Open Access Article
Open Access ArticleOne-step covalent hydrophobic/hydrophilic functionalization of chemically exfoliated molybdenum disulfide nanosheets with RAFT derived polymers†
Andriana
Plantzopoulou
a,
Anastasios
Stergiou
 a,
Martha
Kafetzi
a,
Raul
Arenal
a,
Martha
Kafetzi
a,
Raul
Arenal
 *bcd,
Stergios
Pispas
*bcd,
Stergios
Pispas
 *a and
Nikos
Tagmatarchis
*a and
Nikos
Tagmatarchis
 *a
*a
aTheoretical and Physical Chemistry Institute, National Hellenic Research Foundation, 48 Vassileos Constantinou Avenue, Athens 11635, Greece. E-mail: tagmatar@eie.gr
bLaboratorio de Microscopias Avanzadas (LMA), Universidad de Zaragoza, Mariano Esquillor s/n, Zaragoza 50018, Spain
cInstituto de Nanociencia y Materiales de Aragon (INMA), CSIC-U. de Zaragoza, Calle Pedro Cerbuna 12, Zaragoza 50009, Spain
dARAID Foundation, Zaragoza 50018, Spain
First published on 9th December 2021
Abstract
The covalent functionalization of chemically exfoliated molybdenum disulfide (ce-MoS2) with hydrophobic poly(methyl methacrylate) and hydrophilic poly(acrylic acid) polymers, in a single-step without additives, is presented. The nature of chemical modification and the impact on the structure of ce-MoS2 were spectroscopically investigated. Complexation of Eu3+ was accomplished on grafted polycarboxylate chains on MoS2.
Molecular functionalization of exfoliated MoS2 nanosheets has gained increased interest.1–6 In particular, chemically exfoliated MoS2 nanosheets (ce-MoS2) derived by treating the bulk semiconducting MoS2 powder with organolithium compounds7 are a proven candidate towards stable covalently modified nanosheets with small molecules.6,8–10 However, covalent functionalization of ce-MoS2 nanosheets with polymers remains in its infancy. Recently, via a 2-step approach, poly(perfluoralkyl) chains have been covalently grafted by combining diazonium chemistry followed by a free-radical polymerization.11 Reversible addition fragmentation transfer (RAFT) polymerization is reportedly utilized in multi-step strategies as a chemical tool towards covalent MoS2–polymer ensembles.12–14 Nevertheless, insights into the reaction mechanisms taking place between the ce-MoS2 nanosheets and RAFT-derived polymers, as well as the impact on the structure of the nanosheets are missing or insufficiently described.
In this communication, we present the covalent functionalization of ce-MoS2 with hydrophobic poly(methyl methacrylate), PMMA, and hydrophilic poly(acrylic acid), PAA, polymers derived by RAFT polymerization. The reaction proceeds in a single step in aqueous media without additives. The major advantage of the method is the chemical coupling of well-defined and characterized counterparts in a single step, in contrast to multi-step routes and uncontrolled in situ radical polymerization reactions. We investigated the nature of the chemical modification spectroscopically by FT-IR and spatial Raman spectroscopy mapping, shedding light on the impact of functionalization on the structure of ce-MoS2. Moreover, exploiting the polycarboxylate nature of MoS2–PAA nanosheets and their superior stability in water, we investigated the complexation dynamics for water-soluble fluorescent Eu3+ with the aid of photoluminescence (PL) spectroscopy.
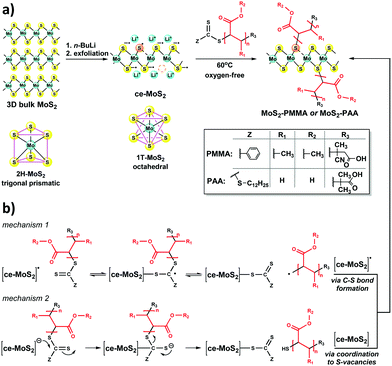
To start with, ce-MoS2 was prepared via chemical intercalation with n-butyllithium, followed by ultrasonic-assisted exfoliation in distilled water. The success of the intercalation/exfoliation process was initially assessed via UV-vis spectroscopy (Fig. S1, ESI†), where the characteristic excitonic transitions of the semiconducting 2H (trigonal prismatic) bulk MoS2 were absent, suggesting symmetry transition to the octahedral 1T-MoS2 phase. Specifically, the recorded electronic absorption features located at 250 and 305 nm are characteristic of the metallic 1T-MoS2 polytype and also verify that the prepared ce-MoS2 nanosheets have surface sulfur sites with electron surplus stabilized by lithium cations (Fig. 1a). Freshly prepared oxygen-protected ce-MoS2 suspensions were used for the covalent decoration of the nanosheets with RAFT-derived PMMA and PAA polymer chains to fully exploit these highly reactive electron-rich exfoliated MoS2 nanosheets. Notably, RAFT-derived polymers retain the thiocarbonylthio group of the utilized chain transfer agent (CTA) or RAFT agent at their ω-terminus constituting dormant macro-RAFT agents, being suitable for synthesizing block copolymers, and preparing end-functional polymers via re-activation of the thiocarbonylthio groups.15,16 In this context, ce-MoS2, possessing electron surplus, was utilized for the ω-terminal functionalization of RAFT-derived PMMA and PAA polymers towards covalently formed MoS2–PMMA and MoS2–PAA ensembles (Fig. 1a). Two main reaction mechanisms are expected to compete: (i) radical and/or (ii) nucleophilic attack to the ω-terminal thiocarbonylthio group of the polymer chain, by reactive radical and/or negatively charged S-sites on the surface of ce-MoS2, respectively. In the case of a radical-mediated reaction, radicals centred around S-defects17 at the surface of ce-MoS2 attack the S-atom of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond of the thiocarbonylthio group releasing polymer bound radicals, which are trapped by neighbouring radicals residing on the surface of ce-MoS2 (mechanism 1, Fig. 1b). According to the second mechanism, reactive nucleophilic anion species centred at S-atoms at the surface of ce-MoS2 attack the C-atom of the C
S bond of the thiocarbonylthio group releasing polymer bound radicals, which are trapped by neighbouring radicals residing on the surface of ce-MoS2 (mechanism 1, Fig. 1b). According to the second mechanism, reactive nucleophilic anion species centred at S-atoms at the surface of ce-MoS2 attack the C-atom of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond of the thiocarbonylthio group releasing thiol-terminated polymer chains, which are then coordinated to S-vacant sites at the surface of ce-MoS2 (mechanism 2, Fig. 1b). To perform the reaction, a water–isopropanol suspension of the freshly prepared ce-MoS2 was mildly heated at 60 °C in the presence of PMMA under oxygen-free conditions. PMMA was also selected as a candidate owing to its functional groups, e.g. methyl esters, which are unable to develop electrostatic interactions with the surface of MoS2. The newly prepared MoS2–PMMA was excessively purified by repeating dispersion/isolation cycles to ensure the removal of trapped and physiosorbed polymer chains, within the nanosheets.
S bond of the thiocarbonylthio group releasing thiol-terminated polymer chains, which are then coordinated to S-vacant sites at the surface of ce-MoS2 (mechanism 2, Fig. 1b). To perform the reaction, a water–isopropanol suspension of the freshly prepared ce-MoS2 was mildly heated at 60 °C in the presence of PMMA under oxygen-free conditions. PMMA was also selected as a candidate owing to its functional groups, e.g. methyl esters, which are unable to develop electrostatic interactions with the surface of MoS2. The newly prepared MoS2–PMMA was excessively purified by repeating dispersion/isolation cycles to ensure the removal of trapped and physiosorbed polymer chains, within the nanosheets.
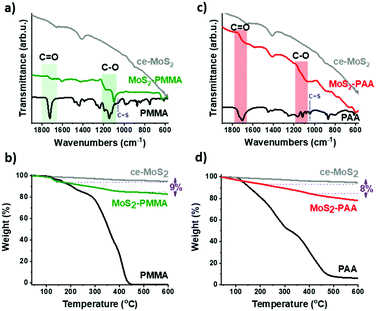
The IR spectra of MoS2–PMMA revealed the characteristic ester C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching mode at 1721 cm−1, appearing as a broad band, as well as the ester C–O stretching mode centred at 1140 cm−1, arising from the covalently grafted PMMA chains (Fig. 2a). In previous reports, it was proposed that polymer chains can be grafted onto ce-MoS2 in the form of thiols14 and alkylthiol-based linkers12 and with ce-MoS2 acting as the Z-functionality of the CTA (cf.Fig. 1).13 Inevitably, the presence of the newly formed C–S bonds between the nanosheets and polymers was indiscernible in the IR spectra. The same stands true for our study. It is, however, noteworthy that the C
O stretching mode at 1721 cm−1, appearing as a broad band, as well as the ester C–O stretching mode centred at 1140 cm−1, arising from the covalently grafted PMMA chains (Fig. 2a). In previous reports, it was proposed that polymer chains can be grafted onto ce-MoS2 in the form of thiols14 and alkylthiol-based linkers12 and with ce-MoS2 acting as the Z-functionality of the CTA (cf.Fig. 1).13 Inevitably, the presence of the newly formed C–S bonds between the nanosheets and polymers was indiscernible in the IR spectra. The same stands true for our study. It is, however, noteworthy that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S bond, originating from the thiocarbonylthio group, was not present in the IR spectrum of MoS2–PMMA. It is reasonable to consider that, due to the reductive environment, the survival of surface disulfide Mo–S–S–(C
S bond, originating from the thiocarbonylthio group, was not present in the IR spectrum of MoS2–PMMA. It is reasonable to consider that, due to the reductive environment, the survival of surface disulfide Mo–S–S–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)–Z species produced via both the proposed mechanisms is unlikely.
S)–Z species produced via both the proposed mechanisms is unlikely.
Thermogravimetric analysis (TGA) under a nitrogen atmosphere for MoS2–PMMA verified a weight loss in the region of 200–400 °C as high as 9%, arising from the grafted polymer chains (Fig. 2b). A weight loss at a low temperature, i.e. <150 °C, was not observed; thus the presence of Mo–S–S–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S)–Z species in the MoS2–PMMA ensembles is excluded. Therefore, from the FT-IR and TGA studies, we conclude that the surface of the nanosheets is exclusively decorated by the polymeric carbon backbone.
S)–Z species in the MoS2–PMMA ensembles is excluded. Therefore, from the FT-IR and TGA studies, we conclude that the surface of the nanosheets is exclusively decorated by the polymeric carbon backbone.
Additional evidence for the covalent insertion of PMMA chains onto MoS2 was provided by investigating the stability of MoS2–PMMA dispersions in water, toluene, methanol and dichloromethane. Interestingly, MoS2–PMMA was found to form the most stable dispersion in toluene, which is an excellent hydrophobic solvent for solubilizing the PMMA polymer, while, in water, which is an excellent solvent to exfoliate and store ce-MoS2, no stable dispersion of MoS2–PMMA was formed (Fig. S2, ESI†). Then, we employed PAA as a hydrophilic polymer counterpart for the covalent modification of ce-MoS2 for generating aqueous-stable MoS2–PAA ensembles. The preparation and characterization of MoS2–PAA were conducted as previously described. Briefly, the FT-IR spectra of the isolated MoS2–PAA displayed the characteristic carboxylic acid C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching mode at 1704 cm−1, as well as the carboxylic acid C–O stretching mode centred at 1116 cm−1, originating from the grafted PAA polymer chains (Fig. 2c). From the TGA thermograms, an 8% weight loss in the region of 200–400 °C was observed, indicating the presence of the PAA chains (Fig. 2d), while MoS2–PAA was found to form highly stable dispersions in water, which is an excellent solvent for both ce-MoS2 and PAA components (Fig. S3, ESI†).
O stretching mode at 1704 cm−1, as well as the carboxylic acid C–O stretching mode centred at 1116 cm−1, originating from the grafted PAA polymer chains (Fig. 2c). From the TGA thermograms, an 8% weight loss in the region of 200–400 °C was observed, indicating the presence of the PAA chains (Fig. 2d), while MoS2–PAA was found to form highly stable dispersions in water, which is an excellent solvent for both ce-MoS2 and PAA components (Fig. S3, ESI†).
In addition, the impact of covalent functionalization on the structure of ce-MoS2 was evaluated with the aid of spatial Raman spectroscopy under 514 and 633 nm laser excitation. For this, 196 spectra collected from a 20 × 20 μm area were analysed. The octahedral 1T symmetry of ce-MoS2 was validated by the presence of the characteristic J1, J2, and J3 phonon modes located at 151, 221 and 329 cm−1, in both 514 and 633 nm laser lines (Fig. 3a and b). Interestingly, the J modes are conserved in the functionalized MoS2–PMMA and MoS2–PAA materials manifesting that the particular covalent functionalization has a stabilizing effect on the metastable octahedral symmetry of MoS2. Furthermore, under 514 nm excitation, an increased full-width-half-maximum (FWHM) in the A1g mode (∼404 cm−1) of ce-MoS2, MoS2-PMMA and MoS2-PAA was evident, as compared to the bulk MoS2, indicating the presence of few layers. This was further elucidated by calculating the frequency difference of A1g–E12g modes under 514 nm excitation. For the bulk 2H-MoS2, a 26 cm−1 value was registered, while for the ce-MoS2 nanosheets the corresponding value was 23 cm−1, implying the delamination of the three-dimensional superstructure towards few-layered nanosheets (<8 layers). Notably, the MoS2–PMMA and MoS2–PAA ensembles displayed A1g–E12g values reaching a maximum value of 24 cm−1; thus, the covalent functionalization proved to shield the nanosheets against restacking to multi-layered non-functionalized structures. The proof of the chemical incorporation of the polymer chains on the surface of the ce-MoS2 nanosheets was given by spatial Raman spectroscopy mapping of the MoS2–PMMA and MoS2–PAA materials and evaluation of the A1g/2LA intensity ratios under 633 nm excitation. The 2LA mode under 633 nm excitation is known to be sensitive to crystal defects and lattice disorders and is actively investigated as a diagnostic tool for chemical functionalization. A recent systematic investigation of the A1g/2LA intensity ratios for defect engineering of ce-MoS2 by thiol coordination showed the potentiality to not only verify the functionalization process, but also allow monitoring of the reactions.18 Herein, we acquired Raman spectra from a 20 × 20 μm area (196 single spot spectra, in raster mode) of ce-MoS2 and the calculated average value of the A1g/2LA intensity ratio was found to be 1.0, with a high homogeneity (Fig. S4, ESI†). The same process was followed for evaluating the A1g/2LA intensity ratios for MoS2–PMMA and MoS2–PAA, where the registered average values were found to be 1.29 and 1.21, respectively, also with a high uniformity (Fig. 3c and d). Evidently, covalent functionalization is responsible for the altered intensity of the A1g and 2LA Raman spectral features. Given the weight loss unveiled by TGA (9% for MoS2–PMMA and 8% for MoS2–PAA), it is obvious that the degree of newly formed covalent bonds is low. It was reported that at the low degree of covalent functionalization via coordination of thiols to S-vacant sites of ce-MoS2, the A1g/2LA intensity ratio tends to decrease, as compared to ce-MoS2,18 while the covalent functionalization of ce-MoS2via C–S bond formation tends to increase it.9 Thus, based on our experimental data, we conclude that the major mechanism for the covalent grafting of PMMA and PAA on the surface of ce-MoS2 is the radical-mediated one (mechanism 1 in Fig. 1b), furnishing covalent C–S bonds between the carbon backbone of the polymer chain and the surface S-atoms of ce-MoS2. However, the possibility of a minor mechanism proceeding via coordination of thiol-terminated polymer chains produced via nucleophilic attack of ce-MoS2 to the thiocarbonylthio ω-terminus of the RAFT-derived polymers cannot be excluded (mechanism 2 in Fig. 1b).
Imaging of MoS2–PMMA and MoS2–PAA ensembles with the aid of high-resolution transmission electron microscopy (HR-TEM) further validated the presence of single- and few-layered nanosheets covered by organic matter (Fig. 3e and f, respectively). Namely the covalently functionalized nanosheets remained suspended without re-aggregation due to the covalent surface coverage by the polymer chains. Additional images of MoS2–PMMA and MoS2–PAA ensembles as well as of ce-MoS2 are shown in the ESI† (Fig. S5).
The described method allowed us to produce hydrophobic MoS2–PMMA and hydrophilic MoS2–PAA ensembles, furnishing stable dispersions in toluene and water, respectively. The latter enabled the utilization of the polycarboxylic nature of MoS2–PAA nanosheets to evaluate the complexation of water-soluble lanthanide cations. Europium(III) cations were selected as fluorescent probes to monitor the complexation dynamics via fluorescence spectroscopy. A solution of Eu(NO3)3 was initially titrated by alkaline PAA (i.e. in the carboxylate form) and the characteristic fluorescence emission peaks of Eu3+ at 591 nm (5D0–7F1 transition) and 615 nm (5D0–7F2 transition) were monitored under 391 nm excitation. During the addition of the PAA carboxylate, the 615 nm peak was significantly increased, while the 591 nm peak was slightly decreased (Fig. 4a). This observation was indicative of the complexation of Eu3+ by the polycarboxylate chains and the formation of an insoluble complex, where the 5D0–7F2 transition became dominant, similarly to the solid Eu(NO3)3 precursor (Fig. 4b). Next, aqueous Eu(NO3)3 was titrated by alkaline MoS2–PAA (i.e. in the polycarboxylate form) and the fluorescence emission spectrum of Eu3+ was found to be gradually quenched by the incremental addition of MoS2–PAA (Fig. 4c). To shed more light on the quenching dynamics, we plotted the change of the fluorescence emission intensity at 591 nm versus the introduced amount of MoS2–PAA polycarboxylate, in analogy to a Stern–Volmer plot. The experimental plot deviates from a linear expression and displays an upward shift, originating from the presence of a static quenching mechanism.19 Static quenching is likely to be driven by the strong ground state electrostatic complexation of Eu3+ by MoS2–PAA. This was also corroborated by the change of the I591nm/I615nm intensity ratio, which was found to decrease from 2.85 to ca. 2.0 and is also attributed to complexation, and likewise the case of complexation of Eu3+ by the free PAA. Collectively, the covalent MoS2–PAA ensembles effectively complexed the lanthanide cations, while the dispersion remained stable in aqueous media.
In summary, we presented a simple one-step synthetic method to obtain well-defined MoS2–polymer nanostructures with tuneable hydrophobicity and hydrophilicity, maintaining the 2D dimensionality and the metallic properties of MoS2. Utilizing the hydrophilic MoS2–PAA ensemble featuring polycarboxylate species, we showed efficient complexation of Eu3+, noting a future use of MoS2–PAA/lanthanide hybrid materials for (opto)electronics.20
The TEM study was performed in the Laboratorio de Microscopias Avanzadas (LMA), Universidad de Zaragoza (Spain). R. A. acknowledges support from the Spanish MICINN (PID2019-104739GB-100/AEI/10.13039/501100011033), the Government of Aragon (projects DGA E13-20R (FEDER, EU)) and the EU H2020 projects “ESTEEM3” (Grant number 823717) and Graphene Flagship (881603).
Conflicts of interest
There are no conflicts to declare.References
- A. Stergiou and N. Tagmatarchis, Chem. – Eur. J., 2018, 24, 18246–18257 CrossRef CAS PubMed.
- S. Bertolazzi, M. Gobbi, Y. Zhao, C. Backes and P. Samorì, Chem. Soc. Rev., 2018, 47, 6845–6888 RSC.
- R. Canton-Vitoria, T. Scharl, A. Stergiou, A. Cadranel, R. Arenal, D. M. Guldi and N. Tagmatarchis, Angew. Chem., Int. Ed., 2020, 59, 3976–3981 CrossRef CAS PubMed.
- I. K. Sideri, R. Arenal and N. Tagmatarchis, ACS Mater. Lett., 2020, 2, 832–837 CrossRef CAS.
- I. K. Sideri, Y. Jang, J. Garces-Garces, A. Sastre-Santos, R. Canton-Vitoria, R. Kitaura, F. Fernandez-Lazaro, F. D'Souza and N. Tagmatarchis, Angew. Chem., Int. Ed., 2021, 60, 9120–9126 CrossRef CAS PubMed.
- A. Stergiou, C. Stangel, R. Canton-Vitoria, R. Kitaura and N. Tagmatarchis, Nanoscale, 2021, 13, 8948–8957 RSC.
- T. H. Le, Y. Oh, H. Kim and H. Yoon, Chem. – Eur. J., 2020, 26, 6360–6401 CrossRef CAS PubMed.
- D. Voiry, A. Goswami, R. Kappera, C. D. C. C. E. Silva, D. Kaplan, T. Fujita, M. Chen, T. Asefa and M. Chhowalla, Nat. Chem., 2015, 7, 45–49 CrossRef CAS PubMed.
- K. C. Knirsch, N. C. Berner, H. C. Nerl, C. S. Cucinotta, Z. Gholamvand, N. McEvoy, Z. Wang, I. Abramovic, P. Vecera, M. Halik, S. Sanvito, G. S. Duesberg, V. Nicolosi, F. Hauke, A. Hirsch, J. N. Coleman and C. Backes, ACS Nano, 2015, 9, 6018–6030 CrossRef CAS PubMed.
- E. Er, H.-L. Hou, A. Criado, J. Langer, M. Möller, N. Erk, L. M. Liz-Marzán and M. Prato, Chem. Mater., 2019, 31, 5725–5734 CrossRef CAS.
- I. Gomez-Munoz, S. Laghouati, R. Torres-Cavanillas, M. Morant-Giner, N. V. Vassilyeva, A. Forment-Aliaga and M. Gimenez-Marques, ACS Appl. Mater. Interfaces, 2021, 13, 36475–36481 CrossRef CAS PubMed.
- H. Cheng, N. Dong, T. Bai, Y. Song, J. Wang, Y. Qin, B. Zhang and Y. Chen, Chem. – Eur. J., 2016, 22, 4500–4507 CrossRef CAS PubMed.
- Z. Souri, M. Adeli and E. Mehdipour, New J. Chem., 2020, 44, 17961–17969 RSC.
- L. Shen, X. Han, J. Qian and D. Hua, RSC Adv., 2017, 7, 10791–10797 RSC.
- G. Moad, E. Rizzardo and S. H. Thang, Polym. Int., 2011, 60, 9–25 CrossRef CAS.
- H. Willcock and R. K. O'Reilly, Polym. Chem., 2010, 1, 149–157 RSC.
- X. S. Chu, A. Yousaf, D. O. Li, A. A. Tang, A. Debnath, D. Ma, A. A. Green, E. J. G. Santos and Q. H. Wang, Chem. Mater., 2018, 30, 2112–2128 CrossRef CAS.
- X. Chen, P. Denninger, T. Stimpel-Lindner, E. Spiecker, G. S. Duesberg, C. Backes, K. C. Knirsch and A. Hirsch, Chem. – Eur. J., 2020, 26, 6535–6544 CrossRef CAS PubMed.
- D. Genovese, M. Cingolani, E. Rampazzo, L. Prodi and N. Zaccheroni, Chem. Soc. Rev., 2021, 50, 8414–8427 RSC.
- J. C. Bunzli and C. Piguet, Chem. Soc. Rev., 2005, 34, 1048–1077 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, UV-vis spectra, digital photos, spatial Raman spectroscopy mapping and TEM images. See DOI: 10.1039/d1cc06195j |
| This journal is © The Royal Society of Chemistry 2022 |