2D Network overtakes 3D for photocatalytic hydrogen evolution†
Abstract
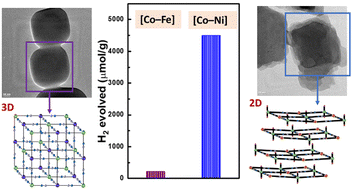
3-Dimensional (3D) cyanide coordination polymers, typically known as Prussian blue Analogues (PBAs), have received great attention in catalysis due to their stability, easily tuned metal sites, and porosity. However, their high crystallinities and relatively low number of surface-active sites significantly hamper their intrinsic catalytic activities. Herein, we report the utilization of a 2-dimensional (2D) layered cobalt tetracyanonickelate, [Co–Ni], for the reduction of protons to H2. Relying on its exposed facets, layered morphology, and abundant surface-active sites, [Co–Ni] can efficiently convert water and sunlight to H2 in the presence of a ruthenium photosensitizer (Ru PS) with an optimal evolution rate of 30 029 ± 590 μmol g−1 h−1, greatly exceeding that of 3D Co–Fe PBA [Co–Fe] and Co–Co PBA [Co–Co]. Furthermore, [Co–Ni] retains its structural integrity throughout a 6 hour photocatalytic cycle, which is confirmed by XPS, PXRD, and Infrared analysis. This recent work reveals the excellent morphologic properties that promote [Co–Ni] as an attractive catalyst for the hydrogen evolution reaction (HER).


 Please wait while we load your content...
Please wait while we load your content...