Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Janus faced fluorocyclohexanes for supramolecular assembly: synthesis and solid state structures of equatorial mono-, di- and tri alkylated cyclohexanes and with tri-axial C–F bonds to impart polarity†
Thomas J.
Poskin
a,
Bruno A.
Piscelli
b,
Keigo
Yoshida
c,
David B.
Cordes
 a,
Alexandra M. Z.
Slawin
a,
Alexandra M. Z.
Slawin
 a,
Rodrigo A.
Cormanich
a,
Rodrigo A.
Cormanich
 b,
Shigeyuki
Yamada
b,
Shigeyuki
Yamada
 c and
David
O'Hagan
c and
David
O'Hagan
 *a
*a
aUniversity of St Andrews, School of Chemistry, North Haugh, St Andrews, Fife KY16 9ST, UK. E-mail: do1@st-andrews.ac.uk; Fax: 01334 463800; Tel: 01334 467171
bUniversity of Campinas, Chemistry Institute, Monteiro Lobato Street, Campinas, Sao Paulo, Brazil 13083-862. E-mail: cormanich@unicamp.br
cFaculty of Molecular Chemistry and Engineering, Kyoto Institute of Technology, Matsugasaki, Sakyo-ku, Kyoto, 606-8585, Japan
First published on 20th June 2022
Abstract
Concise and general synthesis protocols are reported to generate all-syn mono-, di- and tri-alkylated cyclohexanes where a single fluorine is located on the remaining carbons of the ring. The alkyl groups are positioned to lie equatorially and to have triaxial C–F bonds imparting polarity to these ring systems. Intermolecular electrostatic interactions in the solid-state structure of the trialkylated systems are explored and the resultant supramolecular order opens up prospects for design in soft materials.
We have demonstrated that certain arrangements in selectively fluorinated cyclohexanes possess highly polar properties, particularly if the fluorines are strategically located on one face of the cyclohexane ring.1 All-syn-1,2,3,4,5,6-hexafluorocyclohexane 1 is the flagship molecule in this regard, and its unusual polarity is evinced by a very high melting point (mp ∼208 °C) as well as a high molecular dipole of μ = 5.89 D.2 The origin of this polarity lies predominantly in the tri-axial alignment of three of the six C–F bonds, a situation that is always maintained in interconverting chair conformations. This imparts a polarity to the molecule and generates an electronegative (fluorine) and electropositive (hydrogen) face and the rings tends to associate electrostatically (hydrogen to fluorine faces) in the solid-state, with fluorine to hydrogen contacts, rather than fluorous clustering.3,4 The term ‘Janus faced’ cyclohexane5 has been applied to such cycloalkanes for this reason.
Given that the alignment of the axial C–F bonds is particularly significant in terms of inducing polarity in these systems, it can be anticipated that if the equatorial C–F bonds are replaced with alkyl groups, the polarity should be maintained. This was exemplified recently in the solid-state structures of a range of monoalkylated pentafluorocyclohexyl derivatives, where it emerged that the rings adopt the most polar conformation with triaxial C–F bonds and an equatorial alkyl substituent.6 Theory offers partial support where the methyl substituent in, for example, cyclohexane 2 illustrated in Fig. 1, has a small energy preference (−0.51 kcal mol−1) for the equatorial methyl conformation.6a In this paper we demonstrate an increasing preference for the triaxial C–F conformer with di- and tri-alkylated derivatives. This is predicted by the computational outcomes presented in Fig. 1 for cyclohexanes 3 and 4. Progressive methylation at alternate positions around the ring is predicted to increase the triaxial C–F preference and increase the bias towards the more polar arrangements. The calculated7 molecular dipole moments (μ) for the conformers of 4 indicate that it is the triaxial arrangement of the fluorines which contributes most significantly to molecular polarity (μ = 4.65 D), the ring inverted conformer with equatorial fluorines is much less polar (μ = 1.01 D). With this background it became an objective to prepare a range of all-syn 2,4,6-trialkylated-1,3,5-trifluorocyclohexanes 16, to assess their structures and properties, particularly as they are predicted to be strongly trifluoro-triaxial with the more polar conformations dominating. A general route was devised to this class of compounds which also allowed mono- and di-alkylated cyclohexane rings of this class to be prepared as shown in Scheme 1.
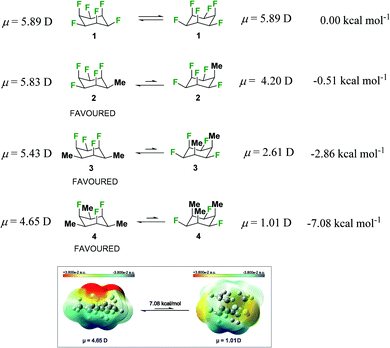 | ||
| Fig. 1 Structures of 1–4 optimized at the B3LYPD3/def2-TZVP theory level, with calculated conformational Gibbs free energy differences and molecular dipole moments between the chair conformations of some selectively fluorinated all-syn fluorocyclohexanes.7 The lower image shows a surface electrostatic potential map for the conformers of 4. | ||
The syntheses sequences start with Sonogashira protocols8 coupling the appropriate commercially available fluoroiodobenzene 5, 9 or 13 with terminal acetylenes. The resultant aryl-acetylenes 6, 10a–c or 14a–f were then hydrogenated under standard conditions over Pd/C to generate alkyl-fluoroaryls 7, 11a–c or 15a–f respectively with saturated side chains. These products were then subject to aryl ring hydrogenations under the conditions recently reported by Glorius8 for the preparation of fluorocyclohexanes from aromatic precursors, using the [CAAC]-COD Rh catalyst 17.9 Although these aryl hydrogenation reactions proved to be slow (H2, 50–70 bar, 1–10 d) conversions overall are moderate to good and it offered a direct approach to this class of compounds generating products 8, 12a–b and 16a–f as illustrated in Fig. 2. Some of the products were amenable to X-ray structure analysis, such as the monosubstituted derivative 8, the disubstituted derivative 12c and trisubstituted trifluorocyclohexanes 16d, 16e and 16f. The offset in the stacking arrangement of the fluorocyclohexyl rings, as observed for the monosubstituted derivatives2,6 was not observed for the di- and tri-substituted systems.
 | ||
| Fig. 2 Structures of the product mono-8, di-12a–b and tri-16a–f fluoroalkylated cyclohexanes prepared in this study. | ||
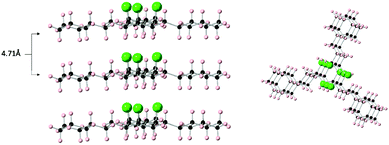
On the other hand, in the cases of the di- and tri-substituted derivatives 12c, 16d and 16e, the cyclohexane rings stack directly, one on top of another as illustrated in Fig. 3 for 16e (see also Fig. SI6–SI8, ESI†). It is also interesting to note that the alkyl substituents in the di- and tri-substituted systems lie in the plane of the stacked rings very different to the monosubstituted all-syn pentafluorocyclohexyl systems such as 8 and as previously disclosed in other cases where there is an angular herringbone arrangement of the side chains to the rings in the solid-state.6a The more ordered stacking arrangement of the trialkyls 16 as a class was explored computationally in the dimeric and trimeric molecular assemblies for compound 4 as a model.
The surface electrostatic potential map for the triaxial arrangement of the fluorines in 4 as illustrated in Fig. 1, highlights the Janus face character of this cyclohexane, with a highly negative (red) electrostatic potential associated with the triaxial fluorine face and a highly positive (blue) electrostatic potential associated with the triaxial hydrogens. On the other hand, the polarization diminishes considerably upon ring inversion, and the Janus face characteristic is lost for the triaxial alkyl groups in compound 4. It is notable that the stacking of the cyclohexane rings in the tri-substituted derivatives further increases the attractive intermolecular interaction between positive and negative ring faces, as evidenced by the surface electrostatic potential maps for the dimer and trimer as illustrated in Fig. 4a, and by the interactions obtained through QTAIM and NCI calculations between axial fluorines and axial hydrogens (Fig. 4b and c, respectively).10 The strong attractive F–H interactions significantly stabilize these arrangements, with calculated complexation energies for the dimer and trimer of −8.04 kcal mol−1 and −17.40 kcal mol−1 respectively, the latter value essentially being the sum of two such intermolecular interactions. Therefore, it can be anticipated that it is the array of F⋯H contacts which are responsible for stabilizing the intermolecular cyclohexane interactions, leading to stabilization of the crystalline supramolecular assembly of the trialkyl-substituted cyclohexanes.
 | ||
| Fig. 4 (a) Surface electrostatic potential maps for dimeric and trimeric arrangements of the polar conformer of 4 obtained at the PBE0/def2-TZVP theory level. Both dimer and trimer are characterized by the electrostatic attraction between the hydrogen and fluorine faces. (b) QTAIM molecular graphics obtained from the PBE0/def2-TZVP electron density, with bond critical points (BCPs) represented by green spheres and ring critical points (RCPs) and cage critical points (CCPs) represented by small red and blue spheres, respectively. (c) NCI iso-surfaces obtained from PBE0/def2-TZVP electron density using reduced density gradient (RDG) = 0.5 and blue-green-red colour scale ranging from −0.015 < sign(λ2)ρ(r) < 0.015 a.u. Complexation energies were calculated at B3LYP-D3/def2-TZVP optimised geometries using the HFLD method and with the aug-cc-pVTZ basis set.10 | ||
Only in the trisubstituted structures 16 are the ring stacks completely insulated from each other by the alkyl substituents. In the mono- and di-substituted systems e.g.8 and 12c there is lateral contact between fluorocyclohexane ring stacks (see ESI†) whereas there is a complete insulation of ring stacks in the solid-state structure of the trisubstituted compounds as illustrated for 16e in Fig. 5. This suggests a characteristic of the trisubstituted systems in the solid-state.
 | ||
| Fig. 5 The solid-state packing arrangements in the structure of 16e showing that the all-syn fluorocyclohexane stacks are isolated and insulated laterally from each other by the hydrocarbon side chains. This is not the case for the mono- and di-substituted alkyl systems e.g.8 and 12c (see ESI†). | ||
Differential scanning calorimetry (DSC) and polarising optical microscopy (POM) analyses were carried out to assess if these materials were predisposed to polymorphism and/or liquid crystalline phases.11 The analyses for compounds 16a–f is presented in the ESI† and illustrated here for compound 16d in Fig. 6. DSC analysis indicated that for some of the compounds only a single phase transition (melting point) was apparent, however for others, such as 16d, there is clear polymorphic behaviour with a distinct transition at 97 °C prior to melting at 141 °C. The POM image of this polymorphic phase for 16d has a clearly contrasted and highly orientated fibrous aspect typical of an ordered supramolecular structure, and this is found in several of the other systems too (see ESI† for 16a & 16e) although characteristics vary with different alkyl functionality.
 | ||
| Fig. 6 DSC trace of 16d illustrating polymorphic phases (mp = 141 °C) during the 1st cooling and 2nd heating cycle. | ||
In conclusion we have presented a synthesis approach to selectively alkylated all-syn alkylated cyclohexanes that also contain triaxial C–F bonds, and we profile the alternating 2,4,6-trialkyl motifs in particular. The parallel orientation of the C–F bonds imparts a high polarity to these ring systems, and in selected cases the alkyl chains completely insulate the Janus ring stacks from each other, an arrangement which is not observed for the mono- and di-alkylated systems. The predisposition of the polar Janus rings to assemble in stacks due to electrostatic attraction between the alternating faces of the rings holds promise for ordering supramolecular assemblies with polar properties and offers an approach to designing next generation soft materials such as polar liquid crystals.
We thank the EPSRC for a studentship (TJP) through the CRITICAT Doctoral Training Centre. FAPESP is also gratefully acknowledged for a studentship (BAP, #2021/09716-5) and a Young Researcher Award (RAC, #2018/03910-1). CENAPAD-SP, CESUP and SDumont are also acknowledged for the computational resources used in theory calculations.
Conflicts of interest
There are no conflicts to declare.References
- (a) C. Yu, A. Kütt, G.-V. Röschenthaler, T. Lebl, D. B. Cordes, A. M. Z. Slawin, M. Bűhl and D. O’Hagan, Angew. Chem., Int. Ed., 2020, 59, 19905–19909 CrossRef CAS PubMed; (b) D. O’Hagan, Chem. – Eur. J., 2020, 26, 7981–7997 CrossRef PubMed; (c) T. Bykova, N. Al-Maharik, A. M. Z. Slawin, M. Bühl, T. Lebl and D. O’Hagan, Chem. – Eur. J., 2018, 24, 13290–13296 CrossRef CAS PubMed; (d) T. Bykova, N. Al-Maharik, A. M. Z. Slawin and D. O’Hagan, Beilstein J. Org. Chem., 2017, 13, 728–733 CrossRef CAS PubMed; (e) M. Salah Ayoup, D. B. Cordes, A. M. Z. Slawin and D. O’Hagan, Beilstein J. Org. Chem., 2015, 11, 2671–2676 CrossRef PubMed.
- N. S. Keddie, A. M. Z. Slawin, T. Lebl, D. Philp and D. O’Hagan, Nat. Chem., 2015, 7, 483–488 CrossRef CAS PubMed.
- (a) R. A. Cormanich, N. Keddie, R. Rittner, D. O’Hagan and M. Bühl, Phys. Chem. Chem. Phys., 2015, 44, 29475–29478 RSC; (b) B. E. Ziegler, M. Lecours, R. A. Marta, J. Featherstone, E. Fillion, W. S. Hopkins, V. Steinmetz, N. S. Keddie, D. O’Hagan and T. B. McMahon, J. Am. Chem. Soc., 2016, 138, 7460–7463 CrossRef CAS PubMed; (c) M. J. Lecours, R. A. Marta, V. Steinmetz, N. Keddie, E. Fillion, D. O’Hagan, T. B. McMahon and W. S. Hopkins, J. Phys. Chem. Lett., 2017, 8, 109–113 CrossRef CAS PubMed.
- (a) O. Shyshov, S. V. Haridas, L. Pesce, H. Y. Qi, A. Gardin, D. Bochicchio, U. Kaiser, G. M. Pavan and M. von Delius, Nat. Commun., 2021, 12, 3134 CrossRef CAS PubMed; (b) A. Theodoridis, G. Papamokos, M. P. Wiesenfeldt, M. Wollenburg, K. Mullen, F. Glorius and G. Floudas, J. Phys. Chem. B, 2021, 125, 3700–3709 CrossRef CAS PubMed; (c) R. Mondal, M. Agbaria and Z. Nairoukh, Chem. – Eur. J., 2021, 27, 7193–7213 CrossRef CAS PubMed.
- N. Santschi and R. Gilmour, Nat. Chem., 2015, 7, 467–468 CrossRef CAS PubMed.
- (a) J. L. Clark, A. Taylor, A. Geddis, R. M. Neyyappadath, B. A. Piscelli, C. Yu, D. B. Cordes, A. M. Z. Slawin, R. A. Cormanich, S. Guldin and D. O’Hagan, Chem. Sci., 2021, 12, 9712–9719 RSC; (b) J. L. Clark, R. M. Neyyappadath, C. Yu, A. M. Z. Slawin, D. B. Cordes and D. O’Hagan, Chem. – Eur. J., 2021, 27, 16000–16005 CrossRef CAS PubMed.
- H. Tsuji, G. Cantagrel, Y. Ueda, T. Chen, L. J. Wan and E. Nakamura, Chem. – Asian J., 2013, 8, 2377–2382 CrossRef CAS PubMed.
- (a) T. Charvillat, P. Bernardelli, M. Daumas, X. Pannecoucke, V. Ferey and T. Besset, Chem. Soc. Rev., 2021, 50, 8178–8192 RSC; (b) Z. Nairoukh, M. Wollenburg, C. Schlepphorst, K. Bergander and F. Glorius, Nat. Chem., 2019, 11, 264–270 CrossRef CAS PubMed; (c) M. P. Wiesenfeldt, Z. Nairoukh, W. Li and F. Glorius, Science, 2017, 357, 908–912 CrossRef CAS PubMed.
- (a) X. Zhang, L. Ling, M. Luo and X. Zeng, Angew. Chem., Int. Ed., 2019, 58, 16785–16789 CrossRef CAS PubMed; (b) Y. Wei, B. Rao, X. Cong and X. Zeng, J. Am. Chem. Soc., 2015, 137, 9250–9253 CrossRef CAS PubMed.
- (a) R. F. W. Bader, Atoms in Molecules: A Quantum Theory, Claredon, Oxford, 1990 Search PubMed; (b) J. Contreras-García, E. R. Johnson, S. Keinan, R. Chaudret, J. Piquemal, D. N. Beratan and W. Yang, J. Chem. Theory Comput., 2011, 7(3), 625–632 CrossRef PubMed; (c) A. Altun, F. Neese and G. Bistoni, J. Chem. Theory Comput., 2019, 15(11), 5894–5907 CrossRef CAS PubMed.
- S. Yamada, M. Morita, Y. Wang, Q. Zhang, D. O’Hagan, T. Agou, H. Fukumoto, T. Kubota, M. Hara and T. Konno, Crystals, 2021, 11, 450 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2174305–2174309. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc03010a |
| This journal is © The Royal Society of Chemistry 2022 |