Open Access Article
Open Access ArticleSynthesis of stack plate covalent organic framework nanotubes using a self-assembled acid as a soft template†
Dan
Wu‡
ab,
Dandan
Han‡
c,
Yong
Zhou
a,
Zhen
Zhang
b,
Hang
Qian
b,
Qian
Zhou
c,
Yongsheng
Zhang
b,
Andrei Y.
Khodakov
 *a and
Vitaly V.
Ordomsky
*a and
Vitaly V.
Ordomsky
 *a
*a
aUniv. Lille, CNRS, Centrale Lille, ENSCL, Univ. Artois, UMR 8181-UCCS-Unité de Catalyse et Chimie du Solide, F-59000 Lille, France. E-mail: vitaly.ordomsky@univ-lille.fr; andrei.khodakov@univ-lille.fr
bSchool of Chemical Engineering, Zhengzhou University, Zhengzhou, 450001, People's Republic of China
cCollege of Science, Henan Agricultural University, Zhengzhou, Henan 450002, People's Republic of China
First published on 19th July 2022
Abstract
COF-LZU1 with nanotube-like morphology has been synthesized with high crystallinity and pore volume in the presence of trimesic acid as a template. The as-synthesized COF nanotubes consist of a stack of plates with a diameter of about 100 nm with a hollow channel inside of about 20 nm.
Covalent organic frameworks (COFs) are kinds of purely organic crystalline materials with uniform pore size and tailored functional moieties.1–4 Since the first introduction by Yaghi and co-workers in 2005,5,6 COFs have been widely used in adsorption, separation, catalysis and electronics due to their ordered structure, high surface areas, low density, thermal and chemical stability, and easy functionalization.7,8 In the past decade, a large number of COFs with different structures have been fabricated based on various covalent bonds (B–O, C–O, C–N, C
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, C
N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, etc.).9,10 Most of the research interest for COF synthesis focuses on tunable structures11,12 and designable functionalities13,14 to improve their physical and chemical properties. However, despite the benefits of tuning the structure and chemical functionalities of COFs, morphology-control of COFs, which is an extremely important factor in absorption and catalysis, is still a challenge due to the highly sensitive crystallization process.15
C, etc.).9,10 Most of the research interest for COF synthesis focuses on tunable structures11,12 and designable functionalities13,14 to improve their physical and chemical properties. However, despite the benefits of tuning the structure and chemical functionalities of COFs, morphology-control of COFs, which is an extremely important factor in absorption and catalysis, is still a challenge due to the highly sensitive crystallization process.15
It is well known that COFs are synthesized by reversible dynamic covalent-bond formation.16 A crystalline and ordered COF structure can only be constructed in a narrow synthetic window.17 Such a trial-and-error synthetic process makes it hard to control the morphologies of COFs during crystallization. Currently, there are only limited methods to tune the morphology of COFs without affecting the crystallinity. For example, a series of COF spheres, fibers and films could be obtained by the condensation of 1,3,5-benzenetricarbaldehyde (BTCA) and 1,3,5-tris(4-aminophenyl)benzene (TAPB) in the presence of a monofunctional amine and aldehyde added as competitors during the crystallization.18 Besides, hollow tubular porous COFs have been synthesized by template-assisted replication of ZnO-nanorods,19 while microtubular COFs can be synthesized from monomers consisting of diketopyrrolopyrrole (DPP) and tetraphenylporphyrin (TPP) moieties.20 A two-step amorphous-to-crystalline strategy has also been well studied for the construction of different morphologies of COFs such as microspheres and fibers.21,22 However, these strategies above either highly rely on the properties of the monomers or suffer from the use of costly hard templates and generally result in amorphous walls of COFs with overlapped or interconnected 2D layers. Thereby, a bottom-up strategy for the morphology-controlled synthesis of highly crystalline COFs regardless of the properties of the monomers is greatly desirable.
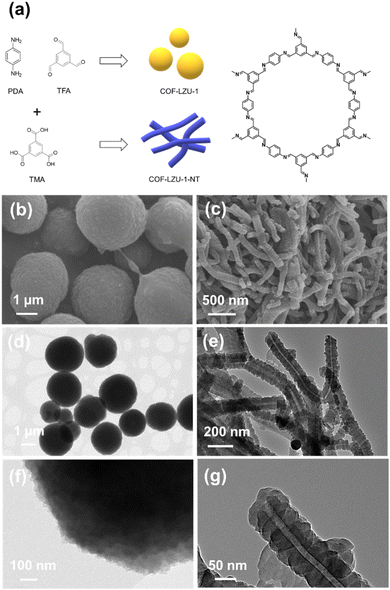
Herein, we present a novel facile strategy to control the morphology of COFs with well-defined crystalline walls. COF nanotubes have been synthesized by the condensation of 1,3,5-triformylbenzene (TFA) and p-phenylenediamine (PDA) in the presence of trimesic acid (TMA) under ambient conditions (Fig. 1a). Inter-molecular assemblies of TMA generate a rod-like soft template. In situ crystallization of COFs around the template leads to the formation of COF plates perpendicularly to the rods of TMA in the form of a nanotube. This work provides a general strategy for the synthesis of COF nanotubes with a well-defined structure of the walls, which would significantly extend the applications of COF materials.
First of all, COF-LZU1 has been synthesized following the conventional procedure via the condensation of TFA and PDA in dioxane solvent at room temperature.23 In agreement with previous reports,24 the obtained COF-LZU1 showed spherical morphologies with an average particle size of around 2–4 μm (Fig. 1b, d and f). To control the morphology of COF-LZU1, TMA was introduced into the 1,4-dioxane solvent and co-crystallized with TFA and PDA. Surprisingly, a fiber-like morphology was fabricated as indicated by SEM analysis with a diameter of the fibers in the range of 100–200 nm (Fig. 1c). Further characterizations by transmission electron microscopy (TEM) analysis demonstrated an interesting structure of the material prepared in the presence of TMA (Fig. 1e) with stacked COF plates arranged perpendicularly to the hollow channel of about 20 nm in diameter (Fig. 1g).
The XRD analysis of both samples demonstrates typical COF-LZU1 structures with high crystallinity. The strongest peak at 4.7° arises from the (100) facet and a set of weak peaks at 8.2°, 9.4° and 12.7° corresponds to the (110), (200) and (210) facets, respectively (Fig. 2a).25,26 The absence of (001) reflection corresponding to stacking between the layers in the XRD spectrum of COF-LZU1-NT is most probably due to the variation of the distances between the layers resulting in the broadening of the peak. The COF-LZU1 materials also show similar Fourier transform infrared (FTIR) spectra with a peak at 1615 cm−1 attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond (Fig. 2b). Meanwhile, the intensity of the peak originating from the aldehyde group (1695 cm−1) of TFA has decreased a lot (Fig. S1, ESI†), confirming the condensation of TFA and PDA.23,25 A similar chemical structure is also confirmed by Raman spectroscopy (Fig. 2c). The absorption peak at 1624 cm−1 was attributed to the C
N bond (Fig. 2b). Meanwhile, the intensity of the peak originating from the aldehyde group (1695 cm−1) of TFA has decreased a lot (Fig. S1, ESI†), confirming the condensation of TFA and PDA.23,25 A similar chemical structure is also confirmed by Raman spectroscopy (Fig. 2c). The absorption peak at 1624 cm−1 was attributed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration in the linkages.27 The adsorption peaks at 1578, 1158, and 992 cm−1 were assigned to C
N stretching vibration in the linkages.27 The adsorption peaks at 1578, 1158, and 992 cm−1 were assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching, C–H bending and C–H plane deformation in the benzene ring, respectively.27 Solid–state cross-polarization magic-angle spinning 13C nuclear magnetic resonance (13C CP/MAS NMR) was used to check the chemical environment of both samples (Fig. 2d). The 13C NMR peak at ∼156 ppm corresponds to the C atom of the C
C stretching, C–H bending and C–H plane deformation in the benzene ring, respectively.27 Solid–state cross-polarization magic-angle spinning 13C nuclear magnetic resonance (13C CP/MAS NMR) was used to check the chemical environment of both samples (Fig. 2d). The 13C NMR peak at ∼156 ppm corresponds to the C atom of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds, confirming the condensation of TFA and PDA. A set of resonance peaks at ∼148, ∼136, ∼130, and ∼121 ppm can be attributed to the carbon atoms of the phenyl groups.28,29 The minor resonance at ∼189 ppm is assigned to the carbon atom of C
N bonds, confirming the condensation of TFA and PDA. A set of resonance peaks at ∼148, ∼136, ∼130, and ∼121 ppm can be attributed to the carbon atoms of the phenyl groups.28,29 The minor resonance at ∼189 ppm is assigned to the carbon atom of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in the terminal TFA moieties.25 The chemical analysis also shows similar N, H, C and O contents for both samples (Table 1). A slightly higher O content in COF-LZU1-NT could be ascribed to the residual TMA molecules inside the COF nanotubes. Thus, the characterization techniques indicate the similar chemical structure of the COF nanotubes and the conventional COF-LZU1 materials. This means that the addition of TMA mostly changes the morphology of the COFs with no effect on the composition and structure.
O bonds in the terminal TFA moieties.25 The chemical analysis also shows similar N, H, C and O contents for both samples (Table 1). A slightly higher O content in COF-LZU1-NT could be ascribed to the residual TMA molecules inside the COF nanotubes. Thus, the characterization techniques indicate the similar chemical structure of the COF nanotubes and the conventional COF-LZU1 materials. This means that the addition of TMA mostly changes the morphology of the COFs with no effect on the composition and structure.
 | ||
| Fig. 2 Characterizations of COF-LZU1 and COF-LZU1-NT. (a) XRD, (b) FTIR, (c) Raman, (d) 13C solid-state NMR, (e) N2 adsorption–desorption isotherms and corresponding (f) pore size distribution. | ||
| Samples | S BET (m2 g−1) | V micro (cm3 g−1) | V meso (cm3 g−1) | Elemental analysis (%) | |||
|---|---|---|---|---|---|---|---|
| C | H | N | O | ||||
| COF-LZU1 | 508 | 0.23 | 0.04 | 76.3 | 4.6 | 14.5 | 4.5 |
| COF-LZU1-NT | 560 | 0.22 | 0.31 | 74.5 | 4.7 | 14.5 | 6.2 |
The information obtained from the N2 adsorption–desorption isotherms also pointed to the different sample morphology. As shown in Fig. 2e, COF-LZU1 exhibits a type I isotherm of N2 with a steep increase in adsorption below p/po = 0.1 suggesting the presence of intrinsic micropores of COF-LZU1.25 Interestingly, different from COF-LZU1, an obvious N2 uptake at p/po > 0.8 was observed in COF-LZU1-NT corresponding to the Type II IUPAC classification typical for CNTs.30 This phenomenon is attributed to the capillary condensation of N2 in the channels of the COF nanotubes, in agreement with TEM analysis.31,32 The Brunauer–Emmett–Teller (BET) surface areas were calculated to be 508 m2 g−1 for COF-LZU1 and 560 m2 g−1 for COF-LZU1-NT (Table 1). The slightly higher BET surface area of COF-LZU1-NT could be ascribed to the presence of voids inside the nanotubes.33 The cumulative pore volume of both samples was calculated by nonlocal density functional theory (NLDFT) and both samples show a similar pore volume for the micropores (0.23 vs. 0.22 cm3 g−1). However, COF-LZU1-NT exhibited a much higher mesoporous volume than COF-LZU1 (Table 1, 0.31 vs. 0.04 m3 g−1). The pore size distribution shown in Fig. 2f demonstrates the presence of mesopores in the range of 20–60 nm in COF-LZU1-NT in agreement with TEM analysis (Fig. 1f).
The thermal stability of COF-LZU1-NT was checked by thermal gravimetric (TG) analysis. As shown in Fig. S2 (ESI†), both COF-LZU1 and COF-LZU1-NT showed good thermal stability (up to 350 °C), which agrees with the literature.25 Notably, after calcination at 250 °C for 1 h, the crystal structure and morphologies of COF-LZU1-NT did not change (Fig. S3, ESI†). The chemical stability of the as-synthesized COF-LZU1-NT has been examined by stirring the material in 1 M HCl and 1 M NaOH for 24 h. COF-LZU1-NT demonstrates preservation of the structure in a base (NaOH) and decomposition in the presence of an acid (HCl, Fig. S4, ESI†).
It has been recently reported that TMA can form fiber-like self-assemblies in the THF solvent.34 Thus, the addition of TMA in the solvent was expected to act as a soft template for regulating the growth of COFs. In order to confirm the role of TMA in the formation of COF nanotubes, pure TMA was firstly dissolved in dioxane and drop-cast on a copper grid for SEM and TEM analysis. As shown in Fig. S5 (ESI†) and Fig. 3b, a rod-like morphology of TMA self-assemblies was formed, demonstrating the feasibility of the TMA template for directing the growth of COF nanotubes. The acid sites of TMA oriented perpendicularly to the rods could act as nucleation sites for the crystallization of COF plates. The formation of the COF nanotubes was illustrated in Fig. 3a. Firstly, a rod-like TMA assembly was generated as a soft template (Fig. 3b). Then, the COF precursors of TFA and PDA encircle the TMA rods. This phenomenon is also confirmed by TEM (Fig. 3c). The presence of the TMA template inside the COF nanotubes was also confirmed by the XRD and FTIR analysis of the sample before washing (Fig. S6 and S7, ESI†). Hydrogen-bonding between –COOH (TMA) and –NH2 (PDA) and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (TFA) results in the condensation of COF monomers perpendicular to the TMA rod. For example, the use of a heteropolyacid in the crystallization of COF has been reported earlier.35 The removal of TMA by washing leads to the generation of the void space inside of the nanotubes (Fig. 3d). It is worth mentioning that the layer distance between the TMA nanorods has been reported to be 3.7 Å,34 which corresponds to the layer distance of COF-LZU1 (Fig. 3a).25 The layer distance matching could be the possible reason for the crystalline COF wall formation.
O (TFA) results in the condensation of COF monomers perpendicular to the TMA rod. For example, the use of a heteropolyacid in the crystallization of COF has been reported earlier.35 The removal of TMA by washing leads to the generation of the void space inside of the nanotubes (Fig. 3d). It is worth mentioning that the layer distance between the TMA nanorods has been reported to be 3.7 Å,34 which corresponds to the layer distance of COF-LZU1 (Fig. 3a).25 The layer distance matching could be the possible reason for the crystalline COF wall formation.
Different solvents and reaction temperatures resulted in insufficient COF formation (Fig. S8, ESI†). These results indicate that the formation of COF nanotubes is highly dependent on the reaction conditions. This can be attributed to the sensitivity of TMA morphology and interaction with monomers as function of the reaction conditions.
Furthermore, the synthesized COF nanotubes were further used as an oil adsorbent for the removal of oil contamination from water. The adsorption of relatively large molecules present in the oils is facilitated by the hierarchical structure of the COF nanotubes containing both micropores and mesopores. As shown in Fig. 4a, COF-LZU1-NT shows a lower density than COF-LZU1 (0.08 vs. 0.56 g cm−3). This difference could be ascribed to the abundance of voids inside the nanotubes. As shown in Fig. 4c, five common oils of different origins have been selected for the adsorption tests. The adsorption capacity of COF-LZU1-NT is much higher than that of COF-LZU1 for all the oils. While initial COF-LZU1 absorbed the amount of oil corresponding to its pore volume (Table 1), COF-LZU1-NT can adsorb a 3-times higher amount. In comparison with the conventional strategy of making COF coatings on melanin foams,36 we demonstrate here that morphology-controlled synthesis of hierarchical COF nanotubes is an efficient way to enhance the adsorption capacity of COF materials. This kind of enhancement could be ascribed to the abundant voids inside the COF nanotubes, which act as the reservoir to accommodate more oils. Furthermore, we applied COF-LZU1 and COF-LZU1-NT in the adsorption of an oil-in-water emulsion by filtration. As shown in Fig. 4c, COF-LZU1-NT shows obvious efficiency for absorption of oil in the water compared to COF-LZU1. Moreover, the filtrate has been weighed and analyzed by gas chromatography. Specifically, when 3 g of the oil-in-water emulsion was used, the amount of oleic acid in the filtrate was only 3.3 mg for COF-LZU1-NT, while 18 mg for COF-LZU1, demonstrating the high oil adsorption selectivity of COF-LZU1-NT. It is worth mentioning that the absorption of oil droplets in the water demands the adsorbent to possess not only high adsorption capacity but also high selectivity for oils. As confirmed by water contact angle (CA) measurement (Fig. S9, ESI†), the COF-LZU1-NT has an increased CA compared to the initial COF-LZU1 (91° vs. 64°), thus ensuring a higher selectivity to oil adsorption than water.
We demonstrated here a general strategy for the morphology-control of COFs and synthesis of hierarchical micro and mesoporous COF nanotubes with a well-defined crystalline wall structure. The utilization of trimesic acid as a soft template has been proposed as an effective way to regulate the morphology of COFs. The formation of trimesic acid rod-like templates and adsorption and growth of COFs around the template lead to the unique nanotube morphology of stacked plates. The abundant mesoporous voids in the hollow channels within COF nanotubes endow the materials with high capacity for adsorption of oily contamination from water. This work affords an alternative way to improve the pore volume and diffusion properties of COF materials, which would significantly promote their further applications in separation, adsorption, and catalysis using relatively large molecules.
The manuscript was written with the contributions of all authors. All authors have given approval to the final version of the manuscript.
Conflicts of interest
There are no conflicts to declare.Notes and references
- S. Das, P. Heasman, T. Ben and S. Qiu, Chem. Rev., 2017, 117, 1515–1563 CrossRef CAS PubMed.
- N. Huang, P. Wang and D. Jiang, Nat. Rev. Mater., 2016, 1, 16068 CrossRef CAS.
- A. G. Slater and A. I. Cooper, Science, 2015, 348, aaa8075 CrossRef PubMed.
- X. Feng, X. Ding and D. Jiang, Chem. Soc. Rev., 2012, 41, 6010–6022 RSC.
- A. P. Cote, A. I. Benin, N. W. Ockwig, M. O'Keeffe, A. J. Matzger and O. M. Yaghi, Science, 2005, 310, 1166–1170 CrossRef CAS PubMed.
- C. S. Diercks and O. M. Yaghi, Science, 2017, 355, eaal1585 CrossRef PubMed.
- P. Poizot, J. Gaubicher, S. Renault, L. Dubois, Y. Liang and Y. Yao, Chem. Rev., 2020, 120, 6490–6557 CrossRef CAS PubMed.
- Z. Wang, S. Zhang, Y. Chen, Z. Zhang and S. Ma, Chem. Soc. Rev., 2020, 49, 708–735 RSC.
- S. Kandambeth, K. Dey and R. Banerjee, J. Am. Chem. Soc., 2019, 141, 1807–1822 CrossRef CAS PubMed.
- P. J. Waller, F. Gandara and O. M. Yaghi, Acc. Chem. Res., 2015, 48, 3053–3063 CrossRef CAS PubMed.
- S. Cao, B. Li, R. Zhu and H. Pang, Chem. Eng. J., 2019, 355, 602–623 CrossRef CAS.
- Y. Song, Q. Sun, B. Aguila and S. Ma, Adv. Sci., 2019, 6, 1801410 CrossRef PubMed.
- K. Geng, T. He, R. Liu, S. Dalapati, K. T. Tan, Z. Li, S. Tao, Y. Gong, Q. Jiang and D. Jiang, Chem. Rev., 2020, 120, 8814–8933 CrossRef CAS PubMed.
- J. L. Segura, S. Royuela and M. Mar Ramos, Chem. Soc. Rev., 2019, 48, 3903–3945 RSC.
- S. Kandambeth, V. Venkatesh, D. B. Shinde, S. Kumari, A. Halder, S. Verma and R. Banerjee, Nat. Commun., 2015, 6, 6786 CrossRef CAS PubMed.
- R. K. Sharma, P. Yadav, M. Yadav, R. Gupta, P. Rana, A. Srivastava, R. Zbořil, R. S. Varma, M. Antonietti and M. B. Gawande, Mater. Horiz., 2020, 7, 411–454 RSC.
- T. Ma, L. Wei, L. Liang, S. Yin, L. Xu, J. Niu, H. Xue, X. Wang, J. Sun, Y. B. Zhang and W. Wang, Nat. Commun., 2020, 11, 6128 CrossRef CAS PubMed.
- S. Wang, Z. Zhang, H. Zhang, A. G. Rajan, N. Xu, Y. Yang, Y. Zeng, P. Liu, X. Zhang, Q. Mao, Y. He, J. Zhao, B.-G. Li, M. S. Strano and W.-J. Wang, Matter, 2019, 1, 1592–1605 CrossRef.
- P. Pachfule, S. Kandmabeth, A. Mallick and R. Banerjee, Chem. Commun., 2015, 51, 11717–11720 RSC.
- B. Gole, V. Stepanenko, S. Rager, M. Grune, D. D. Medina, T. Bein, F. Wurthner and F. Beuerle, Angew. Chem., Int. Ed., 2018, 57, 846–850 CrossRef CAS PubMed.
- J. Tan, S. Namuangruk, W. Kong, N. Kungwan, J. Guo and C. Wang, Angew. Chem., Int. Ed., 2016, 55, 13979–13984 CrossRef CAS PubMed.
- W. Kong, W. Jia, R. Wang, Y. Gong, C. Wang, P. Wu and J. Guo, Chem. Commun., 2019, 55, 75–78 RSC.
- Y. Peng, W. K. Wong, Z. Hu, Y. Cheng, D. Yuan, S. A. Khan and D. Zhao, Chem. Mater., 2016, 28, 5095–5101 CrossRef CAS.
- S. Hao, S. Li and Z. Jia, J. Nanopart. Res., 2020, 22, 270 CrossRef CAS.
- S. Y. Ding, J. Gao, Q. Wang, Y. Zhang, W. G. Song, C. Y. Su and W. Wang, J. Am. Chem. Soc., 2011, 133, 19816–19822 CrossRef CAS PubMed.
- H. Fan, M. Peng, I. Strauss, A. Mundstock, H. Meng and J. Caro, J. Am. Chem. Soc., 2020, 142, 6872–6877 CrossRef CAS PubMed.
- T. Jadhav, Y. Fang, W. Patterson, C. H. Liu, E. Hamzehpoor and D. F. Perepichka, Angew. Chem., Int. Ed., 2019, 58, 13753–13757 CrossRef CAS PubMed.
- S. Y. Ding, X. H. Cui, J. Feng, G. Lu and W. Wang, Chem. Commun., 2017, 53, 11956–11959 RSC.
- Y. Li, Q. Wu, X. Guo, M. Zhang, B. Chen, G. Wei, X. Li, X. Li, S. Li and L. Ma, Nat. Commun., 2020, 11, 599 CrossRef CAS PubMed.
- X. Gu, W. Qi, X. Xu, Z. Sun, L. Zhang, W. Liu, X. Pan and D. Su, Nanoscale, 2014, 6, 6609–6616 RSC.
- M. Kruk and M. Jaroniec, Chem. Mater., 2001, 13, 3169–3183 CrossRef CAS.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CrossRef CAS.
- B. P. Biswal, S. Chandra, S. Kandambeth, B. Lukose, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2013, 135, 5328–5331 CrossRef CAS PubMed.
- S. Tothadi, K. Koner, K. Dey, M. Addicoat and R. Banerjee, ACS Appl. Mater. Interfaces, 2020, 12, 15588–15594 CrossRef CAS PubMed.
- T. Gao, Z. Yan, V. Ordomsky and S. Paul, ChemCatChem, 2022 DOI:10.1002/cctc.202101450.
- Q. Sun, B. Aguila, J. A. Perman, T. Butts, F.-S. Xiao and S. Ma, Chem, 2018, 4, 1726–1739 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2cc01735k |
| ‡ These authors contributed equally to this work and should be considered co-first authors. |
| This journal is © The Royal Society of Chemistry 2022 |