 Open Access Article
Open Access ArticleA novel electrochemical sandwich-like immunosensor based on carboxyl Ti3C2Tx MXene and rhodamine b/gold/reduced graphene oxide for Listeria monocytogenes
Huimin
Niu†
ab,
Shumei
Cai†
ab,
Xueke
Liu†
ab,
Xiaoming
Huang
ab,
Juan
Chen
ab,
Shuiliang
Wang
 *ab and
Shenghang
Zhang
*ab and
Shenghang
Zhang
 *ab
*ab
aFujian Key Laboratory of Aptamers Technology, Affiliated Dongfang Hospital of School of Medicine, Xiamen University, Fuzhou, Fujian 350025, PR China. E-mail: fzzyyzsh@126.com; shuiliang.wang@xmu.edu.cn
bDepartment of Clinical Laboratory Medicine, Fuzhou General Clinical Medical School (the 900th Hospital), Fujian Medical University, Fuzhou, Fujian 350025, PR China
First published on 27th January 2022
Abstract
Listeria monocytogenes (LM) is one of the most common food-borne pathogens and can induce a series of diseases with a high mortality rate to humans; hence, it is very necessary to develop a highly sensitive method for LM detection. Based on this need, a new sandwich-like electrochemical immunosensing platform was developed herein by preparing carboxyl Ti3C2Tx MXene (C-Ti3C2Tx MXene) as the sensing platform and rhodamine b/gold/reduced graphene oxide (RhB/Au/RGO) as the signal amplifier. The high conductivity and large surface area of C-Ti3C2Tx MXene make it a desirable nanomaterial to fix the primary antibody of LM (PAb), while the prepared Au/RGO/RhB nanohybrid is dedicated to assembling the secondary antibody (SAb) of LM, offering an amplified response signal. Through the use of RhB molecule as the signal probe, the experiments showed that the peak currents of RhB increase along with an increase in the concentration of LM from 10 to 105 CFU mL−1, and an extremely low limit of detection (2 CFU mL−1) was obtained on the basis of the proposed immunosensing platform after optimizing various conditions. Hence, it is confirmed that the developed sandwich-like immunosensor based on C-Ti3C2Tx MXene and RhB/Au/Gr has great application in the detection of LM and other analytes.
1. Introduction
As one of the most common food-borne pathogens, Listeria monocytogenes (LM) can induce various serious diseases (e.g. meningitis, febrile gastroenteritis, abortion and septicemia) to human health.1–3 LM can survive easily in a series of foods including water, cheese, milk, meats and raw vegetables.4,5 In the USA, a zero-tolerance policy was formed for the presence of LM in food; in Canada, only 100 CFU g−1 was allowed.6,7 Consequently, developing effective methods to achieve sensitive detection of LM is considerably important. Up to now, several conventional methods (e.g. dynamic light scattering immunoassay, lateral-flow enzyme immunoconcentration, enzyme-linked immunosorbent assay and quartz crystal microbalance immunosensors) have been developed for LM detection.4,8–11 There is no doubt that these methods are very important in detecting LM, but they suffer from drawbacks which include time-consuming process, expensive equipment or complex operation. Compared to the general methods, electrochemical technology has many incomparable merits since it is very simple, low-cost, easy to operate, and time-saving.12 In the last few years, there have been some interesting electrochemical sensing methods proposed to detect LM,13–15 while developing a sensing platform with higher performance is still a major challenge.Resulting from the superior signal amplification feature, which causes improvement in sensitivity, the sandwich-like electrochemical immunosensing strategy has aroused considerable attention in recent years.16–18 As one of the crucial factors, designing suitable materials as sensing platform, which is dedicated to immobilize antibodies coupled with improved electron transfer, is of great significance.19,20 MXenes, known as transition metal carbides/carbonitrides, are an emerging representative 2D nanomaterials in recent years due to their excellent properties.21–23 MXenes were discovered via exfoliating the “A” laminar component from the MAX phase, which represents the corresponding components in compounds, where “M” reflects the transition metal, “A” refers to the elements in group IIIA/IVA, and “X” is assigned to C/N.24–29 The high electrical conductivity and large surface area of MXenes make MXenes-based nanomaterials have important applications in many fields.30–32 In fact, presently MXenes have been applied widely in energy storage and conversion, sensors and biomedicine,33–36 but there are only a few reports focused on their application in electrochemical immunosensors.
Another key factor in the sandwich-like immunosensor is designing a desirable nanostructure as signal amplifier, which is also very important for improving sensing sensitivity. As another star material, reduced graphene oxide (RGO) possesses many excellent advantages such as large surface area, high electrical conductivity and chemical stability; moreover, RGO can easily be functionalized.37–40 Presently, various RGO-based nanostructures have been designed as signal amplifiers to construct electrochemical immunosensors.41,42 For instance, Wang et al.43 prepared horseradish peroxidase (HRP)/gold nanoparticles (Au) on TiO2/graphene as signal amplifier for constructing an immunosensor of carcinoembryonic antigen based on the electrocatalytic current of HRP/H2O2 toward hydroquinone reduction. In addition, similar ways for signal amplification based on RGO and electrocatalysis of HRP were also developed for squamous cell carcinoma antigen,44 prostate specific antigen45 and cardiac troponin I.46 Obviously, the preparation methods and sensing mechanisms of these signal amplifiers are relatively complex.
Herein, carboxyl Ti3C2Tx MXene (C-Ti3C2Tx MXene) and gold/RGO/rhodamine b (Au/RGO/RhB) nanostructures were prepared respectively as sensing platform and signal amplifier. By functionalizing Ti3C2Tx MXene with carboxyl, the formed C-Ti3C2Tx MXene can immobilize LM primary antibody (PAb) via a simple amidation reaction to capture LM targets. As for Au/RGO/RhB, RhB was non-covalently functionalized on the Au/RGO surface via π–π stacking to be used as a desirable probe since it exhibits sensitive electrochemical oxidation current, and the secondary antibody (SAb) was assembled on the Au surface. On the basis of the above principle, a new sandwich-like electrochemical immunosensing platform was constructed for detecting LM (Scheme 1) coupled with an excellent sensing performance under the optimized conditions.
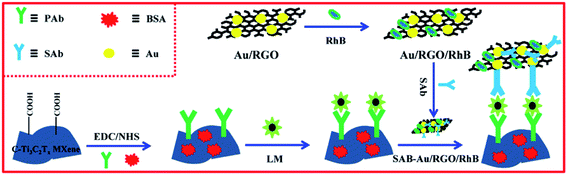 | ||
| Scheme 1 Illustrations for constructing the sandwich-like immunosensor of LM based on C-Ti3C2Tx MXene and RhB/Au/RGO. | ||
2. Experimental section
2.1 Preparation of C-Ti3C2Tx MXene
Ti3C2Tx MXene was first prepared by etching the aluminum layer from Ti3AlC2 according to a previous method.47 Briefly, 1.0 g Ti3AlC2 powder was added slowly to 20.0 mL HF solution under stirring at ∼35 °C and maintained for ∼24 h. After centrifuging, washing and drying of the precipitate, Ti3C2Tx MXene was obtained successfully.Next, C-Ti3C2Tx MXene was prepared according to a previous work with some modifications.48 In brief, 0.16 g 4-aminobenzoic acid and 0.14 g sodium hydroxide were first added to 50.0 mL water (∼2 °C), and then 0.26 g sodium nitrite (7.6 mM) was added slowly. Subsequently, 6.0 mL HCl solution was quickly added to this solution under stirring and kept for ∼40 min. Then, the desired diazonium salt solution was added to 20.0 mL of Ti3C2Tx solution (5.0 mg mL−1) and allowed to stir for ∼5 hours. Finally, the solution was centrifuged, washed and dried to obtain the C-Ti3C2Tx MXene product.
2.2 Preparation of Au/RGO/RhB
Before the preparation of Au/RGO/RhB, Au/RGO was prepared via a common method.49,50 For preparing RhB/Au/RGO, 20.0 mL of Au/RGO dispersion (0.5 mg mL−1) was mixed with 20.0 mL of RhB solution (0.25 mg mL−1) and stirred continuously for ∼30 min. Owing to the π–π stacking between RhB and RGO, the RhB molecules were functionalized non-covalently on the RGO surface. After centrifugation, rinsing and vacuum-drying, the Au/RGO/RhB product was obtained successfully.2.3 Construction of the sandwich-like immunosensor
SAb (30.0 μL) was added to 4.0 mL Au/RGO/RhB dispersion (0.5 mg mL) and incubated at room temperature for ∼2 hour to assemble SAb on Au surface, forming SAb-Au/RGO/RhB. Next, 10.0 μL C-Ti3C2Tx MXene suspension of (0.5 mg mL−1) was dropped onto a cleaned glassy carbon electrode (GCE) and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (0.1 M) with N-hydroxysuccinimide mixture (0.2 M) was used to activate the carboxyl group for 30 min. Sequentially, PAb solution (8.0 μL) was dropped and assembled on the activated C-Ti3C2Tx MXene/GCE surface, denoting the formed electrode as PAb/Ti3C2Tx MXene/GCE. Phosphate buffer solution (PBS) was then used to rinse PAb/Ti3C2Tx MXene/GCE to remove the unbound PAb, and 4.0 μL BSA (0.1 g mL−1) was used to prevent nonspecific adsorption by incubating PAb/Ti3C2Tx MXene/GCE with BSA to form BSA/PAb/Ti3C2Tx MXene/GCE. For LA sensing, the constructed BSA/PAb/Ti3C2Tx MXene/GCE reacted with LM at different concentrations and dipped in PBS to eliminate nonspecific LM cell. Finally, LM/BSA/PAb/Ti3C2Tx MXene/GCE was combined with SAb-Au/RGO/RhB to produce a sandwich-like structure, which was then measured by an electrochemical method.
1 mixture of 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide hydrochloride (0.1 M) with N-hydroxysuccinimide mixture (0.2 M) was used to activate the carboxyl group for 30 min. Sequentially, PAb solution (8.0 μL) was dropped and assembled on the activated C-Ti3C2Tx MXene/GCE surface, denoting the formed electrode as PAb/Ti3C2Tx MXene/GCE. Phosphate buffer solution (PBS) was then used to rinse PAb/Ti3C2Tx MXene/GCE to remove the unbound PAb, and 4.0 μL BSA (0.1 g mL−1) was used to prevent nonspecific adsorption by incubating PAb/Ti3C2Tx MXene/GCE with BSA to form BSA/PAb/Ti3C2Tx MXene/GCE. For LA sensing, the constructed BSA/PAb/Ti3C2Tx MXene/GCE reacted with LM at different concentrations and dipped in PBS to eliminate nonspecific LM cell. Finally, LM/BSA/PAb/Ti3C2Tx MXene/GCE was combined with SAb-Au/RGO/RhB to produce a sandwich-like structure, which was then measured by an electrochemical method.
3. Results and discussion
3.1 Characterization of C-Ti3C2Tx MXene and Au/RGO/RhB
Fig. 1 shows the scanning electron microscopy (SEM) images of Ti3AlC2 MAX phase precursor and Ti3C2Tx MXene. It is noted that the pristine Ti3AlC2 particles exhibit densely aligned layered structures with smooth surface (Fig. 1A). After etching with HF, the produced Ti3C2Tx layered nanostructures become very evident and exhibit loosely packed accordion-like morphology compared to the initial Ti3AlC2 sample (Fig. 1B). Fourier transform infrared (FT-IR) spectroscopy was used to characterize the formation of C-Ti3C2Tx MXene (Fig. 1C). Compared with Ti3C2Tx MXene, C-Ti3C2Tx MXene possesses a typical and strong characteristic peak of carboxyl presented at ∼1680 cm−1. | ||
| Fig. 1 The SEM images of (A) Ti3AlC2 and (B) Ti3C2Tx; (C) FT-IR spectra of Ti3C2Tx MXene (a) and C-Ti3C2Tx MXene (b). | ||
Fig. 2A shows the transmission electron microscopy (TEM) images of Au/RGO hybrids; it was found that Au nanoparticles were distributed uniformly on the RGO surface with an average diameter of ∼5.0 nm (Fig. 2B), and ICP-AES demonstrated that the content of Au is ∼5.7%. The abundant and uniform Au present on the Au/RGO surface is very important to assemble SAb and improve the sensing capability. The functionalization of RhB towards Au/RGO can be easily demonstrated by cyclic voltammetry (CV). As shown in Fig. 2C, there is no peak current present between 0.4 and 1.0 V, while Au/RGO/RhB shows a remarkable oxidation peak current at ∼0.87 V since RhB is an electroactive molecule.
 | ||
| Fig. 2 (A) The TEM image of Au/RGO hybrid, (B) the particle size distribution of AuNPs, and (C) the CV responses of Au/RGO (a) and Au/RGO/RhB (b). | ||
3.2 Electrochemical evaluation of the sandwich-like immunosensor
Firstly, electrochemical impedance spectroscopy (EIS) was introduced to evaluate the procedures in constructing the immunosensing platform, which was performed in a 5.0 mM [Fe(CN)6]3−/4−solution (Fig. 3). Generally, biomolecules including antibody, LM cells and BSA are non-conductive and could restrain electron channel to increase the RCT values. As shown in Fig. 3, the charge-transfer resistance (RCT) at C-Ti3C2Tx MXene/GCE is smaller than that of bare GCE due to the high conductivity of Ti3C2Tx MXene. When C-Ti3C2Tx MXene/GCE was assembled successively with PAB, LM and SAb-Au/RGO/RhB/LM, the RCT values at PAb/Ti3C2Tx MXene/GCE, BSA/PAb/Ti3C2Tx MXene/GCE, LM/BSA/PAb/Ti3C2Tx MXene/GCE and SAb-Au/RGO/RhB/LM/BSA/PAb/Ti3C2Tx MXene/GCE in turn show an increase. These results indicate that the developed immunosensing layers were fabricated successfully.The feasibility of the proposed immunosensor was studied by differential pulse voltammetry (DPV) technique. As shown in Fig. 4 (LM concentration, 106 CFU mL−1), there is no peak current observed at LM/BSA/PAb/Ti3C2Tx MXene/GCE in 0.1 M PBS (pH 7.0). As for SAb-Au/RGO/RhB/LM/BSA/PAb/Ti3C2Tx MXene/GCE, it exhibits an obvious peak current of RhB due to the capture of SAb-Au/RGO/RhB resulting from the antigen–antibody reaction, indicating that the developed immunosensing method is feasible for LM detection. In order to achieve high sensitivity in sensing LM, a series of controlled experiments were performed.
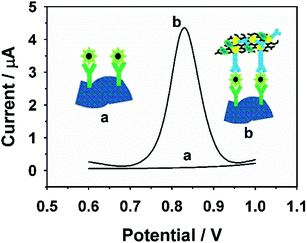 | ||
| Fig. 4 The DPV response of LM/BSA/PAb/Ti3C2Tx MXene/GCE (a) and SAb-Au/RGO/RhB/LM/BSA/PAb/Ti3C2Tx MXene/GCE (b) in 0.1 M PBS. | ||
In general, excessive acidity or alkalinity could enable various proteins to become unstable, thus the effect from PBS needs to be evaluated. As shown in Fig. 5A, the DPV peak currents of SAb-Au/RGO/RhB/LM/BSA/PAb/Ti3C2Tx MXene/GCE increase along with the increase in pH value from 4.0, and the maximum peak current presents at pH value of 7.0, thus PBS with the value of 7.0 was selected for all the experiments. Meanwhile, the C-Ti3C2Tx MXene amount coated on the GCE surface is important for the sensing capability, and Fig. 5B shows that the peak currents of RhB increase along with the increase in the amount from 2.0 to 10.0 μL. When the used amount exceeds 10.0 μL, there is little change in the RhB peak currents, indicating that 10.0 μL C-Ti3C2Tx MXene dispersion was enough. Furthermore, the incubation time between LM with different antibodies was studied. As shown in Fig. 5C, the studies demonstrated that the optimum length for reacting LM with PAb was 50 min, while the time for reacting LM with SAb was 40 min. The concentration of LM used for the controlled experiments was 3 × 104 CFU mL−1. In addition, the amounts of PAb and SAb used in the construction of the immunosensor were also studied, and it can be observed from Fig. 5D that the best amounts of PAb and SAb were 8.0 and 30.0 μL, respectively.
Under the above optimized controlled conditions, the quantitative sensing of LM was performed using DPV based on the proposed SAb-Au/RGO/RhB/LM/BSA/PAb/Ti3C2Tx MXene sandwich-like immunosensor. From Fig. 6, it is observed that the peak currents of RhB probe linearly increase along with the increase in concentration of LM from 10 and 105 CFU mL−1. The linear equation expression is Ip (μA) = 0.281 + 0.003C (CFU mL−1) (R = 0.998) and limit of detection (LOD) is calculated to be 2 ± 0.3 CFU mL−1 (S/N, 3). Compared with most previous works, the present work exhibits better sensing performance including linearity range (LR) and LOD (Table 1); this can attributed mainly to the desirable properties of Ti3C2Tx MXene (large surface area and conductivity) and the signal amplification capacity of Au/RGO/RhB.
| Electrodes | Assay label | Measuring method | Linear range/CFU mL−1 | LOD/CFU mL−1 | Reference |
|---|---|---|---|---|---|
| Gold electrode | HRP | Amperometry | 102 to 106 | 102 | 6 |
| Interdigitated array microelectrode | Urease | EIS | 1.9 × 103 to 1.9 × 106 | 1.6 × 103 | 51 |
| TiO2 nanowire bundle gold microelectrode | Label-free | EIS | __ | 102 | 52 |
| Au NPs/screen-printed carbon electrode | HRP | Amperometry | 2.25 × 10 to 2.25 × 105 | 2.25 × 102 | 53 |
| CNT fibers electrode | HRP | Cyclic voltammetry | 102 to 105 | 1.07 × 102 | 54 |
| C-Ti3C2Tx MXene/GCE | RhB | DPV | 10 to 105 | 2 ± 0.3 | This work |
3.3 Specificity, reproducibility and stability of the developed immunosensing platform
For further evaluating the specificity of the developed sandwich-like immunosensor, the response signals of RhB were studied through selecting other pathogens other than LM. As exhibited in Fig. 7A, the obtained results revealed a significant peak current of RhB observed only from LM (104 CFU mL−1); as for the other pathogens including Escherichia coli (Es), Vibrio parahaemolyticus (Vp), Shigella (Sh), Salmonella enteritidis (Se) and Staphylococcus aureus (Sac) coupled with a high concentration (5 × 104 CFU mL−1), the presented peak currents of RhB are nearly negligible, demonstrating that the developed sandwich-like immunosensor offers excellent specificity for detecting LM.Next, for evaluating the reproducibility of the immunosensor, the electrochemical responses of 8 fabricated sensors were measured parallelly (Fig. 7B), and the results display that the relative standard deviation value from their current response was 4.36%. Furthermore, in order to study the immunosensor stability, the modified electrodes and SAb-Au/RGO/RhB were stored at 4 °C for further use; the research results showed almost no decrease in the current response (∼5.32%) after storing for 32 days (Fig. 7C). These results reveal that the proposed immunosensor possesses satisfactory reproducibility and good stability.
4. Conclusion
By preparing C-Ti3C2Tx MXene and Au/RGO/RhB respectively as the sensing platform and signal amplifier, a new sandwich-like electrochemical immunosensing platform was constructed herein for the sensitive determination of LM. Owing to the superior properties of Ti3C2Tx MXene and Au/RGO/RhB, the developed SAb-Au/RGO/RhB/LM/BSA/PAb/Ti3C2Tx MXene sandwich-like immunosensor possesses wider LR and lower LOD than most previous works for detecting LM; thus it is believed that this sensor has great potential for application in LM analysis. Furthermore, through selecting other suitable antibodies other than LM antibodies, the sandwich-like immunosensor based on the prepared C-Ti3C2Tx MXene and Au/RGO/RhB shows broad application prospects for other targets.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported in part by Research Project of Military Logistics of PLA of China (No. CLB20J024), Foreign cooperation projects of Fujian Natural Science Foundation (No. 2019I0025), National Natural Science Foundation of China (No. 81772848), Joint Funds for the Innovation of Science and Technology from Fujian Province (No. 2017Y9127), and Fund from the 900th Hospital of Joint Logistics Support Force (No. 2020Q01, 2020Q06, 2020Z15, and 2020L17).References
- N. F. D. Silva, M. M. P. S. Neves, J. M. C. S. Magalhães, C. Freire and C. Delerue-Matos, Trends Food Sci. Technol., 2020, 99, 621–633 CrossRef CAS.
- J. Ding, J. Lei, X. Ma, J. Gong and W. Qin, Anal. Chem., 2014, 86, 9412–9416 CrossRef CAS PubMed.
- D. Patel, Y. Zhou and R. P. Ramasamy, J. Electrochem. Soc., 2021, 168, 057523 CrossRef CAS.
- X. Qi, Z. Wang, R. Lu, J. Liu, Y. Li and Y. Chen, Food Chem., 2021, 338, 127837 CrossRef CAS PubMed.
- S. Eissa and M. Zourob, Microchim. Acta, 2020, 187, 486 CrossRef CAS PubMed.
- C. Cheng, Y. Peng, J. Bai, X. Zhang, Y. Liu, X. Fan, B. Ning and Z. Gao, Sens. Actuators, B, 2014, 190, 900–906 CrossRef CAS.
- Q. Chen, J. Lin, C. Gan, Y. Wang, D. Wang, Y. Xiong, W. Lai, Y. Li and M. Wang, Biosens. Bioelectron., 2015, 74, 504–511 CrossRef CAS PubMed.
- B. Giménez, N. Graiver, L. Giannuzzi and N. Zaritzky, Food Control, 2021, 121, 107602 CrossRef.
- B. B. Tasbasi, B. C. Guner, M. Sudagidan, S. Ucak, M. Kavruk and V. C. Ozalp, Anal. Biochem., 2019, 587, 113449 CrossRef CAS PubMed.
- S. Wachiralurpan, K. Chansiri and P. A. Lieberzeit, Sens. Actuators, B, 2020, 308, 127678 CrossRef CAS.
- Y. Zhao, S. Bu, C. Wang, C. Ma, Z. Li, W. Zhang and J. Wan, Anal. Lett., 2021, 54, 1603–1615 CrossRef CAS.
- J. Riu and B. Giussani, Trac. Trends Anal. Chem., 2020, 126, 115863 CrossRef CAS.
- X. Niu, W. Zheng, C. Yin, W. Weng, G. Li, W. Sun and Y. Men, J. Electroanal. Chem., 2017, 806, 116–122 CrossRef CAS.
- A. Liu, L. Shen, Z. Zeng, M. Sun, Y. Liu, S. Liu, C. Li and X. Wang, Food Anal. Methods, 2018, 11, 215–223 CrossRef.
- N. F. D. Silva, M. M. P. S. Neves, J. M. C. S. Magalhães, C. Freire and C. Delerue-Matos, Talanta, 2020, 216, 120976 CrossRef CAS PubMed.
- Q. Guo, X. Zhang, F. Zhao, Q. Song, G. Su, Y. Tan, Q. Tao, T. Zhou, Y. Yu, Z. Zhou and C. Lu, ACS Nano, 2020, 14, 2788–2797 CrossRef CAS PubMed.
- Y. Li, H. Shao, Z. Lin, J. Lu, L. Liu, B. Duployer, P. O. Å. Persson, P. Eklund, L. Hultman, M. Li, K. Chen, X.-H. Zha, S. Du, P. Rozier, Z. Chai, E. Raymundo-Piñero, P.-L. Taberna, P. Simon and Q. Huang, Nat. Mater., 2020, 19, 894–899 CrossRef CAS PubMed.
- M. Zhou, S. Ning, J. Liu, G. I. N. Waterhouse, L. Li, J. Dong and S. Ai, Anal. Lett., 2021, 54, 1769–1782 CrossRef CAS.
- J. Chen, P. Tong, L. Huang, Z. Yu and D. Tang, Electrochim. Acta, 2019, 319, 375–381 CrossRef CAS.
- G. Cai, Z. Yu, P. Tong and D. Tang, Nanoscale, 2019, 11, 15659–15667 RSC.
- L. Wu, X. Lu, Dhanjai, Z.-S. Wu, Y. Dong, X. Wang, S. Zheng and J. Chen, Biosens. Bioelectron., 2018, 107, 69–75 CrossRef CAS PubMed.
- P. K. Kalambate, Dhanjai, A. Sinha, Y. Li, Y. Shen and Y. Huang, Microchim. Acta, 2020, 187, 402 CrossRef CAS PubMed.
- Y. Xie, F. Gao, X. Tu, X. Ma, Q. Xu, R. Dai, X. Huang, Y. Yu and L. Lu, J. Electrochem. Soc., 2019, 166, B1673–B1680 CrossRef CAS.
- Y. Yi, D. Zhang, Y. Ma, X. Wu and G. Zhu, Anal. Chem., 2019, 91, 2908–2915 CrossRef CAS PubMed.
- X. Tu, F. Gao, X. Ma, J. Zou, Y. Yu, M. Li, F. Qu, X. Huang and L. Lu, J. Hazard. Mater., 2020, 396, 122776 CrossRef CAS PubMed.
- H. Zhang, Z. Wang, F. Wang, Y. Zhang, H. Wang and Y. Liu, Anal. Chem., 2020, 92, 5546–5553 CrossRef CAS PubMed.
- P. K. Kalambate, N. S. Gadhari, X. Li, Z. Rao, S. T. Navale, Y. Shen, V. R. Patil and Y. Huang, Trac. Trends Anal. Chem., 2019, 120, 115643 CrossRef CAS.
- Y. Yi, Y. Ma, F. Ai, Y. Xia, H. Lin and G. Zhu, Chem. Commun., 2021, 57, 7790–7793 RSC.
- Y. Xia, Y. Ma, Y. Wu, Y. Yi, H. Lin and G. Zhu, Microchim. Acta, 2021, 188, 1–9 CrossRef PubMed.
- Y. Xia, Y. Zhao, F. Ai, Y. Yi, T. Liu, H. Lin and G. Zhu, J. Hazard Mater., 2021, 127974 Search PubMed.
- R. Zeng, W. Wang, M. Chen, Q. Wan, C. Wang, D. Knopp and D. Tang, Nano Energy, 2021, 82, 105711 CrossRef CAS.
- R. Zeng, Z. Luo, L. Zhang and D. Tang, Anal. Chem., 2018, 90, 12299–12306 CrossRef CAS PubMed.
- Y. Yao, L. Lan, X. Liu, Y. Ying and J. Ping, Biosens. Bioelectron., 2020, 148, 111799 CrossRef CAS PubMed.
- M. Mohammadniaei, A. Koyappayil, Y. Sun, J. Min and M.-H. Lee, Biosens. Bioelectron., 2020, 159, 112208 CrossRef CAS PubMed.
- Y. Cai, J. Shen, G. Ge, Y. Zhang, W. Jin, W. Huang, J. Shao, J. Yang and X. Dong, ACS Nano, 2018, 12, 56–62 CrossRef CAS PubMed.
- D. Huang, Y. Wu, F. Ai, X. Zhou and G. Zhu, Sens. Actuators, B, 2021, 342, 130074 CrossRef CAS.
- L. Jothi, S. K. Jaganathan and G. Nageswaran, Mater. Chem. Phys., 2020, 242, 122514 CrossRef CAS.
- P. Zhao, M. Ni, Y. Xu, C. Wang, C. Chen, X. Zhang, C. Li, Y. Xie and J. Fei, Sens. Actuators, B, 2019, 299, 126997 CrossRef CAS.
- K. Kunpatee, P. Chamsai, E. Mehmeti, D. M. Stankovic, A. Ortner, K. Kalcher and A. Samphao, J. Electroanal. Chem., 2019, 855, 113630 CrossRef CAS.
- R. Zhang, C. Zhang, F. Zheng, X. Li, C.-L. Sun and W. Chen, Carbon, 2018, 126, 328–337 CrossRef CAS.
- S. Lv, K. Zhang, Y. Zeng and D. Tang, Anal. Chem., 2018, 90, 7086–7093 CrossRef CAS PubMed.
- Q. Zhou, Y. Lin, K. Zhang, M. Li and D. Tang, Biosens. Bioelectron., 2018, 101, 146–152 CrossRef CAS PubMed.
- Y. Chen, Y. Li, D. Deng, H. He, X. Yan, Z. Wang, C. Fan and L. Luo, Biosens. Bioelectron., 2018, 102, 301–306 CrossRef CAS PubMed.
- Y. Liu, H. Ma, J. Gao, D. Wu, X. Ren, T. Yan, X. Pang and Q. Wei, Biosens. Bioelectron., 2016, 79, 71–78 CrossRef CAS PubMed.
- M. Li, P. Wang, F. Li, Q. Chu, Y. Li and Y. Dong, Biosens. Bioelectron., 2017, 87, 752–759 CrossRef CAS PubMed.
- H. Lv, X. Zhang, Y. Li, Y. Ren, C. Zhang, P. Wang, Z. Xu, X. Li, Z. Chen and Y. Dong, Microchim. Acta, 2019, 186, 1–10 CrossRef CAS PubMed.
- H. Wu, M. Almalki, X. Xu, Y. Lei, F. Ming, A. Mallick, V. Roddatis, S. Lopatin, O. Shekhah, M. Eddaoudi and H. N. Alshareef, J. Am. Chem. Soc., 2019, 141, 20037–20042 CrossRef CAS PubMed.
- P. Zhang, L. Wang, K. Du, S. Wang, Z. Huang, L. Yuan, Z. Li, H. Wang, L. Zheng and Z. Chai, J. Hazard Mater., 2020, 396, 122731 CrossRef CAS PubMed.
- F. Cui and X. Zhang, J. Electroanal. Chem., 2012, 669, 35–41 CrossRef CAS.
- G. Goncalves, P. A. A. P. Marques, C. M. Granadeiro, H. I. S. Nogueira, M. K. Singh and J. Grácio, Chem. Mater., 2009, 21, 4796–4802 CrossRef CAS.
- D. Wang, Q. Chen, H. Huo, S. Bai, G. Cai, W. Lai and J. Lin, Food Control, 2017, 73, 555–561 CrossRef CAS.
- R. Wang, C. Ruan, D. Kanayeva, K. Lassiter and Y. Li, Nano Lett., 2008, 8, 2625–2631 CrossRef CAS PubMed.
- D. Davis, X. Guo, L. Musavi, C. S. Lin, S. H. Chen and V. C. H. Wu, Ind. Biotechnol., 2013, 9, 31–36 CrossRef CAS.
- Y. Lu, Y. Liu, Y. Zhao, W. Li, L. Qiu and L. Li, J. Nanomater., 2016, 2016, 16 Search PubMed.
Footnote |
| † These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |




