Terpenoids from the medicinal mushroom Antrodia camphorata: chemistry and medicinal potential†
Yi
Kuang
,
Bin
Li
 ,
Zilong
Wang
,
Xue
Qiao
,
Zilong
Wang
,
Xue
Qiao
 * and
Min
Ye
* and
Min
Ye
 *
*
State Key Laboratory of Natural and Biomimetic Drugs, School of Pharmaceutical Sciences, Peking University, 38 Xueyuan Road, Beijing 100191, China. E-mail: qiaoxue@bjmu.edu.cn; yemin@bjmu.edu.cn; Fax: +86-10-8280-2024; Tel: +86-10-8280-1516
First published on 28th July 2020
Abstract
Covering: up to February 2020
Antrodia camphorata is a medicinal mushroom endemic to Taiwan for the treatment of intoxication, liver injury, cancer, and inflammation. Owing to its rare occurrence and potent pharmacological activities, efforts have been devoted to identify its bioactive constituents, especially terpenoids. Since 1995, a total of 162 terpenoids including triterpenoids, meroterpenoids, sesquiterpenoids, diterpenoids, and steroids have been characterized. The ergostane-type triterpenoids (antcins) and meroterpenoids (antroquinonols) are characteristic constituents of A. camphorata. The terpenoids show anti-cancer, hepatoprotective, anti-inflammatory, anti-diabetic, and neuroprotective activities. This review summarizes the research progress on terpenoids in A. camphorata during 1995–2020, including structural diversity, resources, biosynthesis, pharmacological activities, metabolism, and toxicity. The medicinal potential of the terpenoids is also discussed.
1 Introduction
Antrodia camphorata (Polyporaceae family) is a rare and precious medicinal fungus in Taiwan, well known by local people as “Niu-Chang-Chih” (Fig. 1). It grows restrictedly on the decaying empty trunk of Cinnamomum kanehirai Hayata (Lauraceae), an endangered aromatic tree also endemic to Taiwan. This fungus was firstly reported in 1990 as Ganoderma comphoratum, and was then renamed as Antrodia cinnamomea.1,2 In 1997, G. comphoratum and A. cinnamomea were found to be conspecific, and this fungus was given the new name Antrodia camphorata (M. Zang & C. H. Su) Sheng H. Wu, Ryvarden & T. T. Chang.3 In 2004, morphological and genetic features indicated this species belonged to a new genus Taiwanofungus, and it was named Taiwanofungus camphoratus.4 This genus was then reinstated as Antrodia by analyzing the ITS region of the ribosomal RNA gene (rDNA).5 Now, the widely accepted official name for this mushroom is Antrodia camphorata, while A. cinnamomea and T. camphoratus are also used.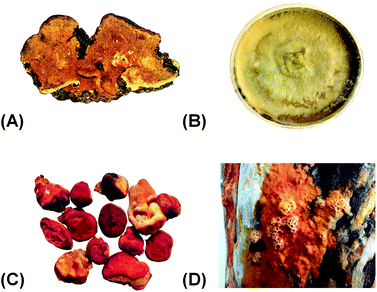 | ||
| Fig. 1 Pictures of Antrodia camphorata. (A) Wild fruiting bodies; (B) dish cultures; (C and D) cutting wood cultures. | ||
A. camphorata has been used as a folk medicine in Taiwan by the aborigines for alcohol intoxication treatment for a long history.6 It is also used as a herbal medicine or dietary supplement to treat various diseases including cancer, liver injury, inflammation, diarrhea, abdominal pain, hypertension, and itchy skin.7 For example, 12% cancer patients in Taiwan tend to use A. camphorata as an adjuvant therapeutic agent or nutrient during cancer treatment. The wild A. camphorata is very rare because C. kanehirai is the only host. The price for the wild fruiting bodies could reach $15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–25
000–25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 per kg.8 Thus, several cultivation techniques have been developed to meet the increasing market demands.9
000 per kg.8 Thus, several cultivation techniques have been developed to meet the increasing market demands.9
The chemical constituents of A. camphorata include terpenoids, maleic and succinic acid derivatives, benzenoids, benzoquinone derivatives, lignans, and polysaccharides.10 Among them, the terpenoids possess noticeable medicinal potential. For instance, the meroterpenoid antroquinonol is under phase II clinical trial against non-small cell lung cancer (ClinicalTrials.gov identifier: NCT02047344) (Table S1†).11 Previous reviews have summarized the bioactive constituents, biological activities, biosynthesis, or fermentation techniques of A. camphorata.6–15 The present review article focuses on the chemistry and medicinal potential of terpenoids in A. camphorata. Research progress during 1995–2020 in structural diversity, resources, biosynthesis, pharmacological activities, metabolism, and toxicity is summarized, and future perspectives are discussed.
2 Structural diversity
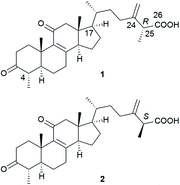
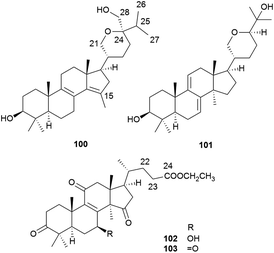
To date, a total of 162 terpenoids (1–162) have been reported from A. camphorata. Most of them were isolated from the fruiting bodies and mycelia, and a few were from the culture broth. According to the chemical skeletons, they can be classified into ergostane-type triterpenoids (1–75), lanostane-type triterpenoids (76–103), meroterpenoids (104–129), sesquiterpenoids (130–133), diterpenoids (134–140), and steroids (141–162). Among them, the most characteristic compounds for A. camphorata are ergostane-type triterpenoids (antcins) and meroterpenoids (antroquinonol and derivatives).2.1 Ergostane-type triterpenoids
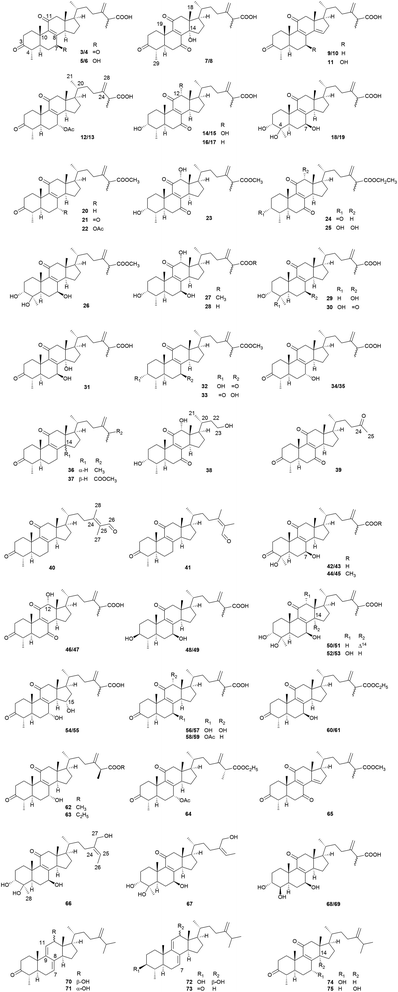
Most Antrodia ergostanes possess a unique C29 skeleton with an α-CH3 group at C-4, and a 24-exo-methylene-26-oic acid side chain at C-17β. Moreover, they usually occur as C-25 epimeric pairs (25R- and 25S-forms) (Fig. 2).Antcins A, B, and C were reported as the first ergostanes from A. camphorata in 1995.16 Thus far, a total of 75 ergostane-type triterpenoids (1–75) have been isolated and identified (Table 1). The most abundant ones are 25R/S-antcin B (3/4), 25R/S-antcin C (5/6), 25R/S-antcin H (14/15), and 25R/S-antcin K (18/19).17 For most of the Antrodia ergostanes, the A/B and C/D rings are both trans-fused (Fig. 3). The structural diversity lies in the oxidative substitutions. C-3, C-7, and C-11 are the most frequently oxidated positions, with a ketone group or α/β-OH at C-3, ketone or α/β-OH(OAc) at C-7, and ketone at C-11. C-4, C-12, and C-15 are occasionally substituted with a β-OH, α-OH (β-OH in a few cases), and α-OH, respectively. Some of them occur as methyl or ethyl esters at C-26. Most of the ergostanes possess a Δ8 double bond, with a few exceptions containing conjugated Δ7,9(11) double bonds. The ergostanes could be classified according to the oxidation degree of the tetracyclic skeleton.
| No. | Compounds | Formula | MW | Source | References |
|---|---|---|---|---|---|
| a *The compounds were isolated as C-25 epimeric mixtures. F, fruiting body; M, mycelium; D, dish culture. | |||||
| 1 | (25R)-Antcin A | C29H42O4 | 454 | F&M | 16 and 17 |
| 2 | (25S)-Antcin A | C29H42O4 | 454 | F&M | 16 and 17 |
| 3 | (25R)-Antcin B (zhankuic acid A) | C29H40O5 | 468 | F | 16 and 23 |
| 4 | (25S)-Antcin B (zhankuic acid A) | C29H40O5 | 468 | F | 16 and 23 |
| 5 | (25R)-Antcin C | C29H42O5 | 470 | F | 16 and 23 |
| 6 | (25S)-Antcin C | C29H42O5 | 470 | F | 16 and 23 |
| 7 | (25R)-Antcin D (zhankuic acid F) | C29H40O6 | 484 | F&D | 18 and 19 |
| 8 | (25S)-Antcin D (zhankuic acid F) | C29H40O6 | 484 | F&D | 18 and 19 |
| 9 | (25R)-Antcin E | C29H40O4 | 452 | F&D | 18 and 19 |
| 10 | (25S)-Antcin E | C29H40O4 | 452 | F&D | 18 and 19 |
| 11 | Antcin F* | C29H40O5 | 468 | F&D | 18 and 19 |
| 12 | (25R)-Antcin G | C31H44O6 | 512 | F&D | 18 and 24 |
| 13 | (25S)-Antcin G | C31H44O6 | 512 | F&D | 18 and 24 |
| 14 | (25R)-Antcin H (zhankuic acid C) | C29H42O6 | 486 | F | 23 and 25 |
| 15 | (25S)-Antcin H (zhankuic acid C) | C29H42O6 | 486 | F | 23 and 25 |
| 16 | (25R)-Antcin I (zhankuic acid B) | C29H42O5 | 470 | F&M | 17 and 25 |
| 17 | (25S)-Antcin I (zhankuic acid B) | C29H42O5 | 470 | F&M | 17 and 25 |
| 18 | (25R)-Antcin K | C29H44O6 | 488 | F&M | 23 and 28 |
| 19 | (25S)-Antcin K | C29H44O6 | 488 | F&M | 23 and 28 |
| 20 | Methyl antcinate A* | C30H44O4 | 468 | F | 20 |
| 21 | Methyl antcinate B* | C30H42O5 | 482 | F | 20 |
| 22 | Methyl antcinate G* | C32H46O6 | 526 | F | 19 |
| 23 | Methyl antcinate H* | C30H44O6 | 500 | F | 19 |
| 24 | Ethyl anticinate B* (zhankuic acid D) | C31H44O5 | 496 | F | 26 |
| 25 | Ethyl anticinate H* (zhankuic acid E) | C31H46O6 | 514 | F | 26 |
| 26 | Methyl antcinate K* | C30H46O6 | 502 | F | 29 |
| 27 | Methyl antcinate N* | C30H46O6 | 502 | F | 29 |
| 28 | Camphoratin A* (antcin N) | C29H44O6 | 488 | F | 21 |
| 29 | Camphoratin B* | C29H44O5 | 472 | F | 21 |
| 30 | Camphoratin C* | C29H42O6 | 486 | F | 21 |
| 31 | Camphoratin D* | C29H42O6 | 486 | F | 21 |
| 32 | Camphoratin E* | C30H44O5 | 484 | F | 21 |
| 33 | Camphoratin F* | C30H44O5 | 484 | F | 21 |
| 34 | (25R)-Camphoratin G | C29H42O5 | 470 | F&D | 18 and 21 |
| 35 | (25S)-Camphoratin G | C29H42O5 | 470 | F&D | 18 and 21 |
| 36 | Camphoratin H | C29H44O2 | 424 | F | 21 |
| 37 | Camphoratin J* | C30H44O4 | 468 | F | 21 |
| 38 | Antcamphin A | C24H36O5 | 404 | F | 22 |
| 39 | Antcamphin B | C26H36O4 | 412 | F | 22 |
| 40 | Antcamphin C | C29H42O3 | 438 | F | 22 |
| 41 | Antcamphin D | C29H42O3 | 438 | F | 22 |
| 42 | Antcamphin E (25S) | C29H42O6 | 486 | F | 22 |
| 43 | Antcamphin F (25R) | C29H42O6 | 486 | F | 22 |
| 44 | Antcamphin G (25S) | C30H44O6 | 500 | F | 22 |
| 45 | Antcamphin H (25R) | C30H44O6 | 500 | F | 22 |
| 46 | Antcamphin I (25R) | C29H40O6 | 484 | F | 22 |
| 47 | Antcamphin J (25S) | C29H40O6 | 484 | F | 22 |
| 48 | Antcamphin K (25S) | C29H44O5 | 472 | F | 22 |
| 49 | Antcamphin L (25R) | C29H44O5 | 472 | F | 22 |
| 50 | (25R)-Antcamphin N | C29H42O6 | 486 | D | 18 |
| 51 | (25S)-Antcamphin N | C29H42O6 | 486 | D | 18 |
| 52 | (25R)-Antcamphin O | C29H44O7 | 504 | D | 18 |
| 53 | (25S)-Antcamphin O | C29H44O7 | 504 | D | 18 |
| 54 | (25R)-Antcamphin P | C29H42O6 | 486 | D | 18 |
| 55 | (25S)-Antcamphin P | C29H42O6 | 486 | D | 18 |
| 56 | (25R)-Antcamphin Q | C29H42O6 | 486 | D | 18 |
| 57 | (25S)-Antcamphin Q | C29H42O6 | 486 | D | 18 |
| 58 | (25R)-Antcamphin R | C31H44O6 | 512 | D | 18 |
| 59 | (25S)-Antcamphin R | C31H44O6 | 512 | D | 18 |
| 60 | (25R)-Antcamphin S | C31H46O5 | 498 | D | 18 |
| 61 | (25S)-Antcamphin S | C31H46O5 | 498 | D | 18 |
| 62 | Antcamphin T | C30H44O5 | 484 | D | 18 |
| 63 | Antcamphin U | C31H46O5 | 498 | D | 18 |
| 64 | Antcamphin V | C33H48O6 | 540 | D | 18 |
| 65 | Antcamphin W* | C30H40O5 | 480 | D | 18 |
| 66 | Antcamphorol B | C28H44O5 | 460 | D | 27 |
| 67 | Antcamphorol C | C28H44O5 | 460 | D | 27 |
| 68 | Antcamphorol D (25R) | C28H42O6 | 474 | D | 27 |
| 69 | Antcamphorol E (25S) | C28H42O6 | 474 | D | 27 |
| 70 | Antcamphorol F | C29H44O2 | 424 | D | 27 |
| 71 | Antcamphorol G | C29H44O2 | 424 | D | 27 |
| 72 | Antcamphorol H | C29H46O2 | 426 | D | 27 |
| 73 | Antcamphorol I | C29H44O | 408 | D | 27 |
| 74 | Antcamphorol J | C29H44O3 | 440 | D | 27 |
| 75 | Antcamphorol K | C29H44O3 | 440 | D | 27 |
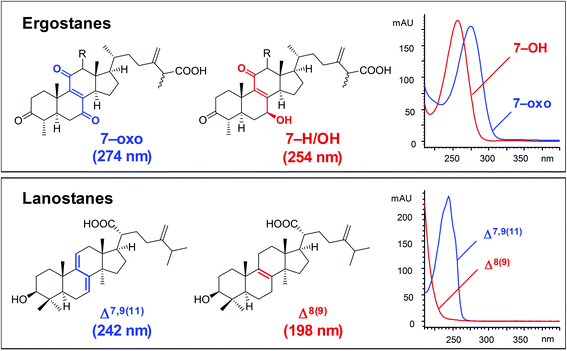 | ||
| Fig. 4 Typical UV spectra of ergostanes and lanostanes.30 | ||
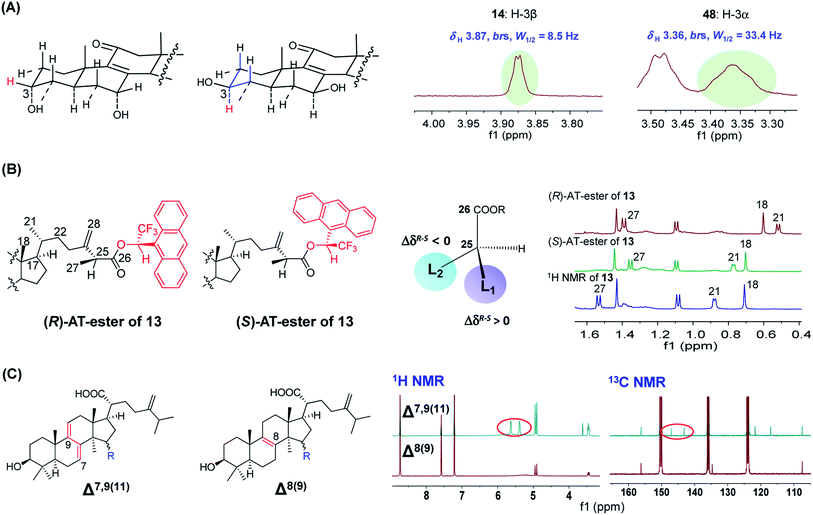 | ||
| Fig. 5 The applications of NMR spectroscopy for structural characterization of Antrodia triterpenoids. (A) 1H NMR to determine the stereo-configuration of H-3 of ergostanes. (B) 1H NMR combined with a chiral derivatizing reagent (AT) to determine the absolute configuration of C-25 of (25S)-antcin G (13). (C) 1H and 13C NMR to differentiate Δ7,9(11) and Δ8(9) lanostanes.22,23 | ||
The C-25 epimers of Antrodia ergostanes exhibit very similar physicochemical properties. They were obtained as a mixture in many early literatures. These epimers could be separated by supercritical fluid chromatography (SFC) using chiral or achiral packing materials.30 The epimers of hydrophilic ergostanes like antcin K (18/19) could also be separated by reversed-phase HPLC. In recent publications, most new ergostanes were isolated as pure optical forms using these techniques.
The absolute configurations for C-25 were determined using a chiral derivatizing reagent-based method for the first time by Du et al. in 2012.23 The carboxyl group at C-26 reacts with (R)- and (S)-1-(9-anthryl)-2,2,2-trifluoroethanonyl (AT) to obtain a pair of diasteromeric esters, and the absolute configuration of C-25 could be established by comparing the 1H NMR signals for surrounding protons of the two esters. For instance, for (25S)-antcin G (13), the signals for CH3-18 and CH3-21 (L2 group) of the (R)-AT-ester shifted upfield more significantly than signals of the (S)-AT-ester due to the anisotropic effect of AT anthracene group, thus leading to ΔδR–S < 0 (Fig. 5B).31
2.2 Lanostane-type triterpenoids
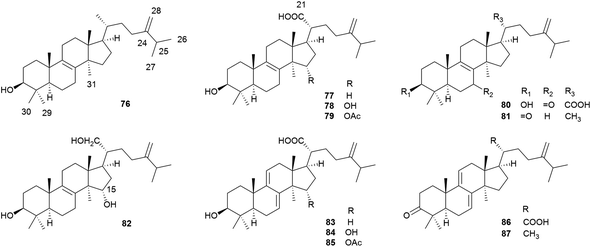
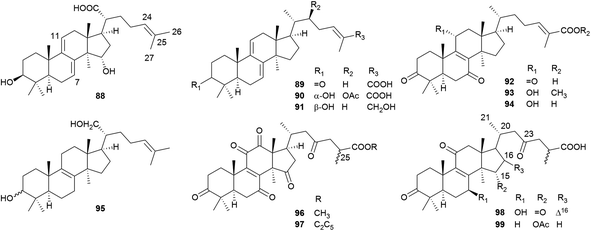
Lanostane-type triterpenoids are a group of tetracyclic triterpenoids derived from lanosterol, which is widely present in fungal species including Ganoderma lucidum, Poria cocos, Laetiporus sulphureus, Inonotus obliquus, and Daedalea dick-insii.32 They possess trans-junctions of A/B and C/D rings, a gem-dimethyl group at C-4, β-oriented methyl groups at C-10 and C-13, an α-oriented methyl group at C-14, and a β-oriented side chain at C-17. Most of the Antrodia lanostanes possess a C31 or C30 skeleton, with a hydroxyl or ketone group at C-3, and a carboxyl group at C-21 or C-26. They usually contain Δ8 and Δ7,9(11) double bonds, and occur as a pair, which are difficult to be separated. For some compounds, C-7 is substituted with a hydroxyl or ketone group, and C-15 has an α-OH/OAc group. The Antrodia lanostanes have lower degrees of oxidation than Ganoderma lanostanes like ganoderic acids, though their structures are very similar.33Thus far, a total of 28 lanostanes (76–103) have been isolated from A. camphorata (Table 2). Eburicol (76) is the first one reported from this species, and the most abundant ones are eburicoic acid (77), sulphurenic acid (78), dehydroeburicoic acid (83), and dehydrosulphurenic acid (84).
| No. | Compounds | Formula | MW | Source | References |
|---|---|---|---|---|---|
| a *The compounds were isolated as C-25 epimeric mixtures. F, fruiting body; M, mycelium; D, dish culture. | |||||
| 76 | Eburicol (24-methylenedihydrolanosterol) | C31H52O | 440 | F | 20 |
| 77 | Eburicoic acid | C31H50O3 | 470 | F | 28 |
| 78 | Sulphurenic acid | C31H50O4 | 486 | F | 28 |
| 79 | Versisponic acid D | C33H52O5 | 528 | F | 28 |
| 80 | Antcamphin X | C31H48O4 | 484 | D | 18 |
| 81 | Eburicone | C31H50O | 438 | D | 18 |
| 82 | 24-Methylenelanost-8-ene-3β,15α,21-triol | C31H52O3 | 472 | F | 22 |
| 83 | Dehydroeburicoic acid | C31H48O3 | 468 | F | 26 |
| 84 | Dehydrosulphurenic acid | C31H48O4 | 484 | F | 26 |
| 85 | 15α-Acetyl-dehydrosulphurenic acid | C33H50O5 | 526 | F | 26 |
| 86 | Dehydroeburiconic acid | C33H48O5 | 524 | D | 18 |
| 87 | 24-Methylenelanosta-7,9(11)-diene-3-one | C31H48O | 436 | D | 18 |
| 88 | 3β,15α-Dihydroxylanosta-7,9(11),24-trien-21-oic acid | C30H46O4 | 470 | F | 28 |
| 89 | Ganoderic acid Sz | C30H44O3 | 452 | F&M | 34 |
| 90 | Ganoderic acid S | C32H48O5 | 512 | F&M | 34 |
| 91 | Ganodermadiol | C30H48O2 | 440 | F&M | 34 |
| 92 | 3,7,11-Trioxo-5α-lanosta-8,24(E)-dien-26-oic acid | C30H42O5 | 482 | F&M | 34 |
| 93 | Methyl 11α-hydroxy-3,7-dioxo-5α-lanosta-8,24(E)-dien-26-oate | C31H46O5 | 498 | F&M | 34 |
| 94 | 11α-Hydroxy-3,7-dioxolanost-8,24(E)-dien-26-oic acid | C30H44O5 | 484 | F&M | 35 |
| 95 | Lanosta-8,24-diene-3,21-diol | C30H50O2 | 442 | F&M | 34 |
| 96 | Methyl-3,7,11,12,15,23-hexaoxo-5α-lanost-8-en-26-oate* | C31H40O8 | 540 | F&M | 34 |
| 97 | Ethyl-3,7,11,12,15, 23-hexaoxo-5α-lanost-8-en-26-oate* | C32H42O8 | 554 | F&M | 34 |
| 98 | 7β-Hydroxy-3,11,15,23-tetraoxo-27ξ-lanosta-8,16-dien-26-oic acid* | C30H40O7 | 512 | F&M | 35 |
| 99 | 15-O-Acetylganolucidate A* | C32H46O7 | 542 | F&M | 35 |
| 100 | Antcamphorol A | C31H50O3 | 470 | D | 27 |
| 101 | Hypocrellol A | C30H48O3 | 456 | D | 18 |
| 102 | Ethyl lucidenate A | C29H42O6 | 486 | F&M | 35 |
| 103 | Ethyl lucidenate F | C29H40O6 | 484 | F&M | 35 |
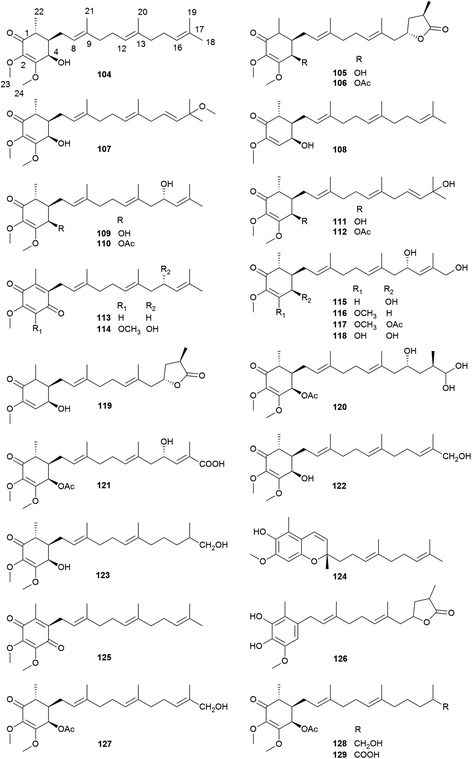
2.3 Meroterpenoids (antroquinonol and derivatives)
Antroquinonols are characteristic constituents of A. camphorata. They may originate from the combination of an orsellinic acid-derived C7 cyclohexenone and a C15 sesquiterpene unit, and thus belong to meroterpenoids. So far, 26 antroquinonols (104–129) have been isolated from the n-hexane extract of this mushroom (Table 3).36–46 Antroquinonol (104) was the first compound of this type reported from A. camphorata in 2007, followed by antroquinonol B (105) and 4-acetyl-antroquinonol B (106).36–38 Similar structures have also been reported from other fungi.39 Antroquinonol and its derivatives have attracted extensive attention due to their significant antitumor activities, which have been tested in clinical trials. Recently, the total synthesis of (±)-antroquinonol has been achieved, through a concise and efficient route.40,41| No. | Compounds | Formula | MW | Source | References |
|---|---|---|---|---|---|
| a F, fruiting body; M, mycelium; B, culture broth. | |||||
| 104 | Antroquinonol | C24H38O4 | 390 | F&M | 36 |
| 105 | Antroquinonol B | C24H36O6 | 420 | M | 37 |
| 106 | 4-Acetylantroquinonol B | C26H38O7 | 462 | B | 37 |
| 107 | Antroquinonol C | C25H40O5 | 420 | M | 42 |
| 108 | Antroquinonol D | C23H36O3 | 360 | F&M | 43 |
| 109 | Antrocamol LT1 | C24H38O5 | 406 | M | 44 |
| 110 | Antrocamol LT2 | C26H40O6 | 448 | M | 44 |
| 111 | Antrocamol LT3 | C24H38O5 | 406 | M | 44 |
| 112 | 4-Acetylantrocamol LT3 (4-acetylantroquinonol H) | C26H40O6 | 448 | M | 45 |
| 113 | Antroquinonol L | C23H32O3 | 356 | B | 38 |
| 114 | Antroquinonol N | C24H34O5 | 402 | B | 38 |
| 115 | Antroquinonol O | C23H36O5 | 392 | B | 38 |
| 116 | Antroquinonol P | C24H38O6 | 422 | B | 38 |
| 117 | Antroquinonol Q | C26H40O7 | 464 | B | 38 |
| 118 | Antroquinonol R | C23H36O6 | 408 | B | 38 |
| 119 | Antroquinonol S | C23H34O5 | 390 | B | 38 |
| 120 | Antroquinonol T | C26H42O8 | 482 | B | 38 |
| 121 | Antroquinonol U | C24H36O7 | 436 | B | 38 |
| 122 | Antroquinonol V | C24H38O5 | 406 | B | 38 |
| 123 | Antroquinonol W | C24H40O5 | 408 | B | 38 |
| 124 | Antroquinonol X | C23H32O3 | 356 | B | 38 |
| 125 | Ubiquinone Q3 | C24H34O4 | 386 | B | 38 |
| 126 | Antroubiquinol A | C23H32O5 | 388 | M | 46 |
| 127 | 4-Acetylantroquinonol I | C26H40O6 | 448 | M | 46 |
| 128 | 4-Acetylantroquinonol J | C26H42O6 | 450 | M | 46 |
| 129 | 4-Acetylantroquinonol K | C26H40O7 | 464 | M | 46 |
Most of the antroquinonols bear a cyclohexenone ring, a methoxyl group at C-2, a methyl at C-6, and Δ2, Δ8 and Δ12 double bonds (Fig. 9). Among them, 105, 106, 119, and 126 contain a γ-lactone ring at the side chain, and 106 is one of the most abundant meroterpenoids in A. camphorata. Compounds 113, 114 and 125 possess a 1,4-benzoquinone ring, while 124 contains a benzopyran ring.
2.4 Sesquiterpenoids, diterpenoids, and steroids
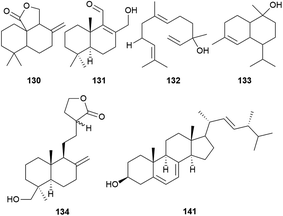
A total of four sesquiterpenoids (130–133) including a sesquiterpene lactone, a naphthalenecarboxaldehyde, a nerolidol, and a cadinol, and seven labdane diterpenoids (134–140) have been reported from A. camphorata (Fig. 10, Table S2 and Fig. S1†). As a unique drimane-type sesquiterpenoid with potent anticancer activities, the total synthesis of antrocin (130) has been reported.47 While steroids and terpenoids are generally considered as two different classes of natural products, the 22 steroids (141–162) from A. camphorata were collected into this review article due to the close biosynthetic relationships. Among the steroids, ergosterol (141) is a major constituent of A. camphorata.3 Resources and quality evaluation
Due to the increasing demands and special growing conditions, different cultivation techniques for A. camphorata have been developed, including cutting wood culture, dish culture, submerged fermentation, and solid support culture.8 Different cultivation techniques and conditions may lead to remarkably different chemical profiles (Fig. 11).17,48 The cutting wood cultures grow on the wood of Cinnamomum trees. The chemical constituents are the most similar to the wild growing mushroom, though the cultivation may take 2–3 years and the cost is high. The submerged fermentations use mixed nutrients and natural extracts as the media, and the solid support cultures use grains or agricultural by-products as the substrates. Their cultivation duration is short (several weeks). It is generally considered that these two types of cultures contain low amounts of triterpenoids and high amounts of polysaccharides. However, the addition of certain nutrients may remarkably increase the production of triterpenoids.9 The dish cultures usually grow on agar media containing different nutrients in petri dishes, and the cultivation duration is relatively short. More importantly, its chemical constituents are similar to those of cutting wood cultures, and it is suitable for large-scale production. Moreover, chemical constituents for the fruiting bodies and the mycelia of the fungus may also vary remarkably. Thus, it is important to evaluate the quality of Antrodia samples.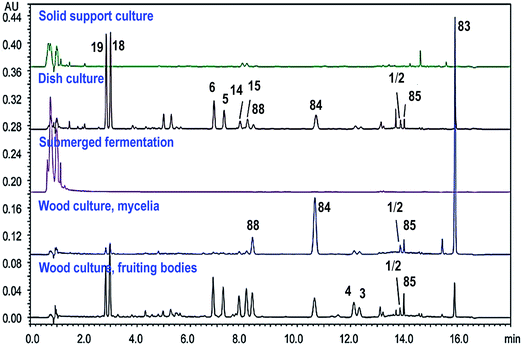 | ||
| Fig. 11 UPLC/UV chromatograms of triterpenoids in different cultures of Antrodia camphorata (250 nm). Adapted from ref. 17. | ||
For sample extraction, aqueous organic solvents like 50% methanol may be used to extract the ergostanes, and methanol or ethanol needs to be used for the lanostanes, because the Antrodia lanostanes usually have lower polarities than the ergostanes.17 A number of methods have been developed for the analysis of triterpenoids in A. camphorata. The C-25 epimeric ergostane mixtures could be separated by supercritical fluid chromatography, capillary electrophoresis, or reversed-phase liquid chromatography.23,30,49 Our group developed a UHPLC/DAD/qTOF-MS method to characterize 49 triterpenoids in A. camphorata, and 31 compounds were confirmed by comparing with reference standards. Moreover, the contents of 18 major triterpenoids in 15 batches of A. camphorata samples were determined by UPLC/UV or SFC/MS. The highest contents for the most important compounds are listed as follows: 25R/S antcin B (3/4, 17.8 mg g−1), 25R/S antcin C (5/6, 20.4 mg g−1), 25R/S antcin H (14/15, 31.3 mg g−1), 25R/S antcin K (18/19, 53.5 mg g−1), eburicoic acid (77, 13.9 mg g−1), sulphurenic acid (78, 23.8 mg g−1), dehydroeburicoic acid (83, 30.1 mg g−1), and dehydrosulphurenic acid (84, 26.6 mg g−1). This was the first time that the contents of pure optical forms for 25R-/S-ergostane epimers were quantitatively determined.17
4 Biosynthesis of triterpenoids and antroquinonols
4.1 Genome and transcriptome analysis
The first genome draft (32.15 Mb, 9254 genes) of A. camphorata was reported in 2014.50 A new 29.7 Mb genome analysis was published in 2018.51 The genomes of related mushrooms like Postia placenta, Ganoderma lucidum, and Ganoderma australe have also been sequenced.52–54 Although A. camphorata is morphologically and chemically similar to Ganoderma species, it is evolutionarily closer to the brown-rot fungus P. placenta. The genome data (730.7 Mb) of Cinnamomum kanehirae, the native host of A. camphorata, has been published recently.55 Two peroxidases may contribute to the wood degradation and colonization capacity of the fungus in this unique habitat.56 The transcriptomes of both fruiting body and mycelium of Antrodia camphorata have been reported.50,51,57 These data are valuable to study the growth, development, biosynthesis, and parasitic mechanisms of A. camphorata.4.2 Biosynthesis of antroquinonols
Antroquinonols have a highly similar structural skeleton to coenzyme Q (CoQ).58 They may originate from the combination of an orsellinic acid-derived C7 cyclohexenone and a C15 sesquiterpene, which are from the polyketide or shikimate pathway and the mevalonate pathway, respectively (Fig. 12). The polyketide synthase gene pks63787 may participate in the biosynthesis of orsellinic acid, a possible precursor for the benzoquinone ring (Table S3†).59,60 On the other hand, 4-hydroxybenzoic acid from the shikimate pathway was reported as a precursor for antroquinonol (104).61Ac-mvd, a mevalonate pyrophosphate decarboxylase gene, may be involved in its upstream biosynthesis.62 The γ-lactone ring of 4-acetylantroquinonol B (106) was proposed to form before the aromatic farnesylation, by condensation of the terminal carboxyl group with the hydroxyl group, or by cyclization of the oxidized terpene.58,63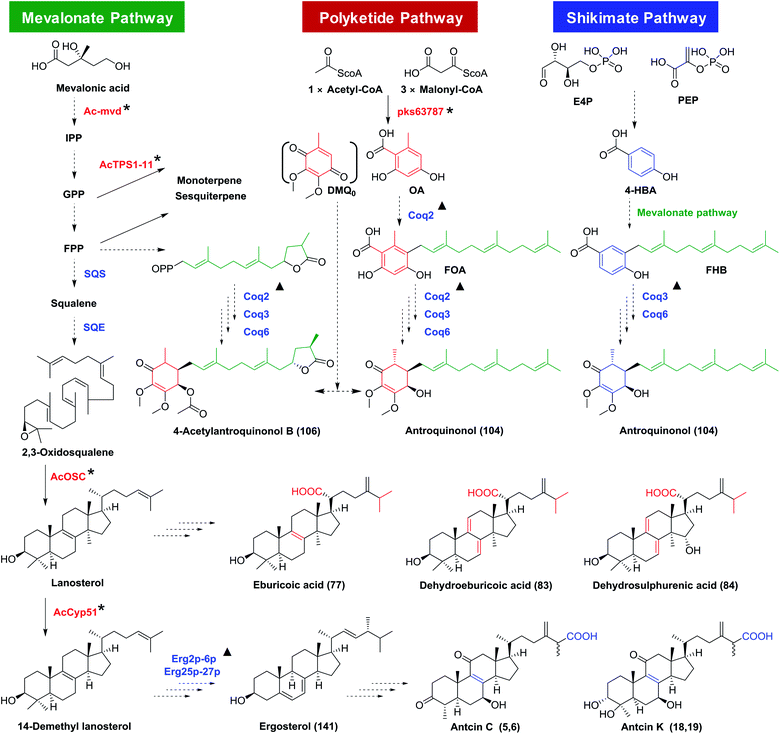 | ||
| Fig. 12 Proposed biosynthesis pathways for antroquinonols and triterpenoids in Antrodia camphorata. SQS, squalene synthase; SQE, squalene monooxygenase or epoxidase; Coq2/3/6, coenzyme Q2/3/6; IPP, isopentenyl diphosphate; GPP, geranyl diphosphate; FPP, farnesyl diphosphate; OA, orsellinic acid; DMQ0, demethoxy-coenzyme Q0; 4-HBA, 4-hydroxybenzoic acid; FOA, 3-farnesyl-orsellinic acid; FHB, 3-farnesyl-4-hydroxybenzoic acid. *, genes characterized from A. camphorata; ▲, similar genes from yeast.15,58,61 | ||
4.3 Biosynthesis of triterpenoids
The triterpenoids are derived from the mevalonate pathway. AcOSC is a 2,3-oxidosqualene cyclase (OSC) of A. camphorata catalyzing the formation of lanosterol. Lanosterol could be further oxidized to form lanostane-type triterpenoids (Table S3†).64 The biosynthetic genes for ergostanes are not clear yet, though they have been proposed to be derived from lanostanes. AcCyp51, encoding a sterol 14α-demethylase, was reported as a functional gene in the biosynthesis of ergostanes.65 Besides, ten potential triterpenoid P450 genes have been discovered for high expression during the formation of fruiting bodies, though their functions have not been characterized.66 The proposed biosynthetic pathway for triterpenoids in A. camphorata is shown in Fig. 12.4.4 Biosynthesis of other terpenoids
Ten terpenoid synthases have been characterized from A. camphorata. They could utilize GPP or FPP to form mono- and sesqui-terpenoids, respectively. They may be involved in the biosynthesis of antrocin (130).674.5 Elicitors for triterpenoid and antroquinonol production
Many strategies have been reported to improve the terpenoids production in A. camphorata. The content of 4-acetylantroquinonol B (106) was significantly improved by adding to the culture medium precursor compounds, including 4-hydroxybenzoic acid, vanillic acid, 2,4-dihydroxybenzoic, 2,4,5-trimethoxybenzaldehyde, coenzyme Q0, coenzyme Q10, or nerolidol.68,69 The production of antroquinonols could also be improved when orsellinic acid, camphorwood leach liquor, soybean oil, or H2O2 were added.70,71 The content of antroquinonol (104) in the submerged fermentation could reach 80.1 mg L−1.72 Moreover, monoterpene, tangerine oil, red light, or α-terpineol could act as elicitors for the biosynthesis of triterpenoids.73–755 Pharmacological activities and medicinal potential
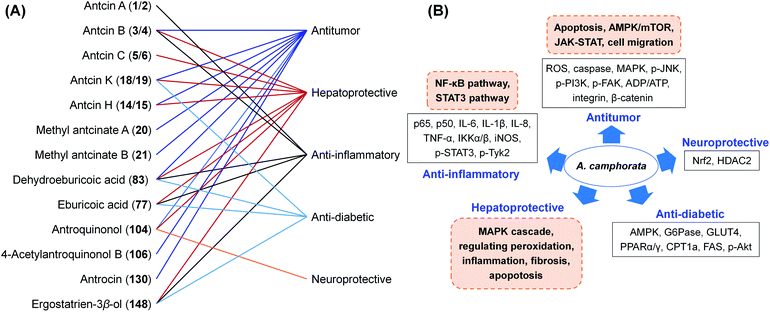
A. camphorata has been used for hepatoprotection and alcohol intoxication treatment by Taiwan local residents for a long history. A plenty of investigations have been reported on the pharmacological activities and mechanisms of A. camphorata extracts and the major compounds. These researches were summarized here with special focus on triterpenoids and antroquinonols with medicinal potential (Fig. 13).5.1 Anti-cancer activities
The extracts and compounds of A. camphorata exhibited a broad spectrum of anticancer activities on different types of cancer, including bladder cancer, breast cancer, liver cancer, lung cancer, pancreatic cancer, squamous cell carcinoma, leukemia, and adenocarcinoma (Table S4†). For in vitro experiments, the IC50 values ranged from 2.36–170 μg mL−1.76–84 Some results were proved by in vivo experiments. For example, the treatment with an ethanol extract of fruiting bodies (0.9 g kg−1, p.o., for 2 weeks) could inhibit the proliferation and migration of murine leukemia WEHI-3 cells and their tumorigenicity in a BALB/c mice allograft tumor model, via reducing p-ERK1/2, p-Akt and MMP-9, and upregulating p21 and p27.79 A clinical research reported that the dish cultured fruiting bodies (5 to 10 g d−1 for 6 months) prolonged survival of an 88-year-old patient with small cell lung cancer.85Antroquinonol (104), 4-acetylantroquinonol B (106), and antroquinonol D (108) exhibited anticancer effects against many cell lines, including colon cancer, lung cancer, liver cancer, breast cancer, and glioblastoma cancer, mainly through AMPK/mTOR and JAK-STAT pathways.38,43,86–90 For instance, 4-acetylantroquinonol B (2.5 mg kg−1, i.p., for 6 weeks) exhibited anticancer effects in colorectal cancer xenograft nude mice, via regulation of Lgr5/Wnt/β-catenin and JAK-STAT pathways.88 Due to its potent antitumor activities, a number of clinical studies on antroquinonol have been conducted (Table S1†). The phase I clinical trial of Hocena® (the trade name of antroquinonol) against NSCLC started in 2010, and the phase II trial started in 2013 in the US and Taiwan (ClinicalTrial.gov: NCT01134016, NCT02047344). The study indicated that antroquinonol at up to 600 mg daily for 4 weeks was generally safe.91
Triterpenoids and sesquiterpenoids from A. camphorata also showed potent anticancer effects. Dehydroeburicoic acid (83) possessed cytotoxicities against HL-60 and U87MG cancer cells with IC50 values of 3.4 and 25.7 μM, respectively.92,93 It could suppress tumor size in HL60 xenografted female immune-deficient athymic mice through DNA damaging and mitochondrial dysfunction. Antcins B, H, K (3/4, 14/15, 18/19) and methyl antcinates A, B (20, 21) exhibited promising cytotoxic activities against liver, colon, oral, and breast cancer cells with IC50 values of 22.3–78.0 μM.94–100 The potential mechanism was through suppression of apoptosis via endoplasmic reticulum stress, and suppression of integrin-mediated adhesion, migration, and invasion (Fig. 14). Antrocin (130) exhibited antitumor effects on lung cancer and breast cancer cells both in vitro and in vivo.101,102
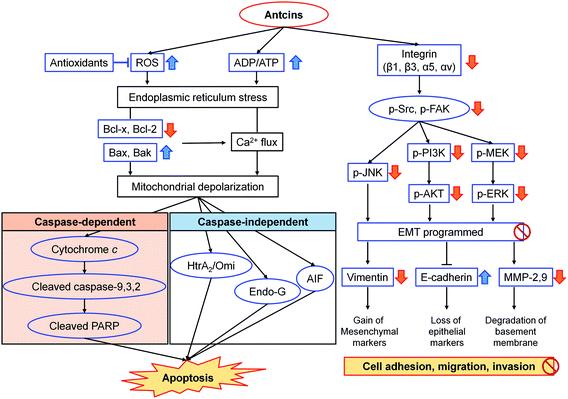 | ||
| Fig. 14 Proposed mechanism of action of antcins B (3/4), H (14/15), K (18/19), and methyl antcinate A (20) in the suppression of cancer.95–99 | ||
Furthermore, A. camphorata possessed synergistic anti-tumor effects with chemotherapeutic agents like mitomycin, cisplatin, trichostatin A, amphotericin B, paclitaxel, sorafenib, 5-fluorouracil, and methotrexate.103–109 For instance, an ethanol extract of A. camphorata significantly increased the tumor inhibition efficacy of sorafenib to 52% on Huh-7 xenograft NOD-SCID mice (100 mg kg−1 extract and 2.5 mg kg−1 sorafenib, i.p., 7 weeks), by targeting mitogen-activated protein (MAP) kinases, modulating cyclin proteins expression, and inhibiting cancer cell invasion.108 Antcin H (14/15, 20 mg kg−1, intratumorally, every other day for 24 days) could enhance the cytotoxicity of low-dose methotrexate (0.5 mg kg−1), significantly suppress tumor growth from 43% to 72%, and prolong the survival from 93.5 days to 106.5 days on Raji lymphoma xenograft NOD-SCID mice, via inhibiting LMP1-induced JAK/STAT related signalling.110 Antrocin (130, 100 mg kg−1) effectively enhanced the radiosensitivity on LAPC4-KD xenografted male BALB/c-nu mice, decreasing tumor size from 76.4% to 88.2% through inhibiting PI3K/AKT and MAPK signalling pathways.111 Our group found that (25R)-antcamphin R (58) and dehydrosulphurenic acid (84) at 20 μM could remarkably enhance the cytotoxicity of paclitaxel (10 nM) against HL60 cells, with inhibition rates increased from 19.6% to 58.2% and 70.6%, respectively.18
5.2 Hepatoprotective activities
The hepatoprotective activities of A. camphorata and its terpenoids have been studied extensively using different hepatotoxicity models. These models were induced by alcohol, galactosamine/TNF-α, acetaminophen (APAP), thioacetamide, carbon tetrachloride (CCl4), lipopolysaccharide (LPS), concanavalin A, or 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) (Table S5†). The proposed mechanisms were summarized in Fig. 15.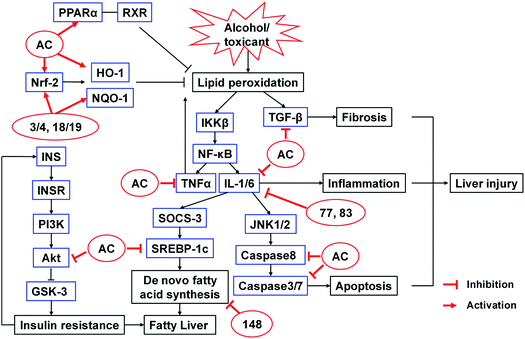 | ||
| Fig. 15 Proposed mechanisms of action of A. camphorata on alcohol or toxicant induced liver injury.112,113,115–119 AC, Antrodia camphorata extract; 3/4, antcin B; 18/19, antcin K; 77, eburicoic acid; 83, dehydroeburicoic acid; 148, ergostatrien-3β-ol. | ||
The fermented mycelia of A. camphorata (0.5–1.0 g kg−1, p.o., for 4 weeks) reduced liver fibrosis induced by CCl4 in rats by scavenging free radical formation.112 Petri-dish cultured A. camphorata (0.3, 0.6, 1.2 g kg−1, p.o., for 30 days) could protect alcohol-induced hepatic injury in Kunming mice by reducing the serum levels of triglyceride (TG), aspartate aminotransferase (AST), alanine aminotransferase (ALT), and malondialdehyde (MDA).113
Antcin H (14/15, 25 and 50 mg kg−1, i.p.) could protect against liver injury in both galactosamine/TNF-α and APAP-induced mice models, reducing the ALT and glutathione (GSH) levels, through disruption of the binding of p-JNK to Sab, which interfered with the ROS-dependent self-sustaining activation of MAPK cascade.114 Our group screened the protective activities of 26 Antrodia triterpenoids (20 μM) on CCl4-induced HepG2 cell injury model. The compounds could increase the cell viabilities from 50% to >80%. Among them, antcins B and K (3/4, 18/19) (50 mg kg−1, p.o., for 7 days) ameliorated CCl4-induced liver injury in mice, decreasing ALT and AST levels and the incidence of liver necrosis. The activities were even more potent than silymarin.115 Eburicoic acid (77) and dehydroeburicoic acid (83) (20 mg kg−1, i.p., for 7 weeks) could protect against CCl4-induced acute hepatic damage in male ICR mice via antioxidant and anti-inflammatory mechanisms.116 The in vivo protective effects of antcin C (5/6) and antroquinonol (104) against AAPH and alcohol-induced acute liver damages have also been reported.117,118 Furthermore, Antrodia compounds also exhibited significant hepatoprotective effects on chronic mice models. For instance, antrosterol (148) (1, 5, 10 mg kg−1, p.o., for 4 weeks) reduced serum and liver lipids of alcohol-diet fed mice by regulating lipid homeostasis, antioxidant capability, alcohol metabolism, and anti-inflammation.119 It lowered fibrosis related gene expressions, as well as the serum levels of AST, ALT, TNF-α, and IL-1β.
5.3 Anti-inflammatory activities
Inflammation is caused by a variety of stimuli, and could lead to a plenty of diseases.120A. camphorata possesses promising anti-inflammatory activities. The methanol extracts of both solid-state-cultured mycelia and wood-cultured fruiting bodies (300 mg kg−1, i.p., for 2 weeks) alleviated hyperoxia-induced lung injury in transgenic mice (NF-κB-luciferase+/+), reducing neutrophil infiltration, lung edema, ROS generation, and the expression of IL-6, TNF-α, IL-1β, IL-8, IKKα/β and iNOS.121 The methanol extract of solid-state-cultured mycelia (50, 250 mg kg−1, p.o., for 5 weeks) reduced Dermatogoides pteronyssinus-induced airway hyperresponsiveness in pathogen-free male BALB/c mice. It could reduce total serum IgE level, recruit inflammatory cells to the bronchoalveolar lavage fluid through cytokine downregulation and Th1/Th2/Th17 response modulation, and exert synergistic effects on multiple targets to relieve asthma symptoms.122The total triterpenoids extract (5–20 mg kg−1, p.o., for 35 days) reduced inflammation to promote wound healing on streptozotocin (STZ) induced hyperglycemia-diabetes mice.123 Eburicoic acid, dehydroeburicoic acid, and ergostatrien-3β-ol (148) (10 mg kg−1, i.p., single dose) decreased the paw edema in ICR mice induced by carrageenan, via increasing the activities of catalase (CAT), superoxide dismutase (SOD), and glutathione peroxidase (GPx).124,125 Antcins A and B exhibited potent inhibition against N-formylmethionyl-leucyl-phenylalanine (fMLP)-induced superoxide production with IC50 values of below 10 μM.126 A number of lanostanes significantly suppressed NO production in LPS-treated murine macrophage RAW 264.7 cells with IC50 values of below 10 μM.35
5.4 Anti-diabetic activities
The ethanol extract of A. camphorata increased high glucose-induced insulin secretion dose-dependently through peroxisome proliferator-activated receptor-γ (PPAR-γ) pathway, and protected from ER stress-induced apoptosis in MIN6 cells.127 The nanoencapsulated triterpenoid extracts of dish-cultured A. camphorata were evaluated in high-fat diet (HFD) and STZ-induced diabetic rats.128 Oral treatment of 4–20 mg kg−1 for 6 weeks significantly reduced fasting glucose and serum insulin levels.The triterpenoids, such as antcin K, eburicoic acid, dehydroeburicoic acid, sulphurenic acid (78), and ergostatrien-3β-ol (148) also displayed antidiabetic and antihyperlipidemic effects in palmitate-treated C2C12 myotubes and in HFD or STZ-induced diabetic mice.129–133 Eburicoic acid could significantly prevent the decrease of phospho-Akt (p-Akt) expression levels in the C2C12 myotubes caused by palmitate. It could also decrease the relative weights of epididymal white adipose tissue and visceral fat from 6–8% to 3–4% (10–40 mg kg−1, p.o., for 4 weeks), reduce the levels of blood glucose, triglyceride, total cholesterol, insulin and leptin, and increase adiponectin levels in HFD mice.129 Dehydroeburicoic acid (10–40 mg kg−1, p.o., for 4 weeks) could markedly decrease blood glucose levels by 42.6–46.5% and reduce the levels of triglyceride and total cholesterol in STZ-induced diabetic mice, via regulation of GLUT4, PPARα, FAS, and phosphorylation of AMPK.132 A number of Antrodia triterpenoids (20 μM) exhibited significant ROS scavenging activities (63.9–70.5%) in high glucose-induced HUVECs.27
5.5 Neuroprotective activities
The extract of A. camphorata fruiting bodies showed significant neuroprotective activities in Aβ40-treated PC-12 cells. Furthermore, the extract (79.45 mg kg−1, p.o., for 28 days) could improve memory and learning abilities of Aβ40 infusion induced Alzheimer's disease (AD) model rats.134 Antroquinonol (7.2, 34.2 mg kg−1, p.o., for 2 months) could improve learning and memory in the Morris water maze test of amyloid precursor protein transgenic AD model mice, reduce hippocampal Aβ levels, and reduce the degree of astrogliosis. It increased Nrf2 expression and reduced histone deacetylase 2 (HDAC2) expression in the hippocampus.135 Moreover, a number of Antrodia diterpenoids exhibited neuroprotective activities in vitro by protecting neurons from Aβ damage.1365.6 Miscellaneous activities
Immunomodulatory and anti-angiogenic activities have been reported for A. camphorata. While the immunomodulatory activities are mainly related with polysaccharides,137,138 methyl antcinate K (26, 50 μM) was reported as the first natural triterpenoid to promote the ability of dendritic cells (DCs) to prime Th2 responses, and can potentially be used to enhance immune responses and modulate DC function in immunotherapy.29 The ethanol extract of A. camphorata produced anti-angiogenic effects in mice by inhibiting the vascular endothelial growth factor receptor 2 (VEGFR2) signalling pathway.1395.7 Metabolism and pharmacokinetics
The metabolism and pharmacokinetics of triterpenoids in A. camphorata were firstly reported in 2015 by our group. After oral administration of the ethanol extract, 18 triterpenoids and 8 biotransformed metabolites were detected in rats plasma by UHPLC/qTOF-MS.140 The ergostanes and Δ7,9(11) lanostanes, but not the Δ8 lanostanes, could be absorbed into circulation. High-polarity ergostanes (antcins H and K) and Δ7,9(11) lanostanes were metabolically stable. Meanwhile, low-polarity ergostanes (antcins B and C) undertook hydrogenation (C-3 or C-7 carbonyl groups) or hydroxylation reactions to produce polar metabolites. Moreover, the pharmacokinetics of 14 triterpenoids and 2 metabolites were simultaneously monitored by LC/MS/MS in rats after oral administration of the ethanol extract (1.0 g kg−1). The ergostanes were absorbed and eliminated rapidly, whereas the lanostanes remained in the plasma at a low concentration for a relatively long time. In a mice xenograft S180 tumor model, antcin H (14/15) could be detected in the tumor tissue following oral administration of A. camphorata extract.141 A Caco-2 cell monolayer model was used to test the intestinal absorption properties of 14 triterpenoids from A. camphorata.142 Most ergostanes exhibited high permeability. Antcins A, B, C, H and K could pass through the monolayer via passive transcellular diffusion, and showed high PAB and PBA values (>2.5 × 10−5 cm s−1). Meanwhile, the lanostanes including dehydrosulphurenic acid, 15α-acetyldehydrosulphurenic acid, dehydroeburicoic acid, and eburicoic acid exhibited poor permeability.The metabolism and pharmacokinetics of antroquinonol have been studied in human and rats. A clinical study evaluated the pharmacokinetics of antroquinonol in patients with metastatic non-small-cell lung cancer after oral administration. These patients received escalating doses (50–600 mg) of once-daily antroquinonol in 4 week cycles (up to 3 cycles).91 Antroquinonol was rapidly eliminated following single and multiple administrations (T1/2 1.30–4.33 h). The Tmax of plasma concentration was 1.00–3.70 h after a single-dose, and 1.92–4.05 h after a multiple-dose treatment. In rats, four metabolites of antroquinonol were isolated from the urine after oral treatment, and their structures were identified by NMR spectroscopic analysis. All the metabolites had a cleaved side chain with a terminal carboxyl group. This catabolic reaction was similar to the cleavage of cholesterol side chain.143
5.8 Toxicity
A. camphorata showed no obvious toxicity in several animal toxicity studies.144–146 Male and female BALB/c mice were orally treated with a water extract of A. camphorata (up to 1666.67 mg per kg per day) for 90 consecutive days, and no significant differences were observed between the control and treatment groups.144 In another study, the 90 day oral toxicity of submerged cultures of A. camphorata was assessed in male and female Sprague-Dawley rats. No significant toxicities were observed for A. camphorata at a dose of 3000 mg per kg per day.145Hocena® (antroquinonol) showed a safe dosage from 50 to 600 mg daily for 4 weeks in 13 NSCLC subjects.147 No all-cause mortality or serious adverse events were reported. Other adverse events included diarrhea, vomiting, and nausea. Another study reported 36 patients with metastatic non-small-cell lung cancer that received escalating doses of once-daily antroquinonol in 4 week cycles.91 The study indicated that antroquinonol at up to 600 mg daily was basically safe. No mortalibites or serious adverse events were reported, and the most common adverse events were diarrhea, vomiting, and nausea.
Thus far, no reports are available on the toxicity of ethanol extracts of A. camphorata, which mainly consist of triterpenoids. The toxicity of major bioactive triterpenoids such as antcins B, C, H and K, and dehydroeburicoic acid has yet to be studied.
6 Conclusions and future perspectives
A. camphorata is a popular dietary supplement and a promising natural medicine. In the past three decades, 162 terpenoids have been identified from A. camphorata. Among them, ergostane- and lanostane-type triterpenoids, as well as antroquinonols (meroterpenoids) are the major bioactive constituents. Chemical or enzymatic modifications could be used to expand structural diversity of the terpenoids, and to improve their physicochemical and pharmacological properties as drug candidates. For example, hydroxylation or glycosylation may improve solubility, bioavailability, and bioactivity of the triterpenoids.148Based on the bioactivities, pharmacokinetics, and natural abundances, the most promising terpenoids in A. camphorata for medicinal development include the anti-cancer agents antroquinonol (104), 4-acetylantroquinonol B (106), and antrocin (130), together with the hepatoprotective compounds antcin B (3/4), antcin C (5/6), antcin H (14/15), antcin K (18/19), eburicoic acid (77), sulphurenic acid (78), dehydroeburicoic acid (83), dehydrosulphurenic acid (84), and ergostatrien-3β-ol (148). Potential of the triterpenoids for the treatment of cancer, diabetes, and hyperlipidemia is also noticeable. However, our understanding on detailed mechanisms of actions and molecular targets of the pure compounds is still limited, particularly the mechanisms different from those of currently known drugs. In-depth basic research will benefit the development and clinical use of A. camphorata. It is encouraging that A. camphorata and antroquinonol show good safety in both animal and human studies. Toxicities of the major bioactive triterpenoids need to be evaluated in the future.
It is also encouraging that different cultivation techniques have been developed to produce A. camphorata at a large scale. This is important for sustainable development of this precious mushroom. Preparation of the pure compounds still mainly relies on traditional extraction and isolation. Fortunately, efficient chemical synthetic routes have been established for antroquinonols and antrocin. For the production of complex triterpenoids, synthetic biology may be a promising approach, though the biosynthetic studies are still in an early stage.
7 Conflicts of interest
The authors declare that there are no conflicts of interest.8 Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81725023, 81891010/81891011, 81921001), the National Key Research and Development Program of China (No. 2017YFC1700405), and Beijing Natural Science Foundation (No. JQ18027). We wish to thank PhD students Ya-qun Zhang and Hui-fei Su of the Ye group for critically reading the manuscript, and Mr Jen-Yu Lo at Honest & Humble Biotechnology Co., Ltd. (New Taipei City, Taiwan) for providing the samples shown in Fig. 1.9 References
- M. Zang and Q. Su, Acta Bot. Yunnanica, 1990, 12, 395–396 Search PubMed.
- T. T. Chang and W. N. Chou, Mycol. Res., 1995, 99, 756–758 CrossRef.
- S. H. Wu, L. Ryvarden and T. T. Chang, Bot. Bull. Acad. Sin., 1997, 38, 273–275 Search PubMed.
- S. H. Wu, Z. H. Yu, Y. C. Dai, C. T. Chen, C. H. Su, L. C. Chen, W. C. Hsu and G. Y. Hwang, Fung. Sci., 2004, 19, 109–116 Search PubMed.
- H. H. Chiu, Antonie van Leeuwenhoek, 2007, 91, 267–276 CrossRef CAS.
- Z. H. Ao, Z. H. Xu, Z. M. Lu, H. Y. Xu, X. M. Zhang and W. F. Dou, J. Ethnopharmacol., 2009, 121, 194–212 CrossRef.
- M. Geethangili and Y. M. Tzeng, Evidence-Based Complementary Altern. Med., 2011, 2011, 212641 Search PubMed.
- M. C. Lu, M. El-Shazly, T. Y. Wu, Y. C. Du, T. T. Chang, C. F. Chen, Y. M. Hsu, K. H. Lai, C. P. Chiu, F. R. Chang and Y. C. Wu, Pharmacol. Ther., 2013, 139, 124–156 CrossRef CAS.
- B. B. Zhang, Y. Y. Guan, P. F. Hu, L. Chen, G. R. Xu, L. Liu and P. C. K. Cheung, Crit. Rev. Biotechnol., 2019, 39, 541–554 CrossRef CAS.
- N. Ganesan, R. Baskaran, B. K. Velmurugan and N. C. Thanh, J. Food Biochem., 2019, 43, 12936 CrossRef.
- V. Angamuthu, M. Shanmugavadivu, G. Nagarajan and B. K. Velmurugan, Chem.-Biol. Interact., 2019, 297, 8–15 CrossRef CAS.
- P. Y. Yue, Y. Y. Wong, K. Y. Wong, Y. K. Tsoi and K. S. Leung, Chin. Med., 2013, 8, 21 CrossRef.
- C. Wang, W. Zhang, J. H. Wong, T. Ng and X. Ye, Appl. Microbiol. Biotechnol., 2019, 103, 7843–7867 CrossRef CAS.
- B. B. Zhang, P. F. Hu, J. Huang, Y. D. Hu, L. Chen and G. R. Xu, J. Agric. Food Chem., 2017, 65, 10395–10405 CrossRef CAS.
- K. J. S. Kumar, M. G. Vani, C. Y. Chen, W. W. Hsiao, J. Li, Z. X. Lin, F. H. Chu, G. C. Yen and S. Y. Wang, J. Food Drug Anal., 2020, 28, 38–59 CrossRef.
- I. H. Cherng, H. C. Chiang, M. C. Cheng and Y. Wang, J. Nat. Prod., 1995, 58, 365–371 CrossRef CAS.
- X. Qiao, W. Song, Q. Wang, K. D. Liu, Z. X. Zhang, T. Bo, R. Y. Li, L. N. Liang, Y. M. Tzeng, D. A. Guo and M. Ye, RSC Adv., 2015, 5, 47040–47052 RSC.
- B. Li, Y. Kuang, M. Zhang, J. B. He, L. L. Xu, C. H. Leung, D. L. Ma, J. Y. Lo, X. Qiao and M. Ye, Org. Chem. Front., 2020, 7, 768–779 RSC.
- I. H. Cherng, D. P. Wu and H. C. Chiang, Phytochemistry, 1996, 41, 263–267 CrossRef CAS.
- D. P. Wu and H. C. Chiang, J. Chin. Chem. Soc., 1995, 42, 797–800 CrossRef CAS.
- S. J. Wu, Y. L. Leu, C. H. Chen, C. H. Chao, D. Y. Shen, H. H. Chan, E. J. Lee, T. S. Wu, Y. H. Wang, Y. C. Shen, K. Qian, K. F. Bastow and K. H. Lee, J. Nat. Prod., 2010, 73, 1756–1762 CrossRef CAS.
- Y. Huang, X. H. Lin, X. Qiao, S. Ji, K. D. Liu, C. T. Yeh, Y. M. Tzeng, D. A. Guo and M. Ye, J. Nat. Prod., 2014, 77, 118–124 CrossRef CAS.
- Y. C. Du, T. Y. Wu, F. R. Chang, W. Y. Lin, Y. M. Hsu, F. T. Cheng, C. Y. Lu, M. H. Yen, Y. T. Tsui, H. L. Chen, M. F. Hou, M. C. Lu and Y. C. Wu, J. Pharm. Biomed. Anal., 2012, 58, 182–192 CrossRef CAS.
- T. Y. Lin, C. Y. Chen, S. C. Chien, W. W. Hsiao, F. H. Chu, W. H. Li, C. C. Lin, J. F. Shaw and S. Y. Wang, J. Agric. Food Chem., 2011, 59, 7626–7635 CrossRef CAS.
- C. H. Chen, S. W. Yang and Y. C. Shen, J. Nat. Prod., 1995, 58, 1655–1661 CrossRef CAS.
- S. W. Yang, Y. C. Shen and C. H. Chen, Phytochemistry, 1996, 41, 1389–1392 CrossRef CAS.
- B. Li, Y. Kuang, J. B. He, R. Tang, L. L. Xu, C. H. Leung, D. L. Ma, X. Qiao and M. Ye, J. Nat. Prod., 2020, 83, 45–54 CrossRef CAS.
- C. C. Shen, Y. C. Kuo, R. L. Huang, L. C. Lin, M. J. Don, T. T. Chang and C. J. Chou, J. Chin. Med., 2003, 14, 247–258 CrossRef.
- Y. L. Yu, I. H. Chen, K. Y. Shen, R. Y. Huang, W. R. Wang, C. J. Chou, T. T. Chang and C. L. Chu, Eur. J. Immunol., 2009, 39, 2482–2491 CrossRef CAS.
- X. Qiao, R. An, Y. Huang, S. Ji, L. Li, Y. M. Tzeng, D. A. Guo and M. Ye, J. Chromatogr. A, 2014, 1358, 252–260 CrossRef CAS.
- J. M. Seco, E. Quinoá and R. Riguera, Chem. Rev., 2004, 104, 17–117 CrossRef CAS.
- J. L. Ríos, I. Andújar, M. C. Recio and R. M. Giner, J. Nat. Prod., 2012, 75, 2016–2044 CrossRef.
- S. Baby, A. J. Johnson and B. Govindan, Phytochemistry, 2015, 114, 66–101 CrossRef CAS.
- H. C. Huang, C. C. Liaw, H. L. Yang, Y. C. Hseu, H. T. Kuo, Y. C. Tsai, S. C. Chien, S. Amagaya, Y. C. Chen and Y. H. Kuo, Phytochemistry, 2012, 84, 177–183 CrossRef CAS.
- C. C. Liaw, Y. C. Chen, G. J. Huang, Y. C. Tsai, S. C. Chien, J. H. Wu, S. Y. Wang, L. K. Chao, P. J. Sung, H. C. Huang and Y. H. Kuo, J. Nat. Prod., 2013, 76, 489–494 CrossRef CAS.
- T. H. Lee, C. K. Lee, W. L. Tsou, S. Y. Liu, M. T. Kuo and W. C. Wen, Planta Med., 2007, 73, 1412–1415 CrossRef CAS.
- S. S. Yang, G. J. Wang, S. Y. Wang, Y. Y. Lin, Y. H. Kuo and T. H. Lee, Planta Med., 2009, 75, 512–516 CrossRef CAS.
- M. C. Chen, T. Y. Cho, Y. H. Kuo and T. H. Lee, J. Nat. Prod., 2017, 80, 2439–2446 CrossRef CAS.
- G. Yatsu, Y. Kino, H. Sasaki, J. Satoh, K. Kinoshita and K. Koyama, J. Nat. Prod., 2019, 82, 1797–1801 CrossRef CAS.
- M. T. Villaume, E. Sella, G. Saul, R. M. Borzilleri, J. Fargnoli, K. A. Johnston, H. Zhang, M. P. Fereshteh, T. G. M. Dhar and P. S. Baran, ACS Cent. Sci., 2016, 2, 27–31 CrossRef CAS.
- X. M. Wang, C. Du, B. K. Hong and X. G. Lei, Org. Biomol. Chem., 2019, 17, 1754–1757 RSC.
- R. S. Sulake, Y. F. Jiang, H. H. Lin and C. Chen, J. Org. Chem., 2014, 79, 10820–10828 CrossRef CAS.
- S. C. Wang, T. H. Lee, C. H. Hsu, Y. J. Chang, M. S. Chang, Y. C. Wang, Y. S. Ho, W. C. Wen and R. K. Lin, J. Agric. Food Chem., 2014, 62, 5625–5635 CrossRef CAS.
- I. C. Yen, C. W. Yao, M. T. Kuo, C. L. Chao, C. Y. Pai and W. L. Chang, Fitoterapia, 2015, 102, 115–119 CrossRef CAS.
- I. C. Yen, S. Y. Lee, K. T. Lin, F. Y. Lai, M. T. Kuo and W. L. Chang, Molecules, 2017, 22, 747 CrossRef.
- J. Z. Chen, J. Z. Huang, Y. L. Lin and C. B. Cai, Compounds from A. camphorata mycelium and the use, Patent application No. CN103570531A, 2012.
- H. Shi, L. Fang, C. Tan, L. Shi, W. Zhang, C. C. Li, T. Luo and Z. Yang, J. Am. Chem. Soc., 2011, 133, 14944–14947 CrossRef CAS.
- J. Y. Lin, T. Z. Wu and J. C. Chou, Bot. Stud., 2006, 47, 267–272 Search PubMed.
- E. Majid, K. B. Male, Y. M. Tzeng, J. O. Omamogho, J. D. Glennon and J. H. T. Luong, Electrophoresis, 2009, 30, 1967–1975 CrossRef CAS.
- M. Y. J. Lu, W. L. Fan, W. F. Wang, T. C. Chen, Y. C. Tang, F. H. Chu, T. T. Chang, S. Y. Wang, M. Y. Li, Y. H. Chen, Z. S. Lin, K. J. Yang, S. M. Chen, Y. C. Teng, Y. L. Lin, J. F. Shaw, T. F. Wang and W. H. Li, Proc. Natl. Acad. Sci. U. S. A., 2014, 11, 4743–4752 CrossRef.
- L. Y. Yang, R. L. Guan, Y. X. Shi, J. M. Ding, R. H. Dai, W. X. Ye, K. Xu, Y. Chen, L. Shen, Y. Y. Liu, F. M. Ding, C. He and H. Meng, Mycol. Prog., 2018, 17, 871–883 CrossRef.
- D. Martinez, J. Challacombe, I. Morgenstern, D. Hibbett, M. Schmoll, C. P. Kubicek, P. Ferreira, F. J. Ruiz-Duenas, A. T. Martinez, P. Kersten, K. E. Hammel, A. V. Wymelenberg, J. Gaskell, E. Lindquist, G. Sabat, S. S. BonDurant, L. F. Larrondo, P. Canessa, R. Vicuna, J. Yadav, H. Doddapaneni, V. Subramanian, A. G. Pisabarro, J. L. Lavin, J. A. Oguiza, E. Master, B. Henrissat, P. M. Coutinho, P. Harris, J. K. Magnuson, S. E. Baker, K. Bruno, W. Kenealy, P. J. Hoegger, U. Kues, P. Ramaiya, S. Lucash, A. Salamov, H. Shapiro, H. Tu, C. L. Chee, M. Misra, G. Xie, S. Teter, D. Yaver, T. James, M. Mokrejs, M. Pospisek, I. V. Grigoriev, T. Brettin, D. Rokhsar, R. Berka and D. Cullen, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 1954–1959 CrossRef CAS.
- S. L. Chen, J. Xu, C. Liu, Y. J. Zhu, D. R. Nelson, S. G. Zhou, C. F. Li, L. Z. Wang, X. Guo, Y. Z. Sun, H. M. Luo, Y. Li, J. Y. Song, B. Henrissat, A. Levasseur, J. Qian, J. Q. Li, X. Luo, L. C. Shi, L. He, L. Xiang, X. L. Xu, Y. Y. Niu, Q. S. Li, M. V. Han, H. X. Yan, J. Zhang, H. M. Chen, A. P. Lv, Z. Wang, M. Z. Liu, D. C. Schwartz and C. Sun, Nat. Commun., 2012, 3, 913 CrossRef.
- D. Agudelo-Valencia, P. T. Uribe-Echeverry and J. F. Betancur-Pérez, Genomics, 2020, 112, 930–933 CrossRef CAS.
- S. M. Chaw, Y. C. Liu, Y. W. Wu, H. Y. Wang, C. Y. Lin, C. S. Wu, H. M. Ke, L. Y. Chang, C. Y. Hsu, H. T. Yang, E. Sudianto, M. H. Hsu, K. P. Wu, L. N. Wang, J. H. Leebens-Mack and I. J. Tsai, Nat. Plants, 2019, 5, 63–73 CrossRef CAS.
- S. T. Huang, S. S. Tzean, B. Y. Tsai and H. J. Hsieh, Microbiology, 2009, 155, 424–433 CrossRef CAS.
- H. X. Li, Z. M. Lu, Q. Zhu, J. S. Gong, Y. Geng, J. S. Shi, Z. H. Xu and Y. H. Ma, Proteomics, 2017, 17, 1700256 CrossRef.
- K. C. C. Chou, S. H. Yang, H. L. Wu, P. Y. Lin, T. L. Chang, F. Sheu, K. H. Chen and B. H. Chiang, J. Agric. Food Chem., 2017, 65, 74–86 CrossRef CAS.
- P. W. Yu, Y. C. Chang, R. F. Liou, T. H. Lee and S. S. Tzean, J. Nat. Prod., 2016, 79, 1485–1491 CrossRef CAS.
- P. W. Yu, T. Y. Cho, R. F. Liou, S. S. Tzean and T. H. Lee, Appl. Microbiol. Biotechnol., 2017, 101, 4701–4711 CrossRef CAS.
- K. C. C. Chou, H. L. Wu, P. Y. Lin, S. H. Yang, T. L. Chang, F. Sheu, K. H. Chen and B. H. Chiang, Phytochemistry, 2019, 161, 97–106 CrossRef CAS.
- J. Li, X. J. Lin, E. S. Shao and Z. X. Lin, MATEC Web Conf., 2016, 64, 03001 CrossRef.
- R. Geris and T. J. Simpson, Nat. Prod. Rep., 2009, 26, 1063–1094 RSC.
- Y. L. Lin, Y. R. Lee, N. W. Tsao, S. Y. Wang, J. F. Shaw and F. H. Chu, J. Nat. Prod., 2015, 78, 1556–1562 CrossRef CAS.
- C. H. Lee, K. H. Hsu, S. Y. Wang, T. T. Chang, F. H. Chu and J. F. Shaw, J. Agric. Food Chem., 2010, 58, 4800–4807 CrossRef CAS.
- K. H. Hsu, Y. R. Lee, Y. L. Lin and F. H. Chu, Int. J. Med. Mushrooms, 2011, 13, 513–523 CrossRef CAS.
- Y. L. Lin, L. T. Ma, Y. R. Lee, J. F. Shaw, S. Y. Wang and F. H. Chu, J. Agric. Food Chem., 2017, 65, 1874–1886 CrossRef CAS.
- S. H. Yang, Y. W. Lin and B. H. Chiang, Biochem. Eng. J., 2017, 117, 23–29 CrossRef CAS.
- C. C. Chiang, T. N. Huang, Y. W. Lin, K. H. Chen and B. H. Chiang, J. Agric. Food Chem., 2013, 61, 9160–9165 CrossRef CAS.
- Y. D. Hu, R. Q. Lu, X. R. Liao, B. B. Zhang and G. R. Xu, Biotechnol. Appl. Biochem., 2016, 63, 398–406 CrossRef CAS.
- Y. D. Hu, B. B. Zhang, G. R. Xu, X. R. Liao and P. C. K. Cheung, J. Funct. Foods, 2016, 25, 70–79 CrossRef.
- Y. J. Xia, X. Zhou, G. Q. Wang, B. B. Zhang, G. R. Xu and L. Z. Ai, J. Sci. Food Agric., 2017, 97, 595–599 CrossRef CAS.
- Z. M. Lu, Y. Geng, H. X. Li, Q. Sun, J. S. Shi and Z. H. Xu, FEMS Microbiol. Lett., 2014, 358, 36–43 CrossRef CAS.
- Y. T. Zhang, D. Y. Li, Z. Wang, W. T. Zang, P. Rao, Y. X. Liang and Y. X. Mei, Food Funct., 2018, 9, 6517–6525 RSC.
- T. W. Ma, Y. T. Lai, L. T. Chen and F. C. Yang, J. Taiwan Inst. Chem. Eng., 2016, 58, 210–218 CrossRef CAS.
- Y. L. Hsu, Y. C. Kuo, P. L. Kuo, L. T. Ng, Y. H. Kuo and C. C. Lin, Cancer Lett., 2005, 221, 77–89 CrossRef CAS.
- Y. L. Hsu, P. L. Kuo, C. Y. Cho, W. C. Ni, T. F. Tzeng, L. T. Ng, Y. H. Kuo and C. C. Lin, Food Chem. Toxicol., 2007, 45, 1249–1257 CrossRef CAS.
- Y. Y. Chen, P. Y. Chou, Y. C. Chien, C. H. Wu, T. S. Wu and M. J. Sheu, Phytomedicine, 2012, 19, 768–778 CrossRef CAS.
- F. C. Liu, M. T. Lai, Y. Y. Chen, W. H. Lin, S. J. Chang, M. J. Sheu and C. H. Wu, Phytomedicine, 2013, 20, 874–882 CrossRef CAS.
- C. I. Lee, C. C. Wu, S. L. Hsieh, C. L. Lee, Y. P. Chang, C. C. Chang, Y. Z. Wang and J. J. Wang, Food Funct., 2014, 5, 3224–3232 RSC.
- K. M. Shang, T. H. Su, W. L. Lee, W. W. Hsiao, C. Y. Chiou, B. Y. Ho, S. Y. Wang and L. F. Shyur, Phytomedicine, 2017, 24, 39–48 CrossRef CAS.
- D. H. Tsai, C. H. Chung and K. T. Lee, Sci. Rep., 2018, 8, 17424 CrossRef.
- C. H. Wu, F. C. Liu, C. H. Pan, M. T. Lai, S. J. Lan, C. H. Wu and M. J. Sheu, Int. J. Mol. Sci., 2018, 19, 791–805 CrossRef.
- Y. C. Chen, Y. C. Liu, M. El-Shazly, T. Y. Wu, J. G. Chang and Y. C. Wu, Int. J. Mol. Sci., 2019, 20, 833 CrossRef CAS.
- H. Long, C. T. Hu and C. F. Weng, Medicina, 2019, 55, 640 CrossRef.
- P. C. Chiang, S. C. Lin, S. L. Pan, C. H. Kuo, I. L. Tsai, M. T. Kuo, W. C. Wen, P. Chen and J. H. Guh, Biochem. Pharmacol., 2010, 79, 162–171 CrossRef CAS.
- Y. W. Lin and B. H. Chiang, J. Agric. Food Chem., 2011, 59, 8625–8631 CrossRef CAS.
- T. C. Chang, C. T. Yeh, B. O. Adebayo, Y. C. Lin, L. Deng, Y. K. Rao, C. C. Huang, W. H. Lee, A. T. Wu, M. Hsiao, C. H. Wu, L. S. Wang and Y. M. Tzeng, Toxicol. Appl. Pharmacol., 2015, 288, 258–268 CrossRef CAS.
- H. C. Lin, M. H. Lin, J. H. Liao, T. H. Wu, T. H. Lee, F. L. Mi, C. H. Wu, K. C. Chen, C. H. Cheng and C. W. Lin, J. Agric. Food Chem., 2017, 65, 51–59 CrossRef CAS.
- H. W. Liu, Y. K. Su, O. A. Bamodu, D. Y. Hueng, W. H. Lee, C. C. Huang, L. Deng, M. Hsiao, M. H. Chien, C. T. Yeh and C. M. Lin, Cancers, 2018, 10, E491 CrossRef.
- Y. C. Lee, C. L. Ho, W. Y. Kao and Y. M. Chen, Mol. Clin. Oncol., 2015, 3, 1375–1380 CrossRef CAS.
- Y. C. Du, F. R. Chang, T. Y. Wu, Y. M. Hsu, M. El-Shazly, C. F. Chen, P. J. Sung, Y. Y. Lin, Y. H. Lin, Y. C. Wu and M. C. Lu, Phytomedicine, 2012, 19, 788–796 CrossRef CAS.
- J. Y. Deng, S. J. Chen, G. M. Jow, C. W. Hsueh and C. J. Jeng, Chem. Res. Toxicol., 2009, 22, 1817–1826 Search PubMed.
- C. T. Yeh, Y. K. Rao, C. J. Yao, C. F. Yeh, C. H. Li, S. E. Chuang, J. H. Luong, G. M. Lai and Y. M. Tzeng, Cancer Lett., 2009, 285, 73–79 CrossRef CAS.
- Y. C. Hsieh, Y. K. Rao, C. C. Wu, C. Y. Huang, M. Geethangili, S. L. Hsu and Y. M. Tzeng, Chem. Res. Toxicol., 2010, 23, 1256–1267 Search PubMed.
- Y. C. Hsieh, Y. K. Rao, J. W. Peng, C. Y. Huang, S. K. Shyue, S. L. Hsu and Y. M. Tzeng, J. Agric. Food Chem., 2011, 59, 10943–10954 CrossRef CAS.
- Y. L. Huang, Y. L. Chu, C. T. Ho, J. G. Chung, C. I. Lai, Y. C. Su, Y. H. Kuo and L. Y. Sheen, J. Agric. Food Chem., 2015, 63, 4561–4569 CrossRef CAS.
- K. Y. Chiu, T. H. Chen, C. L. Wen, J. M. Lai, C. C. Cheng, H. C. Liu, S. L. Hsu and Y. M. Tzeng, Evidence-Based Complementary Altern. Med., 2017, 2017, 5052870 Search PubMed.
- C. I. Lai, Y. L. Chu, C. T. Ho, Y. C. Su, Y. H. Kuo and L. Y. Sheen, J. Tradit. Complement. Med., 2016, 6, 48–56 CrossRef.
- W. C. Tsai, Y. K. Rao, S. S. Lin, M. Y. Chou, Y. T. Shen, C. H. Wu, M. Geethangili, C. C. Yang and Y. M. Tzeng, Bioorg. Med. Chem. Lett., 2010, 20, 6145–6148 CrossRef CAS.
- C. T. Yeh, W. C. Huang, Y. K. Rao, M. Ye, W. H. Lee, L. S. Wang, D. T. Tzeng, C. H. Wu, Y. S. Shieh, C. Y. Huang, Y. J. Chen, M. Hsiao, A. T. Wu, Z. Yang and Y. M. Tzeng, Carcinogenesis, 2013, 34, 2918–2928 CrossRef CAS.
- J. H. Chen, T. H. A. Wu, T. W. D. Tzeng, C. C. Huang, Y. M. Tzeng and T. Y. Chao, Phytomedicine, 2019, 52, 70–78 CrossRef CAS.
- C. Y. Chang, Z. N. Huang, H. H. Yu, L. H. Chang, S. L. Li, Y. P. Chen, K. Y. Lee and J. J. Chuu, J. Ethnopharmacol., 2008, 118, 387–395 CrossRef CAS.
- M. C. Lu, Y. C. Du, J. J. Chuu, S. L. Hwang, P. C. Hsieh, C. S. Hung, F. R. Chang and Y. C. Wu, Arch. Toxicol., 2009, 83, 121–129 CrossRef CAS.
- F. S. Liu, P. Y. Yang, D. N. Hu, Y. W. Huang and M. J. Chen, Int. J. Gynecol. Cancer, 2011, 21, 1172–1179 Search PubMed.
- L. Y. Chen, M. T. Sheu, D. Z. Liu, C. K. Liao, H. O. Ho, W. Y. Kao, Y. S. Ho, W. S. Lee and C. H. Su, J. Agric. Food Chem., 2011, 59, 11255–11263 CrossRef CAS.
- T. H. Huang, Y. H. Chiu, Y. L. Chan, H. Wang, T. L. Li, C. Y. Liu, C. T. Yang, T. Y. Lee, J. S. You, K. H. Hsu and C. J. Wu, Oncotarget, 2015, 6, 25741–25754 CrossRef.
- W. D. Wu, P. S. Chen, H. A. Omar, E. A. Arafa, H. W. Pan, J. Jeng and J. H. Hung, Sci. Rep., 2018, 8, 12914–12926 CrossRef.
- Y. J. Huang, V. K. Yadav, P. Srivastava, A. T. H. Wu, T. T. Huynh, P. L. Wei, C. Y. F. Huang and T. H. Huang, Biomolecules, 2019, 9, 306 CrossRef.
- Y. F. Chen, C. H. Chang, Z. N. Huang, Y. C. Su, S. J. Chang and J. S. Jan, Leuk. Lymphoma, 2019, 60, 1193–1203 CrossRef CAS.
- Y. A. Chen, D. T. W. Tzeng, Y. P. Huang, C. J. Lin, U. G. Lo, C. L. Wu, H. Lin, J. T. Hsieh, C. H. Tang and C. H. Lai, Cancers, 2018, 11, E34 CrossRef.
- W. C. Lin, S. C. Kuo, W. L. Lin, H. L. Fang and B. C. Wang, World J. Gastroenterol., 2006, 12, 2369–2374 CrossRef CAS.
- Y. Wu, W. J. Tian, S. Gao, Z. J. Liao, G. H. Wang, J. M. Lo, P. H. Lin, D. Q. Zeng, D. R. Qiu, X. Z. Liu, M. Zhou, T. Lin and H. F. Chen, Chin. J. Nat. Med., 2019, 17, 33–42 Search PubMed.
- Y. Huo, S. Win, T. A. Than, S. Yin, M. Ye, H. Hu and N. Kaplowitz, Antioxid. Redox Signaling, 2017, 26, 207–220 CrossRef CAS.
- Z. W. Li, Y. Kuang, S. N. Tang, K. Li, Y. Huang, X. Qiao, S. W. Yu, Y. Tzeng, J. Y. Lo and M. Ye, J. Ethnopharmacol., 2017, 206, 31–39 CrossRef CAS.
- G. J. Huang, J. S. Deng, S. S. Huang, C. Y. Lee, W. C. Hou, S. Y. Wang, P. J. Sung and Y. H. Kuo, Food Chem., 2013, 141, 3020–3027 CrossRef CAS.
- M. Gokila Vani, K. J. Kumar, J. W. Liao, S. C. Chien, J. L. Mau, S. S. Chiang, C. C. Lin, Y. H. Kuo and S. Y. Wang, Evidence-Based Complementary Altern. Med., 2013, 2013, 296082 CAS.
- K. J. S. Kumar, F. H. Chu, H. W. Hsieh, J. W. Liao, W. H. Li, J. C. Lin, J. F. Shaw and S. Y. Wang, J. Ethnopharmacol., 2011, 136, 168–177 CrossRef CAS.
- Y. Y. Chang, Y. C. Liu, Y. H. Kuo, Y. L. Lin, Y. S. Wu, J. W. Chen and Y. C. Chen, J. Ethnopharmacol., 2017, 202, 200–207 CrossRef CAS.
- R. Gautam and S. M. Jachak, Med. Res. Rev., 2009, 29, 767–820 CrossRef CAS.
- Y. T. Tung, T. C. Tsai, Y. H. Kuo, C. C. Yen, J. Y. Sun, W. H. Chang, H. L. Chen and C. M. Chen, Phytomedicine, 2014, 21, 1708–1716 CrossRef CAS.
- S. T. Kao, Y. H. Kuo, S. D. Wang, H. J. Hong and L. J. Lin, Cytokine, 2018, 108, 136–144 CrossRef CAS.
- Y. S. Wu and S. N. Chen, Front. Pharmacol., 2016, 7, 154–164 Search PubMed.
- G. J. Huang, S. S. Huang, S. S. Lin, Y. Y. Shao, C. C. Chen, W. C. Hou and Y. H. Kuo, J. Agric. Food Chem., 2010, 58, 7445–7452 CrossRef CAS.
- J. S. Deng, S. S. Huang, T. H. Lin, M. M. Lee, C. C. Kuo, P. J. Sung, W. C. Hou, G. J. Huang and Y. H. Kuo, J. Agric. Food Chem., 2013, 61, 5064–5071 CrossRef CAS.
- J. J. Chen, W. J. Lin, C. H. Liao and P. C. Shieh, J. Nat. Prod., 2007, 70, 989–992 CrossRef CAS.
- C. T. Vong, H. H. Tseng, Y. W. Kwan, S. M. Lee and M. P. Hoi, Front. Pharmacol., 2016, 7, 67–76 Search PubMed.
- S. Sudirman, Y. H. Hsu, A. Johnson, D. Tsou and Z. L. Kon, Int. J. Nanomed., 2018, 13, 5059–5073 CrossRef CAS.
- C. H. Lin, Y. H. Kuo and C. C. Shih, Int. J. Mol. Sci., 2017, 18, 2314–2342 CrossRef.
- Y. H. Kuo, C. H. Lin and C. C. Shih, Int. J. Mol. Sci., 2016, 17, 872–890 CrossRef.
- Y. H. Kuo, C. H. Lin, C. C. Shih and C. S. Yang, Evidence-Based Complementary Altern. Med., 2016, 2016, 4867092 Search PubMed.
- Y. H. Kuo, C. H. Lin and C. C. Shih, J. Agric. Food Chem., 2015, 63, 10140–10151 CrossRef CAS.
- Y. H. Kuo, C. H. Lin and C. C. Shih, J. Agric. Food Chem., 2015, 63, 2479–2489 CrossRef CAS.
- L. C. Wang, S. E. Wang, J. J. Wang, T. Y. Tsai, C. H. Lin, T. M. Pan and C. L. Lee, Appl. Microbiol. Biotechnol., 2012, 94, 1505–1519 CrossRef CAS.
- W. H. Chang, M. C. Chen and I. H. Cheng, Sci. Rep., 2015, 5, 15067–15078 CrossRef CAS.
- C. C. Chen, Y. J. Shiao, R. D. Lin, Y. Y. Shao, M. N. Lai, C. C. Lin, L. T. Ng and Y. H. Kuo, J. Nat. Prod., 2006, 69, 689–691 CrossRef CAS.
- K. J. Liu, S. J. Leu, C. H. Su, B. L. Chiang, Y. L. Chen and Y. L. Lee, Immunology, 2010, 129, 351–362 CrossRef CAS.
- Y. Liu, A. Yang, Y. Qu, Z. Wang, Y. Zhang, Y. Liu, N. Wang, L. Teng and D. Wang, Int. J. Biol. Macromol., 2018, 116, 8–15 CrossRef CAS.
- T. T. Huang, Y. W. Lan, Y. F. Ko, C. M. Chen, H. C. Lai, D. M. Ojcius, J. Martel, J. D. Young and K. Y. Chong, J. Ethnopharmacol., 2018, 220, 239–249 CrossRef.
- X. Qiao, Q. Wang, S. Ji, Y. Huang, K. D. Liu, Z. X. Zhang, T. Bo, Y. M. Tzeng, D. A. Guo and M. Ye, J. Pharm. Biomed. Anal., 2015, 111, 266–276 CrossRef CAS.
- Z. W. Li, S. Ji, B. Li, S. Wang, Y. M. Tzeng, X. Qiao and M. Ye, TMR Mod. Herb. Med., 2018, 1, 40–50 Search PubMed.
- Q. Wang, X. Qiao, Y. Qian, Z. W. Li, Y. M. Tzeng, D. M. Zhou, D. A. Guo and M. Ye, Nat. Prod. Bioprospect., 2015, 5, 237–246 CrossRef CAS.
- C. K. Chen, J. J. Kang, W. C. Wen, H. F. Chiang and S. S. Lee, J. Nat. Prod., 2014, 77, 1061–1064 CrossRef CAS.
- J. B. Chang, M. F. Wu, H. F. Lu, J. Chou, M. K. Au, N. C. Liao, C. H. Chang, Y. P. Huang, C. T. Wu and J. G. Chung, In Vivo, 2013, 27, 739–745 Search PubMed.
- T. I. Chen, C. C. Chen, T. W. Lin, Y. T. Tsai and M. K. Nam, Food Chem. Toxicol., 2011, 49, 429–433 CrossRef CAS.
- T. I. Chen, C. W. Chen, T. W. Lin, D. S. Wang and C. C. Chen, Int. J. Med. Mushrooms, 2011, 13, 505–511 CrossRef.
- ClinicalTrials.gov identifier: NCT01134016.
- K. Li, J. Feng, Y. Kuang, W. Song, M. Zhang, S. Ji, X. Qiao and M. Ye, Adv. Synth. Catal., 2017, 359, 3765–3772 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Tables S1–S5 and Fig. S1. See DOI: 10.1039/d0np00023j |
| This journal is © The Royal Society of Chemistry 2021 |