Adsorption behavior of dodecyl hydroxypropyl sulfobetaine on limestone in high salinity water
Abstract
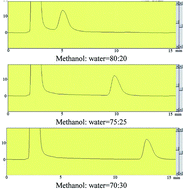
Herein is presented a new methodology to determine the static adsorption of dodecyl hydroxypropyl sulfobetaine (DSB) on limestone with the use of HPLC. The results showed that with the increase of NaCl concentration, adsorption of DSB on the limestone surface decreased due to the increase of zeta potential (less negative). In contrast, with increasing CaCl2 concentration, adsorption of DSB decreased first and then increased, which corresponds to the change in trend of the limestone surface zeta potential. As to the influence of temperature, with an increase of temperature, the adsorption of DSB increased slightly. The behavior of DSB adsorption under different temperatures could be well interpreted by more adsorption of Ca2+ at higher temperature. It was also found that a little addition of inorganic salt could accelerate the formation of vesicles and more salt would inhibit the formation. By comparison of aggregation distribution before and after adsorption, it was found that micelles contribute more to adsorption than vesicles.

 Please wait while we load your content...
Please wait while we load your content...