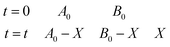Open Access Article
Open Access ArticleThermal stability and pathways for the oxidation of four 3-phenyl-2-propene compounds†
Chang Yu ,
Min Liang,
Su-Yi Dai,
Hai-Jun Cheng,
Li Ma,
Fang Lai,
Xiong-Min Liu
,
Min Liang,
Su-Yi Dai,
Hai-Jun Cheng,
Li Ma,
Fang Lai,
Xiong-Min Liu * and
Wei-Guang Li*
* and
Wei-Guang Li*
College of Chemistry and Chemical Engineering, Guangxi University, Nanning 530004, Guangxi, China. E-mail: xmliu1@gxu.edu.cn; lwgxqz@163.com
First published on 5th October 2021
Abstract
Cinnamaldehyde, cinnamyl alcohol, β-methylstyrene and cinnamic acid are four important biomass 3-phenyl-2-propene compounds. In the field of perfume and organic synthesis, their thermal stability and oxidation pathways deserve attention. This paper reports a new attempt to investigate the thermal stability and reactivity by a custom-designed mini closed pressure vessel test (MCPVT). The pressure and temperature behaviors were measured by MCPVT under nitrogen and oxygen atmosphere. The temperature of initial oxygen absorption (Ta) and rapid oxidation (TR) were calculated. The results showed that four 3-phenyl-2-propene compounds were stable under nitrogen atmosphere. The Ta of cinnamaldehyde, cinnamyl alcohol, β-methylstyrene, and cinnamic acid was 271.25 K, 292.375 K, 323.125 K, and 363.875 K, and their TR was 301.125 K, 332.75 K, 357.91 K, and 385.375 K, respectively. The oxidation reactivity order was derived to be cinnamaldehyde > cinnamyl alcohol > β-methylstyrene > cinnamic acid. The oxidation kinetics were determined using n versus time (n–t) plots, which showed a second-order reaction. Peroxide was determined by iodimetry, and the oxidation products were analyzed by gas chromatography-mass spectrometry (GC-MS). The results showed that the peroxide value of cinnamaldehyde, cinnamyl alcohol, β-methylstyrene, and cinnamic acid reached 18.88, 15.07, 9.62, and 4.24 mmol kg−1 at 373 K for 6 h, respectively. The common oxidation products of four 3-phenyl-2-propene compounds were benzaldehyde, benzoic acid, and epoxide, which resulted from the carbon–carbon double bond oxidation. The substituents' oxidation products were obtained from the oxidation of cinnamaldehyde, cinnamyl alcohol, and β-methylstyrene. In particular, the difference is that no oxidation products of the carboxyl group of cinnamic acid were detected. The common oxidation products of the four 3-phenyl-2-propene compounds were benzaldehyde, benzoic acid, and epoxide, which resulted from the carbon–carbon double bond oxidation. The substituents' oxidation products were caught in the oxidation of cinnamaldehyde, cinnamyl alcohol, and β-methylstyrene. In particular, the difference is that no oxidation products of the carboxyl group of cinnamic acid were detected. According to the complex oxidation products, important insights into the oxidation pathways were provided.
1. Introduction
Cinnamon oil is a natural aldehydic aromatic raw material with high economic value. The main ingredients of cinnamon oil are cinnamaldehyde, cinnamyl alcohol, and cinnamic acid. Cinnamyl alcohol can produce β-methylstyrene through the hydrogenolysis reaction.1 As high-activity biomass compounds, they are widely used in the chemical, medicine, and food industry due to their strong ability of sterilization and antisepsis.2–5 Given their structural similarity, the four compounds are regarded as 3-phenyl-2-propene compounds with bifunctional groups. Owing to the unsaturated carbon–carbon double bonds, the four 3-phenyl-2-propene compounds were oxidized easily to aldehyde and epoxy derivatives, resulting in limited application. Cinnamaldehyde and epoxy cinnamyl alcohol were detected in the autoxidation of cinnamyl alcohol.6 Cinnamic acid reacted with hydroxyl radical (HO) to form the hydroxylation products and the decarboxylation products in a neutral medium.7 For bifunctional compounds, there is a significant fundamental question about how to improve the selectivity of the reaction sites. Much attention have been paid to the selective oxidation to synthesize fine chemicals and intermediates.8–10 The chemoselective oxidation of organics having at least two active functional groups by H2O2 has been studied, which demonstrated the substituent groups' influence on the reactivity.11 trans-β-Methylstyrene oxidation was probed in order to understand the competing reactivity of the bifunctional allylic olefins.12 Studies on the chemoselective competitive oxidation of cinnamyl alcohol and cinnamaldehyde were efficiently realized with the catalyst for epoxidation.13,14 In addition to the carbon–carbon double bonds, the functional groups of four 3-phenyl-2-propene compounds have been extensively investigated. Many studies on the high selectivity for the oxidation of cinnamyl alcohol to cinnamaldehyde have been conducted.15 The aerobic selective oxidation of allylaldehyde provides an efficient way for the synthesis of unsaturated carboxylic acids. Lee16 reported that cinnamaldehyde was directly oxidized to cinnamic acid by silica-supported platinum nanoparticles in base-free media under batch and continuous flow conditions. However, not much information is available on the activation ability of the terminal functional groups to the four 3-phenyl-2-propene compounds. Hence, the study of the relative reactivity of the four 3-phenyl-2-propene compounds is of considerable interest. Based on a consistent assessment of the C–H and C–C bond dissociation energies, the relative reactivity of oxygenated fuels was analyzed.17 A detailed comparison of the ignition behavior of n-alkanes and n-alcohols shows the general trends and influence of the alcohol group.18 The quantitative data about the relative activating ability of different groups in a short series of reactions have been studied.19–21 The relative reactivity of enol ether and allylsilane to the ketene dithioacetal-derived radical cations was studied by competitive and cyclic voltammetry.22 The reactivity of four 3-phenyl-2-propene compounds is dependent on the activity of the carbon–carbon double bonds and terminal functional groups. Moreover, note that more attention should be paid to the thermal stability of four 3-phenyl-2-propene compounds before studying their oxidation reactivity. However, few papers have been published on the initial temperature and oxidation pathways of the four 3-phenyl-2-propene compounds, which decide the storage and application conditions.In the present work, to investigate the thermal stability and oxidation reactivity of the four 3-phenyl-2-propene compounds, the temperature and pressure behaviors of the oxidation process were determined by MCPVT (mini closed pressure vessel test). The temperature of oxygen absorption (Ta), rapid oxidation (TR), and oxidation kinetics of the four compounds were investigated using MCPVT. The effect of different terminal functional groups on the reactivity of the four compounds was analyzed. It is of great interest to study the oxidation products and reaction pathways to understand the difference in the adsorbed oxygen by different functional groups. The purpose of this study is to supply basic data to the thermal stability and the reactivity of the four 3-phenyl-2-propene compounds with oxygen, and to provide the possible methods for the organic synthesis of the compounds such as benzaldehyde. In addition, the data provide guidance for the safe transportation and application of the four 3-phenyl-2-propene compounds.
2. Experiment
2.1 Materials
Cinnamaldehyde (99%), β-methylstyrene (99%), and cinnamic acid (98%) were purchased from Shanghai Macklin Biochemical Technology Co., Ltd, China. Cinnamyl alcohol (99%) was purchased from 9 Ding Industrial Corporation, China. The N2 and O2 (99.99%) gases were supplied by Nanning Zhong yi Chuang gas Co., Ltd, China. KI (99.50%) and Na2S2O3 (99.95%) were obtained from Aladdin Industrial Corporation, China.2.2 Oxidation of the four 3-phenyl-2-propene compounds
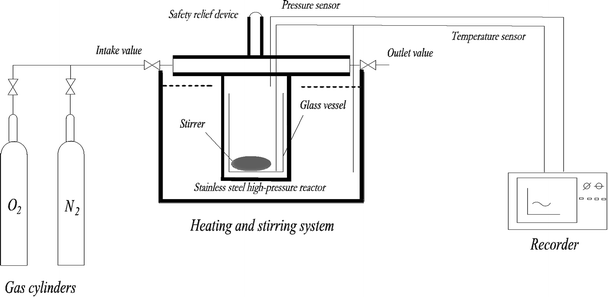
A mini closed pressure vessel test (MCPVT, vessel volume: 35 mL) was designed to trace the reaction process, as shown in Fig. 1. The main components of MCPVT are: (a) pressure sensor, made in Japan; (b) temperature sensor, made in Japan; (c) recorder, made in Japan; (d) stainless steel high-pressure reactor, self-designed; (e) gas cylinder, made in China; (f) heating and stirring system, the system is DF-101S collector type constant temperature heating magnetic stirrer, made in China, and the heating medium is heat conductive oil (glycerol). MCPVT was used to evaluate the thermal stability and define the initial oxidation temperature of the compounds because of the closed system.23,24 MCPVT is sensitive enough to measure the changes in the pressure and temperature; thus, the characteristics of the thermal decomposition reaction can be well understood. MCPVT was used for investigating the thermal stability and oxidation process of the four 3-phenyl-2-propene compounds under nitrogen and oxygen atmosphere. It is worth considering the order of oxidation and thermal decomposition of the four 3-phenyl-2-propene compounds. For understanding the characteristics and reactivity of the four 3-phenyl-2-propene compounds of the oxidation reaction, it is necessary to study the temperature and pressure behavior of the four 3-phenyl-2-propene compounds under oxygen and nitrogen atmospheres.The reactants and magnetic stirrer were put into lined pipes (glass material) of the stainless-steel reactor of MCPVT to avoid the influence of metal on the four 3-phenyl-2-propene compounds' oxidation. Nitrogen/oxygen was directly transmitted from the gas cylinder into the reactor through the intake valve. The oxidation experiments of the four 3-phenyl-2-propene compounds were performed under oxygen atmosphere at different heating modes. The contrast experiments were performed in nitrogen atmosphere. The experimental temperatures were below the boiling point of the four 3-phenyl-2-propene compounds. The changes in the pressure and temperature were recorded by a recorder and a signal conditioner. After the reaction, the products were cooled to the room temperature and collected. In order to reduce the experimental error, three parallel experiments were conducted at each temperature.
2.3 Analysis of the peroxide value
In order to detect the formation of peroxides, the peroxide value of oxidation was determined by iodimetry at different temperatures. About 0.1 g sample obtained from the oxidation was added to aqueous potassium iodide. The peroxides were reduced by potassium iodide (eqn (1)). An equivalent amount of iodine was liberated with starch, and the solution became blue. Then, the solution was titrated with standardized sodium thiosulfate solution until the blue color disappeared (eqn (2)). The experiment was carried out three times in parallel to obtain the average value. The results were quantified as milligrams per kilogram (mmol kg−1) of peroxide.| 2KI + ROOH + H2O = I2 + 2KOH + ROH | (1) |
| I2 + 2Na2S2O3 = Na2S4O6 + 2NaI | (2) |
2.4 Analysis of the oxidation products
GC-MS gas (chromatography-mass spectrometry) is an effectively means to analyze the oxidation products of the four 3-phenyl-2-propene compounds. It is significant for the safe use of four 3-phenyl-2-propene compounds, the analysis of the oxidation mechanism, and the thermal decomposition characteristics. The GC-MS (GCMS-QP2010 Ultra, Shimadzu Corp.) for the analysis of the oxidation products contained an electron impact ionization detector (EID: 70 eV) and a DB-WAX fused silica capillary column (with an inner diameter of 30 m × 0.25 mm, a film thickness of 0.25 mm; J&W Scientific Inc.). The following temperature program was used: maintained 343.15 K for 3 min, which first rose to 450.15 K (8 K min−1) and then rose to 473.15 K (5 K min−1), and finally rose to 513.15 K (20 K min−1). The carrier gas is high-purity helium gas, the column flow rate is 1.53 mL min−1; the injection volume is 0.2 μL, the split ratio is 80.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the inlet temperature is 523.15 K, and the interface temperature is 503.15 K. The scanning range of the mass spectrometer is 20–500 m/z in the full scan mode.
1, the inlet temperature is 523.15 K, and the interface temperature is 503.15 K. The scanning range of the mass spectrometer is 20–500 m/z in the full scan mode.
3. Results and discussion
3.1 Thermal stability of the four 3-phenyl-2-propene compounds' oxidation
The four 3-phenyl-2-propene compounds have two reactive functional groups of unsaturated double bonds and terminal groups, whose structures are shown in Fig. 2.In terms of the molecular structures, they have active chemical properties. Therefore, it is necessary to investigate their thermal stability and oxidation reactivity using MCPVT. The dosages of the four 3-phenyl-2-propene compounds were about 0.9 g. The amount of the initial gas was about 0.007 mol (the initial pressures of cinnamaldehyde and cinnamyl alcohol were about 0.45 MPa at 270 K. The initial pressure of β-methylstyrene and cinnamic acid were about 0.5 MPa at 300 K). In the experiments, cinnamaldehyde and cinnamyl alcohol were heated approximately from 270 K to 370 K, and β-methylstyrene and cinnamic acid were heated from room temperature to 390 K and 410 K, respectively. The heating rate was about 0.5 °C min−1. First, the thermal stability of the four 3-phenyl-2-propene compounds was investigated. The heating experiments of the four 3-phenyl-2-propene compounds were carried out under nitrogen atmosphere; the experimental results of T–t (temperature vs. time) and P–t (pressure vs. time) are shown in Fig. 3 and 4, respectively. Then, the thermal oxidation of the four 3-phenyl-2-propene compounds was performed under oxygen atmosphere. The pressure and internal temperature of the reactor were monitored from 260 K to 423 K slowly; the results of T–t and P–t are shown in Fig. 5 and 6, respectively.
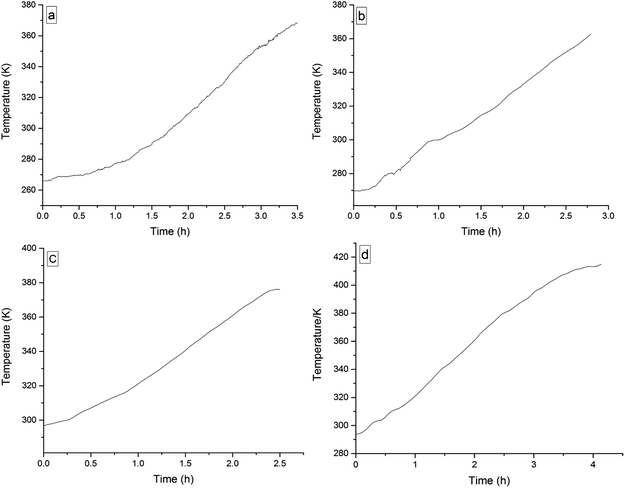 | ||
| Fig. 3 Temperature versus time plots for the four 3-phenyl-1-propene compounds under nitrogen atmosphere, namely, (a) cinnamaldehyde, (b) cinnamyl alcohol, (c) β-methylstyrene, and (d) cinnamic acid. | ||
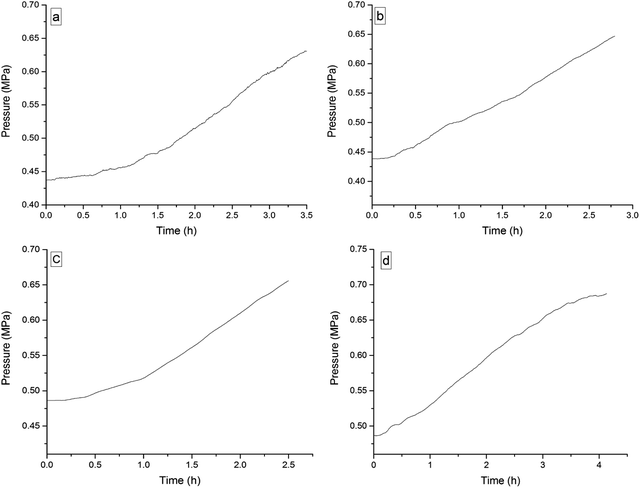 | ||
| Fig. 4 Pressure versus time plots for the four 3-phenyl-1-propene compounds under nitrogen atmosphere, namely, (a) cinnamaldehyde, (b) cinnamyl alcohol, (c) β-methylstyrene, and (d) cinnamic acid. | ||
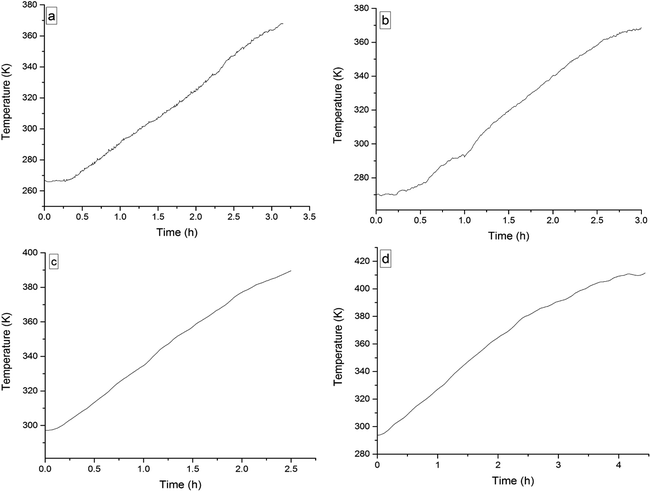 | ||
| Fig. 5 Temperature versus time plots for the four 3-phenyl-1-propene compounds under oxygen atmosphere, namely, (a) cinnamaldehyde, (b) cinnamyl alcohol, (c) β-methylstyrene, (d) and cinnamic acid. | ||
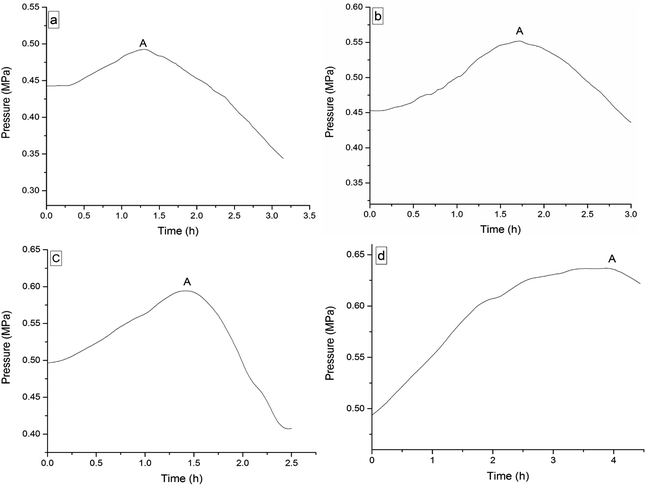 | ||
| Fig. 6 Pressure versus time plots for the four 3-phenyl-1-propene compounds under oxygen atmosphere, namely, (a) cinnamaldehyde, (b) cinnamyl alcohol, (c) β-methylstyrene, (d) and cinnamic acid. | ||
Whether under oxygen or nitrogen atmosphere, the T–t curves are straight lines with no sharp change points in Fig. 3 and 5, which indicated that no exothermic chemical reaction occurred. As shown in Fig. 4, the P–t curves are also straight lines under nitrogen atmosphere, which indicated that no chemical reaction occurred. The P–t curves of Fig. 6 are also not straight lines, which have dramatic drop points (A) under oxygen atmosphere. The autoxidation of cinnamyl alcohol was investigated upon air exposure.6 Cinnamaldehyde could react with oxygen even at room temperature.25 The pressure characteristics showed that the gas was gradually reduced. In other words, oxygen was absorbed and oxidation occurred. The oxidation of the four 3-phenyl-2-propene compounds (P) is shown in reaction (3).
| P + O2 (g) → reaction products | (3) |
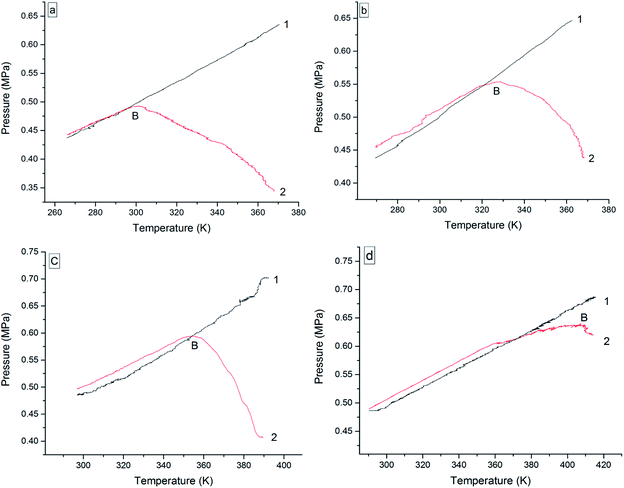
The oxidation reactions of the four 3-phenyl-2-propene compounds are gas–liquid or gas–solid reactions. They reacted with oxygen to form non-gaseous products, resulting in a pressure reduction in the MCPVT. The four 3-phenyl-2-propene compounds are liquid (l) or solid (s) at room temperature, whose boiling points are higher than 445 K at 0.1013 MPa. However, their vapor pressures could not be ignored in the experimental temperature range (260–423 K) and 0.5 MPa pressure. Thus, the pressure behaviors of the oxidation process could not be completely regarded as a change process in the oxygen (g) content. In order to gain insights into the initial point at which oxygen is consumed, i.e., the Ta of the four 3-phenyl-2-propene compounds, the amount of the gaseous substance was calculated using the ideal gas equation (n = PV/RT), where the gas constant R = 8.314 J mol−1 K−1, and T and P were obtained through Fig. 7. The reactor volume V = 35 mL. The results of n–T (number of moles vs. temperature) are shown in Fig. 8.
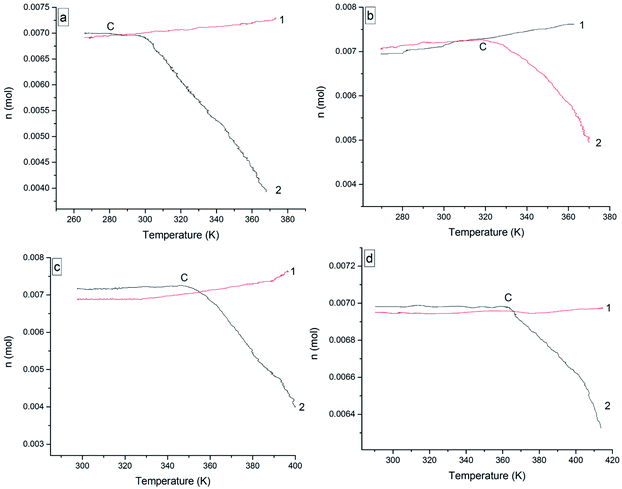 | ||
| Fig. 8 The value for n versus temperature for the four 3-phenyl-1-propene compounds, (a) cinnamaldehyde, (b) cinnamyl alcohol, (c) β-methylstyrene, and (d) cinnamic acid; (1) nitrogen, (2) oxygen. | ||
In Fig. 7, the P–T (pressure vs. temperature) curves were directly obtained from the P–t and T–t relationships. The P–T curves for nitrogen atmosphere are a straight line without apparent change points, i.e., no chemical reaction occurred. However, the P–T curves for the oxygen atmosphere are not a straight line, and contain a pressure drop point (B), which showed that oxidation happened. When T > B, rapid oxidation took place. Insufficiently, the temperature of oxygen absorption could not be reflected in Fig. 7.
In Fig. 8, the value for n increased as the temperature increased under nitrogen atmosphere, which embodied the volatilization process of cinnamaldehyde, cinnamyl alcohol, and β-methylstyrene in the heating process. When the boiling point of the compounds is low, they are more volatile. Unlike the other three 3-phenyl-2-propene compounds, the value for n almost remained constant with increasing temperature. Cinnamic acid is a solid with low volatility, which can be ignored.
Under oxygen atmosphere, the value for n remained constant or slowly increased with temperature up to C and then decreased as the temperature increased in the four 3-phenyl-2-propene compounds system. When the rising rate of the value for n under oxygen atmosphere is less than that of nitrogen, oxygen was absorbed. So, the temperature of oxygen absorption (Ta) of cinnamaldehyde, cinnamyl alcohol, β-methylstyrene, and cinnamic acid was 271.25 K, 292.375 K, 323.125 K, and 363.875 K, respectively. As the experimental temperature rose, the value for n decreased gradually. The four 3-phenyl-2-propene compounds reacted with oxygen rapidly. The temperatures of rapid oxidation (TR) were calculated according to the method of exothermal onset temperature in differential scanning calorimetry (DSC). The TR of cinnamaldehyde, cinnamyl alcohol, β-methylstyrene, and cinnamic acid was 301.125 K, 332.75 K, 357.91 K, and 385.375 K, respectively. The TR is almost consistent with the temperature at which the pressure began to drop. Therefore, the reactions of the four 3-phenyl-2-propene compounds include their evaporation and oxidation with oxygen without thermal decomposition. The Ta and TR of the four 3-phenyl-1-propene compounds are shown in Table 1.
| Compounds | Atmosphere | Ta/K | TR/K |
|---|---|---|---|
| Cinnamaldehyde | N2 | — | — |
| Cinnamyl alcohol | N2 | — | — |
| β-Methylstyrene | N2 | — | — |
| Cinnamic acid | N2 | — | — |
| Cinnamaldehyde | O2 | 271.75 | 301.125 |
| Cinnamyl alcohol | O2 | 292.375 | 332.75 |
| β-Methylstyrene | O2 | 323.125 | 357.91 |
| Cinnamic acid | O2 | 363.875 | 385.75 |
The Ta and TR order for the four 3-phenyl-2-propene compounds was derived to be cinnamic acid > β-methylstyrene > cinnamyl alcohol > cinnamaldehyde. The Ta and TR reflected the reactivity of the four 3-phenyl-2-propene compounds with oxygen. Thus, the following oxidation reactivity order could be derived: cinnamaldehyde > cinnamyl alcohol > β-methylstyrene > cinnamic acid. The chemical structure characteristics were related to the oxidation characteristics, which could explain the reactivity sequence. The activity of the functional groups was related to the reactivity of the four 3-phenyl-2-propene compounds. The reaction sites of the four 3-phenyl-2-propene compounds included the carbon–carbon double bonds and substituents. Undoubtedly, the substituents' activity sequence is CHO > CH2OH > CH3 > COOH. Due to the high activity of the aldehyde groups, they are prone to oxidation to peroxide, which would initiate further oxidation and the reactivity of cinnamaldehyde was improved. Matte17 reported that the electronegativity of the oxygen atoms weakened the closest C–C bond, and the hydroxyl group, especially the carbonyl group, showed a reduced energy of the C–C bond, suggesting that aldehydes have higher reactivity with respect to the corresponding alcohols and alkanes. For low temperature oxidation, aldehyde was produced during the reaction of alcohol with O2, hindering the formation of QOOH,17 which is significant for inducing oxidation. This indicates that the alcohol has lower activity than that of the aldehyde. The mesomeric structures of the unshared pair of electrons in the oxygen singly bonded to the carbonyl carbon allow the ester resonance, thus stabilizing the methyl-butanoate group. Similarly, the carboxyl group of cinnamic acid lowers the reactivity. Moreover, the substituents have a great influence on the reactivity of olefins. The electronic effects of the terminal functional groups affected the activity of the carbon–carbon double bonds. Electron withdrawing groups attached to the alkene double bonds increased the reactivity of the carbon–carbon double bonds with oxygen, while electron donating groups decreased the reactivity. The activation ability of the X group in compound CH2 = CHX is in the descending order of CHO > COOR,26 which provided evidence for the low reactivity of cinnamic acid. However, the hydrogen bond between the cinnamic acid molecules allows it to have a minimum-energy structure, which requires higher activation energy to react. Also, the activation energy of cinnamic acid is the highest, as given in Section 3.2. The high activation energy of the reaction pathways is the “pinning-back” of the alcohol proton by hydrogen bonding, rendering it less likely to take part in any oxidation process.27 To sum up, the oxidation reactivity order was derived as cinnamaldehyde > cinnamyl alcohol > β-methylstyrene > cinnamic acid, which coincided with the MCPVT results.
3.2 Kinetics of oxidation of the four 3-phenyl-2-propene compounds
Chemical kinetics is an important method to study the reactivity and stability of the compounds. The four 3-phenyl-2-propene compounds are unstable under an oxygen atmosphere. Therefore, it is necessary to study the oxidation kinetics of the four 3-phenyl-2-propene compounds to determine the oxidation kinetics in the heating process using MCPVT. It is of great significance to understand the reactivity and thermal stability for the oxidation of the four compounds.It is assumed that the oxidation of the four 3-phenyl-2-propene compounds occurred as follows.
| P (l or s) + O2 (g) → products |
Kinetic equation (eqn (4)):
| dX/dt = k(A − X)α(B − X)β | (4) |
If we make the following assumptions
(1) The oxidation kinetics is a second-order reaction.
(2) Oxygen is an ideal gas, and the remaining number of moles of oxygen can be calculated by the ideal gas equation: nt = (B0 − X) = PV/RT (P is pressure, R is gas constant, T is temperature). For the experiment, nA0 = nB0 = 0.0071 mol.
So, eqn (5) is simplified by the integral eqn (4)
| X/(A − X)A = kt | (5) |
The rate constant:
k = A![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−Ea/RT) exp(−Ea/RT)
| (6) |
Then, eqn (7) can be obtained by the logarithm of eqn (5) and (6).
Y = −Ea/RT + ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) A = ln[X/(A − X)At] A = ln[X/(A − X)At]
| (7) |
Fig. 9 shows the Y ∼ 1/T plots of the four 3-phenyl-2-propene compounds in the heating process, which indicated that Y has a good linear relationship with 1/T. The Ea for the oxidation of the four compounds was obtained from the linear slope. The fitted linear equations and Ea are given in Table 2. The linear oxidation reaction equations of the four 3-phenyl-2-propene compounds are second-order reactions.
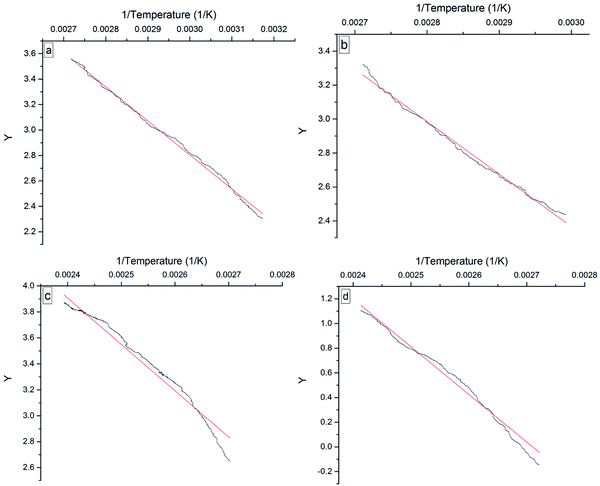 | ||
| Fig. 9 Y versus 1/temperature plots for the four 3-phenyl-1-propene compounds, namely, (a) cinnamaldehyde, (b) cinnamyl alcohol, (c) β-methylstyrene, and (d) cinnamic acid. | ||
| Compound | Equation | Ea/(kJ mol−1) | T/K | R2 |
|---|---|---|---|---|
| Cinnamaldehyde | Y = −2677.25 × (1/T) + 10.83 | 22.26 | 315–368 | 0.9966 |
| Cinnamyl alcohol | Y = −3091.31 × (1/T) + 11.64 | 25.70 | 334–370 | 0.9923 |
| β-Methylstyrene | Y = −3568.76 × (1/T) + 12.47 | 29.67 | 370–404 | 0.9847 |
| Cinnamic acid | Y = −3874.29 × (1/T) + 10.50 | 32.21 | 367–414 | 0.9805 |
The Ea is important to understand the nature and characteristics of the compounds and the chemical reactions, which represents the difficulty of the reaction at a certain temperature. In particular, cinnamaldehyde easily reacted with oxygen even at 304 K because of the low Ea. The thermal runaway of cinnamaldehyde was detected due to oxidation.25 The Ea is consistent with the oxidation reactivity of the four 3-phenyl-2-propene compounds. The Ea also showed that the oxidation reactivity order was cinnamaldehyde > cinnamyl alcohol > β-methylstyrene > cinnamic acid. As important components of special plant resources in Guangxi, the four 3-phenyl-2-propene compounds should be kept away from oxygen and air during storage, transportation, and usage.
3.3 Pressure behavior of the four 3-phenyl-2-propene compounds oxidation under isothermal conditions
To investigate the thermal oxidation behavior of the four 3-phenyl-2-propene compounds, isothermal oxidation experiments were conducted for 6 h by MCPVT under oxygen atmosphere. Equal number of moles of oxygen and the reactant were put into the experiment. The inner temperature and pressure of the reactor were traced when the vessel of cinnamaldehyde was heated to 298 K, 308 K, and 318 K respectively (when the vessel of cinnamyl alcohol was heated to 303 K, 323 K, and 333 K, respectively; when the vessel of cinnamyl alcohol was heated to 343 K, 353 K, and 363 K, respectively; when the vessel of β-methylstyrene was heated to 365 K, 378 K, and 388 K, respectively). The experimental results of the P–t (pressure vs. time) relationship are shown in Fig. 10.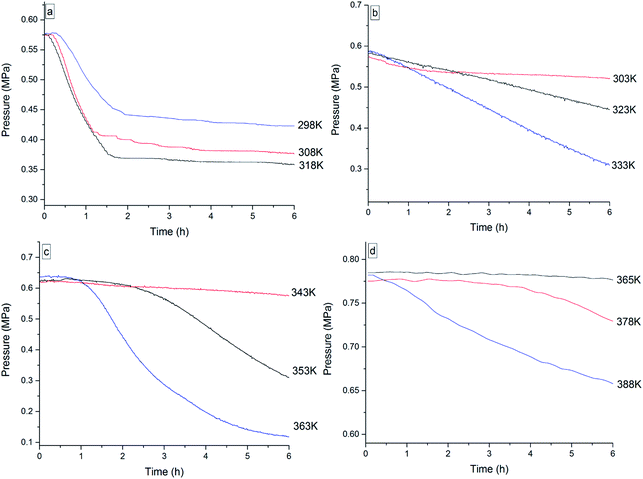 | ||
| Fig. 10 Pressure vs. time plots for the oxidation of the four 3-phenyl-2-propene compounds, namely, (a) cinnamaldehyde, (b) cinnamyl alcohol, (c) β-methylstyrene, and (d) cinnamic acid. | ||
The P–t curve under oxygen atmosphere was not a horizontal line and included the pressure-drop process, indicating that a great deal of oxygen was consumed and that the oxidation process was temperature-dependent. The oxygen consumption during the oxidation of the four 3-phenyl-2-propene compounds was calculated to measure the oxidation degree between the number of moles (n) at the beginning and at the end of the reaction in Table 3.
| (a) Oxygen consumption of cinnamaldehyde | |||
|---|---|---|---|
| Temperature (K) | 298 | 308 | 318 |
| Oxygen consumption (mol × 10−3) | 2.03 | 2.69 | 2.87 |
| (b) Oxygen consumption of cinnamyl alcohol | |||
|---|---|---|---|
| Temperature (K) | 303 | 323 | 333 |
| Oxygen consumption (mol × 10−3) | 7.73 × 10−1 | 1.71 | 3.34 |
| (c) Oxygen consumption of β-methylstyrene | |||
|---|---|---|---|
| Temperature (K) | 343 | 353 | 363 |
| Oxygen consumption (mol × 10−3) | 5.45 × 10−1 | 3.68 | 6.04 |
| (d) Oxygen consumption of cinnamic acid | |||
|---|---|---|---|
| Temperature (K) | 365 | 378 | 388 |
| Oxygen consumption (mol × 10−3) | 9.52 × 10−2 | 5.09 × 10−1 | 1.35 |
The results indicated that oxygen consumption rapidly increased when the reaction temperature increased. A relatively low oxygen absorption process was investigated for cinnamaldehyde at 298 K, cinnamyl alcohol at 308 K, β-methylstyrene at 333 K, and cinnamic acid at 365 K. The peroxide formation process of the four 3-phenyl-2-propene compounds with oxygen is nearly identical. In particular, oxygen consumption rapidly increased near the rapid oxidation temperature. This phenomenon was also observed in the oxidation process of ethers28 and abietic acid,29 which was explained by the mechanism of free radical chain reaction. A large number of free radicals were generated by the thermal decomposition of peroxide, accelerating the oxidation to produce complex secondary oxidation products. The accumulation of the free radical concentration resulted in the deep radical oxidation of the four 3-phenyl-2-propene compounds with oxygen. Thus, the combination of the four 3-phenyl-2-propene compounds with oxygen to generate peroxides is the key. The presence of peroxides could accelerate the reaction, which may cause thermal runaway.30
3.4 Peroxide value of the four 3-phenyl-2-propene compounds
The MCPVT results showed the evidence that oxygen was absorbed in the reaction, which is a complex oxidation process. It deserves careful consideration that some products would be formed in the initial oxidation at a low temperature. The structural characteristics of the four 3-phenyl-2-propene compounds enable the formation of peroxides. Peroxide is a hazardous compound, whose structure and thermal properties indicate that the O–O bond is fragile. Owing to its instability, peroxide is difficult to separate and characterize. To confirm the formation of the peroxide, the peroxide concentrations (peroxide value) were measured by iodimetry. The variation in the peroxide value with the reaction time for the four 3-phenyl-2-propene compounds at 373 K is shown in Fig. 11.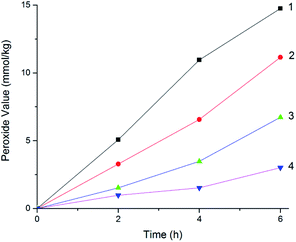 | ||
| Fig. 11 Peroxide value for the four 3-phenyl-2-propene compounds, (1) cinnamaldehyde; (2) cinnamyl alcohol; (3) β-methylstyrene; (4) cinnamic acid. | ||
The peroxide value results proved that peroxides were produced in the oxidation process. The change trend in the peroxide value of the four 3-phenyl-2-propene compounds with the oxidation time was similar. The peroxide value increased with the oxidation time. The order of the peroxide formation rate is as follows: cinnamaldehyde > cinnamyl alcohol > β-methylstyrene > cinnamic acid, which coincided with the chemical structure and the MCPVT results. The peroxide value of cinnamaldehyde, cinnamyl alcohol, β-methylstyrene, and cinnamic acid reached the maximum of 18.88 mmol kg−1, 15.07 mmol kg−1, 9.62 mmol kg−1, and 4.24 mmol kg−1 in 6 h, respectively. Peroxide was easy to form and relatively stable at low temperature. Thus, the peroxides would accumulate, which is reflected in the increase in the peroxide value. The peroxide formation process was analogous to the known mechanism for the auto-oxidation of lipids.31 Peroxide as the oxidation initiator could accelerate the reaction process. In the presence of organic peroxides,28 oxidation occurs easily. On exposure to physical shock or heat, the peroxide was easy to decompose due the vulnerability of the O–O bond. Many thermal runaway incidents have been caused by organic peroxides.32 Therefore, in order to avoid the formation of peroxides, the method of prohibiting oxygen or nitrogen protection should be used in the process of production, storage, and transportation.
3.5 Products of oxidation
The oxidation products are complex and interesting, which are essential to comprehend the characteristics of the oxidation process, reactivity, and mechanism. In order to study the initial oxidation products and explore the pathways for the oxidation of the four 3-phenyl-2-propene compounds, an in-depth study of the oxidation products' composition was undertaken by GC-MS, as shown in Tables 4–7. Because of the four 3-phenyl-2-propene compounds with phenylethenyl structure, benzaldehyde and epoxide as the common oxidation products were detected, which is the result of the unsaturated carbon–carbon bond being attacked by oxygen. The epoxidation pathway most likely proceeds via oxametallacycle intermediates, which are formed through oxygen addition to the carbon–carbon double bond.12 Epoxidation is the interaction between the peroxy radical and the carbon–carbon double bond.33,34 This indicated that epoxidation is an expression of oxidation of the carbon–carbon double bonds. The formation of epoxides was in the descending order of β-methylstyrene > cinnamaldehyde > cinnamyl alcohol > cinnamic acid. The epoxidation rate of olefins is mainly determined by the properties of the functional groups attached to the alkene double bond, and electron donating groups are favorable for epoxidation.11,35 However, in the case of such allylic alcohols having internal C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond or branching adjacent to the C
C bond or branching adjacent to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, the oxidative dehydrogenation of the –CH2OH group is more dominant.13 Alcohols reacted with oxygen mostly to produce the aldehyde at a low temperature.17 This explained the formation of cinnamyl alcohol epoxide with low selectivity in cinnamyl alcohol oxidation.
C bond, the oxidative dehydrogenation of the –CH2OH group is more dominant.13 Alcohols reacted with oxygen mostly to produce the aldehyde at a low temperature.17 This explained the formation of cinnamyl alcohol epoxide with low selectivity in cinnamyl alcohol oxidation.
| No. | Product | Molecular formula | Relative content (%) |
|---|---|---|---|
| 1 | Water | H2O | 0.21 |
| 2 | Carbon dioxide | CO2 | 0.76 |
| 3 | Acetaldehyde | C2H4O | 0.65 |
| 4 | Formic acid | CH2O2 | 2.06 |
| 5 | Acetic acid | C2H4O2 | 0.07 |
| 6 | Benzaldehyde | C7H6O | 25.2 |
| 7 | Phenylacetaldehyde | C8H8O | 7.02 |
| 8 | Acetophenone | C8H8O | 4.31 |
| 9 | Phenylglyoxylic acid | C8H8O2 | 1.48 |
| 10 | Ethyl benzoate | C9H10O2 | 1.46 |
| 11 | 3-Phenyloxirane-2-carbaldehyde (epoxide) | C9H8O2 | 10.28 |
| 12 | Benzoic acid | C7H6O2 | 4.93 |
| 13 | Ethyl phenylacetate | C10H12O2 | 0.47 |
| 14 | 2-Hydroxyacetophenone | C8H8O2 | 4.62 |
| 15 | Cinnamaldehyde | C9H8O | 22.25 |
| 16 | Ethyl cinnamate | C11H12O2 | 6.64 |
| 17 | Cinnamic acid | C9H8O2 | 7.37 |
| 18 | Unknown component | 0.22 |
| No. | Product | Molecular formula | Relative content (%) |
|---|---|---|---|
| 1 | Water | H2O | 2.42 |
| 2 | Methanol | CH4O | 0.25 |
| 3 | Formic acid | CH2O2 | 0.46 |
| 4 | Glycolaldehyde | C2H4O2 | 3.98 |
| 5 | Benzaldehyde | C7H6O | 18.59 |
| 6 | Benzeneacetaldehyde | C8H8O | 0.25 |
| 7 | 2-Hydroxyethyl benzoate | C9H10O3 | 0.72 |
| 8 | Phenylgloxal | C8H6O2 | 0.44 |
| 9 | Benzoic acid | C7H6O2 | 5.16 |
| 10 | Benzenepropanol | C9H12O | 0.38 |
| 11 | 2-Hydroxyacetophenone | C8H8O2 | 0.61 |
| 12 | Cinnamaldehyde | C9H8O | 14.47 |
| 13 | Cinnamyl alcohol | C9H10O | 34.49 |
| 14 | Cinnamyl formate | C10H10O2 | 5.15 |
| 15 | 2,3-Epoxy-3-phenylpropan-1-ol (epoxide) | C9H10O2 | 5.41 |
| 16 | 1-Propanone, 3-hydroxy-1-phenyl | C9H10O2 | 0.42 |
| 17 | Cinnamic acid | C9H8O2 | 1.12 |
| 18 | 1-Phenyl-1,2-ethanediol | C8H10O2 | 4.55 |
| 19 | 1,2-Dibenzoylethane | C16H14O2 | 1.15 |
| 20 | Unknown | 0.1 |
| No. | Product | Molecular formula | Relative content (%) |
|---|---|---|---|
| 1 | Acetaldehyde | C2H4O | 0.38 |
| 2 | Acetic acid | C2H4O2 | 0.13 |
| 3 | Benzaldehyde | C7H6O | 25.11 |
| 4 | β-Methylstyrene | C9H10 | 38.78 |
| 5 | 1,2-Epoxy-1-phenylpropane (epoxide) | C9H10O | 28.17 |
| 6 | Phenylacetone | C9H10O | 0.22 |
| 7 | Propiophenone | C9H10O | 0.41 |
| 8 | 1-Phenyl-1,2-propanedione | C9H8O2 | 0.56 |
| 9 | Benzoic acid | C7H6O2 | 1.53 |
| 10 | 1-Hydroxy-1-phenylacetone | C9H10O2 | 1.46 |
| 11 | Cinnamaldehyde | C9H8O | 2.14 |
| 12 | 1-Phenylpropane-1,2-diol | C9H12O2 | 1.85 |
| 13 | Cinnamic acid | C9H8O2 | 0.37 |
| 14 | Unknown | 0.19 |
| No. | Product | Molecular formula | Relative content (%) |
|---|---|---|---|
| 1 | Formic acid | CH2O2 | 0.13 |
| 2 | Acetic acid | C2H4O2 | 0.37 |
| 3 | Benzaldehyde | C7H6O | 7.14 |
| 4 | Benzeneacetaldehyde | C8H8O | 4.65 |
| 5 | Acetophenone | C8H8O | 0.29 |
| 6 | Benzoic acid | C7H6O2 | 1.71 |
| 7 | Cinnamic acid | C9H8O2 | 82.07 |
| 8 | 2-Oxiranecarboxylic acid, 3-phenyl-(epoxide) | C9H8O3 | 0.35 |
| 9 | Benzophenone | C13H10O | 0.26 |
| 10 | Isostilbene | C14H12 | 0.17 |
| 11 | α-Hydroxybenzeneacetic acid | C8H8O3 | 0.77 |
| 12 | Benzoylformic acid | C8H6O3 | 0.14 |
| 13 | Benzylphenyl ketone | C14H12O | 0.43 |
| 14 | Benzyl benzoate | C14H12O2 | 0.37 |
| 15 | Diphenylethanedione | C14H10O2 | 0.23 |
| 16 | Benzylideneacetophenone | C15H12O | 0.4 |
| 17 | 1,2-Dibenzoylethane | C16H14O2 | 0.31 |
| 18 | Unknown | 0.19 |
Benzaldehyde was obtained from the oxidative cleavage of the C–C bond of the oxirane ring, while phenylacetaldehyde could be oxidized to benzaldehyde.36 Benzaldehyde as the main product was observed in the oxidation. The yield of benzaldehyde increased with the increase in the temperature. The selectivity for benzaldehyde was able to reach 40%. Benzaldehyde is a good natural perfume with a large demand and wide application. Several methods for the synthesis of benzaldehyde from cinnamaldehyde have been reported, which have some limitations such as the use of a catalyst. Yang et al.37 investigated the selective oxidation of cinnamaldehyde to benzaldehyde in the presence of bicarbonate and hydrogen peroxide using β-cyclodextrin polymer as the catalyst. In the study, using oxygen as the green oxidant, the oxidation process was simple and mild, in which no pollution waste liquid was produced. The high selectivity was favorable for improving the utilization of raw materials. If the yield of benzaldehyde is further enhanced, a new thought can be provided for the synthesis of benzaldehyde from cinnamaldehyde (cinnamyl alcohol, β-methylstyrene) at low temperature.
Meanwhile, differential oxidation products were detected due to the difference in the substituent groups of the four 3-phenyl-2-propene compounds. Cinnamaldehyde was detected in the oxidation of cinnamyl alcohol38 and β-methylstyrene.1,22 Cinnamic acid was detected in cinnamaldehyde oxidation.16 However, significant difference was seen in cinnamic acid oxidation. The product of the carboxyl group of cinnamic acid was not detected. This is not surprising as the carboxyl group is relatively stable, which cannot be oxidized by oxygen. Also, the disruption of the oxidation of the propyl chain in cinnamyl alcohol and cinnamic acid would cause the formation of small molecules, such as formic acid. These oxidation products provide a deeper guidance for understanding the reactivity of the four 3-phenyl-2-propene compounds with oxygen, and are important to establish the oxidation pathways.
3.6 Pathways for the oxidation of the four 3-phenyl-2-propene compounds
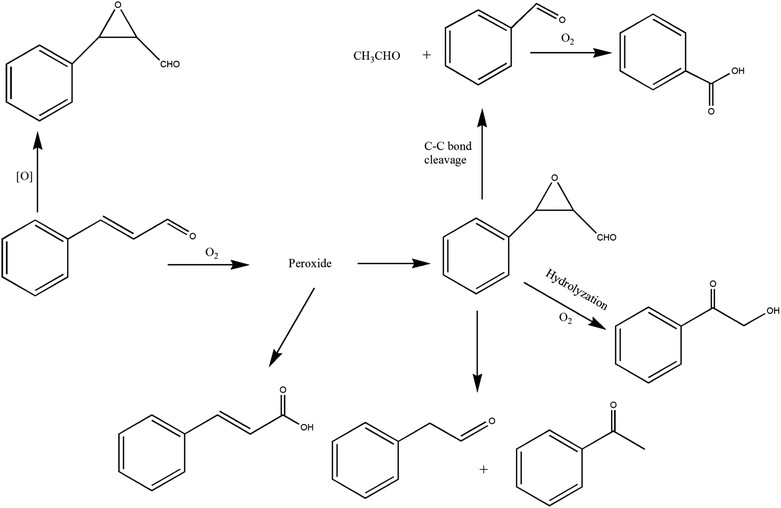
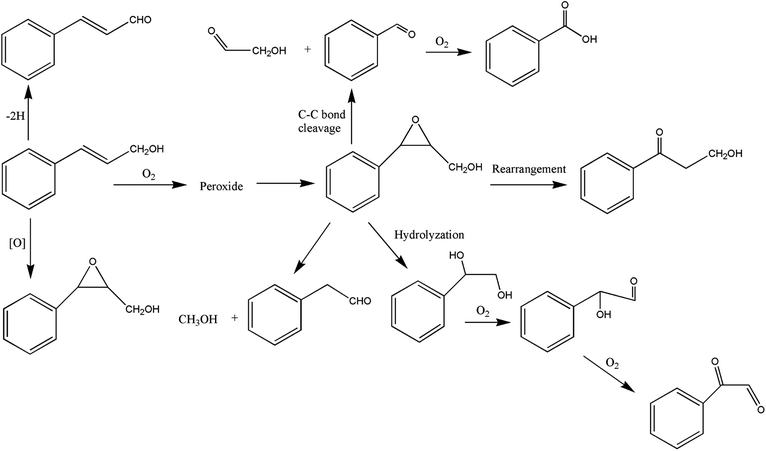
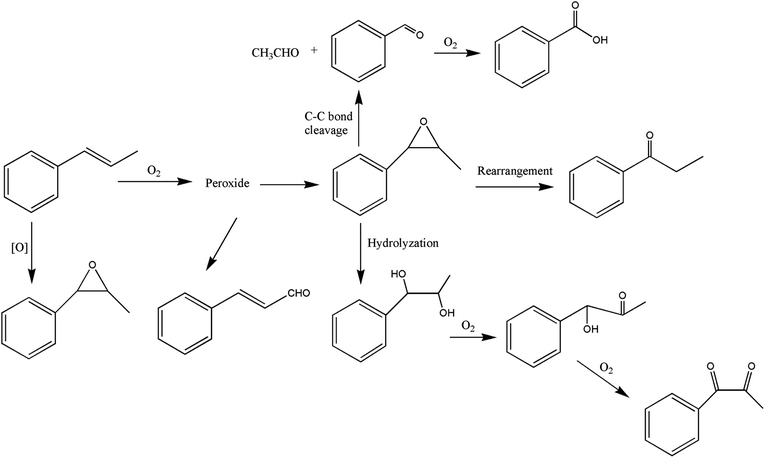
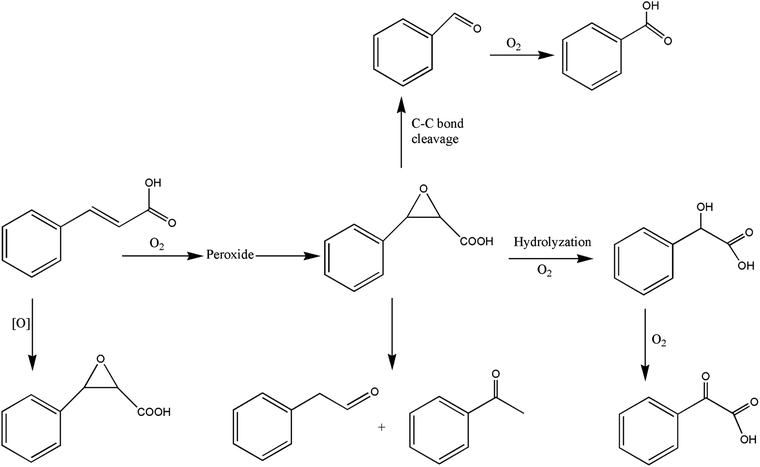
It can be seen from the variety of products that the oxidation of the four 3-phenyl-2-propene compounds is very complicated. The oxidation products give a helpful insight into the oxidation pathways, which are very important to understand the stability and oxidation process of the four 3-phenyl-2-propene compounds with oxygen. Associated with the mechanism reported in the literature, the simplified oxidation pathways of the four 3-phenyl-2-propene compounds in MCPVT are proposed, as shown in Schemes 1–5. The four 3-phenyl-2-propene compounds have two active functional groups. On the one hand, oxidation occurred at the carbon–carbon double bond. The carbon–carbon double bond of the four 3-phenyl-2-propene compounds includes the absorption of oxygen to form peroxide, followed by thermal decomposition and oxidation. Oxygen attacked the C–H bond and C–C double bond of the compounds to form hydroperoxides under certain circumstances.39–41 The hydroperoxides can react further to produce alcohols, ketones, peroxides, and more complex products. The epoxide compounds might be detected as the further products of peroxides.29 The plausible mechanism involves the transformation of cinnamaldehyde to an open peroxide,42 which could be cyclized into a cyclic compound. Furthermore, the epoxidation was ascribed to the addition of peroxyl radicals to the double bonds, followed by unimolecular ring-closure.43 The epoxides of cinnamaldehyde and cinnamyl alcohol were formed by the interaction between the double bonds and the peroxides.33 Similar views on the photooxidation pathway of the phytosterols have also been proposed.34 O2 addition to the allyl radicals generated the pinocarvyl (R(b)OO·) intermediate, which could eliminate the alkoxyl radical to form an epoxide in α-pinene autoxidation.44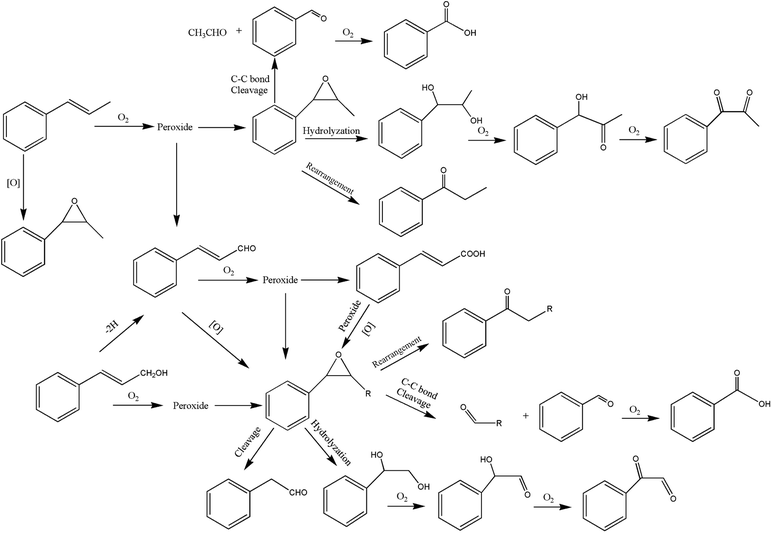 | ||
| Scheme 1 Proposed reaction pathways for the oxidation of the four 3-phenyl-2-propene compounds at a low temperature. | ||
The epoxide compounds as intermediates could produce benzaldehyde in significant amounts through C–C bond cleavage.45 Moreover, the epoxide compounds were converted into the glycol derivative or carbonyl compounds by ring-opening and isomerization reactions,46 such as 1-phenylpropane-1,2-diol and phenylacetone.
On the other hand, the reaction of the terminal substituent groups occurred. Cinnamaldehyde was obtained from the dehydrogenation of cinnamyl alcohol,47 and could also be formed through oxygen insertion into the terminal substituent groups of β-methylstyrene. Moreover, this process is accompanied by the formation of peroxides. The product of the carboxyl of cinnamic acid was not observed, which implied that the oxidation site of cinnamic acid was the carbon–carbon double bond only.
Throughout the whole oxidation processes, the substituent groups could improve the carbon–carbon double bonds reactivity; thus, the peroxides were formed easily, increasing the risk of oxidation. It is of great significance to avoid the exposure of the four 3-phenyl-2-propene compounds to air and heat, and control the storage conditions effectively.
4. Conclusions
The oxidation and the effect of the functional groups of the four 3-phenyl-2-propene compounds were studied by MCPVT. The conclusions are as follows.(1) The thermal stability of the four 3-phenyl-2-propene compounds was investigated. The results showed that the four 3-phenyl-2-propene compounds were stable under nitrogen atmosphere. The four 3-phenyl-2-propene compounds were extremely unstable under oxygen atmosphere. Also, the Ta was investigated, which was 271.25 K, 292.375 K, 323.125 K, and 363.875 K for cinnamaldehyde, cinnamyl alcohol, β-methylstyrene, and cinnamic acid, respectively. The TR of cinnamaldehyde, cinnamyl alcohol, β-methylstyrene, and cinnamic acid was calculated, which was 301.125 K, 332.75 K, 357.91 K, and 385.375 K, respectively.
(2) Staring from Ta, TR, and the effect of the substituent groups, the reactivity of the four 3-phenyl-2-propene compounds with oxygen was discussed. On the whole, the order of reactivity was cinnamaldehyde > cinnamyl alcohol > β-methylstyrene > cinnamic acid.
(3) The oxidation kinetics of the four 3-phenyl-2-propene compounds were determined using n versus time (n–t) of MCPVT, which were second-order reactions.
(4) The peroxides of the four 3-phenyl-2-propene compounds were proved by iodimetry. The peroxide value of cinnamaldehyde, cinnamyl alcohol, β-methylstyrene, and cinnamic acid reached 18.88 mmol kg−1, 15.07 mmol kg−1, 9.62 mmol kg−1, and 4.24 mmol kg−1 at 373 K for 6 h, respectively.
(5) The oxidation products of the four 3-phenyl-2-propene compounds were analyzed by GC-MS. The common oxidation products of the four 3-phenyl-2-propene compounds were benzaldehyde, benzoin acid, and epoxy, which resulted from the oxidation of the carbon–carbon double bond. The substituents' oxidation products of cinnamic acid and cinnamaldehyde were obtained in the oxidation of cinnamaldehyde, cinnamyl alcohol, and β-methylstyrene. However, no oxidation products of the carboxyl group of cinnamic acid were detected. The oxidation reaction pathways of the four 3-phenyl-2-propene compounds were constructed.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by National Natural Science Foundation of China (21776050), National Institute of Advanced Industrial Science and Technology Fellowship of Japan, Major Science and Technology Special Project in Guangxi (AA17204087-20).References
- E. Rucinska, P. J. Miedziak, S. Pattisson, G. L. Brett, S. Iqbal, D. J. Morgan, M. Sankar and G. J. Hutchings, Catal.: Sci. Technol., 2018, 8, 2987–2997 RSC.
- H. J. Cox, J. Li, P. Saini, J. R. Paterson, G. J. Sharples and J. P. S. Badyal, J. Mater. Chem. B, 2021, 9, 2918–2930 RSC.
- S. Bin Fang, H. Y. Ko, S. T. Huang, C. H. Huang, L. T. Li, C. C. Chen, K. C. Wang, C. P. Pai, H. C. Lee and H. W. Fang, RSC Adv., 2015, 5, 22097–22105 RSC.
- M. Somrani, M. C. Inglés, H. Debbabi, F. Abidi and A. Palop, Foods, 2020, 9, 1–12 CrossRef PubMed.
- Y. Wang, Foods, 2019, 423 CrossRef PubMed.
- I. B. Niklasson, T. Delaine, M. N. Islam, R. Karlsson, K. Luthman and A. T. Karlberg, Contact Dermatitis, 2013, 68, 129–138 CrossRef CAS PubMed.
- P. M. P. Santos and A. J. S. C. Vieira, J. Phys. Org. Chem., 2013, 26, 432–439 CrossRef CAS.
- J. Hu, J. Phys. Chem. C, 2013, 117, 16005–16011 CrossRef CAS.
- B. Singh and A. K. Sinha, J. Mater. Chem. A, 2014, 2, 1930–1939 RSC.
- A. Marimuthu, J. Zhang and S. Linic, Science, 2013, 340, 1590–1593 CrossRef PubMed.
- A. Bhaumik, R. Kumar, P. Ratnasamy and N. Delhi, Stud. Surf. Sci. Catal., 1994, 84, 1883–1888 CrossRef CAS.
- X. Liu and C. M. Friend, J. Phys. Chem. C, 2010, 114, 5141–5147 CrossRef CAS.
- N. Srinivas, V. Radha Rani, M. Radha Kishan, S. J. Kulkarni and K. V. Raghavan, J. Mol. Catal. A: Chem., 2001, 172, 187–191 CrossRef CAS.
- S. G. Yang, J. P. Hwang, M. Y. Park, K. Lee and Y. H. Kim, Tetrahedron, 2007, 63, 5184–5188 CrossRef CAS.
- S. Langa, B. C. Nyamunda and J. Heveling, Catal. Lett., 2016, 146, 755–762 CrossRef CAS.
- L. J. Durndell, C. Cucuzzella, C. M. A. Parlett, M. A. Isaacs, K. Wilson and A. F. Lee, Catal. Today, 2019, 333, 161–168 CrossRef CAS.
- M. Pelucchi, C. Cavallotti, E. Ranzi, A. Frassoldati and T. Faravelli, Energy Fuels, 2016, 30, 8665–8679 CrossRef CAS.
- K. A. Heufer, J. Bugler and H. J. Curran, Proc. Combust. Inst., 2013, 34, 511–518 CrossRef CAS.
- M. Friedman and J. A. Romersberger, J. Org. Chem., 1968, 33, 154–157 CrossRef CAS PubMed.
- S. Wall, J. Org. Chem., 1966, 31, 2888–2894 CrossRef.
- H. L. Maurice Morton, J. Am. Chem. Soc., 1952, 3523 CrossRef.
- J. M. Campbell, J. A. Smith, L. Gonzalez and K. D. Moeller, Tetrahedron Lett., 2015, 56, 3595–3599 CrossRef CAS.
- P. Huang, X. Liu, Y. Wada, K. Katoh, M. Arai and M. Tamura, Fuel, 2013, 105, 364–367 CrossRef CAS.
- A. Schreck, A. Knorr, K. D. Wehrstedt, P. A. Wandrey, T. Gmeinwieser and J. Steinbach, J. Hazard. Mater., 2004, 108, 1–7 CrossRef CAS PubMed.
- C. Yu, Y. L. Li, M. Liang, S. Y. Dai, L. Ma, W. G. Li, F. Lai and X. M. Liu, RSC Adv., 2020, 10, 19124–19133 RSC.
- H. Shenhav, Z. Rappoport and S. Patai, J. Chem. Inf. Model., 1981, 53, 1689–1699 Search PubMed.
- I. B. Niklasson, D. J. Ponting, K. Luthman and A. T. Karlberg, Chem. Res. Toxicol., 2014, 27, 568–575 Search PubMed.
- Q. Zhang, M. Kumasaki, X. Liu, F. Ren, Y. Nishiwaki, B. Wang and S. Matsue, Energy Fuels, 2019, 33, 12894–12904 CrossRef CAS.
- Y. Li, X. Xu, M. Niu, J. Chen, J. Wen, H. Bian, C. Yu, M. Liang, L. Ma, F. Lai and X. Liu, Energy Fuels, 2019, 33, 11200–11209 CrossRef CAS.
- C. S. Kao and K. H. Hu, J. Loss Prev. Process Ind., 2002, 15, 213–222 CrossRef.
- Z. A. M. Zielinski and D. A. Pratt, J. Am. Chem. Soc., 2016, 138, 6932–6935 CrossRef CAS PubMed.
- O. R. Valdes, V. Casson Moreno, S. Waldram, L. Véchot and M. Sam Mannan, Process Saf. Environ. Prot., 2016, 102, 251–262 CrossRef CAS.
- Y. Wang, P. Prinsen, F. Mangin, A. Yepez, A. Pineda, E. Rodríguez-Castellón, M. R. Hasan Shah Gilani, G. Xu, C. Len and R. Luque, Mol. Catal., 2019, 474, 110409 CrossRef CAS.
- Y. Zhao, B. Yang, T. Xu, M. Wang and B. Lu, Food Chem., 2019, 288, 162–169 CrossRef CAS PubMed.
- Bittmann, Chem. Informationsdienst, 1981, 12, 34 Search PubMed.
- H. Chen and H. Ji, Chin. J. Chem. Eng., 2011, 19, 972–977 CrossRef CAS.
- Z. Yang, H. Zeng, X. Zhou and H. Ji, Tetrahedron, 2012, 68, 5912–5919 CrossRef CAS.
- G. Wu, G. L. Brett, E. Cao, A. Constantinou, P. Ellis, S. Kuhn, G. J. Hutchings, D. Bethell and A. Gavriilidis, Catal.: Sci. Technol., 2016, 6, 4749–4758 RSC.
- D. E. Clark, Chem. Health Saf., 2001, 12–22 CrossRef CAS.
- G. Maerker, J. Am. Oil Chem. Soc., 1987, 64, 388–392 CrossRef CAS.
- M. Fujita, Y. Yamamoto, K. Watanabe, K. Suzuki and T. Kasahara, Chem. Res. Toxicol., 2021, 34(7), 1749–1758 Search PubMed.
- M. Friedman, N. Kozukue and L. A. Harden, J. Agric. Food Chem., 2000, 48, 5702–5709 CrossRef CAS PubMed.
- J. H. Teles, Applied Homogeneous Catalysis with Organometallic Compounds: A Comprehensive Handbook in Three Volumes, 2012, pp. 465–568 Search PubMed.
- U. Neuenschwander, F. Guignard and I. Hermans, ChemSusChem, 2010, 3, 75–84 CrossRef CAS PubMed.
- S. Antoniotti and E. Duñach, Synthesis, 2003, 2753–2762 CAS.
- D. M. Hodgson and E. Gras, Synthesis, 2002, 1625–1642 CrossRef CAS.
- G. Wu, G. L. Brett, E. Cao, A. Constantinou, P. Ellis, S. Kuhn, G. J. Hutchings, D. Bethell and A. Gavriilidis, Catal.: Sci. Technol., 2016, 6, 4749–4758 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ra04836h |
| This journal is © The Royal Society of Chemistry 2021 |