Open Access Article
Open Access ArticleA gliclazide complex based on palladium towards Alzheimer's disease: promising protective activity against Aβ-induced toxicity in C. elegans†
Amalia
García-García
 a,
Sara
Rojas
a,
Sara
Rojas
 a,
Lorenzo
Rivas-García
a,
Lorenzo
Rivas-García
 b,
María D.
Navarro-Hortal
b,
María D.
Navarro-Hortal
 c,
Jose M.
Romero-Márquez
c,
Jose M.
Romero-Márquez
 c,
José G.
Fernández-Bolaños
c,
José G.
Fernández-Bolaños
 d,
Duane
Choquesillo-Lazarte
d,
Duane
Choquesillo-Lazarte
 e,
Alfonso
Salinas-Castillo
e,
Alfonso
Salinas-Castillo
 f,
Óscar
López
f,
Óscar
López
 *d,
José L.
Quiles
*d,
José L.
Quiles
 *cg and
Antonio
Rodríguez-Diéguez
*cg and
Antonio
Rodríguez-Diéguez
 *a
*a
aDepartment of Inorganic Chemistry, Faculty of Sciences, University of Granada, Av. Fuentenueva S/N, Granada 18071, Spain. E-mail: antonio5@ugr.es
bDepartment of Physiology, University of Granada, Granada 18071, Spain
cInstitute of Nutrition and Food Technology “José Mataix”, Biomedical Research Centre, Department of Physiology, University of Granada, Avda. del Conocimiento s.n, Armilla 18100, Spain
dDepartment of Organic Chemistry, Faculty of Chemistry, University of Seville, Apart. 1203, Seville E-41071, Spain. E-mail: osc-lopez@us.es
eLaboratorio de Estudios Cristalográficos, IACT, CSIC-UGR, Av. Las Palmeras n°4, Granada 18100, Spain
fDepartment of Analytic Chemistry, Faculty of Sciences, University of Granada, Av. Fuentenueva S/N, Granada 18071, Spain
gResearch Group on Foods, Nutritional Biochemistry and Health, Universidad Europea del Atlántico, Isabel Torres, 21, Santander 39011, Spain. E-mail: jlquiles@ugr.es
First published on 16th December 2021
Abstract
A new palladium coordination compound based on gliclazide with the chemical formula [Pd(glz)2] (where glz = gliclazide) has been synthesized and characterised. The structural characterization reveals that this material consists of mononuclear units formed by a Pd2+ ion coordinated to two molecules of the glz ligand, in which palladium ions exhibit a distorted plane-square coordination sphere. This novel material behaves like a good and selective inhibitor of butyrylcholinesterase, one of the most relevant therapeutic targets against Alzheimer's disease. Analysis of the enzyme kinetics showed a mixed mode of inhibition, the title compound being capable of interacting with both the free enzyme and the enzyme–substrate complex. Finally, the palladium compound shows promising protective activity against Aβ-induced toxicity in the Caenorhabditis elegans model, which has never been reported.
Growing evidence suggests that diabetes mellitus (DM) is one of the strongest risk factors for developing Alzheimer's disease (AD).1 However, it remains unclear why DM accelerates AD pathology. Recently, some studies regarding histopathological and biochemical analyses of brain tissues in cynomolgus monkeys with type 2 DM have clarified the relationship between DM and AD pathology.2,3 In particular, DM is a chronic disease characterized by an inefficient metabolism of glucose, which increases its concentration in the blood and could damage some tissues, such as nerves or blood vessels. The current treatments have various limitations such as gastrointestinal discomfort, an increased risk of bladder cancer, edema or distal bone fractures in postmenopausal women, among others. Therefore, research on new pharmacological strategies has exponentially gained attention; specifically, drugs based on organometallic compounds have been demonstrated to be a successful strategy against type 2 DM.4 In this regard, metals such as V, Cr, Mo, Zn, Cu and Mn have been demonstrated to participate in glucose metabolism, but their translation into clinics remains unexplored.5
On the other hand, protein misfolding is associated with a wide number of pathological states in humans and other animals. Concretely, abnormal aggregation of the amyloid-β (Aβ) peptide, α-synuclein, and the human islet amyloid polypeptide (hIAPP) is correlated with AD, Parkinson's disease, and type 2 diabetes, respectively.6 Although these diseases have different target proteins, their molecular mechanisms of action are similar and involve the accumulation of large deposits, this process being generally referred to as amyloidogenesis. Some research studies have demonstrated that metal complexes, such as those formed by Pt and Ru, can act as inhibitors against peptide aggregation primarily through metal coordination.7 Thus, the development of novel coordination compounds may offer novel therapeutic strategies to fight against amyloid protein disorders. Considering all of the above, our research has focused on the preparation of novel action drugs that can interfere with amyloid formation and eliminate misfolded aggregates. Coordination chemistry offers a huge advantage in the design of new therapeutic agents. The synthesis and application of these novel crystalline compounds is feasible due to the possibility to select adequate metal ions and organic ligands to obtain the desired structures. Certainly, metal complexes, such as those of Pt, Ru, V and Cu, show a potential effect against amyloid protein aggregation due to the dual metal coordination–ligand effect. It should be noted that although Pd-based complexes have shown their potential against other important diseases (i.e. cancer or diabetes), their potential against AD remains unexplored.8 Recently, our research group have shown that the synthesis of new coordination compounds with interesting structures and fascinating physical and antidiabetic properties is possible.9 This has opened up new possibilities to design novel multifunctional materials that could be applied in the Alzheimer's research field. In this work, we present a novel coordination compound [Pd(glz)2] based on gliclazide (glz) and Pd. Moreover, we have obtained the first coordination compound with this interesting antidiabetic ligand. In this case, gliclazide is selected here as an ideal drug and moiety due to its nitrogen atoms, which can form coordination bonds with planar-square ions. We have selected both starting building blocks (glz and Pd) considering their potential activity against AD: glz is a normally used antidiabetic drug, while Pd has been demonstrated to be active against diabetes and other important diseases. Glz, a second-generation sulfonylurea, possesses an appropriate antidiabetic activity through the stimulation of the synthesis of insulin in the pancreas. Indeed, this drug blocks the K channels located at the membrane of pancreatic cells and promotes the depolarization and the entry of calcium and, consequently, the insulin secretion. Some alternatives that decrease the side effects and increase the efficacy are the conjugation of the drug with metals to improve its solubility. Furthermore, we have chosen Pd as a metallic ion, since it usually presents square plane coordination and this would be optimal to coordinate with two gliclazide ligands through its nitrogen atoms. Then, in vitro antidiabetic (glucose uptake in C2C12 muscle cells) and anti-Alzheimer studies (inhibition of acetylcholinesterase-AChE and butyrylcholinesterase-BuChE) of this novel compound have been carried out. Finally, the potential effect of the synthesized compound in Alzheimer's disease was tested in vivo using the Caenorhabditis elegans animal model (lethality tests, growth measurements, evaluation of pharyngeal pumping, and analysis of paralysis at the muscle level).
[Pd(glz)2] has been synthetized using a simple route in which the glicazide ligand was reacted with palladium(II) nitrate in ethanol. The palladium compound crystallizes in the monoclinic space group P21/c. Its structure is composed of mononuclear units formed by a Pd2+ ion coordinated to two molecules of the gliclazide ligand (Fig. 1). Table S1 (ESI†) shows the crystallographic data for this material. The palladium ion coordinates to the N1 and N3 nitrogen atoms of two different glz molecules, obtaining a distorted plane-square coordination sphere. The Pd–N bond distances and angles are in the normal range for this type of materials and are listed in Table S2 (ESI†).
There are no crystallization solvent molecules in the structure, but the monomers interact with each other through strong hydrogen bonds N–H⋯O involving the −NH groups of the sulfonyl and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the amide of the neighboring ligand molecules, with values of 2.082 Å (N2B–H2BA⋯O1A) and 2.273 Å (N2A–H2AA⋯O1B).
O of the amide of the neighboring ligand molecules, with values of 2.082 Å (N2B–H2BA⋯O1A) and 2.273 Å (N2A–H2AA⋯O1B).
Thus, one-dimensional supramolecular chains are formed, which propagate along the crystallographic c axis (Fig. S1, ESI†). The distances of the hydrogen bonds are shown in Table S3 (ESI†).
Once the material was designed, synthesized and fully characterized, we decided to analyze its in vitro antidiabetic properties concluding that the treatment with [Pd(glz)2] did not affect the glucose uptake, suggesting the biocompatibility of this compound.
In parallel, and in order to study the potential of [Pd(glz)2] in AD, this novel compound was evaluated as an inhibitor of cholinesterases (ChEs), and hydrolases that catalyze the breakdown of the neurotransmitter acetylcholine (ACh) at the synaptic cleft, selected in this study as targets. Despite the complexity of Alzheimer's disease, with a clear multifactorial aetiology, the modulation of the activity of AChE and BuChE is currently considered as one of the most relevant therapeutic targets to tackle Alzheimer's disease. In fact, three out of the four available drugs (donepezil, rivastigmine and galantamine) are inhibitors of these enzymes.10 The reason is that ACh, correlated with the cognitive functionality, exhibits low levels in the brain of patients; as a result, cognitive impairment is one of the most recognizable hallmarks of Alzheimer's disease.11 It has therefore been stated that inhibition of cholinesterases can restore the levels of the neurotransmitter and contribute to the improvement of the cognitive functionality as a palliative treatment of the disease (cholinergic hypothesis).12
When exposed to [Pd(glz)2] (100 μM), an almost complete blockade of BuChE (91% inhibition) is observed. The IC50 calculation against BuChE (Fig. S3, ESI†) revealed that, although [Pd(glz)2] did not improve the activity found for the drug galantamine (IC50 = 5.5 μM), it exhibited a remarkable activity (IC50 = 27 ± 1 μM). The title compound exhibited a good selectivity against BuChE, as it only showed 58% inhibition when tested against AChE at the same concentration; moreover, it is also worth mentioning that the ligand, glz, completely lacked activity against both enzymes. It should be noted that the selectivity towards the inhibition of BuChE can be of interest for the treatment of Alzheimer's disease in more advanced stages, as the activity of AChE is decreased with the evolution of the disease, whereas BuChE remains unchanged or even slightly increases.13 Then, in order to further understand these results, the inhibition constant (Ki) and the mode of inhibition were obtained (see further details in the ESI†).
Cornish–Bowden plots14 (1/V vs. [I], Fig. S4A, ESI†; [S]/V vs. [I], Fig. S4B, ESI†) revealed a mixed-type inhibition. The [Pd(glz)2] complex is, therefore, capable of interacting with the catalytic site of the free BuChE (Kia = 21 ± 4 μM), but also, with a lower potency, with the enzyme–substrate complex (Kib = 49 ± 9 μM).
In order to evaluate the potential effect of the synthesized compound in Alzheimer's disease in vivo, Caenorhabditis elegans as an experimental model was used. First, the short-term toxicity of the new compound was evaluated by exposing worms to concentrations of [Pd(glz)2] of 0, 0.1, 1, 10 and 100 μg mL−1 during 24 h. As shown in Fig. 2A, in all the assays, the concentrations of [Pd(glz)2] and glz were not lethal to the worms. The next approach to the toxicity investigation was the measurement of the body length and pharyngeal pumping as an overview of the metabolism of the worms and adequate food intake. While the administration of [Pd(glz)2] does not alter the body length of the worms, glz-treated worms were significantly larger than the untreated worms at all dosages (Fig. 2C). Glz is a well-known hypoglycaemic drug that favours insulin secretion, which has been described to have anabolic properties. Although there are no studies evaluating the role of glz in C. elegans, it has been reported that glibenclamide, another member of the sulfonylurea family, loaded into nanoparticles increases insulin like peptide secretion, such as DAF-28.15
DAF-28 acts through a conserved signalling pathway involving the insulin/IGF1 receptor homologue DAF-2, which promotes growth in C. elegans.16 Notwithstanding, worms cultured under high glucose conditions (20–100 μM) have shown a higher body length in comparison with unexposed worms.17 This effect could mask the potential anabolic effect of insulin in the above-mentioned research.
According to this, in the present study, glz treatment could act similarly as glibenclamide or glucose by increasing the body length of worms due to a possible enhancement in the insulin like peptide secretion which could increase the absorption of nutrients under basal conditions.
On the other hand, the pharyngeal pumping rate was slightly reduced when compared with untreated and glz treated worms (Fig. 2B). It is well known that worms with lower pumping rates are smaller than those with higher rates because feeding is one of the most important determinants of growth in C. elegans.18
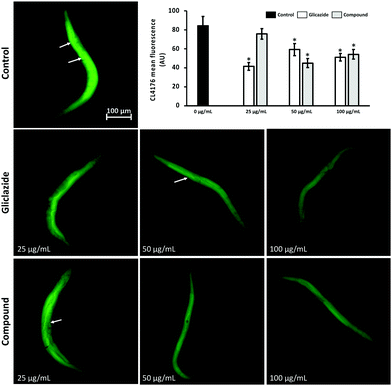
Once the absence of the lethal toxicity of [Pd(glz)2] was confirmed, its potential neuroprotective effect was assayed in CL4176 transgenic strains of C. elegans. Among the histopathological events in AD, Aβ peptide accumulation is one of the most studied features. In this context, the transgenic CL4176 C. elegans strain has proven to be an interesting model for the screening of drugs with neuroprotective activities. CL4176 expresses human 1–42 Aβ in muscle cells which causes a progressive paralysis of nematodes. As shown in Fig. 2D.1, the percentage of non-paralysed worms was significantly higher in the compound-treated group but only in the highest concentration at 30 h after the temperature increased.
Similarly, the protective effect of the compound against Aβ-induced paralysis was observed at 32 h (Fig. 2D.2) and 34 h (Fig. 2D.3) at all the assayed concentrations. In contrast, the unconjugated gliclazide only showed a protective effect in the highest dosage at 34 h after the increased temperature.
In accordance with the paralysis assay, the thioflavin T staining assay showed a lower accumulation of the Aβ oligomer in C. elegans muscle cells with both the studied compounds (glz and [Pd(glz)2]) (Fig. 3 and Fig. S5, ESI†).
Although there is no previous research evaluating the effect of glz in the C. elegans AD model, some authors employed other animal models to preliminarily determine the possible application of the sulfonylurea family in AD with promising results. The sulfonylurea family modulate ATP-sensitive potassium channels, which are involved in Aβ-induced pathology.19 In this context, glibenclamide treatment has been shown to reduce affective disorders,20 memory impairment and neuroinflammation21 as well as Aβ deposition22 in different rodent models of AD.
Similarly, our experiments have proven that glz is an effective treatment against Aβ toxicity and accumulation in C. elegans. Nonetheless, interesting differences were found between [Pd(glz)2] and glz. In this sense, the neuroprotective effect of the organometallic compound against Aβ-toxicity was remarkably higher than the free glz. The coordination of glz to Pd led to a flat-square structure, which might facilitate its interaction with Aβ peptides. In fact, the anti-Alzheimer effect of Pd as the facilitator has previously been reported using Au–Pd nanoparticles loaded with quercetin in a human neuroblastoma cell line.23 These results could explain, at least in part, the improved protective activity of gliclazide against Aβ-toxicity once coordinated to Pd, showing interesting prospects to continue studying the role of this compound in AD.
In summary, we have synthesized a new Pd coordination compound based on glz [Pd(glz)2], in which Pd ions exhibit a distorted plane-square coordination sphere. This novel compound behaves like a good and selective inhibitor of BuChE (IC50 = 27 μM), one of the most relevant therapeutic targets nowadays against AD. Analysis of the enzyme kinetics revealed a mixed mode of inhibition, indicating that the title compound is capable of interacting with both the free enzyme and the enzyme–substrate complex. According to in vivo tests, [Pd(glz)2] did not produce lethal toxicity. The treatment with glz showed for the first time a promising effect against Aβ-induced toxicity in C. elegans. Moreover, the newly synthesized coordination compound [Pd(glz)2] exceeded the protective activity of glz against Aβ-toxicity, opening the possibility for further studies to deepen the characterization and interaction of the compound with other in vivo AD models.
The authors gratefully acknowledge funding support from the Spanish Ministry of Science, Innovation and Universities (PGC2018-102052-B-C21 and PID2020-116460RB-I00) and the Junta de Andalucía (FQM-394 and FQM-134). The authors gratefully acknowledge the funding support of FEDER/Junta de Andalucía Consejería de Economía y Conocimiento, Grant B-AGR-193-UGR18. O. L. and J. G. F. B. also thank Grant PID2020-116460RB-I00 funded by MCIN/AEI/10.13039/501100011033. S. R. acknowledge the Juan de la Cierva Fellowship (IJC2019-038894-I). María D. Navarro-Hortal and Jose M. Romero-Márquez are FPU fellows from the Spanish Ministry of Educación y Formación Profesional.
Conflicts of interest
There are no conflicts to declare.Notes and references
- K. Mittal, R. J. Mani and D. P. Katare, Sci. Rep., 2016, 6, 25589 CrossRef CAS PubMed.
- S. Okabayashi, N. Shimozawa, Y. Yasutomi, K. Yanagisawa and N. Kimura, PLoS One, 2015, 10, e0117362 CrossRef PubMed.
- N. Kimura, Adv. Exp. Med. Biol., 2019, 1128, 133–145 CrossRef CAS PubMed.
- X. Lin, Y. Xu, X. Pan, J. Xu, Y. Ding, X. Sun, X. Song, Y. Ren and P.-F. Shan, Sci. Rep., 2020, 10, 14790 CrossRef CAS PubMed.
- A. R. Khan and F. R. Awan, J. Diabetes Metab. Disord., 2014, 13, 16 CrossRef PubMed.
- G. J. Cooper, A. C. Willis, A. Clark, R. C. Turner, R. B. Sim and K. B. Reid, Proc. Natl. Acad. Sci. U. S. A., 1987, 84, 8628–8632 CrossRef CAS PubMed.
- J. C. Bataglioli, L. M. F. Gomes, C. Maunoir, J. R. Smith, H. D. Cole, J. McCain, T. Sainuddin, C. G. Cameron, S. A. McFarland and T. Storr, Chem. Sci., 2021, 12, 7510–7520 RSC.
- M. Mital, K. Szutkowski, K. Bossak-Ahmad, P. Skrobecki, S. C. Drew, J. Poznański, I. Zhukov, T. Frączyk and W. Bal, Int. J. Mol. Sci., 2020, 21, 9200 CrossRef CAS PubMed.
- M. E. López-Viseras, B. Fernández, S. Hilfiker, C. Sánchez González, J. Llopis González, A. J. Calahorro, E. Colacio and A. Rodríguez-Diéguez, J. Inorg. Biochem., 2014, 131, 64–67 CrossRef PubMed.
- M. Singh, M. Kaur, H. Kukreja, R. Chugh, O. Silakari and D. Singh, Eur. J. Med. Chem., 2013, 70, 165–188 CrossRef CAS PubMed.
- J. Godyń, J. Jończyk, D. Panek and B. Malawska, Pharmacol. Rep., 2016, 68, 127–138 CrossRef PubMed.
- A. Contestabile, Behav. Brain Res., 2011, 221, 334–340 CrossRef CAS PubMed.
- N. H. Greig, D. K. Lahiri and K. Sambamurti, Int. Psychogeriatr., 2002, 14, 77–91 CrossRef PubMed.
- A. Cornish-Bowden, Biochem. J., 1974, 137, 143–144 CrossRef CAS PubMed.
- D. Lucio, M. C. Martínez-Ohárriz, Z. Gu, Y. He, P. Aranaz, J. L. Vizmanos and J. M. Irache, Int. J. Pharm., 2018, 547, 97–105 CrossRef CAS PubMed.
- G. Kao, C. Nordenson, M. Still, A. Rönnlund, S. Tuck and P. Naredi, Cell, 2007, 128, 577–587 CrossRef CAS PubMed.
- J. Alcántar-Fernández, R. E. Navarro, A. M. Salazar-Martínez, M. E. Pérez-Andrade and J. Miranda-Ríos, PLoS One, 2018, 13, e0199888 CrossRef PubMed.
- C. Mörck and M. C. Pilon, BMC Dev. Biol., 2006, 6, 39 CrossRef PubMed.
- F. Maqoud, R. Scala, M. Hoxha, B. Zappacosta and D. Tricarico, CNS Neurol. Disord.: Drug Targets, 2021 DOI:10.2174/1871527320666210119095626.
- M. H. Esmaeili, B. Bahari and A.-A. Salari, Brain Res. Bull., 2018, 137, 265–276 CrossRef CAS PubMed.
- M. H. Esmaeili, M. Enayati, F. Khabbaz Abkenar, F. Ebrahimian and A.-A. Salari, Behav. Brain Res., 2020, 379, 112359 CrossRef CAS PubMed.
- Y.-J. Ju, N. Kim, M. S. Gee, S. H. Jeon, D. Lee, J. Do, J.-S. Ryu and J. K. Lee, Eur. J. Pharmacol., 2020, 884, 173416 CrossRef CAS PubMed.
- Y. Liu, H. Zhou, T. Yin, Y. Gong, G. Yuan, L. Chen and J. J. Liu, Colloid Interface Sci., 2019, 552, 388–400 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2102397. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1cc04404d |
| This journal is © The Royal Society of Chemistry 2022 |