Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Silole and germole complexes of lanthanum and cerium†
Xiaofei
Sun‡
 ,
Luca
Münzfeld‡
,
Luca
Münzfeld‡
 ,
Da
Jin
,
Adrian
Hauser
,
Da
Jin
,
Adrian
Hauser
 and
Peter W.
Roesky
and
Peter W.
Roesky
 *
*
Institute of Inorganic Chemistry, Karlsruhe Institute of Technology (KIT), Engesserstraße 15, 76131, Karlsruhe, Germany. E-mail: roesky@kit.edu
First published on 27th June 2022
Abstract
Using dianionic metallole ligands (silole or germole) and the cyclooctatetraendiide dianion, heteroleptic lanthanide multi-decker complexes have been prepared. Due to the heteroatom of the metallole ligands intermolecular bridging between the sandwich complexes takes place. Our work highlights that different combinations of the lanthanide and heterocycle lead to different intermolecular interactions including a dimeric La-silole sandwich complex, a La-germole ladder-type polymeric species and a Ce-germole coordination polymer.
In organometallic chemistry, sandwich complexes and multi-decker species continue to be a highly attractive compound class not only due to their intriguing structure motifs but also their potential application in catalysis and materials science.1 Specifically, carbocyclic planar π-conjugated ligands with different ring sizes (three to nine) have been utilized and incorporated into such sandwich-type structure motifs.2 Among those, the 6π-aromatic cyclopentadienyl (Cp− = C5H5−) ligand and its derivatives are the most studied ones.3 Numerous sandwich and multi-decker compounds have been synthesized using monoanionic Cp systems, whereas depending on the central atoms and substitution patterns of Cp, distinct types of coordination modes can be achieved. Starting from the s-block of the periodic table, [Li(Cp)]n and [Na(Cp)]n both have nearly linear polymer chains whereas the heavier [K(Cp)]n adopts a zigzag chain.4 The d-block contains countless possible sandwich motifs for example classical metallocenes like ferrocene ([Fe(η5-Cp)2])2a,c or triple-decker species like [Ni(η5-Cp)3]+.5 Very similarly, the p-block features a variety of sandwich compounds such as the bent plumbocene ([Pb(Cp)2])6 and the dicationic tetra-decker complex ([(η5-Cp)Sn(μ-η5:η5-Cp)Sn(μ-η5:η5-Cp)Sn(η5Cp)]2+.7 Also in lanthanide chemistry, a great variety of sandwich complexes arise from substituted and unsubstituted cyclopentadienyl ligands.8 In addition, the utilization of the cyclooctatetraendiide dianion (COT = C8H82−) and sterically encumbered silyl-substituted COT derivatives has enlarged the family of lanthanide multi-decker complexes. Divalent triple-decker Ln complexes [(η5-Cp*)Ln(μ-η8:η8-COT)Ln(η5-Cp*)]9 (Ln = Sm, Eu, Yb) and the trivalent tetra-decker Yb complex [(η5-Cp*)Yb(μ-η8:η8-COTTMS)Yb(μ-η8:η8-COTTMS)Yb(η5-Cp*)]10 (Cp* = C5Me5−, COTTMS = 1,3,6-SiMe3-C8H52−) were synthesized by combining monoanionic and dianionic Cp and COT derivatives. Recently, our group disclosed the missing structure of the homoleptic mixed-valence tetra-decker species [(η8-COTTIPS)Sm(μ-η8:η8-COTTIPS)Sm(μ-η8:η8-COTTIPS)Sm(η8-COTTIPS)] (COTTIPS = 1,4-SiiPr3C8H62−).11
The chemistry of aromatic group 14 dianionic metalloles has reemerged as a topic of interest in the recent years.12 In particular, silole and germole dianions have gained attentions not only due to the potentially aromatic character but also the electronic features acting as ligand. In 2018, the group of Müller reported the synthesis of silyl-substituted silole and germole dianions, which could be applied in gram-scale.12g It has been shown that they are useful building blocks and distinct reactivities have been have been reported through salt metathesis reactions.12j For instance, reactions with organic halides,12l chlorosilanes13 or metallocene dichlorides are known.12f,k,14
Only very lately, such ligands has been successfully introduced into the coordination sphere of rare-earth elements.15 In a recent report, the group of Tan and co-workers synthesized the homoleptic bis-germole and the heteroleptic Cp*-germole complexes of yttrium.15 Around the same time, our group prepared the first examples of heteroleptic lanthanide sandwich-type complexes comprising a plumbole and a bulky COT ligand as decks.16 Such dianionic heterocycles might be ideal starting materials to construct multi-decker lanthanide complexes. Combining the dianionic COT moiety with one heavy group 14 metallole (silole or germole) within the coordination sphere of the larger lanthanide element, resulted in unprecedented coordination modes and coordination polymers.
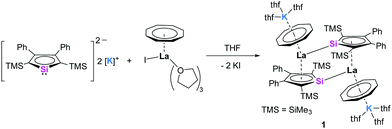
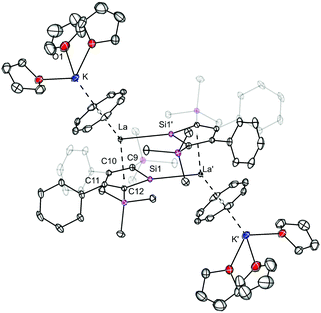
The dipotassium salts of the 1,4-bis-(trimethylsilyl)-2,3-diphenylsilole dianion [K2][LSi] and 1,4-bis-(trimethylsilyl)-2,3-dimethylgermole dianion [K2][LGe] were synthesized in situ according to a synthetic protocol of the group of Müller.12g The necessary lanthanide starting materials [Ln(η8-COT)I(thf)3)] (Ln = La, Ce) were prepared according to a recently established procedure from our group,2j that is based on a protocol from Mashima et al.17 As a first attempt, a THF solution of [K2][LSi] was added to a THF solution of [La(η8-COT)I(thf)3)] dropwise at −88 °C and stirred for 1 h at −88 °C before it was allowed to warm up to room temperature (Scheme 1). Small amounts of an off-white precipitate were formed during the reaction time. A clear solution was collected by filtration and slow evaporation of the THF solution at room temperature afforded the dimeric sandwich complex 1 as dark red crystals in 25% yield. The molecular structure of 1 was determined by X-ray diffraction analysis. Complex 1 crystallizes in the monoclinic space group P21/n with one half of the dimeric complex in the asymmetric unit. As expected, the central La atom is coordinated to the COT ligand in an η8-fashion and to the planar silole ring in a η5-fashion (Fig. 1). The distances to the ring centroids are 2.126 Å (CtCOT) and 2.137 Å (CtLSi), respectively (Ct = ring centroid). The thf-solvated K atom is additionally η8-coordinated to the COT ring with a K-CtCOT distance of 2.528 Å. Due to the presence of a lone pair at the Si atom (orthogonal to the cyclic π-system), an additional La–Si bond (La–Si′ = 3.2908(6) Å) is formed to the neighboring double-decker sandwich species. Thus, resulting in the dimeric structure of 1. The La–Si bond length is about 0.1 Å longer than in another reported La silylene complex (3.1868(8) Å),18 even though a direct comparison of the bond lengths is hampered due to the distinct characteristics of dianionic silole compared to neutral silylene ligands and the different coordination numbers of both species. NMR spectra of 1 were recorded in THF-d8. In the 1H NMR spectrum, the signal of the COT protons is found at δ = 6.22 ppm, and the singlet signals of the SiMe3 protons at δ = −0.02 ppm. In the 29Si{1H} NMR spectrum, two signals were found at δ = 187.9 and −13.9 ppm, the former one could be assigned to the silole silicon nucleus and is significantly downfield shifted compared to the dipotassium silole [K2][LSi] (δ = 148.5).12g
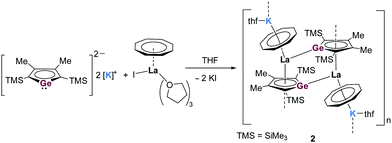
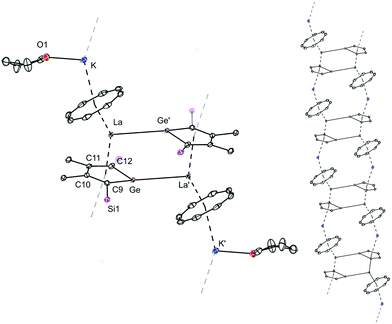
In a similar manner, the in situ-generated dipotassium germole [K2][LGe] was reacted with an equimolar amount of [La(η8-COT)I(thf)3)] and by slow evaporation of the solvents, orange-red crystals of the product 2 were obtained in 34% yield (Scheme 2). X-ray diffraction analysis confirmed that 2 has a novel one-dimensional coordination polymeric structure with repeating dimeric [K(thf)(η8-COT)La(η5-LGe)]2 units, in which the La center is coordinated by the germole dianion and the COT dianion in η5 and η8 coordination motifs, respectively (Fig. 2, left). Each La atom in 2 is additionally coordinated by another germole moiety in a η1 fashion. Due to the bridged double chain, the structure consists of an infinite ladder structure (Fig. 2, right). The dimeric triple-decker chain links are similar to 1. However, in the polymeric chains of 2 the COT and germole moieties are acting as bridging ligands. In contrast to the σ,π-bridging silole ligand, the germole can act as a σ,π,π-triply bridging entity. Compared to 1, in which three THF molecules are coordinated towards the potassium atom, in 2, only one THF molecule is bonded to the K atom. Due to the larger size of Ge, the respective La–Ge′ (3.3048(5) Å) and La–CtLGe (2.567 Å) distances are longer than in the silole analogue 1. In addition, the central sandwich structure of 2 is more bent compared to that in 1 (CtCOT–La–CtLGe = 144.2° and CtCOT–La–CtLSi = 147.7°). It is noteworthy to mention that molecular compounds comprising Ln(III)–Ge bonds are very scarce,15,19 therefore, complex 2 is the rare example of a structurally characterized molecule with La–Ge bonds. In the 1H NMR spectrum, aside from the signals of the coordinated THF molecules, three singlet signals are visible at δ = 6.17, 2.45 and 0.14 ppm, which can be assigned to the protons of COT, Me and SiMe3, respectively.
The successful incorporation of germole and silole ligands into lanthanum sandwich complexes has prompted us to further study the coordination properties of those dianionic ligands. Formation of 2 has shown that the five-membered germole ring can act as a bridging ligand in construction of linear 1D polymeric assemblies, keeping this in mind, it is particularly interesting to see whether a triple-decker complex with germole as middle deck and COT as outer decks can be formed. Therefore, the reaction between [K2][LGe] and [La(η8-COT)I(thf)3)] in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio was carried out, however, only single crystals of complex 2 were obtained.
2 molar ratio was carried out, however, only single crystals of complex 2 were obtained.
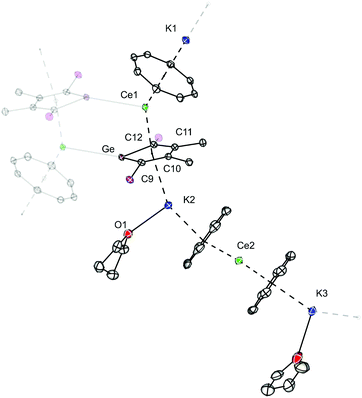
In a next attempt, the reaction between one equivalent of [K2][LGe] and two equivalents of [Ce(η8-COT)I(thf)3)] was tested in THF (Scheme 3). From the resulting purple reaction mixture, small amounts of dark red crystals of 3 were isolated and their identity was confirmed by X-ray diffraction analysis. Interestingly, instead of the expected triple-decker compound, a 2D coordination polymer 3 was obtained in 20% yield. Fig. 3 depicts the repeating unit of the coordination polymer. The asymmetric unit of 3 contains two distinct Ce atoms (Ce1 and Ce2), three different K atoms (K1, K2 and K3), three COT rings as well as one germole ring. K1 and K3 are located on two crystallographically independent symmetry elements and both have an occupancy of 0.5. Each germole ligand is coordinated via its lone pair to the Ce atom of the next polymeric chain, therefore resulting in a 2D polymeric network. Each repeating unit can be considered formally as a heteroleptic anionic sandwich structure [(η8-COT)Ce(η5-LGe)]− and a homoleptic anionic sandwich structure [(η8-COT)Ce(η8-COT)]− that are interlinked by different K cations. If sandwich complexes form coordination polymers, they usually have one kind of sandwich complex as repeating unit. In contrast, compound 3 features two different sandwich complexes as chain links. They are arranged in an ABABAB alternating mode. The structural motif of the heteroleptic coordination polymer of 3 is unique. The bent heteroleptic sandwich motif CtCOT–Ce–CtLGe (angle: 142.8°) is comparable to that in the La-germole complex 2. The homoleptic [(η8-COT)Ce(η8-COT)]− unit is close to be perfectly linear with a CtCOT–Ce–CtCOT angle of 173.6°. The reason of the unexpected formation of 3 remains to be clarified. A possible explanation might be the formation of the polymeric system in order to maximize the number of interlinking Ce–Ge bonds.
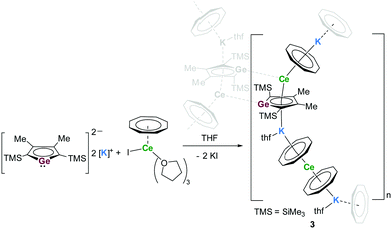 | ||
| Scheme 3 Synthesis of [{K0.5(μ-η8:η8-COT)Ce(μ-η5:η5-LGe)K(thf)(μ-η8:η8-COT)Ce(μ-η8:η8-COT)K0.5(thf)0.5}2]n (3). | ||
Our results highlight that dianionic group 14 metallole ligands are suitable bridging ligands for the synthesis of lanthanide multi-decker species. By using different combinations of the employed elements (La and Si, La and Ge, Ce and Ge), an increase in dimensionality of the respective complexes could be achieved. This allowed us to isolate a dimeric La-silole sandwich complex (1), a 1D La-germole coordination polymer (2) and a 2D Ce-germole coordination polymer (3). Thus, the application of heterocycles as ligands for the construction of sandwich complexes opens the door to new and unprecedented structural motifs.
Deutsche Forschungsgemeinschaft (DFG) is acknowledged for financial support within the Reinhart Koselleck-Projekt 440644676, RO 2008/19-1.
Conflicts of interest
There is no conflict of interest to declare.References
- (a) A. N. Nesmeyanov and N. S. Kochetkova, Russ. Chem. Rev., 1974, 43, 710–715 CrossRef; (b) K. Miyajima, A. Nakajima, S. Yabushita, M. B. Knickelbein and K. Kaya, J. Am. Chem. Soc., 2004, 126, 13202–13203 CrossRef CAS PubMed; (c) X. Zhang, J. Wang, Y. Gao and X. C. Zeng, ACS Nano, 2009, 3, 537–545 CrossRef CAS PubMed; (d) S.-J. Wang, Y. Li, D. Wu, Y.-F. Wang and Z.-R. Li, J. Phys. Chem. A, 2012, 116, 9189–9196 CrossRef CAS PubMed; (e) M. J. Heras Ojea, L. C. H. Maddock and R. A. Layfield, in Organometallic Magnets, ed. V. Chandrasekhar and F. Pointillart, Springer International Publishing, Cham, 2019, pp. 253–280 Search PubMed.
- (a) T. J. Kealy and P. L. Pauson, Nature, 1951, 168, 1039–1040 CrossRef CAS; (b) G. Wilkinson, M. Rosenblum, M. C. Whiting and R. B. Woodward, J. Am. Chem. Soc., 1952, 74, 2125–2126 CrossRef CAS; (c) E. O. Fischer and W. Pfab, Z. Naturforsch., B: Chem. Sci., 1952, 7, 377–379 CrossRef; (d) E. O. Fischer and W. Hafner, Z. Naturforsch., B: Anorg. Chem., Org. Chem., Biochem., Biophys., Biol., 1955, 10, 665–668 CrossRef; (e) A. Streitwieser and U. Mueller-Westerhoff, J. Am. Chem. Soc., 1968, 90, 7364 CrossRef CAS; (f) F. Mares, K. Hodgson and A. Streitwieser, J. Organomet. Chem., 1970, 24, C68–C70 CrossRef CAS; (g) M. D. Walter, G. Wolmershäuser and H. Sitzmann, J. Am. Chem. Soc., 2005, 127, 17494–17503 CrossRef CAS PubMed; (h) K. Kawasaki, R. Sugiyama, T. Tsuji, T. Iwasa, H. Tsunoyama, Y. Mizuhata, N. Tokitoh and A. Nakajima, Chem. Commun., 2017, 53, 6557–6560 RSC; (i) M. Xémard, S. Zimmer, M. Cordier, V. Goudy, L. Ricard, C. Clavaguéra and G. Nocton, J. Am. Chem. Soc., 2018, 140, 14433–14439 CrossRef PubMed; (j) L. Münzfeld, C. Schoo, S. Bestgen, E. Moreno-Pineda, R. Köppe, M. Ruben and P. W. Roesky, Nat. Commun., 2019, 10, 3135 CrossRef PubMed.
- (a) C. Elschenbroich, Organometallics, 3rd edn, Wiley-VCH, Weinheim, Weinheim, 2006 Search PubMed; (b) B. M. Day, F.-S. Guo and R. A. Layfield, Acc. Chem. Res., 2018, 51, 1880–1889 CrossRef CAS PubMed.
- R. E. Dinnebier, U. Behrens and F. Olbrich, Organometallics, 1997, 16, 3855–3858 CrossRef CAS.
- (a) A. Salzer and H. Werner, Angew. Chem., Int. Ed. Engl., 1972, 11, 930–932 CrossRef CAS; (b) H. Werner and A. Salzer, Synth. React. Inorg. Met.-Org. Chem., 1972, 2, 239–248 CrossRef CAS.
- E. O. Fischer and H. Grubert, Z. Anorg. Allg. Chem., 1956, 286, 237–242 CrossRef CAS.
- M. Schleep, C. Hettich, J. Velázquez Rojas, D. Kratzert, T. Ludwig, K. Lieberth and I. Krossing, Angew. Chem., Int. Ed., 2017, 56, 2880–2884 CrossRef CAS PubMed.
- F. T. Edelmann, Metallocenes, 1998, pp. 55–110 DOI:10.1002/9783527619542.ch2.
- (a) W. J. Evans, J. L. Shreeve and J. W. Ziller, Polyhedron, 1995, 14, 2945–2951 CrossRef CAS; (b) W. J. Evans, R. D. Clark, M. A. Ansari and J. W. Ziller, J. Am. Chem. Soc., 1998, 120, 9555–9563 CrossRef CAS; (c) W. J. Evans, M. A. Johnston, M. A. Greci and J. W. Ziller, Organometallics, 1999, 18, 1460–1464 CrossRef CAS.
- A. Edelmann, S. Blaurock, V. Lorenz, L. Hilfert and F. T. Edelmann, Angew. Chem., Int. Ed., 2007, 46, 6732–6734 CrossRef CAS PubMed.
- L. Münzfeld, A. Hauser, P. Hädinger, F. Weigend and P. W. Roesky, Angew. Chem., Int. Ed., 2021, 60, 24493–24499 CrossRef PubMed.
- (a) M. Saito and M. Yoshioka, Coord. Chem. Rev., 2005, 249, 765–780 CrossRef CAS; (b) M. Saito, R. Haga, M. Yoshioka, K. Ishimura and S. Nagase, Angew. Chem., Int. Ed., 2005, 44, 6553–6556 CrossRef CAS PubMed; (c) M. Nakada, T. Kuwabara, S. Furukawa, M. Hada, M. Minoura and M. Saito, Chem. Sci., 2017, 8, 3092–3097 RSC; (d) M. Saito, Acc. Chem. Res., 2018, 51, 160–169 CrossRef CAS PubMed; (e) M. Saito, N. Matsunaga, J. Hamada, S. Furukawa, M. Minoura, S. Wegner, J. Barthel and C. Janiak, Dalton Trans., 2018, 47, 8892–8896 RSC; (f) Z. Dong, K. Bedbur, M. Schmidtmann and T. Müller, J. Am. Chem. Soc., 2018, 140, 3052–3060 CrossRef CAS PubMed; (g) Z. Dong, C. R. W. Reinhold, M. Schmidtmann and T. Müller, Organometallics, 2018, 37, 4736–4743 CrossRef CAS; (h) P. Tholen, Z. Dong, M. Schmidtmann, L. Albers and T. Müller, Angew. Chem., Int. Ed., 2018, 57, 13319–13324 CrossRef CAS PubMed; (i) M. Saito, M. Nakada, T. Kuwabara, R. Owada, S. Furukawa, R. Narayanan, M. Abe, M. Hada, K. Tanaka and Y. Yamamoto, Organometallics, 2019, 38, 3099–3103 CrossRef CAS; (j) Z. Dong, L. Albers and T. Müller, Acc. Chem. Res., 2020, 53, 532–543 CrossRef CAS PubMed; (k) L. Albers, P. Tholen, M. Schmidtmann and T. Müller, Chem. Sci., 2020, 11, 2982–2986 RSC; (l) Z. Dong, L. Albers, M. Schmidtmann and T. Müller, Chem. – Eur. J., 2019, 25, 1098–1105 CrossRef CAS PubMed.
- C. R. W. Reinhold, Z. Dong, J. M. Winkler, H. Steinert, M. Schmidtmann and T. Müller, Chem. – Eur. J., 2018, 24, 848–854 CrossRef CAS PubMed.
- Z. Dong, O. Janka, J. Kösters, M. Schmidtmann and T. Müller, Angew. Chem., Int. Ed., 2018, 57, 8634–8638 CrossRef CAS PubMed.
- J. Liu, K. Singh, S. Dutta, Z. Feng, D. Koley, G. Tan and X. Wang, Dalton Trans., 2021, 50, 5552–5556 RSC.
- L. Münzfeld, X. Sun, S. Schlittenhardt, C. Schoo, A. Hauser, S. Gillhuber, F. Weigend, M. Ruben and P. W. Roesky, Chem. Sci., 2022, 13, 945–954 RSC.
- K. Mashima, Y. Nakayama, A. Nakamura, N. Kanehisa, Y. Kai and H. Takaya, J. Organomet. Chem., 1994, 473, 85–91 CrossRef CAS.
- X. Sun, T. Simler, F. Kraetschmer and P. W. Roesky, Organometallics, 2021, 40, 2100–2107 CrossRef CAS.
- (a) H. Schumann and M. Cygon, J. Organomet. Chem., 1978, 144, C43–C45 CrossRef CAS; (b) B. K. Campion, R. H. Heyn and T. D. Tilley, Organometallics, 1993, 12, 2584–2590 CrossRef CAS; (c) H. Schumann, J. A. Meese-Marktscheffel and L. Esser, Chem. Rev., 1995, 95, 865–986 CrossRef CAS; (d) S.-M. Chen, J. Xiong, Y.-Q. Zhang, F. Ma, H.-L. Sun, B.-W. Wang and S. Gao, Chem. Commun., 2019, 55, 8250–8253 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2173290–2173292. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2cc02810g |
| ‡ Both authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |