Advances in cellulose-based superabsorbent hydrogels
Abstract
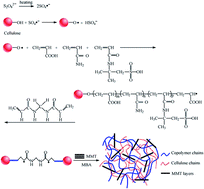
This contribution provides a brief overview of recent progress in cellulose-based superabsorbent hydrogels, fabrication approaches, materials and promising applications. First, different synthesis methods are introduced, including physical, as well as chemical cross-linking. Second, some of the cellulose series original materials were introduced in this work. In addition, some applications and future research in cellulose-based superabsorbent hydrogels are also discussed in this review.

 Please wait while we load your content...
Please wait while we load your content...