IR-808 loaded nanoethosomes for aggregation-enhanced synergistic transdermal photodynamic/photothermal treatment of hypertrophic scars†
Zhixi
Yu‡
a,
Xinxian
Meng‡
a,
Shunuo
Zhang
a,
Xiaodian
Wang
a,
Yunsheng
Chen
*a,
Peiru
Min
*a,
Zheng
Zhang
 *a and
Yixin
Zhang
*ab
*a and
Yixin
Zhang
*ab
aDepartment of Plastic and Reconstructive Surgery, Shanghai Ninth People's Hospital, School of Medicine, Shanghai Jiao Tong University, 639 Zhizaoju Rd, Shanghai 200011, P.R. China. E-mail: yunshengchen@126.com; aru_ren@msn.com; 72300119003@shsmu.edu.cn; Zhangyixin6688@163.com
bShanghai National Engineering Research Center for Nanotechnology, 245 Jiachuan Road, Shanghai 200237, PR China
First published on 12th November 2021
Abstract
Synergistic transdermal photodynamic therapy (PDT)/photothermal therapy (PTT) has emerged as a novel strategy for improving hypertrophic scar (HS) therapeutic outcomes. Herein, a near-infrared heptamethine cyanine dye, named IR-808, has been selected as the desirable photosensitizer owing to its PDT and PTT properties. Benefitting from the transdermal delivery ability of ethosomes (ESs), IR-808 loaded nanoethosomes (IR-808-ES) have been prepared as a novel nanophotosensitizer for the transdermal PDT/PTT of HSs. The special structure of IR-808 aggregate distribution in the ES lipid membrane enhances ROS generation and hyperthermia. The in vitro experiments indicate that the IR-808-ES enhances the PDT/PTT efficacy for inducing the HS fibroblast (HSF) apoptosis via the intrinsic mitochondrial pathway. Furthermore, the in vivo transdermal delivery studies reveal that the IR-808-ES efficiently delivers IR-808 into HSFs in the HS tissue. Systematic assessments in the rabbit ear HS models demonstrate that the enhanced PDT/PTT performance of the IR-808-ES has remarkable therapeutic effects on improving the HS appearance, promoting HSF apoptosis and remodeling collagen fibers. Therefore, the IR-808-ES integrates both the transdermal delivery ability and the aggregation-enhanced PDT/PTT effect, and these features endow the IR-808-ES with significant potential as a novel nanophotosensitizer for the transdermal phototherapy of HSs in the clinical field.
Introduction
Hypertrophic scar (HS) is a serious skin condition caused by excessive proliferation of fibroblasts and over-deposition of the extracellular matrix, which results in persistent dermal fibrosis.1 The conventional clinical therapies include surgical resection, laser treatment, corticosteroid injection and other non-invasive methods.2,3 However, low therapeutic efficacy, high recurrence and painful processes remain the major challenges and hinder their clinical applications. Recently, photodynamic therapy (PDT) has become an attractive non-invasive approach for the HS, owing to its promising therapeutic efficacy and reduced side effects.4,5PDT is a non-invasive therapy involving a photosensitizer and an appropriate exciting light, where the excited photosensitizer generates the reactive oxygen species (ROS) to induce apoptosis of the over-proliferating HS fibroblasts (HSFs) and remodeling of the collagen fibers.6 The most commonly used topical photosensitizer in dermatology is 5-aminolevulinic acid (ALA), approved by the United States Food and Drug Administration (FDA) in 2000.7 However, because of its poor skin penetration ability, 5-ALA for the treatment of HSs is still controversial in its clinical application.8 Although the nano-formulations can help the transdermal delivery process, the limited tissue penetration depth of the laser (wavelength = 632.8 nm) and insufficient therapeutic efficacy in the HS are still great challenges.9
Visible light (from 380 nm to 700 nm) has a shallow penetration depth (<1 mm) owing to its strong absorption by most tissues. In comparison with visible light, the near-infrared light (NIR, 700–1700 nm) exhibits an excellent penetration depth of more than 1 cm, as the longer wavelength undergoes a lower extent of light scattering and tissue absorption.10,11 Therefore, the NIR window, known as the biological transparency window, demonstrates an exceptional advantage in HS phototherapy. Meanwhile, a novel synergistic treatment strategy, the combination of PDT with photothermal therapy (PTT), has attracted extensive interest in recent years.12,13 PTT generally requires a photosensitizer for the conversion of the absorbed light into localized heat energy, thus, leading to the ablation of target cells. In addition, hyperthermia can increase the cellular uptake efficiency of the photosensitizers and enhance ROS generation, resulting in improved PDT.14,15 Therefore, screening the NIR light activated photosensitizer with both PDT and PTT is critical for improving the treatment of HSs.
Recently, a heptamethine cyanine dye, IR-808, has been reported to exhibit a significant photothermal effect and photodynamic properties.16 The near-infrared excitation wavelength (≈800 nm) exhibits a penetration depth of 7.0–11.0 mm, which can reach the deep dermis. Previous studies have reported its preferential tumour accumulation without chemical conjugation of the target ligands along with the mitochondrial targeting ability.17 The synchronous PDT/PTT effects and mitochondrial targeting behaviour provide high PDT/PTT efficacy and exhibit a great potential for HS treatment.14 However, the hydrophobicity of the dye leads to a marked accumulation in the stratum corneum (SC), and thus how to overcome the compact dermal barrier of HS and realize the transdermal delivery to deep dermis present the current obstacles for the topical application of the dye.
Our previous studies have successfully established a nanoethosomal system with a high transdermal delivery efficiency in HS treatment.18,19 The ethosomal (ES) vesicles can disrupt the barrier function of the SC and squeeze through narrow spaces to reach the dermis. Meanwhile, the small fluorescent organic molecules in their solution state generally suffer from a nonradiative decay due to flexible molecular motions resulting in fluorescence quenching.20 The lipid bilayer can provide an ideal environment to form aggregates and thus achieve an aggregation-induced emission enhancement (AIEE).21 Moreover, a few AIE luminogens have been reported to show an enhanced PDT effect in the aggregated state.22 As IR-808 is a liposoluble molecule, the lipid bilayer of the ES increases its solubility, contributes to forming the aggregate state and thus serves as an effective drug carrier theoretically. However, the transdermal delivery efficiency and the membrane stability of ES loaded liposoluble photosensitizers are still unknown.
In this study, the near-infrared photosensitizer, IR-808, was introduced for the synergistic transdermal PDT/PTT of HSs by preparing IR-808 loaded ESs (IR-808-ES). IR-808-ES exhibited the advantages of transdermal delivery of IR-808 and enhanced PDT/PTT with its special structure of IR-808 aggregating distribution in the ES lipid membrane (Scheme 1). The in vitro studies showed that the ES structure could help the accumulation of IR-808 in HSFs. Meanwhile, the intact ES structure played a critical role in improving the PDT/PTT effect and inducing the HSF apoptosis. The in vivo transdermal penetration studies suggest that the ES could enhance IR-808 penetration into the rabbit HS tissue and delivery into the HSFs. Furthermore, the in vivo PDT/PTT evaluation reveal that IR-808-ES showed high PDT/PTT efficacy with decreased scar thickness and collagen remodeling. In conclusion, the results suggest that the ES could facilitate the tissue penetration of IR-808 and enhance the synergistic PDT/PTT effects for HSs. This study selected an ideal photosensitizer and developed a suitable transdermal nanocarrier for further clinical treatment of HSs.
 | ||
| Scheme 1 Schematic illustration of IR-808-ES preparation and its application in the synergistic transdermal PDT/PTT of HSs via aggregation-induced enhancement. | ||
Experimental section
IR-808-ES solution and gel preparation
IR-808 and the carbopol gel matrix were synthesized according to previously reported methods (S1, ESI†).17 The preparation of IR-808-ES is as follows. Phosphatidylcholine (PC, 6.7% w/v, soybean origin) and IR-808 were dissolved in chloroform in a round-bottom flask. After the solvent was removed using a rotary evaporator, PC with IR-808 was hydrated in a hydroalcoholic solution (ethanol/water, 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, v/v) overnight. Finally, the mixture was dispersed using an ultrasonic bath for 20 min (300 W), and IR-808-ES was obtained with the final content of IR-808 at 0.33 mM. The fluorescent ES was labeled using 1-palmitoyl-2-{6-[(7-nitro-2-1,3-benzoxadi-azol-4-yl)amino]hexanoyl}-sn-glycero-3-phosphocholine (NBD-PC, 4 mol%). IR-808-ES gels were prepared by mixing the IR-808-ES solution with the carbopol gel matrix (1
70, v/v) overnight. Finally, the mixture was dispersed using an ultrasonic bath for 20 min (300 W), and IR-808-ES was obtained with the final content of IR-808 at 0.33 mM. The fluorescent ES was labeled using 1-palmitoyl-2-{6-[(7-nitro-2-1,3-benzoxadi-azol-4-yl)amino]hexanoyl}-sn-glycero-3-phosphocholine (NBD-PC, 4 mol%). IR-808-ES gels were prepared by mixing the IR-808-ES solution with the carbopol gel matrix (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) in a sealed container at 500 rpm, where the concentration of IR-808 in the IR-808-ES gels was 0.33 mg mL−1. An IR-808 hydroalcoholic solution (IR-808-HA, 30% ethanol, v/v, 0.67 mg mL−1 IR-808) was used to prepare IR-808-HA gels using the same method as with the IR-808-ES gels.
1, v/v) in a sealed container at 500 rpm, where the concentration of IR-808 in the IR-808-ES gels was 0.33 mg mL−1. An IR-808 hydroalcoholic solution (IR-808-HA, 30% ethanol, v/v, 0.67 mg mL−1 IR-808) was used to prepare IR-808-HA gels using the same method as with the IR-808-ES gels.
Characterization of IR-808-ES
The morphological features of IR-808-ES were observed using a transmission electron microscope (TEM, JEM-2010 JEOL, Japan). The size distribution was assessed using Nanosight 300 systems. The encapsulation efficiency (EE) of IR-808 was determined by ultracentrifugation and demulsification. IR-808-ES (100 μL) was placed in an ultrafiltration tube and centrifuged (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 20 min). The free IR-808 (Qf) was passed through an ultrafiltration tube into a centrifuge tube. Then the total IR-808 (Qt) was obtained through demulsification by adding ethanol (100 μL) to IR-808-ES (100 μL). Then, the EE was calculated as follows:
000 rpm, 20 min). The free IR-808 (Qf) was passed through an ultrafiltration tube into a centrifuge tube. Then the total IR-808 (Qt) was obtained through demulsification by adding ethanol (100 μL) to IR-808-ES (100 μL). Then, the EE was calculated as follows:| EE = (1 − Qf/Qt) × 100%. |
The in vitro release studies were evaluated using the dialysis method as reported in our previous studies.23 The fluorescence intensities were obtained using a Synergy H4 hybrid reader (Bio-Tek, USA, λex = 635 nm/λem = 730 nm). UV-Vis spectra were obtained using a Varian Cary 50 UV-Vis spectrophotometer (PerkinElmer, USA). The fluorescence spectra were recorded using a Hitachi FL-4100 spectrofluorometer.
Singlet oxygen sensor green (SOSG, ThermoFisher, USA) was employed to evaluate the generation of singlet oxygen (1O2). Typically, IR-808-ES or IR-808-HA (1 mL, 10 μM of IR-808 equivalent) and SOSG (final concentration of 2.5 μM) were mixed and irradiated for 120 s (808 nm, 2 W cm−2). Then, the 1O2 was quantified at 525 nm (λex = 504 nm). Analysis of the heating profiles of IR-808-ES or IR-808-HA (10 μM of IR-808 equivalent) was done immediately after laser irradiation (808 nm, 2 W cm−2) using an infrared thermal camera (Thermal Intelligence, Shanghai, China).
In vitro PDT/PTT of HSFs
In vivo PDT/PTT studies
Statistical analysis
All of the data were shown as mean ± standard deviations. GraphPad Prism 9.0 (GraphPad Software, Inc.) was used for the statistical analysis. One-way ANOVA followed by Dunnett's test was performed for multiple comparisons with P < 0.05 as the minimum level of significance (*p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001).Results and discussion
Characterization of IR-808-ES
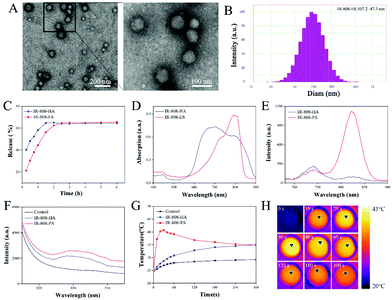
The morphology of the prepared IR-808-ES was first characterized using TEM (Fig. 1A). As observed in the TEM images, IR-808-ES established intact spherical vesicles with a homogeneous size. A high-magnification image with more details exhibited multiple lamellas in even concentric patterns, indicating that IR-808-ES formed the classic multilamellar nanovesicle structure.24 The size distribution analysis provided the precise particle diameter as 107.2 ± 47.3 nm (Fig. 1B). The encapsulation efficiency (EE) of IR-808-ES was measured during preparation and the results show an efficient loading with an EE value of 68.74%.In IR-808-ES, IR-808 is a hydrophobic molecule loaded between the bilayer membrane. Therefore, the stability of IR-808-ES is an important factor and herein it was evaluated by the extent of the release of IR-808 from IR-808-ES (Fig. 1C). In the release profile of IR-808-HA, over 60% of free IR-808 was observed to be rapidly released to the outside medium within 1 h, while IR-808-ES showed a relatively slow drug release pattern with 45% and 60% release of free IR-808 within 1 h and 2 h, respectively. An equilibrium release was achieved after 2.5 h. The observed results indicate that IR-808 was effectively encapsulated in the IR-808-ES.
The influence of the ES on the physico-chemical properties was further studied. Compared with IR-808-HA, IR-808-ES showed a red-shift of the absorption peak (from 705 to 800 nm) with a slight increase in the absorption strength and a significantly increased fluorescence emission intensity at a wavelength of 820 nm (Fig. 1D and E). Being a small fluorescent organic molecule, IR-808 in an aqueous solution suffers from fluorescence quenching. These observed optical enhancements show that the ES structure helps IR-808 to form the aggregate state and to exhibit the aggregation-induced emission enhancement (AIEE) phenomenon.21,22 The assembly of IR-808 in the ES prevented intramolecular rotation or vibration, and thus realizes AIEE through the restriction of intramolecular motions (RIMs).25
As a photosensitizer, the photodynamic and photothermal characteristics of IR-808 are critical for therapeutic effects. Herein, SOSG was used to selectively detect the 1O2 generation under laser irradiation, which is the main therapeutic agent involved in the PDT process (Fig. 1F). Compared with IR-808-HA, the singlet oxygen generation was observed to increase by 1.4-fold. Meanwhile, the photothermal effect was evaluated by detecting the temperature increase of the solution under rated laser intensity and at different times (Fig. 1G and H). The solution temperature of IR-808-ES raised rapidly to over 40 °C within 30 s followed by a decrease to 35 °C in 2 min, while the temperature of the IR-808-HA group slightly increased to 35 °C in 2 min. The fall of the temperature curve was probably attributed to the rupture of the ethosomal structure caused by the rising temperature and molecular motion which further attenuated the hyperthermal effect. These results suggest that the AIEE of the ES could not only decrease the luminescence quenching but also enhance the PDT/PTT effect.
In summary, the ES was confirmed to successfully encapsulate the hydrophobic IR-808 and increase the absorption and emission in the NIR region via the formation of the aggregate state. Moreover, the AIEE enhanced ROS generation and increased the photothermal effect, which was desirable for realizing a satisfactory PDT/PTT effect.
In vitro PDT/PTT of HSFs
The in vitro photocytotoxicity of IR-808-HA and IR-808-ES (0–10 μM) was investigated after incubation for 2 h (Fig. 2B). The cell viability of the IR-808-ES group significantly decreased after laser irradiation from 6 μM, with over 80% of the decrease detected at 10 μM. While the IR-808-HA group did not exhibit any significant effect on the cell viability. Thanks to the aggregate state of IR-808 in ES, the enhanced PDT/PTT effect induced significant phototoxicity. The effect of single PDT or PTT is investigated in the following section.
The IR-808-ES accumulation was further investigated using NBD-labelled ES with incubation periods of 2 and 6 h (Fig. 2C). The intense red (IR-808) and green (ES) fluorescence signals at 2 h indicate that IR-808 was delivered in HSFs by IR-808-ES, while the decayed green fluorescence signal at 6 h suggests the degradation of the ES structure within 6 h. Therefore, based on the results of the cellular uptake, the following experiments were conducted with an incubation period of 2 h. As IR-808 is a mitochondria-targeted photosensitizer, the mitochondrial targeting property of IR-808-ES was investigated using a mitochondrial tracker (Mito-tracker Green) (Fig. 2D).17 The high overlap of the red fluorescence of IR-808 with the green fluorescence of Mito-tracker, indicates that the mitochondrial targeting property was not affected.
To further study the single and synergistic PDT/PTT therapeutic effects, laser irradiation was conducted under ice incubation for single PDT and HSFs were pretreated with N-acetylcysteine (NAC, a ROS inhibitor) for single PTT. The phototoxicity was investigated by calcein AM and propidium iodide (PI) co-staining (Fig. S2† and Fig. 2F). The red fluorescence from PI revealed the dead cells, and the higher mortality of the PDT/PTT group than the single PDT or PTT groups suggested that the synergistic PDT/PTT effect contributed to the enhanced phototoxicity of IR-808-ES. In addition, compared with the result obtained after 2 h of incubation, the cell mortality of the 6 h group obviously decreased with the degradation of the ES. These described results show that the accumulated IR-808-ES in HSFs still retained the aggregate form and showed an enhanced synergistic PDT/PTT effect. Moreover, the degradation of the ES along with the attenuated therapeutic effect confirmed its critical role.
In vivo transdermal delivery studies
Transdermal drug delivery is a critical process in PDT/PTT for HSs. Herein, the transdermal penetration and distribution of IR-808 were evaluated by CLSM using a rabbit HS model. In the previous studies, the carbopol gel had been introduced for continuous topical administration without having any influence on drug delivery and release.19 Herein, the IR-808-HA gel and IR-808-ES gel have been used for in vivo studies. After topical administration for 3 h, in the IR-808-ES group, the red fluorescence of IR-808 was observed to be evenly distributed in the entire dermal layer (Fig. 3A and B). In addition, co-staining with DAPI confirmed the accumulation in the HS tissue. In the IR-808-HA group, although ethanol could disrupt the lipid organization in the SC, IR-808 mainly accumulated in the epidermis due to its liposolubility. The semi-quantitative analysis of the red fluorescence showed that IR-808 accumulation in the dermis of the IR-808-ES group was 12-fold higher than that in the IR-808-HA group (Fig. 3C), which indicates that IR-808-ES possesses a remarkable transdermal delivery ability. Furthermore, the histological characterization of in vivo delivery were explored in detail using TEM (Fig. 3D). IR-808-ES was observed to penetrate through the dense collagen fibers into the deep dermis. It was suggested that the transdermal ability could be attributed to the remarkable deformability and fluidity of the vesicle membrane and confirmed the transdermal penetration via an intercellular pathway.28 The framed and enlarged details of IR-808-ES illustrated the deformed but intact vesicles in both extracellular and intracellular matrices, showing that IR-808-ES has fully entered HSFs, which contributed to reserve the AIEE effect and enhance the following PDT/PTT effect. To sum up, compared to IR-808-HA, IR-808-ES shows the desired transdermal penetration ability and retains the structural integrity during delivery into HSFs.In vivo PDT/PTT efficacy
In vivo PDT/PTT efficacy was assessed using the rabbit HS models (Fig. 4A). After re-epithelialization of the wounds and formation of the HS, the PDT/PTT was conducted once a week. Before treatment, the HS of all of the groups showed a dark-red color and thick texture. After 4 therapeutic sessions, compared to the control group, the HS tissues of IR-808-ES exhibited a faded color and flattened appearance, while the IR-808-HA group revealed a lesser degree of improvement (Fig. 4B). The scar elevation index (SEI), the ratio of the total HS tissue thickness to the normal skin thickness, was calculated to quantify the improvement (Fig. 4C). The SEI value of the IR-808-ES group was observed to significantly decrease from 1.7 to 1.1, indicating a more efficient therapeutic effect.The histological analysis based on Masson's trichrome staining as well as Sirius red staining was chosen to observe the collagen deposition in the HS tissue (Fig. 4D and E). The control group showed abundant collagen deposition with an uneven arrangement of the collagen fibers. After treatment, the collagen deposition remarkably decreased in the IR-808-ES group and was remodeled parallel. Although the IR-808-HA group also showed a small extent of the therapeutic effect, the collagen fibers still showed a bulky appearance. The Sirius red staining further showed the distribution of collagen I (bright yellow) and collagen III (green) through polarized light observation. During the process of wound healing, the ratio of type III to type I collagen plays a crucial role, in which the higher ratio results in reduced scar formation.29 In the control group, the major collagen fibers appeared yellow in color, indicating the major deposition of collagen I. The ratio of collagen I to III significantly decreased in the IR-808-ES group, suggesting the therapeutic efficacy of IR-808-ES leading to improve collagen deposition (Fig. 4F).
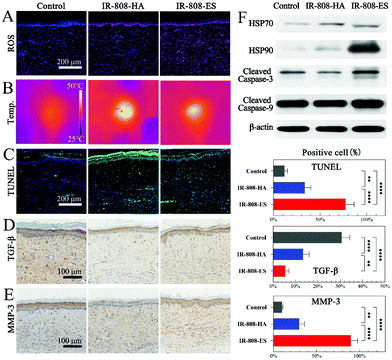
Although IR-808-ES showed a significant enhancement of PDT and PTT in the in vitro studies, the in vivo ROS generation and photothermal effects were different because of the different transdermal delivery ability and drug accumulation in the HS tissue. The in vivo ROS generation and distribution corresponded to the distribution of IR-808, confirming the ROS generation from IR-808 and the retention of the PDT enhancement after penetration in the HS tissue (Fig. 5A and S3†). A superficial temperature elevation was observed immediately after laser irradiation using an infrared thermal camera (Fig. 5B). The control group showed a minor increase from 29.7 °C to 38.7 °C, while IR-808-HA and IR-808-ES groups were elevated to 49.3 °C and 46.1 °C, respectively. The contrasting results from the in vitro studies were due to the extreme accumulation of IR-808 in the epidermis, as revealed by the in vivo transdermal delivery studies. In summary, IR-808-ES retained both enhanced PDT and PTT effects after the transdermal penetration and distribution, while IR-808-HA showed extremely high temperatures because of the excessive accumulation in the epidermis, which would lead to the risk of epidermal damage.
The TUNEL analysis was conducted to further investigate the in vivo apoptosis of HSFs (Fig. 5C). Few TUNEL-positive apoptotic HSFs were observed in the control group. In the IR-808-HA group the apoptotic activities were limited mainly in the epidermis and superficial dermis, while the apoptotic HSFs in the IR-808-ES group were distributed throughout the entire dermis. The described results corresponding to ROS generation and temperature elevation confirmed their therapeutic effects. Meanwhile, the expression of TGF-β and MMP-3 in the HS tissue, the indicators of HSF proliferation and collagenolytic activity, was also evaluated (Fig. 5D and E).30 The lowest TGF-β level in the IR-808-ES group indicated the remarkable inhibition of HSF proliferation via generated ROS and hyperthermia. The significantly increased MMP-3 expression in the IR-808-ES group confirmed the influence of the enhanced PDT/PTT effects on collagen metabolism, which was consistent with the finding from Masson's staining. The western blot analysis showed that cleaved caspase-3 and cleaved caspase-9 were increased after PDT/PTT of IR-808-ES, confirming that the HSF apoptosis in vivo was still mediated by the intrinsic mitochondrial pathway (Fig. 5F). Heat shock protein 90 (HSP90) played an important role in the cell response to hyperthermia, hence the increased expression in the IR-808-ES group and no modification in the IR-808-HA group indicating that the limited hyperthermia in the epidermis provided no therapeutic effects. Meanwhile, some studies reported that HSP90 reduced hypothermic efficacy, suggesting that HSP90 could be a potential therapeutic target to overcome the phototherapy resistance.31
Overall, IR-808-ES was confirmed to exhibit an enhanced PDT/PTT therapeutic effect for the HS in in vivo studies by promoting the HSF apoptosis and remodeling the collagen fibers.
Conclusions
In this study, IR-808-ES was prepared as a novel nanophotosensitizer for an enhanced synergistic transdermal PDT/PTT for HSs. The morphological analysis and release profile confirmed the successful encapsulation of IR-808 in the ES. The physicochemical features indicated that the ES induced the AIEE effect of IR-808 and enhanced the PDT/PTT effect. The in vitro studies revealed the synergistic PDT/PTT effect via inducing the apoptosis of HSFs and confirmed the critical role of the ES structure in the PDT/PTT. As revealed by the in vivo transdermal penetration studies, IR-808-ES could penetrate evenly to the deep dermis via the deformable membrane and enter HSFs with an intact vesical structure. The in vivo PDT/PTT assessment in the rabbit ear HS model showed the enhanced therapeutic effects in improving the HS appearance, promoting HSF apoptosis and remodeling collagen fibers. Therefore, this work suggests IR-808-ES as an efficient photosensitizer for the transdermal PDT/PTT of HSs. Next, IR-808-ES will be further investigated for its potential in clinical applications.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This study was supported by the National Natural Science Foundation of China (81772098, 81801917 and 81801918), the Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (20152227), the Clinical Multi-Disciplinary Team Research Program of ninth People's Hospital, the Shanghai Jiao Tong University School of Medicine (20171007), the Cross Research Project of Ninth People's Hospital, the Shanghai Jiao Tong University School of Medicine (JYJC202009), the Shanghai Health Industry Clinical Research Special Project (20204Y0443) and the Shanghai Municipal Key Clinical Specialty (shslczdzk00901). The authors would like to express their gratitude to EditSprings for the expert linguistic services provided.References
- D. Wolfram, A. Tzankov, P. Pulzl and H. Piza-Katzer, Dermatol. Surg., 2009, 35, 171–181 CrossRef CAS.
- Z. Zhang, J. Chen, J. Huang, Y. Wo, Y. Zhang and X. Chen, Nanoscale Res. Lett., 2018, 13, 26 CrossRef PubMed.
- S. Lin, G. Quan, A. Hou, P. Yang, T. Peng, Y. Gu, W. Qin, R. Liu, X. Ma, X. Pan, H. Liu, L. Wang and C. Wu, J. Controlled Release, 2019, 306, 69–82 CrossRef CAS.
- M. Tosa and R. Ogawa, Scars Burn. Heal., 2020, 6, 2059513120932059 Search PubMed.
- P. Agostinis, K. Berg, K. A. Cengel, T. H. Foster, A. W. Girotti, S. O. Gollnick, S. M. Hahn, M. R. Hamblin, A. Juzeniene, D. Kessel, M. Korbelik, J. Moan, P. Mroz, D. Nowis, J. Piette, B. C. Wilson and J. Golab, Ca-Cancer J. Clin., 2011, 61, 250–281 CrossRef.
- S. Kwiatkowski, B. Knap, D. Przystupski, J. Saczko, E. Kedzierska, K. Knap-Czop, J. Kotlinska, O. Michel, K. Kotowski and J. Kulbacka, Biomed. Pharmacother., 2018, 106, 1098–1107 CrossRef CAS.
- X. Wang, P. Cao, J. Liu, P. Du, Z. Wang, W. Chen, C. Liu and Y. Wu, Med. Sci. Monit., 2017, 23, 46–56 CrossRef CAS.
- R. F. Lopez, N. Lange, R. Guy and M. V. Bentley, Adv. Drug Delivery Rev., 2004, 56, 77–94 CrossRef CAS PubMed.
- Z. Zhang, Y. Chen, H. Xu, Y. Wo, Z. Zhang, Y. Liu, W. Su, D. Cui and Y. Zhang, Nanoscale, 2016, 8, 19270–19279 RSC.
- G. Chen, Y. Cao, Y. Tang, X. Yang, Y. Liu, D. Huang, Y. Zhang, C. Li and Q. Wang, Adv. Sci., 2020, 7, 1903783 CrossRef CAS PubMed.
- G. Hong, A. L. Antaris and H. Dai, Nat. Biomed. Eng., 2017, 1, 1–22 CrossRef.
- Z. Zhang, Y. Chen, J. Ding, C. Zhang, A. Zhang, D. He and Y. Zhang, Nanoscale Res. Lett., 2017, 12, 622 CrossRef PubMed.
- W. Yin, T. Bao, X. Zhang, Q. Gao, J. Yu, X. Dong, L. Yan, Z. Gu and Y. Zhao, Nanoscale, 2018, 10, 1517–1531 RSC.
- S. Liu, X. Pan and H. Liu, Angew. Chem., Int. Ed., 2020, 59, 5890–5900 CrossRef CAS PubMed.
- H. Wu, C. Wang, J. Sun, L. Sun, J. Wan, S. Wang, D. Gu, C. Yu, C. Yang, J. He, Z. Zhang, Y. Lv, H. Wang, M. Yao, W. Qin, C. Wang and H. Jin, ACS Appl. Mater. Interfaces, 2019, 11, 43996–44006 CrossRef CAS.
- S. Luo, Z. Yang, X. Tan, Y. Wang, Y. Zeng, Y. Wang, C. Li, R. Li and C. Shi, ACS Appl. Mater. Interfaces, 2016, 8, 17176–17186 CrossRef CAS.
- S. Luo, X. Tan, S. Fang, Y. Wang, T. Liu, X. Wang, Y. Yuan, H. Sun, Q. Qi and C. Shi, Adv. Funct. Mater., 2016, 26, 2826–2835 CrossRef CAS.
- Y. Chen, Z. Zhang, Y. Xin, Z. Yu, X. Meng, Y. Zhang, D. He and Y. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 7955–7965 CrossRef CAS.
- Z. Zhang, Y. Liu, Y. Chen, L. Li, P. Lan, D. He, J. Song and Y. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 3704–3714 CrossRef CAS.
- S. M. Hart, J. L. Banal, M. Bathe and G. S. Schlau-Cohen, J. Phys. Chem. Lett., 2020, 11, 5000–5007 CrossRef CAS.
- E. R. Sodre, B. C. Guido, P. E. N. de Souza, D. F. S. Machado, V. H. Carvalho-Silva, J. A. Chaker, C. C. Gatto, J. R. Correa, T. A. Fernandes and B. A. D. Neto, J. Org. Chem., 2020, 85, 12614–12634 CrossRef CAS.
- Z. Zheng, T. Zhang, H. Liu, Y. Chen, R. T. K. Kwok, C. Ma, P. Zhang, H. H. Y. Sung, I. D. Williams, J. W. Y. Lam, K. S. Wong and B. Z. Tang, ACS Nano, 2018, 12, 8145–8159 CrossRef CAS.
- Y. Chen, Z. Zhang, Y. Xin, R. Zhou, K. Jiang, X. Sun, D. He, J. Song and Y. Zhang, Nanoscale, 2020, 12, 15435–15442 RSC.
- H. Natsheh and E. Touitou, Molecules, 2020, 25, 2959 CrossRef CAS.
- Y. Tu, Z. Zhao, J. W. Y. Lam and B. Z. Tang, Natl. Sci. Rev., 2021, 8, nwaa260 CrossRef PubMed.
- E. Henderson, M. Kempf, C. Yip, L. Davenport, E. Jones, S. Kong, E. Pearson, A. Kearns and L. Cuttle, Arch. Dermatol. Res., 2021 DOI:10.1007/s00403-021-02217-y.
- A. Mamalis, E. Koo, G. D. Sckisel, D. M. Siegel and J. Jagdeo, Br. J. Dermatol., 2016, 175, 512–519 CrossRef CAS.
- Z. Yu, X. Meng, S. Zhang, Y. Chen, Z. Zhang and Y. Zhang, Molecules, 2021, 26, 3093 CrossRef CAS.
- C. D. Marshall, M. S. Hu, T. Leavitt, L. A. Barnes, H. P. Lorenz and M. T. Longaker, Adv. Wound Care, 2018, 7, 29–45 CrossRef PubMed.
- U. Mirastschijski, B. Lupse, K. Maedler, B. Sarma, A. Radtke, G. Belge, M. Dorsch, D. Wedekind, L. J. McCawley, G. Boehm, U. Zier, K. Yamamoto, S. Kelm and M. S. Agren, Int. J. Mol. Sci., 2019, 20, 5234 CrossRef CAS.
- X. Deng, W. Guan, X. Qing, W. Yang, Y. Que, L. Tan, H. Liang, Z. Zhang, B. Wang, X. Liu, Y. Zhao and Z. Shao, ACS Appl. Mater. Interfaces, 2020, 12, 4265–4275 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Additional experimental details, materials, and methods; flow cytometry results of cellular uptake; fluorescent images of calcein AM/PI co-staining; fluorescent images of in vivo ROS generation. See DOI: 10.1039/d1bm01555a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |