Catalytic asymmetric amination of azlactones with azobenzenes†
Abstract
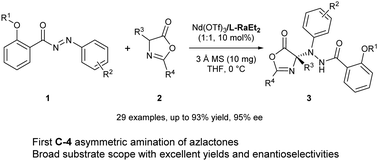
We reported an efficient asymmetric amination of azlactones with N-aryl-N-aroyldiazenes through a chiral N,N′-dioxide-based Lewis acid catalyst. The multicoordination ability of Nd(III) enabled it to simultaneously activate and to locate the two reactants for N-selective addition. Hydrazine-bearing azlactone derivatives were obtained in moderate to good yields with high enantioselectivity.
- This article is part of the themed collection: Chemical Communications HOT Articles 2022


 Please wait while we load your content...
Please wait while we load your content...