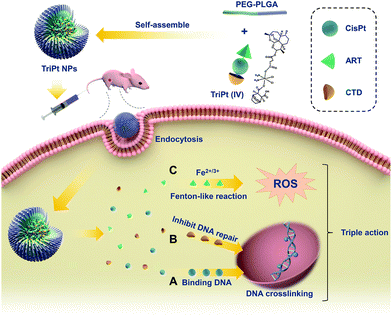
Nanoparticle delivery of a triple-action Pt(IV) prodrug to overcome cisplatin resistance via synergistic effect†
Peng
Xie‡
a,
Qiao
Jin‡
b,
Yifan
Li
c,
Jinbo
Zhang
c,
Xiang
Kang
d,
Jialin
Zhu
e,
Xinzhan
Mao
*a,
Peiguo
Cao
*b and
Chaoyong
Liu
 *c
*c
aDepartment of Orthopedics, The Second Xiangya Hospital, Central South University, Changsha, Hunan 410011, China. E-mail: xinzhan.mao@csu.edu.cn
bDepartment of Oncology, the Third Xiangya Hospital, Central South University, Changsha, 410013, Hunan, China. E-mail: xy3caopg@csu.edu.cn
cCollege of Life Science and Technology and Beijing Advanced Innovation Center for Soft Matter Science and Engineering, Beijing University of Chemical Technology, Beijing, 100029, P. R. China. E-mail: chaoyongliu@mail.buct.edu.cn
dDepartment of Obstetrics and Gynecology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China
eDepartment of Diagnostic and Therapeutic Ultrasonography, Tianjin Medical University Cancer Institute and Hospital, National Clinical Research Center of Cancer, Key Laboratory of Cancer Prevention and Therapy, Tianjin's Clinical Research Center for Cancer, Tianjin, 300060, China
First published on 23rd November 2021
Abstract
Cisplatin is the most widely used chemotherapeutic agent due to its efficacy in the treatment of a broad range of cancer types; while the side effects and drug resistance of cisplatin limit its clincial application. Combination therapy, which contains several types of free drugs, exhibits promising potential in clinical practice. Nevertheless, current combination chemotherapy cannot accurately deliver different drug components into a single tumor cell at the same time. Herein, we report a triple-action nanoplatinum drug based on artesunate and cantharidin to overcome the influence of pharmacokinetics and distribution variation in different drugs. The results show that the triple action nanoplatinum drug enhances ROS generation, leads to DNA damage, and inhibits DNA repair. Therefore, a high-efficiency killing effect is achieved with a triple-action platinum drug in a single tumor cell.
Introduction
Cisplatin, as a well-known chemotherapy drug, has been widely used in cancer treatment. However, the acquired drug resistance limits its clinical application.1,2 Since the occurrence of drug resistance is a complex process involving multiple factors and complex mechanisms,3–5 researchers have now concluded that the drug resistance cannot be overcome by relying solely on cisplatin.6–8 Platinum-based combination therapy, which combines cisplatin with one or more drug components to achieve synergistic effect via different mechanisms, holds great promise to overcome the drug resistance.9–11Combination therapy is mainly based on free drug combinations in clinical practice. Since the pharmacological and pharmacokinetic properties of drugs are different, current combination therapy cannot accurately deliver different drug components into a single tumor cell simultaneously, which compromises the synergistic effect of the combination therapy.12 Furthermore, precise control of the drug ratio in combination therapy remains challenging.13 Although researchers have developed various strategies relying on nanosystems to deliver multiple drugs with a single carrier, batch to batch variation during the fabrication process hinders their clinical translation.14–16 Therefore, a drug delivery system with precise control over the amount of each drug component is highly desired.17,18
Artesunate (ART) is an antimalarial compound isolated from Chinese herb Qinghao. Recently, its anti-tumor effect has already been demonstrated. The mechanism for the anti-tumor effect is based on chemical dynamic therapy (CDT) that reactive oxygen species (ROS) is generated through the Fenton-like reaction with intracellular iron.19,20 Another drug component that is suitable for combination with cisplatin is cantharidin (CTD), which is a toxic monoterpene produced by blister beetle that inhibits DNA repair.21,22 Therefore, it is of great significance to combine cisplatin with ART and CTD as a combination therapy for cancer treatment (Scheme 1).
The discovery of platinum(IV) (Pt(IV)) drugs provides a versatile platform to achieve synergistic effects with the abovementioned three drugs.23–25 Herein, by adding drugs with chemical dynamism or with inhibiting DNA repair ability in the axial direction,26 a three-drug compound containing Pt(IV), ART, and CTD with equal molar ratio was successfully synthesized (Fig. S1†).
Results and discussion
To further enhance the concentration of TriPt in tumor cells, methoxy poly(ethylene glycol)5000-b-poly(lactide-co-glycolide)7600 (mPEG5000-PLGA7600), an FDA approved polymer, was utilized to encapsulate TriPt prodrugs,27 and the obtained nanoparticle was denoted as TriPt NPs. Nanoparticles were prepared using various mass ratios of Pt to polymer from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 1
1 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5. Subsequently, the size and polydispersity index (PDI) of nanoparticles were characterized. As the ratio between Pt to polymer changes, the size and PDI of nanoparticles fluctuate between 37 to 45 nm, and 0.19 to 0.25, respectively (Fig. S2†). Therefore, an optimized Pt to polymer mass ratio of 1
5. Subsequently, the size and polydispersity index (PDI) of nanoparticles were characterized. As the ratio between Pt to polymer changes, the size and PDI of nanoparticles fluctuate between 37 to 45 nm, and 0.19 to 0.25, respectively (Fig. S2†). Therefore, an optimized Pt to polymer mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 was selected and used as the optimal formulation of nanoparticles for the following experiments.
4 was selected and used as the optimal formulation of nanoparticles for the following experiments.
Dynamic light scattering (DLS) and transmission electron microscopy (TEM) were employed to characterize TriPt NP. As shown in Fig. 1A, TriPt NPs have a diameter of 36.9 nm. Representative TEM image revealed that the TriPt NPs are spherically shaped with a diameter of ∼43 nm, which are consistent with the DLS result (Fig. 1B). Since the stability of nanoparticles is essential for their biomedical applications, we continue to evaluate their stability via DLS. TriPt NPs were then incubated with 10% FBS or H2O for 7 days. The size of TriPt NP was monitored by DLS throughout the incubation process (Fig. 1C). No significant change in the diameter of TriPt NPs was found during the whole incubation process, hence demonstrating the excellent stability of TriPt NPs.
Since TriPt only exhibits an antitumor effect after entering the tumor cells, the internalization process of nanoparticles is important. To visualize the cell uptake process, TriPt NPs were labelled with Cy5.5 to form NP@Cy5.5. The cell actin was stained with Alexa fluor 488 to better reveal the intracellular location of nanoparticles. 7404DDP (cisplatin resistance) cells were then incubated with NP@Cy5.5 for 1, 4, and 7 h. Confocal laser scanning microscopy (CLSM) was subsequently employed to study the distribution of nanoparticles. As shown in Fig. 2A, the red fluorescence increased with the treatment time, suggesting a time-dependent cellular uptake process for TriPt NPs.
Then, we used flow cytometry to quantitatively investigate the intracellular uptake of 7404DDP cells. Similarly, the red fluorescence was found to be increasing in a time-dependent manner (Fig. 2B). As shown in Fig. 2C, the red fluorescence signal at 7 h is 3.6 times more than that at 1 h, suggesting a rapid uptake of NPs.
Given that platinum is a metal element, we further employed inductively coupled plasma mass spectrometry (ICP-MS) to quantitatively analyse the cell uptake process. The 7404 and 7404DDP cells were treated with cisplatin, TriPt, and TriPt NPs for 1, 4, and 7 h, respectively (Fig. 2D and E). These results are in good agreement with the observation from flow cytometry, which proved TriPt NPs’ ability in enhancing drug accumulation in cells, as well as their potential in cell-killing effect.
Since the anticancer effect of ART comes from the reactive oxygen species (ROS) generated by chemical dynamic therapy,28 we continued to examine the ROS generation in 7404DDP cells using the 2′,7′-dichlorodihydrofluorescein diacetate (DCHF-DA) probe. CLSM was employed to visualize the generation and distribution of ROS. The green fluorescence indicates the ROS level. As shown in Fig. 3A, noticeable green fluorescence could be found on the TriPt NPs group, hence confirming the effect of Art. However, only negligible green fluorescence signals were observed for cisplatin and CTD treatments. To further confirm the role of iron in ROS generation, 7404DDP cells were pre-treated with 10 μM of iron sulfate, after which a significant increase of the ROS generation was found.
The ROS generation process was then quantitatively studied via flow cytometry (Fig. 3B). As shown in Fig. 3C, TriPt NPs produced a 2.4-times higher ROS level than cisplatin, thus proving the synergistic effect of the drug design. Furthermore, the Fe2+ pre-treatment significantly enhanced the ROS generation, further confirming the role of ART in TriPt prodrug.
Having confirmed the cell uptake and ROS generation, we continued to investigate the anti-cancer effect of TriPt NPs. Since platinum drugs, such as cisplatin, were often used in chemotherapy for clinical treatment of ovarian cancer,29 we first performed an MTT assay on A2780 (cisplatin-sensitive) and A2780DDP (cisplatin-resistant) cells. As shown in Fig. 4A, TriPt NPs showed the best cell-killing effect with the half inhibitory concentrations (IC50) value at 0.32 μM and 1.89 μM on A2780 and A2780DDP cells, respectively. Moreover, similar results were found on 7404 and 7404DDP cells. TriPt NPs exhibit the best cell-killing effect with IC50 value at 0.64 μM and 2.38 μM on 7404 and 7404DDP, respectively, which is 2.8- and 2.1-fold lower than that of their free combination treatment group. The IC50 value for each group was calculated, and the results are summarized in Table S1.† Compared with the free drug combination group, TriPt and TriPt NP exhibited better cell-killing effects, further confirming the synergistic effect for the drug design of TriPt.
To better mimic the in vivo cell microenvironment, 3D multicellular tumor spheroids (MCS) were subsequently utilized to evaluate the efficacy of TriPt NPs. Compared with the traditional 2D cell culture, 3D MCS is more reliable in mimicking the real in vivo environment in terms of cell morphology, proliferation, and signal transduction. Live/dead cell staining was employed to visualize the therapeutic effect. As depicted in Fig. 4B, almost all cells in PBS, CTD, CisPt showed green signals (live cells). Compared to the free drug combination group (CisPt + CTD + ART), TriPt NPs exhibit stronger red fluorescence signal (dead cells) at equal platinum concentration, hence demonstrating their synergistic effect.
The in vitro antiproliferative performance of TriPt NPs was further verified through a colony formation assay performed on 7404DDP cells. The cells were treated with various drugs at an equal drug concentration (0.1 μM). Significant reductions in colony formation were found after 7 days of TriPt and TriPt NPs treatment, further confirming the cell killing effect of TriPt NPs (Fig. S3†).
We continued to explore the mechanism of TriPt NPs, a cell apoptosis study was carried out by flow cytometry, and an Annexin-V staining assay was performed. As shown in Fig. 4C, including both early-stage and late-stage apoptosis, TriPt NPs showed an 80.01% apoptosis rate on 7404DDP cells after 24 h treatment. In contrast, the apoptosis rate of the CisPt + CTD + ART group was only at 36% (Fig. 4D). Hence, a 2.2-fold increase in apoptosis rate was observed in the cell with TriPt NPs treatment, compared with that of the free drug combination group.
To further investigate the anti-tumor effect of TriPt NPs, the 7404DDP tumor model was established. We first investigate the biodistribution of TriPt NPs, Cy7.5 labelled NPs were i.v. injected for in vivo Imaging30 (Fig. S4A†). The results showed that the accumulation of TriPt NPs at the tumor site increased with time. The maximum fluorescence intensity was observed at 24 h post-injection (Fig. S4B†). At the 48 h post-injection, the mouse was sacrificed, and the major organs and tumor were collected for fluorescence quantification (Fig. S4C†). The ex vivo results showed that strong fluorescence signal was observed at the tumor site of the mice treated with Cy7.5 labelled NPs (Fig. S4D†).
Next, the efficacy of TriPt NPs was evaluated. TriPt NPs were administered by i.v. injection for three times. The tumor volume was recorded every two days from the day of the first treatment (day 0) (Fig. 5A). The result showed that an 88% inhibition in TriPt NPs treated group was observed as compared to the PBS group (Fig. 5B). After 14 days, all mice were sacrificed and their tumors were collected and weighed. As shown in Fig. 5C, the tumors of TriPt NPs-treated mice is 0.23 g, which is significantly lower than that of the PBS-treated group (0.83 g) and CisPt-treated group (0.59 g) (Fig. 5D). Finally, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay of tumor was conducted to confirm the anti-tumor effect of TriPt NPs (Fig. 5E). The strongest green fluorescence was observed in TriPt NPs-treated group, thereby demonstrating better anti-tumor effect. In addition, biosafety is a prerequisite for the biomedical application. Comprehensive physiological and biochemical tests were carried out, and the results of UREA, CREA, ALT, AST, and ALP suggested no significant difference among all groups, indicating excellent biosecurity of TriPt NPs (Fig. S5†).
Conclusions
In conclusion, we designed and synthesized a TriPt prodrug for combination therapy with an equal molar ratio of three drugs, including cisplatin, ART, and CTD. To maximize the synergistic effect, TriPt NPs with an average diameter of 36.9 nm were prepared for further application, and the triple action has been well demonstrated in the TriPt NP group. Enhancement of ROS generation, as well as platinum uptake, was found with TriPt NPs. Compared with free drug combination therapy, TriPt NPs significantly improved the drug efficacy. On MTT study, the IC50 values of TriPt NPs on A2780 cells and A2780DDP cells were 0.32 and 1.89 μM, while on 7404 cells and 7404DDP cells were 0.64 and 2.38 μM, respectively. Moreover, the TriPt NPs exhibited a strong anti-tumor effect in the 7404DDP tumor bearing mice. Therefore, TriPt NPs exhibit promising synergistic effect, which holds great potential for clinical application of platinum-based combination therapy.Ethical statement
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Central South University and approved by the Ethics Committee of the Second Xiangya Hospital, Central South University.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Natural Science Foundation of China (81602629), National Natural Science Foundation of China (Grant No. 52073015), and Fundamental Research Funds for the Central Universities (ZY2006).Notes and references
- I. Dagogo-Jack and A. T. Shaw, Nat. Rev. Clin Oncol., 2018, 15, 81–94 CrossRef CAS.
- L. Wang, Y. Yu, D. Wei, L. Zhang, X. Zhang, G. Zhang, D. Ding, H. Xiao and D. Zhang, Adv. Mater., 2021, 33, e2100599 CrossRef PubMed.
- J. C. Marine, S. J. Dawson and M. A. Dawson, Nat. Rev. Cancer, 2020, 20, 743–756 CrossRef CAS.
- Y. G. Assaraf, A. Brozovic, A. C. Goncalves, D. Jurkovicova, A. Line, M. Machuqueiro, S. Saponara, A. B. Sarmento-Ribeiro, C. P. R. Xavier and M. H. Vasconcelos, Drug Resist. Updates, 2019, 46, 100645 CrossRef.
- R. Li, Z. Chen, Z. Dai and Y. Yu, Cancer Biol. Med., 2021, 18(2), 388–400 Search PubMed.
- N. Vasan, J. Baselga and D. M. Hyman, Nature, 2019, 575, 299–309 CrossRef CAS.
- X. Yang, Y. Yu, X. Huang, Q. Chen, H. Wu, R. Wang, R. Qi, Y. Miao and Y. Qiu, Mater. Sci. Eng., C, 2019, 96, 96–104 CrossRef CAS.
- P. Xie, Y. Wang, D. Wei, L. Zhang, B. Zhang, H. Xiao, H. Song and X. Mao, J. Mater. Chem. B, 2021, 9, 5173–5194 RSC.
- N. Chatterjee and T. G. Bivona, Trends Cancer, 2019, 5, 170–182 CrossRef CAS PubMed.
- D. Wei, Y. Yu, Y. Huang, Y. Jiang, Y. Zhao, Z. Nie, F. Wang, W. Ma, Z. Yu, Y. Huang, X. D. Zhang, Z. Q. Liu, X. Zhang and H. Xiao, ACS Nano, 2021, 15, 5428–5438 CrossRef CAS PubMed.
- H. Chen, Y. Wang, Y. Liu, L. Tang, Q. Mu, X. Yin, L. Zheng, Y. Chen and C. Liu, Small, 2021, 17, e2101804 CrossRef.
- L. Miao, S. Guo, C. M. Lin, Q. Liu and L. Huang, Adv. Drug Delivery Rev., 2017, 115, 3–22 CrossRef CAS PubMed.
- J. Pan, K. Rostamizadeh, N. Filipczak and V. P. Torchilin, Molecules, 2019, 24(6), 1035 CrossRef CAS.
- M. Zeinali, S. Abbaspour-Ravasjani, M. Ghorbani, A. Babazadeh, T. Soltanfam, A. C. Santos, H. Hamishehkar and M. R. Hamblin, Drug Discovery Today, 2020, 25, 1416–1430 CrossRef CAS PubMed.
- Y. Wang, Y. Jiang, D. Wei, P. Singh, Y. Yu, T. Lee, L. Zhang, H. K. Mandl, A. S. Piotrowski-Daspit, X. Chen, F. Li, X. Li, Y. Cheng, A. Josowitz, F. Yang, Y. Zhao, F. Wang, Z. Zhao, A. Huttner, R. S. Bindra, H. Xiao and W. M. Saltzman, Nat. Biomed. Eng., 2021, 5(9), 1048–1058 CrossRef CAS.
- J. Hu, X. W. Yuan, F. Wang, H. L. Gao, X. L. Liu and W. Zhang, Chin. Chem. Lett., 2021, 32, 1341–1347 CrossRef CAS.
- S. S. Qi, J. H. Sun, H. H. Yu and S. Q. Yu, Drug Delivery, 2017, 24, 1909–1926 CrossRef CAS PubMed.
- R. Chen, L. Huang and K. Hu, Acta Pharm. Sin. B, 2020, 10, 2140–2155 CrossRef CAS.
- B. Yang, Y. Chen and J. Shi, Chem. Rev., 2019, 119, 4881–4985 CrossRef CAS PubMed.
- Y. Augustin, H. M. Staines and S. Krishna, Pharmacol. Ther., 2020, 216, 107706 CrossRef CAS.
- P. Ma, H. Xiao, C. Yu, J. Liu, Z. Cheng, H. Song, X. Zhang, C. Li, J. Wang, Z. Gu and J. Lin, Nano Lett., 2017, 17, 928–937 CrossRef CAS.
- D. Wei, Y. Yu, X. Zhang, Y. Wang, H. Chen, Y. Zhao, F. Wang, G. Rong, W. Wang, X. Kang, J. Cai, Z. Wang, J. Y. Yin, M. Hanif, Y. Sun, G. Zha, L. Li, G. Nie and H. Xiao, ACS Nano, 2020, 14(12), 16984–16996 CrossRef CAS.
- X. Kang, Y. Wang, Z. Chen, Y. Wu, H. Chen, X. Yang and C. Yu, Chem. Commun., 2020, 56, 11271–11274 RSC.
- Z. Ren, S. Sun, R. Sun, G. Cui, L. Hong, B. Rao, A. Li, Z. Yu, Q. Kan and Z. Mao, Adv. Mater., 2020, 32, e1906024 CrossRef.
- L. Yang, D. Han, Q. Zhan, X. Li, P. Shan, Y. Hu, H. Ding, Y. Wang, L. Zhang, Y. Zhang, S. Xue, J. Zhao, X. Hou, Y. Wang, P. Li, X. Yuan and H. Qi, Theranostics, 2019, 9, 7680–7696 CrossRef CAS PubMed.
- P. G. Pilie, C. Tang, G. B. Mills and T. A. Yap, Nat. Rev. Clin Oncol., 2019, 16, 81–104 CrossRef CAS.
- H. He, L. Liu, E. E. Morin, M. Liu and A. Schwendeman, Acc. Chem. Res., 2019, 52, 2445–2461 CrossRef CAS PubMed.
- Z. Chen, X. Kang, Y. Wu, H. Xiao, X. Cai, S. Sheng, X. Wang and S. Chen, Chem. Commun., 2019, 55, 4781–4784 RSC.
- M. Markman, Drugs, 2019, 79, 1231–1239 CrossRef PubMed.
- Z. Tao, M. D. Muzumdar, A. Detappe, X. Huang, E. S. Xu, Y. Yu, T. H. Mouhieddine, H. Song, T. Jacks and P. P. Ghoroghchian, Nano Lett., 2018, 18, 2195–2208 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1bm01556g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |