There is a hole in my bucky: perspective on a maverick
Fred
Wudl
Department of Materials, University of California, Santa Barbara, California 93106, USA. E-mail: wudl@chem.ucsb.edu
Abstract
This small contribution about Prof. Jan C. (Kees) Hummelen shows a part of his scientific life that involved his stay at UCSB and subsequent developments that were influenced by it. The article concentrates on Kees’ major contributions to fullerene research and to organic materials science, particularly organic electronics.
In the process of writing one's memoir, some colleagues and friends stand out and among them is clearly Jan Cornelis Hummelen (“Kees” Hummelen). It must have been written in some book of fate that Jan C. Hummelen's and my path would cross. He started his formative research with chirality, or more general, asymmetry, progressed to symmetry and ended up in electronic aspects of physical organic chemistry. His work on chirality dealt with asymmetric synthesis of epoxides.1 His work on symmetry applied to highly symmetric molecules, first adamantane derivatives and next the fullerenes. The work on organic electronics was fuelled by his participation in the discovery of bulk heterojunction solar cells. In my case, the professional evolution was very similar, including dealing with asymmetric synthesis. For my PhD dissertation I worked on the preparation of a chiral polymer for the chromatographic optical resolution of amino acids, where the synthesis of the monomer involved an asymmetric synthesis of a sulfinate ester.
In this perspective the contributions to fullerene research in our group at Santa Barbara as well as Kees’ enormous contributions to organic electronics will be presented as a set of “vignettes”, starting with Kees joining our group.
The beginning
One day, I believe in 1993, I received a note from Dr Jan C. Hummelen, a PhD organic chemist who wanted to join our group and who looked like he would be a good match for the research we were doing on fullerenes. I invited him to Santa Barbara and I was pleasantly surprised when he responded to my letter positively.When Kees arrived in Santa Barbara we were heavily involved in all aspects of fullerene science, from synthetic derivatives to solid-state characterization and applications. Kees was already familiar with the isolation and purification of fullerenes because he had a Dutch patent application with him as the sole inventor2 on the chromatographic separation and purification of C60 and C70.
First steps
Kees’ first publication from our group, in collaboration with researchers from Emory University, was on the inhibition of the protease of the HIV virus. In collaboration with another Dutch postdoctoral fellow in the group, Rint Sijbesma, Kees prepared a relatively water-soluble methanofullerene that also exhibited low toxicity in mice.3 His next publication with the group was the discovery that a classical photochemical reaction, the di-π-methane rearrangement, worked surprisingly well to realize the rearrangement of a fulleroid to a methanofullerene.4 Since many addition reactions to fullerene afford the fulleroid as the kinetic control product, this simple rearrangement became useful to convert this product to a methanofullerene, a more stable form. Later, together with Rosario Gonzalez, Kees showed that one can also achieve the rearrangement with acid catalysis.5 Together, these processes opened the access to a large number of methanofullerene derivatives for applications in bio-medicine and organic electronics. Eventually Kees would be involved in 25 additional publications between 1994 and 1999 with our group, and an additional one in 2002.Thinking outside the cage
When Kees and Maurizio Prato applied a self-sensitized photo-oxidation reaction of fullerenes to an N-MEM substituted azafulleroid (C60N-MEM), they had discovered the first systematic cage opening of a fullerene derivative in the form of a keto lactam (C60NO2-MEM) (Fig. 1). When the results were presented in a group meeting, it was a typical Kees’ thinking to come up with a possible title for the publication of this result, “There is a Hole in My Bucky”.6 We, of course, all thought it was a clever suggestion that Peter Stang, the then editor of JACS, would not accept and force us to change it. Much to our surprise, the manuscript was accepted with its title unchanged.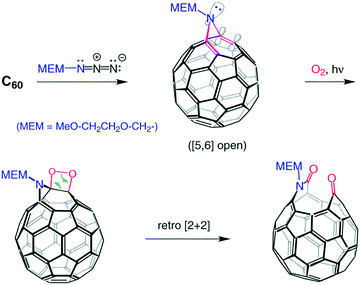 | ||
| Fig. 1 Azafulleroid conversion to “holey bucky”.6 | ||
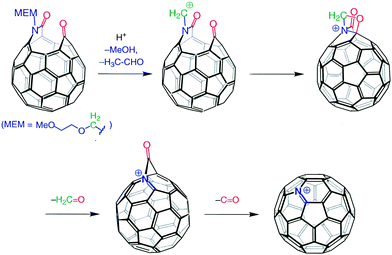
Though this was a beautiful and important demonstration that the C60 cage can be opened in a regiospecific way, it was not as momentous a discovery as the finding that a carbon atom could be replaced by a nitrogen atom chemically (not pyrolytically or by electron bombardment) under mild conditions. This was another very Kees-specific discovery in the sense that, when Kees was presented with what appeared to be a trivial answer, he questioned it until he was satisfied with the solution. So, when the FAB-MS of the “holey bucky” showed a base-peak at m/z 722 (m/z of C60 = 720), the average chemist would have concluded that the peak was due to a doubly protonated fullerene, NOT Kees! He asked for a high-resolution measurement and received m/z = 721.9991; calculated for C59N m/z = 722.03074, for C60H2, m/z = 722.6577. Therefore the base peak, within experimental error, had to be C59N(+). He then set out to try to mimic the gas-phase reaction with a reaction in solution phase and soon found out he could convert the “holey bucky” to the azafullerene cation with a mild acid-catalyzed process (Fig. 2).
 | ||
| Fig. 2 Formation of heterofullerene C59N.7 | ||
Gentle reduction of the cation, in situ, afforded a free radical that was isolated as the azafullerene dimer (NC59–C59N), where the cage–cage bond occurred through the carbon atom α to the nitrogen atom. This keen observation resulted in a Science magazine publication only a few months after the preparation of the “holey bucky”.7 It was clearly an exciting result that showed the way to a fullerene cage system with different properties compared to the original all-carbon cage.
Genuine interdisciplinary researcher and PCBM
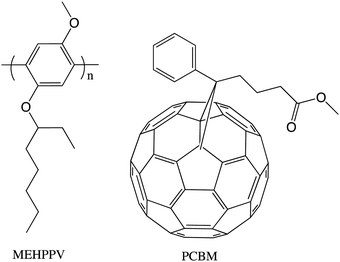
Our group had as a priority truly multidisciplinary research, where physicists from Alan Heeger's group interacted with chemists in our group on a daily basis. So, when Serdar Sariciftci, a visiting scientist in the Heeger lab, needed some pure C60 to see if it would participate as an electron acceptor in a photoinduced electron transfer experiment, he obtained a sample from Kees. Indeed the combination of a conjugated polymer invented in our group (MEHPPV) and Buckminsterfullerene gave a relatively efficient photovoltaic effect.8When Kees joined the group we had a project to make water-soluble fullerene derivatives to investigate their biomedical properties. Kees took an undergraduate student, Florence LePeq, under his wing and they synthesized a carboxylic acid derivative that was expected to be water-soluble as its carboxylate anion. The compound, shortly after its publication,9 became known as PCBM. After the successful experiments detailed in ref. 8, Serdar Sariciftci needed a more soluble derivative of C60 that would be more compatible with MEHPPV, so he asked Kees if he could provide such a derivative and Kees gave him a sample of PCBM. The MEHPPV/PCBM combination gave the highest efficiency organic photovoltaic of the day (Fig. 3).10
The new concept of bulk heterojunction (bhj) solar cells was discovered with this system. From then on, for many years, PCBM and its C70 analog were the “fruit fly” of organic photovoltaics. Not surprisingly, the demand for these PCBMs by physicists, physical chemists and electronic engineers was substantial, so, shortly after his return to the Netherlands, Kees and his group made commercially available samples of PCBM, including fully deuterated samples. It was clear that after the Science magazine publication of the first case of a bhj,10 Kees became one of the world leaders in the physical organic chemistry of organic photovoltaics. He became part of a European group of scientists that made exciting and fundamental contributions to this very important energy conservation research.
Teaching an old dog new tricks
After his return to Groningen, Kees maintained interdisciplinary collaborations with physicists and chemists in Europe. He made very important contributions to the separation and characterization of multi-addends to C60. He became an international exponent in organic electronics but I believe one of his more interesting publications is a beautiful extension of the well-known theoretical approach of valence-bond (resonance) in organic chemistry, to a new concept, namely “omniconjugation”.11 This concept is based on an observation that an apparent cross-conjugated system would actually allow through conjugation to all the peripheral substituents of a π-system if it had the proper topology. I believe that this led him into the area of single molecule electronic devices, a topic that he continued to research until his retirement.Today
Over the years, the field of research of organic materials has resulted in the preparation of organic metals, organic superconductors, organic semiconductors and organic magnets. The last organic solid-state application that remained to be explored was thermoelectrics. Kees' group, in the last couple of years, has made substantial progress in this area, particularly in the microscale. There are a number of semiconductor applications where a high dielectric constant layer is an important component of the device. Kees’ group has also made great inroads, within the last two years, into high dielectric constant materials for device fabrication with the design of specific methanofullerene derivatives containing oligo-PEG and branched oligo-PEG.Today, Kees is enjoying retirement in a bucolic setting outside Groningen with his partner, Gearstje, the academic advisor to the faculty at Groningen. Kees is enjoying, among other interests (jazz piano, etc.), the care of two horses.
References
- R. Helder, J. C. Hummelen, R. W. P. M. Laane, J. S. Wiering and H. Wynberg, Catalytic Asymmetric Induction in Oxidation Reactions. The Synthesis of Optically Active Epoxides, Tetrahedron Lett., 1976, 17, 1831 CrossRef.
- J. C. Hummelen, Liquid chromatography apparatus and method for the recovery of high-purity fullerenes from fullerene mixtures, Neth. Appl., NL 9200341 A 19930916, 1993.
- R. F. Schinazi, A. McMillan, A. S. Juodawlkis, J. Pharr, R. Sijbesma, G. Srdanov, J.-C. Hummelen, F. D. Boudinot, C. L. Hill and F. Wudl, Anti-Human Immunodeficiency Virus, Toxicity in Cell Culture, and Tolerance In Mammals of a Water-Soluble Fullerene, Proc. Electrochem. Soc., 1994, 94, 689 Search PubMed.
- R. A. J. Janssen, J. C. Hummelen and F. Wudl, Photochemical Fulleroid to Methanofullerene Conversion via the Di-π-methane (Zimmerman) Rearrangement, J. Am. Chem. Soc., 1995, 117, 544–545 CrossRef CAS.
- R. Gonzalez, J. C. Hummelen and F. Wudl, The Specific Acid-Catalyzed and Photochemical Isomerization of a Robust Fulleroid to a Methanofullerene, J. Org. Chem., 1995, 60, 2618–2620 CrossRef CAS.
- J. C. Hummelen, M. Prato and F. Wudl, There Is a Hole in My Bucky, J. Am. Chem. Soc., 1995, 117, 7003–7004 CrossRef CAS.
- J. C. Hummelen, B. Knight, J. Pavlovich, R. González and F. Wudl, Isolation of the Heterofullerene C59N as Its Dimer (C59N)2, Science, 1995, 269, 1554–1556 CrossRef CAS PubMed.
- R. A. J. Janssen, J. C. Hummelen, K. Lee, K. Pakbaz, N. S. Sariciftci, A. J. Heeger and F. Wudl, Photoinduced Electron Transfer from π-Conjugated Polymers onto Buckminsterfullerene, Fulleroids and Methanofullerenes, J. Chem. Phys., 1995, 103, 788–793 CrossRef CAS.
- J. C. Hummelen, B. W. Knight, F. LePeq and F. Wudl, Preparation and Characterization of Fulleroid and Methanofullerene Derivatives, J. Org. Chem., 1995, 60, 532–538 CrossRef CAS.
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Polymer Photovoltaic Cells: Enhanced Efficiencies via a Network of Internal Donor-Acceptor Heterojunctions, Science, 1995, 270, 1789–1791 CrossRef CAS.
- M. H. van der Veen, M. T. Rispens, H. T. Jonkman and J. C. Hummelen, Molecules with linear π-conjugated pathways between all substituents: Omniconjugation, Adv. Funct. Mater., 2004, 14, 215 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |