Open Access Article
Open Access ArticleA roadmap for laser optimization of Yb:Ca3(NbGa)5O12-CNGG-type single crystal garnets†
J. O.
Álvarez-Pérez
 ,
J. M.
Cano-Torres
,
J. M.
Cano-Torres
 ,
A.
Ruiz‡
,
M. D.
Serrano
,
A.
Ruiz‡
,
M. D.
Serrano
 ,
C.
Cascales
,
C.
Cascales
 and
C.
Zaldo
and
C.
Zaldo
 *
*
Instituto de Ciencia de Materiales de Madrid, Consejo Superior de Investigaciones Científicas, c/Sor Juana Inés de la Cruz 3, 28049 Madrid, Spain. E-mail: cezaldo@icmm.csic.es
First published on 22nd March 2021
Abstract
The continuous wave laser performance of Yb-doped Ca3(NbGa)5O12-CNGG-type disordered single crystal cubic garnets has been analyzed on the statistical basis of 26 crystals grown by the Czochralski method under different conditions. The low purity of the CaCO3 chemical used for phase synthesis is the major source of colour induced by non-correlated visible and red-near infrared optical absorptions, but the centres responsible for this colouration have a very minor impact on Yb3+ laser performance. The use of high purity (≥99.99%) Yb2O3 is essential to preserve the radiative properties of the Yb3+ laser. The Yb segregation coefficient in CNGG amounts to SYb = 1.12 ± 0.11. Up to 20% of Ca2+ in the dodecahedral garnet site can be substituted by Na+ and Yb3+ with SNa = 0.39 ± 0.05 and SYb = 1.34 ± 0.09, respectively, i.e. Na promotes Yb incorporation. In contrast, tetrahedral Li+ reduces Yb incorporation to SYb = 0.88 ± 0.07. The Yb3+ peak absorption cross section in CNGG amounts to σABS(λ = 971.7nm) = 1.97 ± 0.32 × 10−20 cm2. σABS is sensitive to Na and Li incorporation but no evidence of significant emission or gain cross section changes is found indicating that the majority Yb3+ centre is responsible for the observed fluorescence. Correspondingly, laser performance of crystals containing up to 15 at% of Yb shows only a slight improvement in Li-modified Yb:CLNGG crystals, associated with better crystalline quality. In them laser tuning extends in an unprecedented range up to 60 nm, even beyond 1080 nm in Li and Na co-modified crystals. 7–8 at% Yb doping of Li-modified CLNGG crystals with ≈95% pump absorption show large laser slope efficiency along with maximized output power.
1. Introduction
In the past years there has been very intense and prolific research into the development of mode-locked ultrashort (fs) laser pulses both in oscillators and amplifiers.1–4 Different gain media, including dyes dispersed in liquids, semiconductors, optical fibers and solid state technologies, have been used for this purpose. Even nowadays, the latter is the only option for high peak/average (>1 MW/>100 GW) laser powers.5 In turn, high power solid state lasers comprise glass, single crystal and ceramics optical gain media. In chronological order, single crystals replaced glass due to the low thermal conductivity of the latter, and ceramics are gaining use due to their better mechanical properties and superior processing versatility than single crystals.Although the technology of Ti-sapphire (Ti-sa) laser systems is very well established and presently it is the single crystal of reference for production of fs laser pulses, more recently Yb3+ lasers have attracted a lot of attention because they are in-band pumped with InGaAs diode lasers (DL), the quantum defect of Yb3+ is very small alleviating the thermal problems generally encountered in most solid state lasers, and Yb3+ has no excited state optical absorption losses. These are large advantages for the power scalability of the laser systems.
Ultrashort laser pulses require optical gain media with a large fluorescence bandwidth. Most trivalent lanthanides (Ln3+) do not fulfil this requirement due to the inner character of the 4fn orbital, but the situation is more favourable for Tm3+ (4f12) and Yb3+ (4f13). The near to full filling of the 4f orbitals for these latter ions favours electron interactions with lattice vibrations and their f–f spectroscopic bands are much broader than those observed for the rest of the Ln3+. In spite of this fact conventional Yb-doped single crystals and ceramics, such as Yb:YAG in which Yb (and other Ln3+) has a unique crystallographic position and environment, show time-bandwidth limited laser pulses with a duration longer than 100 fs.6 Only when the spontaneous wavelength emission was hindered could Yb:YAG break down the 100 fs barrier,7 but at the cost of reduced efficiency.
Solid state lasers have been demonstrated for years using either glass or single crystal doped hosts. Although the newer transparent ceramics technology8–10 is gradually replacing the above hosts, there are still many applications which benefit from the use of single crystals, for instance in TW–PW short pulsed lasers the depolarization losses are minimized by crystal orientation11,12 which cannot be achieved in ceramics, when UV Xe lamp pumping is required permanent solarisation of the presently developed YAG ceramics may appear,13 or the larger Rayleigh light scattering of ceramics in the visible region may limit laser implementation in this spectral range. Furthermore, despite grain-oriented anisotropic fluorapatite ceramics being produced with limited optical transmittance for laser applications,14,15 large cross section laser ceramics are basically restricted to optically isotropic media, i.e. cubic materials, which limits host options for optical bandwidth engineering.
The so called “disordered” crystals are presently being actively investigated as an alternative to enlarge the fluorescence bandwidth of Yb3+ and other Ln3+, with the aim of achieving shorter laser pulses. In disordered crystals a variety of crystalline environments around the Ln3+ induces further inhomogeneous broadening with regard to conventional ordered crystals. In fact, this goal has been pursued first in single crystal and later in ceramic garnet hosts by isovalent substitutions, for instance in Yb:(YGd2)Sc2(GaAl2)O12 media reaching 112 fs pulses.16
The {Ca◊}3[NbGa□]2(GaNb¤)3O12 cubic garnet (hereafter shortened as CNGG. ◊, □, and ¤ are cationic vacancies at the corresponding sites) is an outstanding disordered crystal since laser pulses as short as 45 fs have been demonstrated in a 10 at% Yb-doped crystal of this class,17 and it has the potential to be developed as transparent ceramics, although the attempt so far reported was not successful.18 Room temperature thermal conductivity of CNGG is intermediate, reaching 4.4 W m−1 K−1 in undoped CNGG and 3.63 W m−1 K−1 for Li-modified 7.57 at% Yb:CLNGG.19 Although a minor (<5 × 1018 cm−3) amount of Yb3+ can be found in the octahedral [16a] site of this garnet20 the majority of Yb sits at the dodecahedral {24c} site, which is also occupied by Ca2+ and eventually with a small amount (1.5%) of {◊} vacancies, being surrounded by several oxygen polyhedra: four other nearest dodecahedra {24c}, four corner-sharing octahedra [16a] and six tetrahedra (24d), four of the latter sharing corners and the two others, at the shortest distance, sharing edges. In undoped CNGG the octahedral site is mostly filled by Nb5+ (≈62%) with a minor amount of Ga3+ (≈37%) and [□] vacancies (≈0.3%), while the tetrahedral site is mostly filled by Ga3+ (≈82%) with minor occupancy of Nb5+ (≈10%) and (¤) vacancies (≈8%). These occupancies are further sensitive to the Yb doping and to the incorporation of crystal modifiers (non-optically active dopants, such as Na+ or Li+). The crystalline complexity provides a large number of oxygen and cationic environments around Yb3+ with a large impact on its optical band broadening. Furthermore, Na21 and Li22,23 CNGG modifications (hereafter CNNGG and CLNGG crystals, respectively) have been also developed. Na+ enters in the dodecahedral site, potentially helping in the charge compensation of Ca2+ substitution by Yb3+,21 while Li+ exclusively sits at the tetrahedral sites, removing tetrahedral cationic vacancies.19
Laser characterization of Yb:CNGG-type crystals has been demonstrated in continuous wave (cw), Q-switched and mode-locked operation regimes. For a comprehensive description of the work so far reported see the ESI,† Table S1. In particular, rather high laser output powers, 5–10 W, have been obtained in cw operation, as well as short (45 fs) laser pulses in mode-locked operation under DL pumping. However, the activity and conclusions are limited by the scarce variety of available crystals, most often limited to only 5 at% Yb nominal doping. On the other hand, the spectroscopic results so far reported are to some extent contradictory, for instance the Yb3+ 0 → 0′ maximum absorption cross section (at λ ≈ 972 nm) varies in the σABS = 1.4–3.8 × 10−20 cm2 range while its full width at half maximum (FWHM) has been reported in the 2.6–15 nm range and the reported ratio between absorption cross sections at λ = 972 and λ = 933 nm varies in the 1–2.8 range. Furthermore, the average output power achieved for the shortest pulses is modest, ≈30 mW. In some cases improvements in the laser performance associated with Li or Na modifications have been claimed,17,24 but some of these conclusions are doubtful since we shall show that the actual crystal concentration of Na is much lower than previously assumed. Other aspects, such as the influence of crystal colouration, purity of chemical reagents, or the Yb concentration and associated pump absorption on laser performance, have been mostly ignored.
In the present work by using 26 crystals (24 of them doped with Yb) with different compositions and growth procedures we extend the knowledge about optimal material and experimental conditions for cw Yb3+ laser operation, which is a required first step for scaling power in mode-locked laser operation. Also, the improvement of the knowledge of the physicochemical properties of the material may help in successful ceramics development in the future. We carefully monitor Na incorporation into Yb:CNNGG crystals and the Yb concentration for unmodified and Li- or Na-modified crystals. We probe that laser operation occurs up to ≈15 at% of Yb doping of the dodecahedral {24c} site, opening the possibility of thin-disk laser designs.25,26 Among the different material and growth variables considered, the most relevant ones are the CaCO3 reagent purity which determines crystal colouration, the Yb concentration and Yb2O3 reagent purity which determines pump absorption and Yb3+ fluorescence quantum yield, the crystal mosaicity which is found to be similar to that of the commercial Yb:YAG crystal, as well as the presence of crystal modifiers. The impact of these variables on the Yb3+ laser performance is analysed in detail. Finally, we show that the oxygen content in the CNGG-type Czochralski grown crystals is virtually the same as that of the stoichiometric formula and apparently it cannot be modified by after growth oxidation annealing near the melting point, but vacuum thermal annealing even at moderate temperature (≈800 °C) leads to generalized Ga loss which could be the origin of the failure in previous ceramic processing.
2. Experimental techniques
CNGG-type single crystals were grown from melts of precursor ceramic powders synthesized by solid state reaction. Differential scanning calorimetry (DSC) measurements of these precursor powders and grown crystals were made with Setaram equipment, model SETSYS Evolution. Two consecutive 10 °C × min−1 heating/cooling cycles in air were carried out in all cases. These precursor powders were further characterized by powder X-ray diffraction (XRD) θ–2θ scans using a Bruker D8 Advance diffractometer operated with a Cu Kα source and a Lynxeye position sensitive detector.An oxidative atmosphere, either air or O2, and Pt crucibles were used for the Czochralski growth. Crystal growth details can be found in our previous works.19,21 Rocking curves of the 100 XRD of CNGG-type crystals were recorded at room temperature in a Texture Analysis Diffractometer Bruker D8 Advance with Cu Kα radiation.
The incorporation of Yb and Na into the crystal was determined by X-ray fluorescence analyses in a JEOL Superprobe JXA-8900 Electron Probe Micro Analyzer (EPMA). The ZAF method27 and CaO, Nb2O5, Ga2O3, Yb2O3 and Na2O standards were used. Na concentration was also evaluated by Wavelength Dispersive X-Ray Fluorescence (WDXRF) using a MagiX sequential spectrometer and methods described in a previous work.28 For the latter purpose specific Na standards were developed for calibration as explained in the ESI,† see Fig. S5, and the interference between Ga and Na X-ray emissions was explicitly considered, see Fig. S6 (ESI†). Furthermore, single crystal X-ray diffraction (scXRD) refinements of the crystallographic structure, at least in crystals having the largest Yb concentration for each CNGG, CNNGG and CLNGG crystal series, were performed. Details of the scXRD equipment and procedures used can be found in the ESI† and in our previous works.19,21 When required, Yb concentrations were also calculated from the integrated optical absorption of Yb3+ taken as reference EPMA measurements. Table S3 (ESI†) summarizes the growth conditions, composition and other physical properties of the 26 used crystals.
Optical absorption (OA) was recorded in a Varian 5E spectrophotometer with a spectral resolution of 0.15 nm for λ ≈ 900–1150 nm and 0.2 nm for λ ≈ 300–850 nm. The spectra were acquired with a wavelength scanning step of 0.05–0.1 nm and 1–5 s of integration time. To decrease acquisition noise, each near infrared (NIR) spectrum was the average of ten acquisitions. Furthermore, for each crystal composition several sample thicknesses were compared to assure the linear response of the equipment, and thick samples (>10 mm) were used to record the weak Yb3+ OA in the λ = 980–1150 nm spectral region. Photoluminescence (PL) was excited with a Ti-sa laser, dispersed using a Czerny–Turner SPEX monochromator (f = 34 cm) and detected with a 77 K cooled Ge photodiode. All NIR PL measurements were corrected by the spectral response of the equipment. The Yb lifetime was determined from the intensity decay of the λ = 1021 or 1035 nm PL excited with a MOPO laser system tuned at λ = 971 or 972 nm, this PL was dispersed using the above-described monochromator, detected with a Peltier-cooled Hamamatsu photomultiplier model H10330A-75, and recorded with Lecroy 200 MHz or Tektronix 500 MHz oscilloscopes. For all these spectroscopic measurements the sample temperature was set in the 6–300 K range by using a closed-cycle He cryostat equipped with a Lake Shore temperature controller.
Laser properties of the crystals were assessed in a z-shaped optical resonator sketched in the ESI,† Fig. S1. A lens (f = 80 mm) focuses onto the sample the TEM00 mode of a cw Ti-sa laser. It generates a focus with a diameter of 62 μm and a depth of focus 2z0 ≈ 4 mm. TOC = 1, 2.5, 5 and 10% output coupler transmissions were used. Laser spectral distribution was assessed with an APE laser spectrometer, model WaveScan. Uncoated samples were glued with silver paste to a water-cooled (16 °C) copper holder. Samples were set perpendicularly to the pumping beam and the sample Fresnel reflection was evaluated from the refractive index of the crystal.19 Laser tuning was obtained by inserting a SF10 prism in the cavity.
3. Results
3a. Crystal growth, composition and crystallography
As a preliminary step for crystal growth, the melting nature of (Ca1−2xYbxNax)3(Nb0.3375Ga0.6375)5O12 precursor powders synthesized by solid state reaction (two steps of heating to 1330 °C for 6 h with intermediate grinding) was studied by DSC. Results are shown and discussed in detail in the ESI.† For undoped CNGG only a single endo/exothermic peak was observed upon two consecutive heating/cooling cycles, see Fig S2a (ESI†). This situation is maintained up to <20 at% of total Yb + Na cooperative substitution in the garnet formula. For ≥20 at% of Ca substitution new peaks at a temperature lower than that corresponding to the garnet melting are observed in the first and second DSC cycles, see Fig. S2b (ESI†). Thus, it can be anticipated that the total Na + Yb limit for melt stability occurs for Ca substitution in the garnet dodecahedral position of ≈20 at%, even lower for non-modified Yb:CNGG precursor powders.Correspondingly, Yb:CNGG crystals were obtained with good optical quality up to 15 at% of Yb concentration in the melt. Likewise, up to 30 at% of Na in the melt (8.78 at% of Na in the crystal) could be used to grow Na-modified ≈9.4 at% Yb:CNNGG crystals. Furthermore, Li - and Li + Na-modified 8 at% (in the melt) Yb:CLNGG and Yb:CLNNGG crystals were also grown. These crystals have been grown using Yb2O3 of 99.9%, 99.99% or ≥99.998% purity. CaCO3 with 99.5% or 99.99% of purity was used. Other used reagent purities were 99.5% and 99.997% for Na2CO3, 99% and 99.997% for Li2CO3, 99.9% or 99.99% for Nb2O5 and 99.99% for Ga2O3. Some crystals were grown in a pure oxygen (O2) atmosphere and others in air. Further DSC analyses of the grown single crystals show that Yb and Li incorporations increase the melting temperature while Na modification significantly decreases it, see the ESI† Table S2.
In our previous scXRD analyses21 of CNGG-type crystals, combined with high resolution powder neutron diffraction data for Li-modified CLNGG crystals,19 an oxygen stoichiometric composition was assumed for the analysis. Taking into account that some oxide single crystals may be oxygen deficient, as established by careful scXRD refinements in alternative materials,29,30,31 the oxygen occupancy factor (OF) is now refined for undoped CNGG and selected Yb:CNGG-type (either non-modified or modified with Na) crystals to assess its influence on crystal stoichiometry. Structure refinements have been performed in the cubic space group Ia![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d (No. 230), and they include atomic coordinates, the evaluation of cationic and vacancy distributions over dodecahedral {24c}, octahedral [16a], and tetrahedral (24d) garnet crystal sites, and anisotropic thermal displacements. These analyses provide an alternative estimation of crystal compositions, as well as unit cell parameter and interionic distances. A selection of the obtained results is included in Table 1. These results indicate that the studied CNGG-type crystals do not present oxygen deficiency, moreover their comparison with previous data from structure analyses that did not include oxygen OF refinements reveals differences only within the estimated precision in cationic and vacancy distributions. Further details of scXRD refinements as well as crystal data are included in the ESI,† Tables S4–S7.
d (No. 230), and they include atomic coordinates, the evaluation of cationic and vacancy distributions over dodecahedral {24c}, octahedral [16a], and tetrahedral (24d) garnet crystal sites, and anisotropic thermal displacements. These analyses provide an alternative estimation of crystal compositions, as well as unit cell parameter and interionic distances. A selection of the obtained results is included in Table 1. These results indicate that the studied CNGG-type crystals do not present oxygen deficiency, moreover their comparison with previous data from structure analyses that did not include oxygen OF refinements reveals differences only within the estimated precision in cationic and vacancy distributions. Further details of scXRD refinements as well as crystal data are included in the ESI,† Tables S4–S7.
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) d, Z = 8) garnets. a = lattice parameter. OF = occupancy factors. Na/Yb and Li atomic melt compositions (at%) are referenced to the octahedral and tetrahedral sites, respectively. *Previously available data without oxygen OF refinement
d, Z = 8) garnets. a = lattice parameter. OF = occupancy factors. Na/Yb and Li atomic melt compositions (at%) are referenced to the octahedral and tetrahedral sites, respectively. *Previously available data without oxygen OF refinement
| Melt composition (at%) | a (Å) | OF | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| {24c dodecahedral site} | [16a octahedral site] | (24d tetrahedral site) | 96h | ||||||||||
| Ca2+ | Yb3+ | Na+ | ◊ | Nb5+ | Ga3+ | □ | Ga3+ | Nb5+ | Li+ | ¤ | O2− | ||
| CNGG | 12.4969(1) | 0.987(6) | — | — | 0.013 | 0.623(4) | 0.374(4) | 0.003 | 0.82(4) | 0.10(4) | — | 0.08 | 0.994(8) |
| 11.6Yb: CNGG | 12.4548(3) | 0.868(2) | 0.102(2) | — | 0.03 | 0.63(2) | 0.34(2) | 0.03 | 0.922(4) | 0.003(4) | — | 0.075 | 0.999(6) |
| Li-Modified | |||||||||||||
| CLNGG* | 12.50657(2) | 0.998(5) | — | — | 0.002 | 0.66(3) | 0.34(3) | 0 | 0.842(4) | 0.052(4) | 0.079(14) | 0.027 | 1 |
| 9.2Li:8Yb:CLNGG* | 12.48228(2) | 0.931(4) | 0.069(4) | — | 0 | 0.67(3) | 0.33(3) | 0 | 0.810(3) | 0.069(3) | 0.111(15) | 0.01 | 1 |
| Na-Modified | |||||||||||||
| 30Na:8Yb: CNNGG | 12.4883(1) | 0.814(4) | 0.051(1) | 0.135(4) | 0 | 0.550(4) | 0.450(4) | 0 | 0.825(4) | 0.085(4) | — | 0.090 | 0.999(6) |
| 16Na:8Yb:CNNGG | 12.4842(1) | 0.791(4) | 0.093(1) | 0.116(4) | 0 | 0.661(4) | 0.339(4) | 0 | 0.93(4) | 0.01(3) | — | 0.06 | 0.997(7) |
| 12Na:12Yb:CNNGG | 12.4611(1) | 0.783(4) | 0.161(2) | 0.056(4) | 0 | 0.773(4) | 0.227(4) | 0 | 0.94(4) | 0.02(4) | — | 0.04 | 0.993(8) |
| 10Na:10Yb:CNNGG | 12.4618(1) | 0.783(4) | 0.117(2) | 0.059(4) | 0.041 | 0.659(4) | 0.341(4) | 0 | 0.918(4) | 0.022(4) | — | 0.060 | 1.01(1) |
With reference to undoped CNGG crystal the substitution of Ca2+ by Yb3+ induces a reduction of the cell volume. This is compensated when Na+ is simultaneously incorporated in the dodecahedral site. Cationic vacancies over the dodecahedral and octahedral sites are virtually eliminated by the Yb and Na incorporation, but vacancies on the tetrahedral site are reduced to a lesser extent. These are only largely eliminated by Li modification.
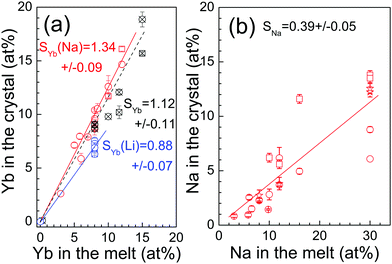
Fig. 1a and b show the relationship between in-the-melt and in-the-crystal Yb and Na concentrations, respectively. Considering all techniques used, the Yb segregation coefficient in CNGG is SYb = 1.12 ± 0.11. When Na is added to the melt in the same amount as Yb, the segregation coefficient of the latter increases slightly up to SYb = 1.34 ± 0.09, while it reduces to SYb = 0.88 ± 0.07 for Yb:CLNGG crystals, which shows the benefit of Na co-doping for Yb incorporation. These results agree qualitatively with those obtained in ref. 32, SYb = 1.19 for 10 at% Na: 10 at% Yb:CNNGG and SYb = 1.07 for 10 at% Na: 10 at% Yb:CLNNGG, which confirms that Li modification reduces Yb incorporation. On the other hand, the segregation coefficient for Na is SNa = 0.39 ± 0.05. The difficult incorporation of Na is the likely reason for there only being a small difference between SYb of Yb:CNGG and Yb:CNNGG crystals and it induces a Na-rich melt as the crystal growth proceeds, which at some point degrades the melt composition and produces growth instabilities.
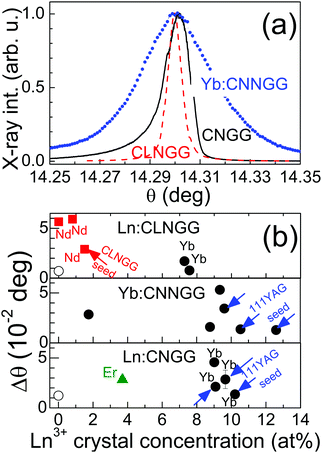
Although single crystals appear macroscopically free of defects, at the microscopic scale they have a mosaicity characterized by the angular width of a selected X-ray reflection in rocking curve experiments. We first analysed the 100 reflection of a 10 at% Yb:YAG acquired from a commercial laser crystal manufacturer. The full width at half maximum (FWHM) of the reflection was Δθ = 0.0153 deg. This was clearly larger than the instrumental resolution that was determined with a 111 Si standard as 0.0071 deg. Fig. 2 shows selected 100 rocking curves obtained for analyzed CNGG-type crystals, including Nd, Er and Yb dopants as well as Li and Na modifiers. The width of the X-ray reflection falls within the Δθ = 0.0065–0.06 deg range depending on the incorporated cations and growth conditions. Undoped CNGG has Δθ = 0.0123 deg. The smallest mosaicity was found for undoped Li-modified CLNGG, Δθ = 0.0065 deg, i.e. in the limit of our instrumental resolution. The incorporation of Yb supposes in all cases a moderate increase of the crystal mosaicity with regards to the corresponding undoped host, which was most evident in Na-modified Yb:CNNGG crystals, see Fig. 2a. However, significant differences are found if the seed used for growth is taken in consideration. Crystals grown using 111 YAG seeds generally have smaller Δθ mosaicity values than those spontaneously nucleated on platinum wires.
Li-modified Yb:CLNGG crystals exhibit a mosaicity similar to that of commercial Yb:YAG with similar Yb content, even when these crystals were spontaneously nucleated on Pt wire. The incorporation of Nd3+ (larger ionic radii than Yb3+) in CLNGG leads to a rapid increase of the mosaicity even for low Nd concentrations, <1.5 at% in the melt, but also in this case the use of a CLNGG crystalline seed reduced significantly the mosaicity, see Fig. 2b.
3b. Crystal colouration
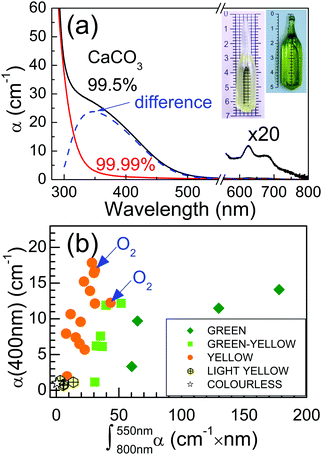
Grown CNGG-type crystals show different colours from intense green to pale yellow, and exceptionally they appear colourless, see Fig. 3a insets for two extreme examples of the crystal colouration obtained. This colouration is commonly reported in the literature for CNGG-type crystals grown by the Czochralski method.21,33–35 Two different OA features determine the observed crystal colour, see Fig. 3a. On one hand a complex but relatively weak OA is present in the λ = 580–800 nm Red-NIR region and on the other hand a pre-edge visible (VIS) OA overlapping the band gap optical transition is observed for λ < 500 nm. The difference between the VIS OA observed in highly and lowly coloured crystals is a band with maximum at λ = 350 nm, see dashed line in Fig. 3a.Fig. 3b shows a plot of the OA in the VIS versus that in the Red-NIR region for the different CNGG-type grown crystals. It is obvious that VIS and Red-NIR OA are not correlated, i.e. they correspond to different optical centres. Crystals grown in a pure oxygen atmosphere, labelled as O2 in Fig. 3b, do not show lesser colouration than those grown in air, which in accord with the above presented crystallographic study (oxygen OF ≈ 1) suggests that oxygen deficiency is not the origin of the observed colouration. A similar conclusion is reached from thermal annealing in air or in vacuum of CNGG-type crystals, see the ESI,† Fig. S7 and S8. Crystals annealed to 1340 °C (i.e. only ≈100 °C below the melting temperature) for 48 h do not show any significant modification of the OA associated to the colouration, and crystals reduced for 50 h in vacuum (2.5 × 10−2 mbar) up to 900 °C don’t show significant changes of the bulk colour, although in this case the crystal surface degrades with evident Ga loss, see Fig. S9 (ESI†).
Furthermore, crystal colouration is not exclusive to Yb-doped crystals, it also appears in undoped/unmodified CNGG crystals, so it is not directly related to the purity of used Yb2O3. Although low purity reagents (99.5% Na2CO3 and 99% Li2CO3) were used to grow some crystals, see Table S3,† their small required amount makes it unlikely that they were the main source of colouration. The use of low purity 99.5% CaCO3 precursor is the main cause of the observed crystal colouration. According to the Analysis Certificate of the 99.5% CaCO3 Alfa Aesar supplier, traces of transition metals are present: Fe, 0.0003%; Ba, 0.0008%; Sr, 0.003%; and Mg, 0.0012%. The presence of these contaminants in our crystal was tested by EPMA but, if present, their concentration is below the detection limit of the technique. Nevertheless, Fe garnets have intense OA bands in the VIS region,36 thus it seems to us likely that the VIS pre-edge absorption could be related to residual Fe impurities, as also suggested for YAG.37 The Red-NIR bands are maybe ascribed to localized electric charge trapped near cationic vacancies present in the structure,38 thus they decrease as vacancies are removed, for instance by doping with Li.
3c. Yb3+ optical cross sections
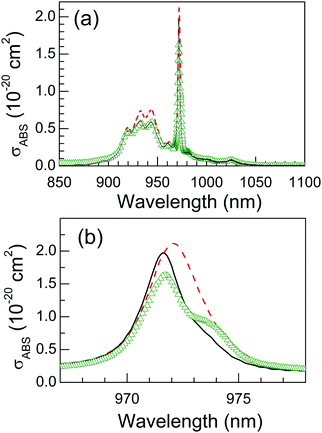
The Yb3+ absorption cross section, defined as σABS = α/[Yb] (where α is the Yb3+ absorption coefficient and [Yb] is the Yb3+ density in the crystal) describes the crystal efficiency for absorption of the optical pumping. In the case of cubic garnet crystals the OA is isotropic. A correct evaluation of σABS is essential in the assessment of laser performance since the emission and gain cross sections (the latter characterizing the efficiency of three level lasers) are obtained from it. For Yb:CNGG we have used seven crystals with different Yb concentrations to average the results, while for Na-modified Yb:CNNGG only the crystal with maximum Na concentration (12.23 at% Na and 7.13 at% Yb in the crystal) is used, and two different crystals with 7.57 and 7.28 at% Yb in the crystal were averaged for the Li-modified Yb:CLNGG case. The Yb incorporation to the CNGG lattice shrinks the garnet cell volume (see Table 1) increasing the Yb density. This effect has been taken into account explicitly in our σABS calculations by using actual cell volumes obtained by XRD analyses for each used crystal. The same procedure has been also used for Yb:CLNGG crystals, however for the Na-modified Yb:CNNGG the cell size reduction induced by Yb3+ is largely compensated by the increase induced by the bigger ionic size of Na+, thus the volume cell of CNGG was used. Fig. 4 shows the σABS results obtained at room temperature.The Yb3+ electronic configuration consists only of the ground 2F7/2 and one excited 2F5/2 multiplets whose degeneracy is lifted by the lattice crystal field into four (mJ = 0, 1, 2, 3) and three (mJ = 0′, 1′, 2′) Stark levels, respectively. Consequently, its OA spectrum is composed of a prominent Yb3+ 0 → 0′ band near to λ = 971.7 nm and of Yb3+ 0 → 1′,2′ related bands at λ = 944.2, 933 and 922 nm. The observation of more bands than expected should be related to the coexistence of several different crystalline environments around Yb and/or phonon coupling effects. Furthermore, at 300 K other replica bands with minor intensity are observed at the low energy side of these and ascribed to thermally assisted Yb3+ 1,2,3 → 0′,1′,2′ transitions. The most significant differences between Yb:CNGG and Na- or Li-modified crystals are: (a) The σABS ratio between λ = 971.7 nm and λ = 933 nm evolves from Yb:CNGG, σABS(971.7 nm)/σABS(932.7 nm) = 3.19, to σABS(971.8 nm)/σABS(933 nm) = 2.85 in Yb:CNNGG, and to σABS(972.1 nm)/σABS(933 nm) = 2.86 for Yb:CLNGG. In the case of Na-modified Yb:CNNGG crystals the decrease is due to the appearance of a new Yb3+ 0 → 0′ absorbance at λ = 973.5 nm, see Fig. 4b and Fig. S10.† In contrast, the decrease observed for the Yb:CLNGG crystal is mainly due to an enhancement of the Yb3+ 0 → 1′,2′ bands at λ = 944 and 933 nm. (b) The shape of the Yb3+ 0 → 0′ band in CNGG crystals at room temperature is observed to be rather symmetric with a FWHM = 2.23 nm, but this changes with crystal modifications. For Na modification the aforementioned band at λ = 973.5 nm appears, and the FWHM of the overlapped bands increases to 3.65 nm, while for Li modification the maximum of the Yb3+ 0 → 0′ band in CLNGG shifts to longer wavelengths, λ = 972.1 nm, and its FWHM increases (with regard to Yb:CNGG) to 3.1 nm. This peak shift is confirmed by the excitation of laser experiments described in later laser results section. (c) The maximum Yb3+σABS of Yb:CNGG amounts to 1.97 ± 0.32 × 10−20 cm2. In the 12.23 at% Na-modified Yb:CNNGG peak σABS decreases to 1.65 × 10−20 cm2, while in Li-modified CLNGG it increases to σABS = 2.12 ± 0.34 × 10−20 cm2. The measured σABS peak value of the narrow band at λ ≈ 972 nm greatly depends on the spectral resolution of the measurements. A large interval of values has been reported in the literature, thus the comparison with previous results based on σABS value at 933 nm is more reliable. On this basis, our results agree (within 50% of deviation) with the conclusions of ref. 21, 32, 39 and 40 and refute those of ref. 24, 41 and 42. It is also worth noting that despite the disordered nature of CNGG single crystals, the Yb3+ peak σABS in it is at least twice larger than that found in Yb:YAG, σABS(300 K) = 0.8 × 10−20 cm2.43
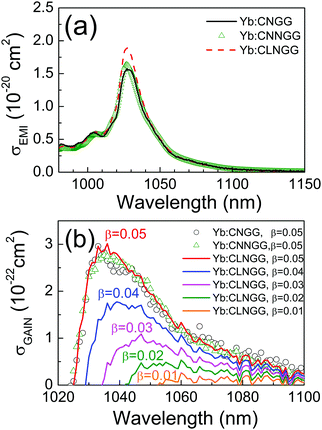
The emission cross section, σEMI, describes the fluorescence efficiency of the Yb3+ ion. Here, we have used the reciprocity method44 for its calculation as σEMI = σABS(Zl/Zu)exp[(Ezl−hν)/kBT]. The partition functions Zl and Zu, Z = Σkexp(−Ek/kBT), for the ground (2F7/2) and excited (2F5/2) Yb3+ multiplets, respectively, have been calculated from the Stark level (Ek) energies obtained from 6 K OA and PL spectroscopies, see the ESI,† Fig. S11 and S12. Due to the uncertainties for the band to Yb energy level assignment, associated with the multisite character of these crystals, a unique Zl/Zu = 1.45 value was used in all cases. Ezl is the energy of the Yb3+ 0 → 0′ transition, hν is the photon energy and kB is the Boltzmann constant. Even though thick (>1 cm) samples were used to reduce OA measurement uncertainty for λ > 1040 nm, measurements beyond λ > 1060 nm were not reliable. Therefore, the 300 K Yb3+ fluorescence normalized at λ = 1027.6 nm was used for the alternative quantification of σEMI at these long wavelengths. Fig. 5a shows the σEMI(λ) distribution obtained for Yb:CNGG and its change in Na- or Li-modified crystals. In all cases σEMI peaks at λ = 1027.6 nm. Although Yb:CLNGG has a slightly larger peak σEMI = 1.87 × 1020 cm2 than Yb:CNGG, σEMI = 1.56 × 1020 cm2, or Yb:CNNGG, σEMI = 1.62 × 1020 cm2, for λ > 1045 nm all three curves collapse into a single one. Taking into account that spontaneous cw laser operation takes place at λ = 1030–1060 nm, see later sections, Yb:CLNGG may have slightly better cw laser efficiency, but for mode-locked laser operation, typically operated with low transmission output couplers (TOC ≤ 1%), the laser emission shifts to larger wavelength values (λ = 1047–1061 nm) and laser performance differences for the three Yb:CNGG crystal types here considered are not anticipated.
An independent assessment of the certainty of the calculated σEMI can be inferred from the comparison of the experimental and calculated Yb3+ radiative lifetime, τRAD. Its value can be experimentally evaluated at low temperature by using a thin crystal with very low Yb concentration, which fully prevents fluorescence reabsorption and Yb–Yb energy diffusion losses. Thus we have used a 0.08 at% (1 × 1019 at Yb cm−3) Yb:CNGG crystal, obtaining τRAD = 816 μs, see the ESI† Fig. S14. This value is slightly larger than (and consistent with) fluorescence lifetimes previously reported for crystals with larger Yb concentration, 791 μs42 for 5.77 at% Yb (2.3 × 1020 cm−3) and 780 μs39 for 2.8 at% Yb (1.1 × 1020 cm−3) doping. On the other hand τRAD may be calculated from the σEMI(λ) by the Füchtbauer–Ladenburg method45 as 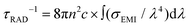 , where n is the refractive index, n(λ = 980 nm) = 1.96,19 and c is the speed of light in the vacuum. The calculated τRAD values using the above emission cross sections are τRAD = 440 μs for Yb:CNGG, τRAD = 464 μs for 12.23 at% Na-modified 7.13 at% Yb:CNNGG, and τRAD = 419 μs for Li-modified Yb:CLNGG. Taking into account the large number of parameters involved in these methodologies and the multisite character of the crystals, we consider that that experimental and calculated τRAD values agree, giving confidence to the present spectroscopic results.
, where n is the refractive index, n(λ = 980 nm) = 1.96,19 and c is the speed of light in the vacuum. The calculated τRAD values using the above emission cross sections are τRAD = 440 μs for Yb:CNGG, τRAD = 464 μs for 12.23 at% Na-modified 7.13 at% Yb:CNNGG, and τRAD = 419 μs for Li-modified Yb:CLNGG. Taking into account the large number of parameters involved in these methodologies and the multisite character of the crystals, we consider that that experimental and calculated τRAD values agree, giving confidence to the present spectroscopic results.
Since the ground 2F7/2 laser level of Yb3+ is electronically populated at room temperature, the laser efficiency is determined by the competition between absorption and emission cross sections. The gain cross section, σGAIN = βσEMI − (1 − β)σABS (β denotes the ratio of inverted ions to the total Yb density), describes this fact. Fig. 5b shows σGAIN(λ) for Yb:CLNGG crystals. Very similar σGAIN distributions have been also found for Yb:CNGG and Yb:CNNGG, see Fig. 5b.
3d. Laser results
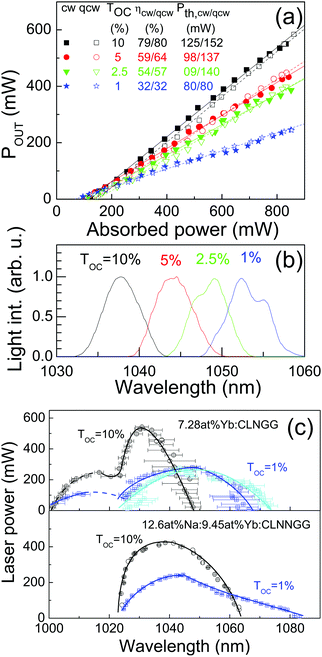
Along with laser experiments the sample pump absorption (either under laser operation or not) was evaluated from the beam passing through one of the cavity folding mirrors (a pumping mirror was used for this purpose). Systematic measurements were performed either under true continuous wave (cw) pumping or by 50% modulation of the pump beam with a chopper, quasi continuous (qcw) pumping.For low Yb concentration, pump absorption limits laser operation, but crystals with 1.74 at% Yb already showed laser action. While for high Yb concentration the radiative losses are the limit, consistently a 18.8 at% Yb:CNGG crystal grown using low purity precursors, i.e. 99.9% Yb2O3, 99.9% Nb2O5 and 99.5% CaCO3 did not show laser action. In between these limits, laser operation was achieved independently of the growth conditions. Fig. 6a shows an example of the linear relation between the laser output power and the absorbed pump power obtained using different output coupler transmissions (TOC). Laser performance is characterized by the slope of this relationship (η) and by the pump power laser threshold (Pth). Under these conditions laser output was unpolarized. For all tested crystals only minor differences were found between cw and qcw operation regimes, thus at the considered power levels, the sample heating was not a relevant factor. The laser slope efficiency increases with TOC up to rather large values (η = 80% for the 7.28 at% Yb:CLNGG monitored crystal with TOC = 10%) and the laser emission spectrum shifts to shorter wavelengths with TOC increase, see Fig. 6b. This latter effect is consistent with the shift observed for σGAIN with increasing β, see Fig. 5b, and it is characteristic of three level lasers.
A broad laser tuning range is a prerequisite for short laser pulses by mode-locking. Laser tuning of the crystals was studied by inserting at the Brewster angle a dispersive prism near to the rear mirror that forces a horizontally polarized laser output. In this case a high reflectance folding mirror was used opposite to the pumping mirror that recycled some of the transmitted pump, leading to slightly higher laser output powers. Fig. 6c shows the tuning range obtained for Yb-doped CLNGG and CLNNGG crystals using different output couplers.
It must be noted that at the peak of the tuning curves the emission is quite stable around a single wavelength (FWHM < 1.5 nm), but at the wings of the curves most typically several laser wavelengths with differences of up to Δλ ≈ 10 nm coexist. This difference is represented in Fig. 6c by horizontal error bars. Consistently with the wavelength shift described above, tuning with TOC = 1% extends more in the NIR (increasing λ) in comparison to the curves obtained with TOC = 10%. With the 7.28 at% Yb:CLNGG crystal tuning ranges from λ = 1000 to 1075 nm thanks to the contribution of a secondary regime for λ < 1020 nm. It must be noted that lasing for λ < 1010 nm is limited by the coating design of the used optics with a cutoff at this wavelength. In the 12.56 at% Na: 9.45 at% Yb:CLNNGG crystal this secondary regime was not observed and laser emission started at λ = 1025 nm but by using TOC = 1% it extends to λ > 1080 nm. These laser tuning spectral ranges widely exceed the 45 nm range (λ = 1030–1075 nm) previously reported with a Na-modified Yb:CNNGG crystal,17 even though in this latter work a TOC = 0.8% was used.
Since laser performance depends on many different material and instrumental parameters, in the following we study the relevance of some of them, such as crystal colouration, purity of used precursors, pump absorption and the effect of crystal modifications with Li or Na.
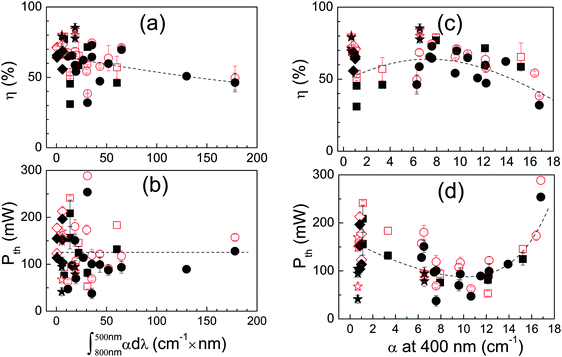
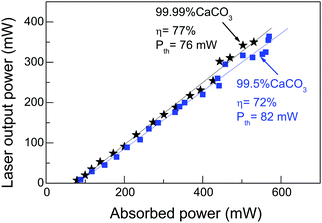
Since laser colouration is mostly related to the purity of the used CaCO3, Fig. 8 shows a comparison of the laser performance for ≈9 at% Yb:CNGG crystals grown using 99.99% or 99.5% CaCO3. No laser performance difference is found despite the fact that the integrated OA of the Red-NIR bands changes from 7.8 to 31 cm−1 × nm and that the pre-edge OA of both crystals was α(400 nm) = 7.9 and 12.1 cm−1, respectively. A similar conclusion was also obtained for Na-modified ≈9 at% Yb:CNNGG, and for Li-modified ≈7.5 at% Yb:CLNGG, see the ESI,† Fig. S16. It can be concluded that, different to other laser crystal hosts, the defects responsible for the CNGG crystal colouration do not have much influence on the Yb3+ laser performance.
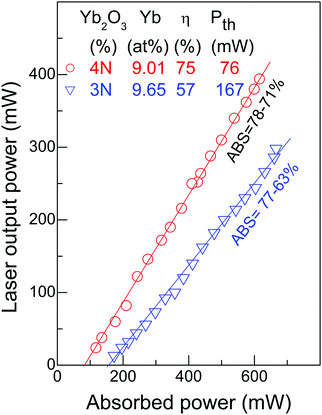
In order to test the influence of Yb2O3 purity on the cw laser performance, two CNNG crystals with Yb concentration near 9.5 at% have been compared under as close as possible experimental conditions. The sample thickness was calculated to compensate the slight variation of the Yb concentration of the two samples in such a way that the pump absorption of the crystals under laser operation was close to each other and always in the 63–78% range. Fig. 9 shows the obtained results. For the crystal grown using 99.9% Yb2O3 reagent the slope efficiency was ηCW = 46% and ηQCW = 57% for the cw and qcw operation regimes, respectively. These magnitudes increase up to ηCW = 77% and ηQCW = 75% when 99.99% Yb2O3 reagent was used. In parallel, Pth decreases with increasing Yb2O3 purity, i.e. Pth = 167 mW and 75 mW for 99.9% and 99.99% Yb2O3 purity, respectively.
Another clue to understanding the influence of the Yb2O3 purity on laser operation is monitoring the Yb3+ lifetime. Generally, the high purity of Yb2O3 preserves the Yb3+ fluorescence yield,48,49 which is beneficial for laser performance, however a decrease of the Yb3+ lifetime is expected with increasing Yb concentration due to Yb–Yb energy migration. This general trend is shown in the ESI,† Fig. S15. Thus it is important to compare only crystals with similar Yb concentration. The Yb3+ lifetime measured for a 9.65 at% Yb:CNGG crystal grown with 99.9% Yb2O3 was τ = 484 μs, but this value recovers to τ = 547 μs in 9.08 at% Yb:CNGG grown with 99.99% Yb2O3 purity. Similarly, the lifetime of a 12.07 at% Yb:CNGG crystal grown with 99.9% Yb2O3 was τ = 406 μs while that for a 12.6 at % Yb:CNNGG crystal grown with 99.99% Yb2O3 amounted to τ = 432 μs. Both, direct laser characterization and spectroscopic Yb3+ lifetimes indicate that as high as possible (at least 99.99%) Yb2O3 purity must be used to optimize laser operation of Yb:CNGG-type crystals.
Fig. 10 shows the input–output laser characteristics of a 7.3 at% Yb-doped CLNGG crystal with several sample thicknesses from d = 0.66 mm to 3.09 mm for TOC = 10%. In all cases the emission was around λ = 1030 nm with a FWHM ≈ 2 nm. The largest slope efficiency (η ≈ 94%) was obtained for the thinnest used crystal, but the total output obtained in this case was limited to about 340 mW due to the low sample absorption (55–70% depending on pump power). For increasing crystal thickness the most evident trend is the increase of the pump power laser threshold. Maximum output power of 555 mW was achieved with a 2.05 mm crystal thickness with η = 79% and Pth = 123 mW for the λ = 972.2 nm pump beam, the absorption of this sample was 95–97%. A larger sample thickness tested, i.e. d = 3.09 mm, absorbs even more the laser pump power (98–99%) but the slope efficiency did not improve and the maximum output power was slightly lower, ≈500 mW. Therefore under the instrumental approach here used, pump absorption during laser operation in the 95–98% range seems optimal, which corresponds to 98% of absorption under low pump irradiation conditions.
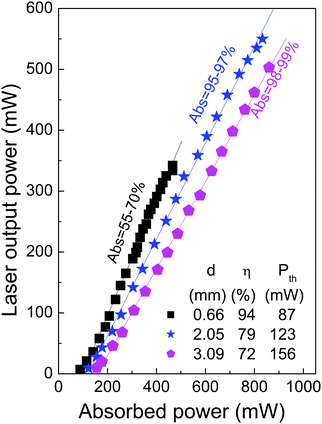 | ||
| Fig. 10 Input–output cw laser characteristics of 7.3 at% Yb:CLNGG crystals with different sample thickness, d. λPUMP = 972.2 nm, TOC = 10%. Abs = pump absorption under laser operation. | ||
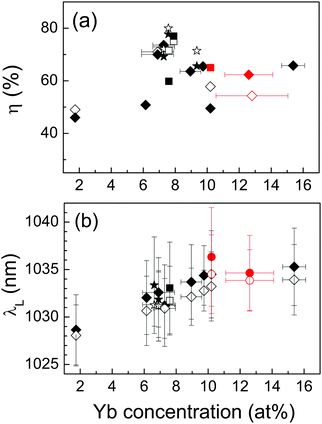
An alternative way to increase laser pump absorption is by using larger Yb density in the crystal, and this is particularly desired if a small crystal thickness is required, however at some point the radiative yield of Yb3+ degrades with increasing Yb concentration, see Fig. S15 (ESI†). Fig. 11a and b show the effect of the Yb concentration on the laser slope efficiency and laser emission wavelength, respectively, for a near to constant pump absorption of 70–80% under laser operation, obtained by thickness selection. All crystals used for this comparison were grown with ≥99.99% of Yb2O3 precursor purity. The laser slope efficiency increases from η = 47% in 1.74 at% Yb:CNNGG crystal to about η = 75% in crystals with 7–8 at% Yb. For larger Yb concentrations, up to about 15.4 at% of Yb doping, the laser slope efficiency decreases but still values around η = 60% were obtained. For all these crystals the laser threshold pump power remains in the Pth = 60–135 mW range, quite independent of the Yb concentration.
The increase of the Yb concentration also has a notable effect on the laser emission wavelength, see Fig. 11b. Although in fact the laser wavelength shifts for ≈1 nm to larger values as the pumping power increases from the threshold to the maximum pumping power available ≈1 W, the increase of the Yb concentration has a more notable effect shifting the spontaneous laser emission from λL = 1028 nm up to about λL = 1035 nm (for TOC = 10%) as the Yb concentration changes from 1.7 at% to ≈15 at%.
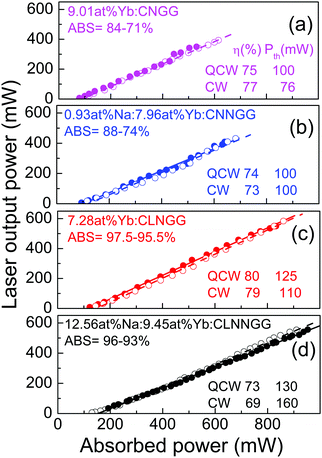
Fig. 12 shows the laser performance of these four crystals. The slope efficiencies observed are in all cases in the η = 70–80% range, with very little difference between the qcw or cw operation regimens, i.e the effect of CNGG modifications on cw laser performance is weak, although still the largest slope efficiency is generally found for crystals modified with Li.
4. Discussion
CNGG-type single crystals are without doubt one of the crystalline laser media with largest emission bandwidths for lanthanides and in fact it is one of the few Yb-doped media that have produced sub 50 fs laser pulses, along with Yb:Ca(Y/Gd)AlO4 (CALGO),50–52 Yb:Ca4YO(BO3)3 (YCOB),53 Yb:NaY(WO4)2,54 and Yb:CaF2,55 thus knowledge about the preparation and characterization of these garnets is relevant both from fundamental and technological view-points. Different to CALGO and YCOB, CNGG is optically isotropic, which is a significant advantage for crystal production and enables ceramic processing. In comparison to the present garnet, laser operation of Yb:NaY(WO4)2 is limited by lower thermal conductivity, κ < 1.8 W m−1 K−1.56 Although the laser capability of the CNGG crystal has been known for more than 30 years,57 the information so far available on preparation, growth and characterization is still scarce due to the complexity of the crystal site filling and in particular, laser results obtained with different crystal compositions and experimental setups are difficult to compare, thus the real influence of the differences found in tested crystals on laser performance is not conclusive.The incorporation of Li+ is the classical crystal modifier.22,23,58 The incorporation of Na+ is a more recent and apparently successful proposal;21 note that the shortest Yb laser pulses reported for these garnets were achieved with a Na-modified Yb:CNNGG crystal.17 Both alkaline metals play an utterly different role in the crystalline structure and thus in the physical properties. Complementary MAS-NMR and neutron diffraction studies19 showed that Li+ incorporates exclusively in the tetrahedral site (not shared with the octahedral position as usually speculated without experimental evidence), while as determined by scXRD measurements Na+ incorporates exclusively in dodecahedral sites, see Table 1 and ref. 21. Na+ and Yb3+ codoping in equal amounts in substitution of two Ca2+ ions was thought to be a charge and elastic stress self-compensation method since the three ions sit on the same crystal site, but Fig. 1 shows that Na and Yb have quite different segregation coefficients, SNa ≈ 0.39 and SYb > 1, respectively. Therefore, the appropriate self-compensation cannot be obtained by using equal amounts of Na and Yb in the melt, as so far tried. Results shown in section 3a indicate that by using a ≈3Na:1Yb molar ratio in the melt, Na and Yb concentrations equalize in the crystal, leading to true self-compensation and this procedure indeed enhances the Yb incorporation to the crystal by slightly increasing the Yb segregation coefficient from SYb = 1.12 in Yb:CNGG crystals to SYb = 1.34 in Yb:CNNGG ones, in both cases larger than the Yb segregation coefficient in YAG, SYb(YAG) = 1.08.59 On the contrary the segregation of Yb in Li-modified CLNGG reduces to SYb = 0.88, which is not so surprising because both Yb3+ and Li+ incorporations suppose extra electric charge with regards to the substituted ions, Ca2+ and neutral tetrahedral vacancies, respectively.
One important consideration in the development of laser crystals is the cost of growth production and manufacturing. In this respect the purity of the required chemicals plays a key role. In Section 3b it was shown that the use of 99.5% CaCO3 purity induces crystal colouration thus the more expensive 99.99% purity is required to minimize it. Other crystal growth procedures, particularly the growth in non-oxidative atmosphere required by the use of iridium crucibles, may also contribute with additional crystal colouration.35 In our experience the colouration described here in Section 3b, Fig. 3, is permanent as it was not removed by after growth crystal annealing in air upon cooling, or by subsequent 1340 °C annealing in air (see the ESI,† Fig. S7). We have even observed that 1150 °C air annealing of Yb:CNGG-type ground crystals leads systematically to a decrease of the Yb3+ fluorescence lifetime, see the ESI† Fig. S17. However, in light of the results presented in Fig. 7 and 8 for the Yb:CNGG-type crystals the impact of this colouration in the Yb laser performance is minor. This can be attributed to several facts: Firstly, neither the pump (λPUMP ≈ 972 nm) nor laser emission (λL = 1030–1080 nm) wavelengths characteristic of Yb3+ lasers overlap the defect-related VIS (λ = 300–500 nm) or Red-NIR (λ = 580–800 nm) OA bands responsible for the crystal colouration. This conclusion must be taken with care when considering other laser lanthanides, such as NIR Tm3+ and Nd3+ lasers (pumped at λ ≈ 800 nm),60,61 or VIS lasers based in Pr3+ (λEXC = 485 nm and λEMI = 595 and 640 nm), Tb3+ (λEXC = 488 nm and λEMI = 542 and 587 nm) or Dy3+ (λEXC = 425–475 nm and λEMI≈ 580nm).62 Secondly, the Yb3+ lifetime reduction observed with increasing crystal colouration is smaller than 10%,19 thus it likely has a minor effect on the Yb3+ fluorescence yield and on laser performance. Thirdly, the cubic centrosymmetric garnet structure lacks the electro-optic Pockels effect thus any microscopic electric field caused by electron photo-excitation and diffusion has virtually no effect on the beam propagation controlled by a single refractive index value, only the very weak Kerr effect would contribute to refractive index changes. Fourthly, any thermal gradient induced by light absorption in these colour centres is symmetric, thus it must be easier to handle than for highly anisotropic crystals, e.g. monoclinic Yb:KLn(WO4)2 lasers.63
Undoped CNGG-type crystals can be grown with a microscopic crystallographic quality as good as that of commercial YAG crystals, see Fig. 2b. However, while full Yb solubility is found in the YAG case, i.e. Y can be completely substituted by Yb leading to YbAG crystals, in the CNGG case DSC and XRD results presented in section 3a have shown that the maximum substitution of Ca is about 20% of the atomic content of the dodecahedral position. This limit could be maybe enhanced by incorporation in the dodecahedral site of other divalent cations with ionic radius smaller than Ca2+, but at present 15 at% Yb incorporation is a real limit for Yb:CNGG-type crystals with laser quality. Furthermore, Yb incorporation generally increases crystal mosaicity (see Fig. 3b), enhances fluorescence reabsorption and reduces the Yb3+ fluorescence quantum efficiency (fluorescence lifetime reduction with increasing Yb concentration, see Fig. S15, ESI†). Thus specific care must be paid to Yb incorporation in CNGG grown for laser purposes.
What makes a significant difference in laser performance is the Yb2O3 purity used for crystal growth. Fig. 9 shows the reduced laser slope efficiency found for 9.65 at% Yb:CNGG crystals grown with 99.5% Yb2O3 purity and, as mentioned previously, the only crystal which did not operate as a laser was a 18.8 at% Yb:CNGG crystal grown with 99.5% Yb2O3 purity. For such high Yb concentrations the fluorescence lifetime reduces to less than half of the radiative value, see Fig. S14 and S15 (ESI†). Care must be taken with some lifetime values reported in the Yb:CNGG literature32 because despite the large Yb concentration of the used crystals and the use of pin hole methods they exceed the radiative value most likely due to fluorescence reabsorption.
The impact of the crystal modifications on qcw/cw laser performance of 7.28–9.45 at% Yb:CNGG-type crystals here examined is rather small. The observed differences could be attributed to difficulty in controlling day-to-day fluctuations in the experimental conditions or in sample preparation, for instance perfection of the cavity alignment, fluctuations of the Ti-sa beam quality, accuracy of sample face flatness and parallelism or quality of the sample face polishing. Nevertheless, the comprehensive analysis of all the results here presented suggests that under similar conditions Li-modified Yb:CLNGG crystals have slightly better laser performances than the others, see Fig. 11a and Fig. 12, which agrees with the conclusion of ref. 24. This prevalence has not an optical spectroscopic support since emission and gain cross sections have been found independent of the Yb:CNGG Li or Na modifiers, see Fig. 5a and b, which points to the fact that the 300 K Yb3+ fluorescence is controlled by the main Yb3+ centre, in all cases a dodecahedral Yb3+ surrounded by six GaO4 tetrahedra. A better crystalline quality could be the likely reason for the slightly improved Yb:CLNGG laser performance. It must be noted that despite its spontaneous nucleation on Pt wires, Li-modified Yb:CLNGG crystals exhibit a mosaicity lower than that observed in other Yb:CNGG-type compositions with similar Yb concentration, see Fig. 2b.
Mode-locking laser experiments using semiconductor saturable absorption mirrors (SESAM) as rear high reflectors benefit from low transmission output couplers (TOC ≤ 1%) to increase the optical fluence onto the SESAM. Fig. 6b shows that this shifts the spontaneous emission laser wavelength towards larger values. Accordingly, previous Yb:CNGG-type crystals have shown mode-locking pulses in the λ = 1047.5–1061 nm region, see Table S1 (ESI†). Fig. 6c shows an unprecedented large cw laser tuning range for 12.56 at% Na:9.45 at% Yb:CLNNGG crystals, extending beyond λ > 1080 nm with a smooth variation of the output power in the long wavelength wing. The shortest pulses (τ = 45 fs) so far reported for Yb:CNGG-type crystals17 were achieved with a TOC = 0.8% and a Yb:CNNGG crystal doped in the melt with 10 at% Na (≈4 at% Na in the crystal in light of our results) and 10 at% Yb, they were centred at 1061 nm with a FWHM of 26.8 nm, i.e. near to the long wavelength limit of the tuning curves shown in Fig. 6c for TOC = 1%, however the average laser output was only 30 mW. While σGAIN > 0 is still found for λ = 1100 nm, see Fig. 5b, for λ ≥ 1040 nm its spectral distribution is not modified by Li or Na incorporations, thus further improvements (shorter pulses and larger average output power) should be still expected by optimizing the crystal laser properties along the lines here described, i.e. maximizing laser performance by selecting Yb concentration in the 7–8 at% range, pump absorption to ≈95%, and reducing crystal mosaicity by the use of high crystalline quality seeds for growth and Li incorporation. Also, common Ti-sa laboratory lasers are limited to <2 W for λ = 972 nm, thus the use of Distributed Bragg Reflector DL (DBR-DL) with stabilized emission near the 972 nm would benefit the slightly larger peak σABS shown in Fig. 5a while providing the high fluence needed by the used SESAM.
Thin-disk lasers25,26 use crystal wafer thickness in the order of d = 100 μm for efficient cooling. One important aspect in this respect is the efficient absorption of the pumping without the need to increase the sample thickness. According to the results shown in Fig. 11a this seems possible in CNGG by using Yb doping levels between 10 and 15 at%, but direct Yb incorporation increases crystal mosaicity, Fig. 2b. This mosaicity can be reduced by Li modification, but this in turn reduces Yb incorporation. Although Na incorporation also increases the crystal mosaicity it is still a useful strategy to promote Yb incorporation to high levels, ≈15 at%, thus Li and Na co-modified Yb:CLNNGG crystals seem a promising direction for future improvements of the efficiency of these lasers.
Transparent ceramic processing of CNGG garnets remains challenging,18 but in light of the results presented in Fig. S9 (ESI†), vacuum annealing at high temperature should be avoided in order to minimize Ga loss that presumably gives rise to foreign crystallographic phases at the grain boundaries.
5. Conclusions
CNGG single crystals can be grown by the Czochralski method up to ≈20 at% of Ca substitution by Yb and Na. The segregation coefficients for Yb and Na are rather different, namely SYb = 1.12 ± 0.11 for Yb (SYb = 1.34 ± 0.09 in Yb and Na codoped crystals) and SNa = 0.39 ± 0.05 for Na. Li reduces the Yb segregation coefficient below one, SYb = 0.88 ± 0.07. Similar Na and Yb densities in the crystal require a 3Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1Yb atomic ratio in the melt. The incorporation of Yb and Na into the garnet structure of CNGG crystals leads to a slight increase of the crystalline mosaicity, which can be compensated by Li incorporation as well as by the use of seeds with high crystalline quality, for instance YAG. Li and Na are complementary modifications of the garnet CNGG structure that help to simultaneously control the Yb incorporation and crystalline quality. Slightly better cw laser performance has been found in Li-modified Yb:CLNGG crystals while NIR extended laser tuning was observed in Li and Na co-modified Yb:CLNNGG crystals.
1Yb atomic ratio in the melt. The incorporation of Yb and Na into the garnet structure of CNGG crystals leads to a slight increase of the crystalline mosaicity, which can be compensated by Li incorporation as well as by the use of seeds with high crystalline quality, for instance YAG. Li and Na are complementary modifications of the garnet CNGG structure that help to simultaneously control the Yb incorporation and crystalline quality. Slightly better cw laser performance has been found in Li-modified Yb:CLNGG crystals while NIR extended laser tuning was observed in Li and Na co-modified Yb:CLNNGG crystals.
The purity of chemical precursors, in particular CaCO3, is crucial to reduce the sample colouration associated with broad optical absorption bands appearing in the λ = 580–800 nm spectral range and with a band in the pre-edge (λ = 300–500 nm, peaking at λ = 350 nm) of the band gap transition. These two optical absorptions correspond to different optical centres apparently not related to oxygen deficiency, but their presence does not have a great influence on the cw laser performance of Yb3+ in the studied garnet host. However, the use of high purity Yb2O3 chemical is critical to preserve the radiative properties of Yb3+ thus to maximize the cw laser slope efficiency and to reduce the pump power laser threshold.
A statistical assessment of the Yb3+ absorption cross section provides peak values of σABS(λ = 971.7 nm) = 1.97 ± 0.32 × 10−20 cm2 for Yb:CNGG, σABS(λ = 971.8 nm) = 1.65 × 10−20 cm2 for 12.23 at% Na-modified Yb:CNNGG and σABS(λ = 972.1 nm) = 2.12 ± 0.34 × 10−20 cm2 for Li-modified Yb:CLNGG. No evidence of significant emission or gain cross section changes with the incorporation of Li or Na modifiers is found. σGAIN > 0 is found even for λ ≥ 1100 nm. By using different output coupler transmissions and crystal compositions, laser tuning was possible in a very broad spectral range, from λ = 1000 nm to beyond λ = 1080 nm, suggesting that the full capability of these crystals for the production of ultrashort laser pulses has not yet been completely exploited.
Author contributions
All authors contributed to Conceptualization, Investigation, Data analysis and Funding acquisition. The manuscript was written through contributions of all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Work supported by MICINN under project number RTI2018-094859-B-I00. EPMA analyses have been performed by A. Fernández Larios at Centro Nacional de Microscopía Electrónica of Universidad Complutense de Madrid. CSIC support for Open Access publication as well as that of P. Ortega and M. J. Velasco (ICV-CSIC) in WDXRF and J. Perles and M. Ramírez (SIdI-UAM) in scXRD measurements are acknowledged.References
- U. Keller, Appl. Phys. B: Lasers Opt., 2010, 100, 15 CrossRef.
- V. C. Coffey, Opt. Photonics News, 2014, 30 Search PubMed.
- M. Arrigoni, S. Butcher and J. Henrich, Laser Focus Word, 2020, 56 Search PubMed.
- M. Smrž, O. Novák, J. Mužík, H. Turčičová, M. Chyla, S. S. Nagisetty, M. Vyvlečka, L. Roškot, T. Miura, J. Černohorská, P. Sikocinski, L. Chen, J. Huynh, P. Severová, A. Pranovich, A. Endo and T. Mocek, Appl. Sci., 2017, 7, 1016 CrossRef.
- W. Sibbett, A. A. Lagatsky and C. T. A. Brown, Opt. Express, 2012, 20, 6989 CrossRef CAS PubMed.
- T. Südmeyer, C. Kränkel, C. R. E. Baer, O. H. Heckl, C. J. Saraceno, M. Golling, R. Peters, K. Petermann, G. Huber and U. Keller, Appl. Phys. B: Lasers Opt., 2009, 97, 281 CrossRef.
- S. Uemura and K. Torizuka, Jpn. J. Appl. Phys., 2011, 50, 010201 CrossRef.
- A. Ikesue and Y. L. Aung, J. Am. Cer. Soc., 2006, 89, 1936 CrossRef.
- J. Sanghera, W. Kim, G. Villalobos, B. Shaw, C. Baker, J. Frantz, B. Sadowski and I. Aggarwal, Materials, 2012, 5, 258 CrossRef PubMed.
- S. Chen and Y. Wu, Am. Ceram Soc. Bull., 2013, 92, 32 Search PubMed.
- O. Puncken, H. Tünnermann, J. J. Morehead, P. Weßels, M. Frede, J. Neumann and D. Kracht, Opt. Express, 2010, 18, 20461 CrossRef PubMed.
- D. Albach and J. Chanteloup, Opt. Express, 2015, 23, 570 CrossRef PubMed.
- L. Zhang, J. Wu, P. Stepanov, M. Haseman, T. Zhou, D. Winarski, P. Saadatkia, S. Agarwal, F. A. Selim, H. Yang, Q. Zhang, Y. Wang, C. Wong and H. Chen, Photonics Res., 2019, 7, 549 CrossRef.
- J. Akiyama, Y. Sato and T. Taira, Opt. Lett., 2010, 23, 3598 CrossRef.
- H. Furuse, N. Horiuchi and B. Kim, Sci. Rep., 2019, 9, 10300 CrossRef PubMed.
- M. Tokurakawa, A. Shirakawa, K. Ueda, H. Yagi, T. Yanagitani and A. A. Kaminskii, CLEO/IQEC, 2009, CFO3 Search PubMed.
- J. Ma, Z. Pan, J. Wang, H. Yuan, H. Cai, G. Xie, L. Qian, D. Shen and D. Tang, Opt. Express, 2017, 25, 14968 CrossRef PubMed.
- V. Lupei, A. Lupei, C. Gheorghe, L. Gheorghe, A. Achim and A. Ikesue, J. Appl. Phys., 2012, 112, 063110 CrossRef.
- M. D. Serrano, J. O. Álvarez-Pérez, C. Zaldo, J. Sanz, I. Sobrados, J. A. Alonso, C. Cascales, M. T. Fernández-Díaz and A. Jezowski, J. Mater. Chem. C, 2017, 5, 11481 RSC.
- J. O. Álvarez-Pérez, J. M. Cano-Torres, M. D. Serrano, C. Cascales and C. Zaldo, J. Mater. Chem. C, 2020, 8, 7882 RSC.
- E. Castellano-Hernández, M. D. Serrano, R. J. Jiménez-Riobóo, C. Cascales and C. Zaldo, Cryst. Growth Des., 2016, 16, 1480 CrossRef.
- A. A. Sobol, Proc. Indian Natl. Sci. Acad., 1991, 57, 125 Search PubMed.
- Y. K. Voronko, A. A. Sobol, A. Y. Karasik, N. A. Eskov, P. A. Rabochkina and S. N. Ushakov, Opt. Mater., 2002, 20, 197 CrossRef.
- J. Liu, Y. Wan, Z. Zhou, X. Tian, W. Han and H. Zhang, Appl. Phys. B: Lasers Opt., 2012, 109, 183 CrossRef.
- A. Giesen, H. Hugel, A. Voss, K. Wittig, U. Brauch and H. Opower, Appl. Phys. B: Lasers Opt., 1994, 58, 365 CrossRef.
- A. Giesen and J. Speiser, IEEE J. Sel. Top. Quantum Electron., 2007, 13, 598 Search PubMed.
- J. I. Goldstein, D. E. Newbury, P. Echlin, D. C. Joy, A. Roming, C. Lyman, C. Fiori and E. Lifshin, Scanning Electron Microscopy and X-ray Microanalysis, Plenum. New York, 1992 Search PubMed.
- F. J. Valle, P. Ortega, A. de Pablos, C. Zaldo, F. Esteban-Betegón and M. D. Serrano, X-Ray Spectrom., 2009, 38, 287 CrossRef.
- G. M. Kuz’micheva, I. A. Kaurova, A. A. Brykovskiy, V. B. Rybakov, Y. N. Gorobets, A. N. Shekhovtsov and A. Cousson, Mater. Res. Bull., 2016, 78, 134 CrossRef.
- I. A. Kaurova, G. M. Kuz’micheva, V. B. Rybakov, A. B. Dubovskii and A. Cousson, Inorg. Mater., 2010, 46, 988 CrossRef.
- G. M. Kuz'micheva, I. A. Kaurova, V. B. Rybakov, P. A. Eistrikh-Geller, E. V. Zharikov, D. A. Lis and K. A. Subbotin, CrystEngComm, 2016, 18, 2921 RSC.
- Z. Pan, P. Loiko, J. M. Serres, H. Yuan, X. Dai, H. Cai, M. Aguiló, F. Díaz, P. Camy, Y. Wang, U. Griebner, V. Petrov and X. Mateos, Proc. SPIE, 2020, 11259, 1125911 Search PubMed.
- Z. Pan, P. Loiko, Y. Wang, Y. Zhao, H. Yuan, K. Tang, X. Dai, H. Cai, J. M. Serres, S. Slimi, E. B. Salem, E. Dunina, A. Kornienko, L. Fomicheva, J. Doualan, P. Camy, W. Chen, U. Griebner, V. Petrov, M. Aguiló, F. Díaz, R. M. Solé and X. Mateos, J. Alloys Compd., 2021, 853, 157100 CrossRef CAS.
- L. Gheorghe, M. Greculeasa, F. Voicu, C. Gheorghe, S. Hau, A. M. Vlaicu, K. N. Belikov, E. Yu Bryleva and O. V. Gaiduk, Opt. Mater., 2018, 84, 335 CrossRef CAS.
- Q. Fu, X. Liu, Y. Chen, C. Hong, X. Hu, N. Zhuang and J. Chen, J. Alloys Compd., 2016, 660, 471 CrossRef CAS.
- D. L. Wood and J. P. Remeika, J. Appl. Phys., 1967, 38, 1038 CrossRef CAS.
- C. R. Varney and F. A. Selim, AIMS Mater Sci., 2015, 2, 560 CAS.
- O. F. Schirmer, J. Phys., 1980, 41, C6.479 Search PubMed.
- J. M. Serres, V. Jambunathan, P. Loiko, X. Mateos, H. Yu, H. Zhang, J. Liu, A. Lucianetti, T. Mocek, K. Yumashev, U. Griebner, V. Petrov, M. Aguiló and F. Díaz, Opt. Mater. Express, 2015, 6, 46 CrossRef.
- V. E. Shukshin, Phys. Wave Phenom., 2009, 17, 165 CrossRef.
- J. Liu, H. Yang, H. Zhang, X. Mateos, V. Petrov and J. Wang, Laser Phys. Lett., 2008, 5, 874 CrossRef CAS.
- H. Zhang, J. Liu, J. Wang, J. Fan, X. Tao, X. Mateos, V. Petrov and M. Jiang, Opt. Express, 2007, 15, 9464 CrossRef CAS PubMed.
- J. Koerner, C. Vorholt, H. Liebetrau, M. Kahle, D. Kloepfel, R. Seifert, J. Hein and M. C. Kaluza, J. Opt. Soc. Am. B, 2012, 29, 2493 CrossRef CAS.
- D. E. McCumber, Phys. Rev. A: At., Mol., Opt. Phys., 1964, 136, A954 CrossRef.
- B. F. Aull and H. P. Jenssen, IEEE J. Quantum Electron., 1982, 18, 925 CrossRef.
- D. Sugak, A. Matkovskii, A. Durygin, A. Suchocki, I. Solskii, S. Ubizskii, K. Kopczynski, Z. Mierczyk and P. Potera, J. Lumin., 1999, 82, 9 CrossRef CAS.
- X. Han, M. Rico, M. D. Serrano, C. Cascales and C. Zaldo, Laser Phys. Lett., 2013, 10, 045808 CrossRef CAS.
- V. Müller, V. Peters, E. Heumann, M. Henke, K. Petermann and G. Huber, Adv. Sol. St. Lasers Tech. Dig., 2002, 153 Search PubMed.
- F. Esteban-Betegón, C. Zaldo and C. Cascales, Chem. Mater., 2010, 22, 2315 CrossRef.
- F. Pirzio, S. D. di Dio Cafiso, M. Kemnitzer, A. Guandalini, F. Kienle, S. Veronesi, M. Tonelli, J. A. der Au and A. Agnesi, Opt. Express, 2015, 23, 9790 CrossRef CAS.
- Z. Gao, J. Zhu, J. Wang, Z. Wei, X. Xu, L. Zheng, L. Su and J. Xu, Photonics Res., 2015, 3, 335 CrossRef CAS.
- S. Manjooran and A. Major, Opt. Lett., 2018, 43, 2324 CrossRef CAS PubMed.
- A. Yoshida, A. Schmidt, V. Petrov, C. Fiebig, G. Erbert, J. Liu, H. Zhang, J. Wang and U. Griebner, Opt. Lett., 2011, 36, 4425 CrossRef CAS PubMed.
- J. Ma, H. Huang, H. Yu, H. Zhang and D. Tang, IEEE Photon Tech. Lett., 2016, 28, 1298 CAS.
- W. P. Sévillano, G. Machinet, R. Dubrasquet, P. Camy, J. L. Doualan, R. Moncorgé, P. Georges, F. Druon, D. Descamps and E. Cormier, Adv. Sol. St. Lasers Tech. Dig., 2013, AF3A.6 Search PubMed.
- M. D. Serrano, C. Cascales, X. Han, C. Zaldo, A. Jezowski, P. Stachowiak, N. Ter-Gabrielyan, V. Fromzel and M. Dubinskii, PLoS One, 2013, 8, e59381 CrossRef CAS.
- A. A. Kaminski, E. L. Belokoneva, A. V. Butashin, K. Kurbanov, A. A. Markosyan, B. V. Mill, O. K. Nikol'sakaya and S. E. Sarkisov, Inorg. Mater., 1986, 22, 1061 Search PubMed.
- Y. M. Yu, V. I. Chani, K. Shimamura and T. Fukuda, J. Cryst. Growth, 1997, 171, 463 CrossRef CAS.
- X. Xu, Z. Zhao, J. Xu and P. Deng, J. Cryst. Growth, 2003, 255, 338 CrossRef CAS.
- J. M. Cano-Torres, M. Rico, X. Han, M. D. Serrano, C. Cascales, C. Zaldo, V. Petrov, U. Griebner, X. Mateos, P. Koopmann and C. Kränkel, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 174207 CrossRef.
- A. García-Cortés, C. Cascales, A. de Andrés, C. Zaldo, E. V. Zharikov, K. A. Subbotin, S. Bjurshagen, V. Pasiskevicius and M. Rico, IEEE J. Quantum Electron., 2007, 43, 157 Search PubMed.
- C. Kränkel, D. Marzahl, F. Moglia, G. Huber and P. W. Metz, Laser Photonics Rev., 2016, 10, 548 CrossRef.
- E. Castellano-Hernández, X. Han, M. Rico, L. Roso, C. Cascales and C. Zaldo, Opt. Express, 2015, 23, 11135 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: (i) Laser setup. (ii) Summary of previous laser achievements. (iii) Crystal growth, analysis of phases present in solid state synthesized precursors and crystal melting. (iv) Inventory of crystals. (v) Crystallographic data. (vi) WDXRF of Na. (vii) Effect of thermal annealing on OA. (viii) σABS of CNNGG. (ix) Low temperature Yb3+ spectroscopy. (x) Yb3+ lifetime measurements. (xi) Role of Yb2O3 purity on laser performance. (xii) Effect of air annealing on Yb3+ lifetime. See DOI: 10.1039/d0tc05718e |
| ‡ In memoriam of Ana Ruiz y Ruiz de Gopegui (Avilés, July 31st 1960 – Madrid, February 14th 2021). |
| This journal is © The Royal Society of Chemistry 2021 |