Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Bismuth-based mixed-anion compounds for anode materials in rechargeable batteries†
Prashant
Kumar
a,
Wandi
Wahyudi
 a,
Abhinav
Sharma
a,
Youyou
Yuan
b,
George T.
Harrison
a,
Murali
Gedda
a,
Xuan
Wei
a,
Abdulrahman
El-Labban
a,
Shahzad
Ahmad
c,
Vinod
Kumar
a,
Abhinav
Sharma
a,
Youyou
Yuan
b,
George T.
Harrison
a,
Murali
Gedda
a,
Xuan
Wei
a,
Abdulrahman
El-Labban
a,
Shahzad
Ahmad
c,
Vinod
Kumar
 d,
Vincent
Tung
a and
Thomas D.
Anthopoulos
d,
Vincent
Tung
a and
Thomas D.
Anthopoulos
 *a
*a
aKAUST Solar Center, King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Saudi Arabia. E-mail: thomas.anthopoulos@kaust.edu.sa
bCore Labs, King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Saudi Arabia
cDepartment of Chemistry, Zakir Husain Delhi College, University of Delhi, Delhi 110002, India
dSpecial Center for Nanoscience, Jawaharlal Nehru University, Delhi 110067, India
First published on 7th February 2022
Abstract
A facile solvothermal synthesis approach for chemical composition control in ternary Bi–S–I systems is reported by simply controlling the sulfide concentration. We demonstrate the application of these bismuth-based ternary mixed-anion compounds as high capacity anode materials in rechargeable batteries. Cells utilising Bi13S18I2 achieved an initial capacity value of 807 mA h g−1, while those with BiSI/Bi13S18I2 a value of 1087 mA h g−1 in lithium-ion battery systems.
Mixed-anion compounds are subjects of immense interdisciplinary interest compared to mono-anion compounds due to them inheriting properties of the different constituent anions in a single molecular material along with several new and interesting properties of additional orbital hybridization.1–3 These properties can further be tuned by controlling the stoichiometry of the anions and charge rebalancing, thus finding potential applications in a wide range of fields, including electrical, magnetic, optical, energy storage and transport devices.1,4 Bismuth sulfide iodide systems (Bi–S–I), a class of ternary mixed anion compounds, possess a quite complex phase diagram ranging from metastable/intermediate compositions,5 along with thermodynamically stable phases of BiSI and Bi19S27I3.5–7 In particular, the structure of Bi19S27I3 was debatable for a long time,7 with the recent structural analysis by Groom et al.8 referred to as Bi12.67S18I2 or Bi13S18I2 for the sake of convenience. It is noteworthy to mention that Bi13S18I2 has a unique structure in which ribbon like subunits of (Bi4S6)∞, i.e., [(Bi2S3)2]∞, form six spokes around a central hexagonal channel at the corners of the unit cell.8 BiSI and Bi13S18I2 are termed as sulfur-deficient and sulfur-rich compositions depending on the sulfide stoichiometry present.
Bi2S3 has been regarded as a promising host for lithium-ion storage, but enormous volume expansion takes place upon the charging–discharging process, which leads to pulverization of the Bi2S3 particles, thus compromising its performance as an electrode for lithium-ion batteries (LIBs).9,10 To overcome this crucial challenge, several Bi2S3-based composites have been introduced, such as yolk–shell Bi2S3@C, core–shell Bi2S3@C, Bi2S3/C nanorods, Bi2S3–CNT, and Bi2S3–MoS2.9–14 Compared to a composite, using a molecular compound is advantageous because of its homogeneity, while components in the composite remain separate and distinct within the finished structure, which makes it relatively difficult to understand. Therefore, the exploration of inorganic/organic ternary mixed-anion compounds such as bismuth-based materials and their use as an integral part of battery electrodes are desirable.
Here, we present a facile synthesis of bismuth-based materials and demonstrate their applications as electrodes in LIBs. Our obvious choices of these ternary mixed-anion materials are Bi13S18I2 and BiSI. Besides the aforementioned structural properties of Bi13S18I2, BiSI possesses a layered structural arrangement that is also suitable for ion migration. In particular, the structure of the [(BiSI)∞]2 ribbons in BiSI is held together by weak ionic or van der Waals-type forces.7
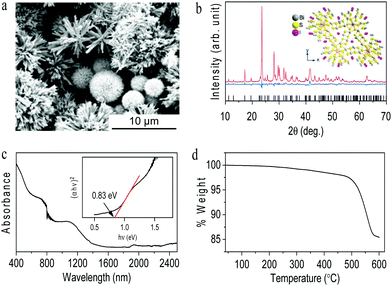
We employed BiI3 as the molecular precursor for bismuth and iodine in place of using two separate precursors for each element, while thiourea was used as the source of sulfur. Both the precursors, i.e., BiI3 and thiourea, are quite stable compounds. To prevent the evolution of toxic gases, the reactions were performed under a closed atmosphere using ethanol as the reaction medium, as both the precursors show an excellent solubility in ethanol (see Experimental section, ESI†). In a typical synthesis of sulfur-rich bismuth sulfide iodide, i.e., Bi13S18I2, 4 mmol of thiourea was first mixed with 1 mmol of BiI3 in an 82 mL autoclave using 60 mL of ethanol at 60 °C for 7 hours to ensure that both the compounds were well mixed and then the solution was heated at 120 °C for 24 hours. After the reaction, a black color compound was separated and washed several times using a centrifuge at 2000 rpm at room temperature and washed several times using ethanol and isopropanol followed by drying at room temperature. The morphologies of the synthesized particles were first observed by field emission scanning electron microscopy (FE-SEM). Fig. 1a shows the morphology of the synthesized Bi13S18I2 by the reaction of excess thiourea to BiI3 according to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio, showing that rod-like particles assembled in dandelion flower shapes. Elemental mapping shows the homogeneity of Bi, S and I across the particles (Fig. S1, ESI†). Energy dispersive spectroscopy (EDS) attached with SEM confirms that Bi, S and I are present according to the composition Bi6.35S9.12I, i.e., Bi12.71S18.24I2 (Fig. S2, ESI†). Fig. 1b shows the Rietveld refined powder X-ray diffraction (XRD) pattern of the synthesized bismuth sulfide iodide, which matches with trigonal Bi13S18I2 in the P3 space group (ICSD 243731).8 The estimated lattice parameters from the Rietveld refinement of Bi13S18I2 are a = 15.703(4) Å and c = 4.035(1) Å with Rexp = 2.4920, Rwp = 7.1516, Rp = 5.5369, χ2 = 1.694 and GOF = 2.869. The inset of Fig. 1b shows the crystal structure of the trigonal Bi13S18I2. Fig. 1c shows the vis-NIR absorbance spectrum of the synthesized compound, showing the absorption of a complete visible range with a band gap calculated using the Kubelka–Munk function of 0.83 eV (inset Fig. 1c), which is consistent with previously reported values.8 The thermal stability of the synthesized Bi13S18I2 was characterized by thermogravimetric analysis (TGA) under a nitrogen atmosphere, which shows a single-step dissociation (Fig. 1d). After the thermal treatment, the obtained product was further characterized by XRD, which matches well with orthorhombic Bi2S3 (Fig. S3, ESI†).5
1 molar ratio, showing that rod-like particles assembled in dandelion flower shapes. Elemental mapping shows the homogeneity of Bi, S and I across the particles (Fig. S1, ESI†). Energy dispersive spectroscopy (EDS) attached with SEM confirms that Bi, S and I are present according to the composition Bi6.35S9.12I, i.e., Bi12.71S18.24I2 (Fig. S2, ESI†). Fig. 1b shows the Rietveld refined powder X-ray diffraction (XRD) pattern of the synthesized bismuth sulfide iodide, which matches with trigonal Bi13S18I2 in the P3 space group (ICSD 243731).8 The estimated lattice parameters from the Rietveld refinement of Bi13S18I2 are a = 15.703(4) Å and c = 4.035(1) Å with Rexp = 2.4920, Rwp = 7.1516, Rp = 5.5369, χ2 = 1.694 and GOF = 2.869. The inset of Fig. 1b shows the crystal structure of the trigonal Bi13S18I2. Fig. 1c shows the vis-NIR absorbance spectrum of the synthesized compound, showing the absorption of a complete visible range with a band gap calculated using the Kubelka–Munk function of 0.83 eV (inset Fig. 1c), which is consistent with previously reported values.8 The thermal stability of the synthesized Bi13S18I2 was characterized by thermogravimetric analysis (TGA) under a nitrogen atmosphere, which shows a single-step dissociation (Fig. 1d). After the thermal treatment, the obtained product was further characterized by XRD, which matches well with orthorhombic Bi2S3 (Fig. S3, ESI†).5
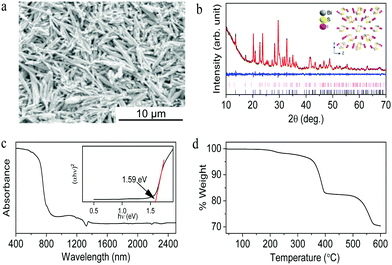
The morphology of the synthesized bismuth sulfide iodide obtained by reacting 1 mmol of thiourea with 1 mmol of BiI3 is shown in Fig. 2a, showing a rod-like microstructure with a random arrangement, unlike Bi13S18I2. Elemental mapping shows the homogeneity of Bi, S and I across the microstructure (Fig. S4, ESI†), which are present according to the average composition BiS0.98I1.03 (Fig. S5, ESI†). The observed reflections in the powder XRD pattern closely resemble those of orthorhombic BiSI in the Pnma space group (ICSD 023631) along with the secondary phase trigonal Bi13S18I2 in the P3 space group (Fig. 2b). The estimated lattice parameters from the Le Bail refinement are a = 8.501(5) Å, b = 10.256(0) Å and c = 4.176(1) Å with Rwp = 0.0395, Rp = 0.0295, χ2= 1.602 and GOF =1.27. The secondary phase of trigonal Bi13S18I2 was also incorporated into the Le Bail refinement and its % mass contribution was found to be approx. 23%. Inset of Fig. 2b shows the crystal structure of orthorhombic BiSI. Fig. 2c shows the vis-NIR absorbance spectrum of the synthesized BiSI/Bi13S18I2 with a calculated band gap of 1.59 eV by using Kubelka–Munk functions (inset Fig. 2c), which is close to the previously reported BiSI band gap value of 1.57–1.59 eV.4 TGA analysis of the synthesized BiSI/Bi13S18I2 in Fig. 2d shows two major steps in temperature-dependent dissociation. The TGA result is consistent with previous reports that indicate the formation of intermediate phase Bi13S18I2 and Bi2S3 as the final product, while a few percent of weight loss at around 230 °C might be due to intriguing chemistry between BiSI and Bi13S18I2.7,15
Based on our results, both sulfur-rich and sulfur-deficient compositions of mixed-anion bismuth-based compounds could be directly obtained by simply controlling the reactive sulfur concentration under identical experimental conditions. The presence of thiourea also ensures a reducing atmosphere. Because of a low boiling point and a high vapour pressure, ethanol was used for low temperature reactions. The use of excess sulfur leads to sulfur-rich compositions, i.e., Bi13S18I2, while equivalent/less sulfur favours a sulfur deficient composition, i.e., BiSI. Bismuth shows a high oxygen affinity and to avoid any kind of oxygen contamination, a reducing atmosphere in a non-aqueous solvent is recommended for bismuth chalcohalide synthesis. Thus, the presented facile synthesis of ternary Bi–S–I compounds by simply varying the sulfur concentration is favourable, which enables us to control the chemical composition under mild reaction conditions.
We demonstrated the performance of the synthesized Bi13S18I2 and BiSI/Bi13S18I2 as the anodes in LIBs by depositing the compounds onto a carbon cloth (CC) current collector. The 2032 type half-cells were assembled by employing lithium metal as the counter electrode and a commercial electrolyte of 1.0 molar LiPF6 in an ethylene carbonate/dimethyl carbonate solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol%). Fig. 3a represents the open circuit voltages (OCVs) of the cells ranging from 2.9 to 3.1 V recorded for 2.0 hours prior to the electrochemical measurements, the stable OCVs indicate good compatibility of the electrodes as there is no self-discharge observed within the cells.16 The electrochemical impedance spectroscopy (EIS) measurements (inset Fig. 3a) show comparable resistivity of Bi13S18I2 and BiSI, i.e., ∼260 Ω. The observed resistivity value is much lower compared to that of the pristine CC (∼440 Ω), as confirmed by the smaller semicircles of the cells employing Bi13S18I2 and BiSI than that of pristine CC. Fig. 3b shows the cyclic voltammetry (CV) curves of the cells, recorded at a scan rate of 0.2 mV s−1 after the OCV and EIS measurements. Bismuth containing compounds are known for alloy formation with lithium.17,18 The cathodic peaks in the CV curves (Fig. 3b) between 1.5 V and 1.9 V suggest the conversion of Bi13S18I2 and BiSI/Bi13S18I2 to metallic Bi upon the lithiation process,17,18 which is confirmed by the formation of plateaus upon the first galvanostatic discharge of the corresponding electrodes in Fig. 3c. Cathodic peaks between 0.5 V and 0.6 V shown in Fig. 3b can be assigned to the alloying process of metallic Bi with Li.17,18 The ex situ XRD analysis of the electrodes after discharging reveals that the conversion of Bi13S18I2 and BiSI/Bi13S18I2 results in Li3Bi, and Li3Bi along with LiBi, respectively, as presented in Fig. S6 (ESI†). Similar to the desodiation process in sodium-ion batteries from Na3Bi to NaBi to Bi,19 anodic scans (Fig. 3b) for Bi13S18I2 and BiSI/Bi13S18I2 show two peaks, one broad peak in the range of 0.2 V – 0.5 V and a second around 0.9 V, corresponding to delithiation of Li3Bi to LiBi to Bi. The XRD patterns of charged Bi13S18I2 and BiSI/Bi13S18I2 match with that of metallic Bi, obtained after the dealloying process. The conversion is also consistent with the formation of plateaus of vthe oltage profiles upon charging at around 0.9 V (Fig. 3c).17,18 Based on charge–discharge analysis, CV profiles, and XRD analysis, the reaction pathway in the Bi13S18I2–Li and BiSI/Bi13S18I2–Li cells during the discharge–charge process is shown below.
1 vol%). Fig. 3a represents the open circuit voltages (OCVs) of the cells ranging from 2.9 to 3.1 V recorded for 2.0 hours prior to the electrochemical measurements, the stable OCVs indicate good compatibility of the electrodes as there is no self-discharge observed within the cells.16 The electrochemical impedance spectroscopy (EIS) measurements (inset Fig. 3a) show comparable resistivity of Bi13S18I2 and BiSI, i.e., ∼260 Ω. The observed resistivity value is much lower compared to that of the pristine CC (∼440 Ω), as confirmed by the smaller semicircles of the cells employing Bi13S18I2 and BiSI than that of pristine CC. Fig. 3b shows the cyclic voltammetry (CV) curves of the cells, recorded at a scan rate of 0.2 mV s−1 after the OCV and EIS measurements. Bismuth containing compounds are known for alloy formation with lithium.17,18 The cathodic peaks in the CV curves (Fig. 3b) between 1.5 V and 1.9 V suggest the conversion of Bi13S18I2 and BiSI/Bi13S18I2 to metallic Bi upon the lithiation process,17,18 which is confirmed by the formation of plateaus upon the first galvanostatic discharge of the corresponding electrodes in Fig. 3c. Cathodic peaks between 0.5 V and 0.6 V shown in Fig. 3b can be assigned to the alloying process of metallic Bi with Li.17,18 The ex situ XRD analysis of the electrodes after discharging reveals that the conversion of Bi13S18I2 and BiSI/Bi13S18I2 results in Li3Bi, and Li3Bi along with LiBi, respectively, as presented in Fig. S6 (ESI†). Similar to the desodiation process in sodium-ion batteries from Na3Bi to NaBi to Bi,19 anodic scans (Fig. 3b) for Bi13S18I2 and BiSI/Bi13S18I2 show two peaks, one broad peak in the range of 0.2 V – 0.5 V and a second around 0.9 V, corresponding to delithiation of Li3Bi to LiBi to Bi. The XRD patterns of charged Bi13S18I2 and BiSI/Bi13S18I2 match with that of metallic Bi, obtained after the dealloying process. The conversion is also consistent with the formation of plateaus of vthe oltage profiles upon charging at around 0.9 V (Fig. 3c).17,18 Based on charge–discharge analysis, CV profiles, and XRD analysis, the reaction pathway in the Bi13S18I2–Li and BiSI/Bi13S18I2–Li cells during the discharge–charge process is shown below.
(I) Discharge process:
(II) Charge process:
Fig. 3d shows that the cell employing BiSI/Bi13S18I2 achieves an initial capacity of 1087 mA h g−1 and retains 703 mA h g−1 after 25 cycles, while the cell employing Bi13S18I2 achieves capacities of 807 mA h g−1 and 601 mA h g−1, respectively. Interestingly, both the ternary BiSI and Bi13S18I2 compounds exhibit a stable cycling performance and achieve a higher capacity retention compared to the previously reported binary bismuth-based materials, as summarized in Table S1 (ESI†). To date, the application of bismuth-based materials as battery electrodes still lacks study, but researchers have found that these materials are promising for Li+, Na+, and K+ storages.9–14,17–22 Importantly, the class of ternary bismuth-based materials, such as Bi–S–I in this study and previously reported BiSbS3,11 suggest outstanding performance as battery electrodes over the binary bismuth-based materials and the state-of-the-art graphite anode (theoretical capacity of 372 mA h g−1).23,24 Furthermore, these materials merit further investigations, which include applications in other types of batteries, such as multivalent metal ion (e.g., Zn2+, Mg2+, and Al3+) battery systems.
In summary, we reported the controlled synthesis of Bi13S18I2 and BiSI by simply tuning the sulfur concentration in a non-aqueous medium and demonstrate their potential as anode materials for rechargeable batteries. Our facile synthesis strategy enables variation of mixed-anion compounds beyond the Bi–S–I systems. Furthermore, the exploration of a new class of mixed-anion ternary Bi13S18I2 and BiSI compounds as anode materials in LIBs paves the way to new research in the development of future electrodes for electrochemical energy storage systems, e.g., Li+, Na+, K+, Zn2+, Mg2+, and Al3+ batteries.
The authors are grateful to King Abdullah University of Science and Technology (KAUST) for the financial support. P. K. thanks Yuliar Firdaus for valuable suggestions.
Conflicts of interest
The authors declare no conflicts of interest.References
- H. Kageyama, K. Hayashi, K. Maeda, J. P. Attfield, Z. Hiroi, J. M. Rondinelli and K. R. Poeppelmeier, Nat. Commun., 2018, 9, 772 CrossRef PubMed.
- M. Amsler, L. Ward, V. I. Hegde, M. G. Goesten, X. Yi and C. Wolverton, Phys. Rev. Mater., 2019, 3, 035404 CrossRef CAS.
- Daniel W. Davies, K. T. Butler, J. M. Skelton, C. Xie, A. R. Oganov and A. Walsh, Chem. Sci., 2018, 9, 1022–1030 RSC.
- S. Li, L. Xu, X. Kong, T. Kusunose, N. Tsurumachi and Q. Feng, J. Mater. Chem. C, 2020, 8, 3821–3829 RSC.
- Z. S. Aliev, S. S. Musayeva, F. Y. Jafarli, I. R. Amiraslanov, A. V. Shevelkov and M. B. Babanly, J. Alloys Compd., 2014, 610, 522–528 CrossRef CAS.
- G. Miehe and V. Kupčík, Naturwissenschaften, 1971, 58, 219 CrossRef CAS.
- W. Haase-Wessel, Naturwissenschaften, 1973, 60, 474 CrossRef CAS.
- R. Groom, A. Jacobs, M. Cepeda, R. Drummey and S. E. Latturner, Chem. Mater., 2017, 29, 3314–3323 CrossRef CAS.
- L. Zhao, H.-H. Wu, C. Yang, Q. Zhang, G. Zhong, Z. Zheng, H. Chen, J. Wang, K. He, B. Wang, T. Zhu, X. C. Zeng, M. Liu and M.-S. Wang, ACS Nano, 2018, 12, 12597–12611 CrossRef CAS PubMed.
- J. Ni, Y. Zhao, T. Liu, H. Zheng, L. Gao, C. Yan and L. Li, Adv. Energy Mater., 2014, 4, 1400798 CrossRef.
- S. Wen, J. Zhao, J. Chen, J. Yang and J. Xu, Dalton Trans., 2019, 48, 10448–10454 RSC.
- Y. Zhao, T. Liu, H. Xia, L. Zhang, J. Jiang, M. Shen, J. Ni and L. Gao, J. Mater. Chem. A, 2014, 2, 13854–13858 RSC.
- Y. Qin, Y. Zhang, J. Wang, J. Zhang, Y. Zhai, H. Wang and D. Li, ACS Appl. Mater. Interfaces, 2020, 12, 42902–42910 CrossRef CAS PubMed.
- W. Sun, X. Rui, D. Zhang, Y. Jiang, Z. Sun, H. Liu and S. Dou, J. Power Sources, 2016, 309, 135–140 CrossRef CAS.
- H. Sun, G. Yang, J. Chen, C. Kirk and N. Robertson, J. Mater. Chem. C, 2020, 8, 13253–13262 RSC.
- W. Wahyudi, Z. Cao, P. Kumar, M. Li, Y. Wu, M. N. Hedhili, T. D. Anthopoulos, L. Cavallo, L.-J. Li and J. Ming, Adv. Funct. Mater., 2018, 28, 1802244 CrossRef.
- P. Kumari, K. Awasthi, S. Agarwal, T. Ichikawa, M. Kumar and A. Jain, RSC Adv., 2019, 9, 29549–29555 RSC.
- C. Chen, P. Hu, X. Hu, Y. Mei and Y. Huang, Chem. Commun., 2015, 51, 2798–2801 RSC.
- P. Xiong, P. Bai, A. Li, B. Li, M. Cheng, Y. Chen, S. Huang, Q. Jiang, X. H. Bu and Y. Xu, Adv. Mater., 2019, 31, 1904771 CrossRef CAS PubMed.
- C. Lu, Z. Li, L. Yu, L. Zhang, Z. Xia, T. Jiang, W. Yin, S. Dou, Z. Liu and J. Sun, Nano Res., 2018, 11, 4614–4626 CrossRef CAS.
- H. Liang, J. Ni and L. Li, Nano Energy, 2017, 33, 213–220 CrossRef CAS.
- B. Hu, X. Wang, Q. Wei, H. Shu, X. Yang, Y. Bay, H. Wu, Y. Song and L. Liu, J. Alloys Compd., 2013, 579, 18–26 CrossRef CAS.
- J. Ming, Z. Cao, W. Wahid, M. Li, P. Kumar, Y. Wu, J.-Y. Hwang, M. N. Hedhili, L. Cavallo, Y.-K. Sun and L.-J. Li, ACS Energy Lett., 2018, 3, 335–340 CrossRef CAS.
- W. Wahyudi, V. Ladelta, L. Tsetseris, M. M. Alsabban, X. Guo, E. Yengel, H. Faber, B. Adilbekova, A. Seitkhan, A.-H. Emwas, M. N. Hedhili, L.-J. Li, V. Tung, N. Hadjichristidis, T. D. Anthopoulos and J. Ming, Adv. Funct. Mater., 2021, 31, 2101593 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and additional results. See DOI: 10.1039/d1cc06456h |
| This journal is © The Royal Society of Chemistry 2022 |