 (3) and its O-methylated derivative, NORD-2 (4). Unexpectedly, we found that 2 and 4 afforded NO, but not NO+, more efficiently and quickly than 1 and 3 in the presence of 1 equivalent of sodium ascorbate (SA), a biologically ubiquitous reductant. In ex vivo assay using rat aorta strips, 2 induced vasodilation more quickly than 1, and was effective even in the absence of exogenously added ascorbic acid.
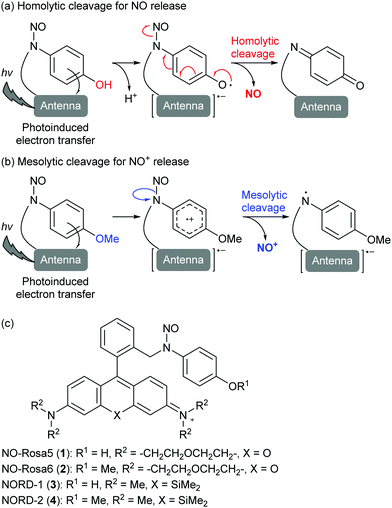 | ||
| Fig. 1 Plausible mechanisms for photoinduced release of NO (a), and NO+ (b); (c) structures of NO-Rosa5 (1), NO-Rosa6 (2), NORD-1 (3), and NORD-2 (4). | ||
We previously found that light irradiation induced little release of NO from 2, but nevertheless, decomposition of 2 was faster than that of 1.5c Decomposition product analysis revealed that 2 released NO+ instead of NO in vitro. Since NO+ can directly cause S- or N-nitrosylation, controlled NO+ release is expected to be a good approach for biological investigation of nitrosylated proteins and other biological nitrosylated molecules.7 Thus, we considered that 4, which is an O-methylated derivative of the red-light-controllable NO releaser 3, might also be a photocontrollable NO+ releaser based on the structural analogy with 2. So, we prepared these compounds according to the previous method (see Supporting Information) and confirmed that these compounds efficiently harvest green or red light, respectively (Fig. S1, ESI†).
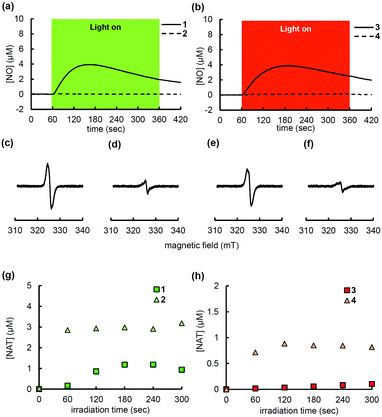
The NO-releasing ability of these compounds was investigated using an NO electrode. A solution of each compound (10 μM) was irradiated with green (568 nm) or red (660 nm) LED light and the NO concentration was recorded by using an electrochemical detector equipped with an NO electrode. As shown in Fig. 2a and b, 1 and 3 released NO efficiently, whereas 2 and 4 did not. It was also confirmed that the light irradiation did not induce any amperometric response in the absence of these compounds (Fig. S2, ESI†). The proposed radical intermediates formed in the PeT process were detected by means of electron spin resonance (ESR) spectroscopy. The signal of 2 (Fig. 2d) is assigned to the charge-separated (CS) state generated by intramolecular PeT. The signal of 1 (Fig. 2c) was stronger than that of 2. This indicates that the signal of the phenoxyl radical [1(–H˙)] generated by deprotonation of the donor radical cation moiety overlaps with the signal of the short-lived CS state. The SOMO of 1(–H˙) consists of the N–N antibonding orbital (Fig. S3m and n, ESI†). The NO release occurred by cleavage of the N–N bond of 1(–H˙) (d(N–N) = 1.357 Å), which is elongated compared to that of 2 (1.333 Å) (Fig. S3a and b, ESI†).8 Similar behaviors were observed in the cases of 3 and 4 (Fig. 2e, f and Fig. S3c, d, ESI†). On the other hand, as shown in Fig. S4 (ESI†), the absorbance of 2 or 4 during photoirradiation decreased more quickly than that of 1 or 3, respectively. This result suggests that 2 and 4 released a different species, that is NO+, and the resulting amino group of the aniline moiety attacked the 9-position of the xanthene moiety to form a colorless closed (spiro) form (Scheme S1a, ESI†). In contrast, 1 and 3 released NO, and the resulting semiquinone radical would disproportionate to form a colorless closed compound and a still-colored aldehyde (Scheme S1b, ESI†). Next, to investigate the NO+-releasing ability of these PeT-driven compounds, 2,3-diaminonaphthalene (DAN) was employed as an NO+ fluorescence probe.9 DAN has also been utilized as an alternative fluorescence NO probe to Griess reagent; it reacts with NO+ derived from NO2− under acidic conditions to form 2,3-naphthotriazole (NAT), which shows strong fluorescence. DAN can be employed for the direct detection of NO+ without acidic treatment. A solution of each PeT-driven compound (10 μM) and DAN (10 μM) was photoirradiated and then the fluorescent products were analyzed by HPLC. As shown in Fig. 2g, h and Fig. S5 (ESI†), a larger amount of NAT was found in the irradiated solution of 2 or 4, as compared with 1 or 3, respectively. These results indicated that O-methylation converted the PeT-driven NO releasers into photocontrollable NO+ releasers. In terms of the reaction mechanism, it is assumed that NO+ release is not due to destabilization of the N–N bond via formation of a radical intermediate such as 1 and 3, but rather is due to the strong polarization of the N–N bond caused by radical cation formation after PeT.
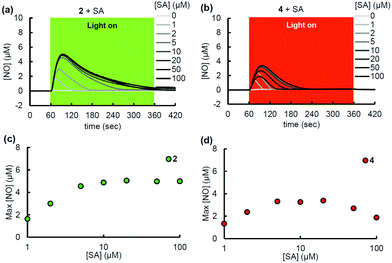
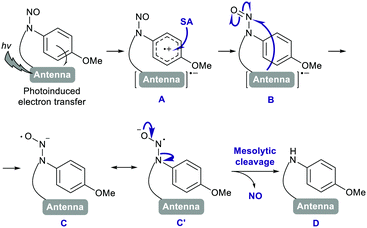
Various redox-active substances exist in the biological milieu, and we next examined the effects of some of them on the photoreactivity of the PeT-driven compounds working via radical mechanisms. First, the effect of SA, a major one-electron reductant that exists both inside and outside cells was examined.10 While SA hardly affected the NO release from 1 and 3, significant NO release was unexpectedly observed from 2 and 4 in the presence of SA (Fig. 3a, b and Fig. S6, ESI†). Other biological reductants examined, glutathione (GSH), nicotine adenine dinucleotide (NADH), and tocopherol, did not affect the NO release from PeT-driven compounds (Fig. S7, ESI†). The ascorbate-assisted NO release was SA-concentration-dependent, and reached a plateau at 10 μM SA (Fig. 3c and d). At SA concentrations over 10 μM, the maximum NO concentration derived from 2 was unaffected, while that derived from 4 decreased with increasing concentration of ascorbate. The ascorbate-assisted NO release from 2 and 4 seemed quick and efficient, so we determined the quantum yield ΦNO using an NO electrode and a chemical actinometer based on ammonium tetrathiocyanatodiamminechromate(III), known as Reinecke's salt (Table S2, ESI†).11 As shown in Table S3 (ESI†), the ΦNO values of 2 (9.84 × 10−3) and 4 (4.97 × 10−3) in the ascorbate-assisted reaction were greater than those of 1 (0.919 × 10−3) and 3 (3.30 × 10−3), respectively, and SA hardly affected the ΦNO values of 1 and 3. Notably, the ascorbate-assisted ΦNO of 2 was about ten times larger than that of 1. To confirm whether NO+ reacts directly with SA, nitrosonium tetrafluoroborate (NOBF4) was added to a solution of SA, and the NO concentration was monitored. As shown in Fig. S8a (ESI†), no increase of NO was observed when a solution of NOBF4 in MeCN was added to an aqueous solution of SA. Further, no NO increment was observed when a solution of sodium nitrite (NaNO2), which would be formed after reaction of NO+ and H2O, was added to a solution of SA (Fig. S8b, ESI†). These results indicate that SA did not react directly with NO+ or NO2−, but instead reacted with the photoreaction intermediate of 2 or 4, resulting in the release of NO. The SA addition did not affect either the absorption spectrum or the fluorescence quantum yield Φf of each compound, suggesting that SA did not interact with the ground or excited state of the antenna moiety but rather with the CS state (Fig. S9, ESI†). A plausible mechanism for NO release from 2 and 4 is shown in Fig. 4. After photoinduced electron transfer, the cation radical A would undergo one-electron reduction from SA. Then, the radical intermediate B of the antenna moiety would mediate one-electron reduction of N-nitrosoaniline to form oxyhydrazinyl radical C or C′. This intermediate C′ would release NO in a mesolytic cleavage process to form D. In the case of the red-light-controllable compound 4, Si-rhodamine could undergo two-electron reduction more easily than 10-oxa-rhodamine, so that excess SA might induce over-reduction, which would compete with the NO-releasing pathway (Scheme S2, ESI†).12 Further, the nature of the photodecomposition products explains why 2 and 4 showed larger ΦNO values than 1 and 3: the main photodecomposition products of 1 and 3 are both colorless closed-form products and still-colored aldehydes (Fig. S4b, ESI†), whereas those of 2 and 4 are only colorless closed-form products (Scheme S4a, ESI†). In other words, inner filter effects could interfere with photoexcitation of remaining 1 and 3.13 Indeed, photodecomposition was faster at lower concentrations of 1 or 3, probably because of a smaller inner filter effect (Fig. S10, ESI†).
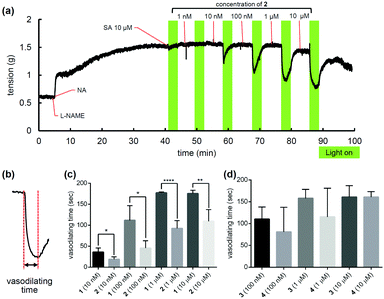
NO can induce vasodilation of arteries by activating soluble guanylyl cyclase to promote production of cyclic GMP.2 Therefore, we examined the vasorelaxant activity of these compounds by monitoring their effect on the tension of a rat aorta strip. Since the blood of healthy people contains at least 10 μM SA, the photovasodilation experiments was conducted in the presence of 10 μM SA.14 The strip was pre-constricted with noradrenaline (NA, 10 μM), then SA (10 μM) was added, and 40 mW cm−2 light irradiation was repeated with increasing concentrations of each PeT-driven compound (Fig. 5a). As shown in Fig. S11 (ESI†), the maximum vasodilation reached 60–80% of the NA-induced constriction for each compound. The EC50 values of vasodilation at 40 mW cm−2 light irradiation were calculated to be approximately 10–100 nM. These values are consistent with previous reports that NO causes vasodilation in the 10–100 nM range.15 The faster NO release from 2 than 1 was reflected in the vasodilating time, which is defined as the time to reach minimum tension in response to NO release (Fig. 5b). As shown in Fig. 5c and d, the vasodilating time of 2 was shorter than that of 1, while there was no significant difference between those of 3 and 4. This is probably because the ΦNO of 2 was nearly 10 times higher than that of 1, while that of 4 was only about 1.3 times than that of 3. Overall, the in vitro finding that SA made 2 and 4 better NO releasers is consistent with the outcomes of the ex vivo experiment. We also conducted a similar experiment using 2 without addition of SA, and 2 still showed potent vasodilation, as shown in Fig. S12 (ESI†). This can be explained by the presence of endogenous ascorbate in the cells of the aorta. These results indicate that the PeT-driven NO+ releasers could act as efficient photovasodilators even without exogenous addition of SA. To confirm the mechanism of this vasodilation of 2, we added a sGC inhibitor, ODQ, and confirmed that ODQ inhibited vasorelaxation induced by 2. Importantly, the addition of a solution of NaNO2, the final decomposition product of NO+, induced no vasodilation at 10 μM, and had little effect even at 100 μM (Fig. S13, ESI†), suggesting that NO, but not NO2−, derived from 2 induced sGC-mediated vasodilation. 1 and 2 showed no significant cytotoxicity at 10 μM, though 3 and 4 suppressed cell proliferation, likely due to the lipophilicity of the dye (Fig. S14, ESI†).
We found that O-methylation of PeT-driven NO releasers converts them into NO+ releasers, and also that these NO+ releasers rapidly and efficiently release NO in the presence of SA at physiological concentrations. The NO release efficiency, ΦNO, of the O-methylated compounds was greater than those of previously reported NO releasers. Our results indicate that the higher ΦNO is due to a reduced inner filter effect. This finding could be helpful to improve the photoreaction efficiency in the development of other photoresponsive compounds. In an ex vivo photovasodilation test, these compounds could induce 60–80% of maximum vasodilation in response to light. The EC50 values were in the 10–100 nM range. The photovasodilation of 2 was faster than that of 1, and 2 was active even in the absence of additional (non-endogenous) SA. The structure-activity relationship data obtained in this study should be helpful in the development of compounds to control blood flow in vivo.
This work was supported in part by JSPS KAKENHI grant numbers 19H03354 (H. N.), 20K05752 (N. I.), 20K09583 (Y. H.), 21K19576 (K. K.) and 20H02779 (K. O.) as well as by the JST ACT-X Grant Number JPMJAX2011 (N. I.), Japan, and by Research Equipment Sharing Center at Nagoya City University.
Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) H. K. Eltzschig and T. Eckle, Nat. Med., 2011, 17, 1391 CrossRef CAS; (b) K. Kisler, A. R. Nelson, A. Montagne and B. V. Zlokovic, Nat. Rev. Neurosci., 2017, 18, 419 CrossRef CAS; (c) M. Cannistrà, M. Ruggiero, A. Zullo, G. Gallelli, S. Serafini, M. Maria, A. Naso, R. Grande, R. Serra and B. Nardo, Int. J. Surg., 2016, 33, S50 Search PubMed; (d) T. Thenappan, M. L. Ormiston, J. J. Ryan and S. L. Archer, BMJ, 2018, 360, j5492 CrossRef PubMed; (e) F. A. Yafi, L. Jenkins, M. Albersen, G. Corona, A. M. Isidori, S. Goldfarb, M. Maggi, C. J. Nelson, S. Parish, A. Salonia, R. Tan, J. P. Mulhall and W. J. G. Hellstrom, Nat. Rev. Dis. Primers, 2016, 2, 16003 CrossRef PubMed.
- L. J. Ignarro, G. M. Buga, K. S. Wood, R. E. Byrns and G. Chaudhuri, Proc. Natl. Acad. Sci. U. S. A., 1987, 84, 9265 CrossRef CAS PubMed.
- (a) L. R. Makings and R. Y. Tsien, J. Biol. Chem., 1994, 269, 6282 CrossRef CAS PubMed; (b) S. Namiki, T. Arai and K. Fujimori, J. Am. Chem. Soc., 1997, 119, 3840 CrossRef CAS; (c) S. Sortino, S. Petralia, G. Compagnini, S. Conoci and G. Condorelli, Angew. Chem., Int. Ed., 2002, 41, 1914 CrossRef CAS; (d) S. Wecksler, A. Mikhailovsky and P. C. Ford, J. Am. Chem. Soc., 2004, 126, 13566 CrossRef CAS PubMed; (e) T. Suzuki, O. Nagae, Y. Kato, H. Nakagawa, K. Fukuhara and N. Miyata, J. Am. Chem. Soc., 2005, 127, 11720 CrossRef CAS PubMed; (f) F. Karaki, Y. Kabasawa, T. Yanagimoto, N. Umeda, Firman, Y. Urano, T. Nagano, Y. Otani and T. Ohwada, Chem. – Eur. J., 2012, 18, 1127 CrossRef CAS PubMed; (g) M. Blangetti, A. Fraix, L. Lazzarato, E. Marini, B. Rolando, F. Sodano, R. Fruttero, A. Gasco and S. Sortino, Chem. – Eur. J., 2017, 23, 9026 CrossRef CAS PubMed; (h) E. Y. Zhou, H. J. Knox, C. J. Reinhardt, G. Partipilo, M. J. Nilges and J. Chan, J. Am. Chem. Soc., 2018, 140, 11686 CrossRef CAS PubMed; (i) H. He, Y. Xia, Y. Qi, H. Y. Wang, Z. Wang, J. Bao, Z. Zhang, F. G. Wu, H. Wang, D. Chen, D. Yang, X. Liang, J. Chen, S. Zhou, X. Liang, X. Qian and Y. Yang, Bioconjugate Chem., 2018, 29, 1194 CrossRef CAS PubMed.
- S. Namiki, F. Kaneda, M. Ikegami, T. Arai, K. Fujimori, S. Asada, H. Hama, Y. Kasuya and K. Goto, Bioorg. Med. Chem., 1999, 7, 1695 CrossRef CAS.
- (a) N. Ieda, Y. Hotta, N. Miyata, K. Kimura and H. Nakagawa, J. Am. Chem. Soc., 2014, 136, 7085 CrossRef CAS PubMed; (b) H. Okuno, N. Ieda, Y. Hotta, M. Kawaguchi, K. Kimura and H. Nakagawa, Org. Biomol. Chem., 2017, 13 Search PubMed; (c) N. Ieda, Y. Oka, T. Yoshihara, S. Tobita, T. Sasamori, M. Kawaguchi and H. Nakagawa, Sci. Rep., 2019, 9, 1430 CrossRef PubMed; (d) N. Ieda, Y. Hotta, M. Kawaguchi, K. Kimura and H. Nakagawa, Chem. Pharm. Bull., 2019, 67, 576 CrossRef CAS PubMed; (e) N. Ieda, Y. Hotta, A. Yamauchi, A. Nishikawa, T. Sasamori, D. Saitoh, M. Kawaguchi, K. Kimura and H. Nakagawa, ACS Chem. Biol., 2020, 15, 2958 CrossRef CAS PubMed.
- Q. Zhu, E. C. Gentry and R. R. Knowles, Angew. Chem., Int. Ed., 2016, 55, 9969 CrossRef CAS PubMed.
- (a) D. T. Hess, A. Matsumoto, S. O. Kim, H. E. Marshall and J. S. Stamler, Nat. Rev. Mol. Cell. Biol., 2005, 6, 150 CrossRef CAS PubMed; (b) P. Anand, A. Hausladen, Y. J. Wang, G. F. Zhang, C. Stomberski, H. Brunengraber, D. T. Hess and J. S. Stamler, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 18572 CrossRef CAS PubMed.
- The radical pair generated by PeT are localized in the electron-donor and -acceptor moieties, respectively. The HOMO and LUMO calculated by DFT are perfectly separated (Fig. S3e–l, ESI†).
- P. Damiani and G. Burini, Talanta, 1986, 33, 649 CrossRef CAS.
- J. Du, J. J. Cullen and G. R. Buettner, Biochim. Biophys. Acta, 2012, 1826, 443 CAS.
- E. E. Wegner and A. W. Adamson, J. Am. Chem. Soc., 1966, 88, 394 CrossRef CAS.
- W. Piao, S. Tsuda, Y. Tanaka, S. Maeda, F. Liu, S. Takahashi, Y. Kushida, T. Komatsu, T. Ueno, T. Terai, T. Nakazawa, M. Uchiyama, K. Morokuma, T. Nagano and K. Hanaoka, Angew. Chem., Int. Ed., 2013, 52, 13028 CrossRef CAS PubMed.
- S. E. Braslavsky, Glossary of terms used in photochemistry, 3rd edition (IUPAC Recommendations 2006), Pure Appl. Chem., 2007, 79, 293 CrossRef CAS.
- R. L. Schleicher, M. D. Carroll, E. S. Ford and D. A. Lacher, Am. J. Clin. Nutr., 2009, 90, 1252 CrossRef CAS PubMed.
- Q. Yan, Q. Liu, J. L. Zweier and X. Liu, Pharmacol. Res., 2007, 55, 329 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc03193k |
| This journal is © The Royal Society of Chemistry 2022 |