Self-assembling nitrilotriacetic acid nanofibers for tracking and enriching His-tagged proteins in living cells†
Li-Song
Zhang
a,
Yi-Lun
Yin
a,
Lei
Wang
a,
Ying
Xia
a,
SungJu
Ryu
ab,
Zhen
Xi
c,
Lu-Yuan
Li
*a and
Zhi-Song
Zhang
 *a
*a
aState Key Laboratory of Medicinal Chemical Biology, College of Pharmacy and Tianjin Key Laboratory of Molecular Drug Research, NanKai University, Tianjin 300350, China. E-mail: liluyuan@nankai.edu.cn; zzs@nankai.edu.cn
bInstitute of microbiology, State Academy of Sciences, Pyongyang, North Korea
cState Key Laboratory of Elemento-Organic Chemistry and College of Chemistry, Nankai University, 94 Weijin Road, Tianjin 300071, China
First published on 26th November 2020
Abstract
Specific and expeditious identification and enrichment of target proteins in living cells is often a challenging task. The hexahistidine (6His) tag is frequently used to label artificially engineered proteins produced in prokaryotic or eukaryotic cells. Utilizing the interaction between 6His-tag and nitrilotriacetic acid (NTA) mediated by divalent metal ions (Ni2+, Cu2+, Zn2+ or Co2+), we designed and synthesized a series of Nap-G/Biotin/ANA-FFpYGK-NTA probes that, assisted by alkaline phosphatase (ALP), self-assemble into nanofibers. The probe consists of an NTA group that specifically binds to 6His-tag, an FFpY group that promotes self-assembly facilitated by ALP, and a hydrophobic (Nap-G/ANA/Biotin) capping group for various applications. We demonstrate that the ANA-FFpYGK-NTA(Ni2+) nanofibers are fit for real-time tracking of His-tagged protein in living cells, and the Biotin-FFpYGK-NTA(Ni2+) nanofibers are for isolating His-tagged proteins and other proteins that they interact with.
Fast, accurate, and real-time labelling and tracking of intracellular proteins is a key issue in revealing the protein biological function and the signal network.1 It is particularly difficult, however, to efficiently label proteins in living cells.2 Fluorescent protein-based labelling technology has been widely used,3 with the coding sequences of various fluorescent proteins, such as green fluorescent protein (GFP) and red fluorescent protein (RFP), being fused to the N- or C-terminus of the protein of interest and overexpressed in the cell. The most obvious shortcoming of fluorescent protein tags is their large molecular weight (e.g. the most commonly used GFP is about 27 kDa), which is likely to affect the natural function of the protein of interest.4 There are other protein labelling or tracking methods based on enzymology and substrates with limited application scenarios because of the reaction conditions and substrate distribution properties.5–7 Therefore, the development of simple chemical labeling methods remains an important task.
It is well known that short peptide tags of 5–7 amino acids are unlikely to affect the protein function.8 Poly-histidine tag with 6–10 histidine residues is one of the most widely used tags.9–11 His-tag in the presence of Ni2+ is able to specifically bind to NTA. Ni2+ is in an octahedral coordination in the complex, in which four coordination sites are occupied by NTA, and two coordination sites by His-tagged protein.12 Similar to Ni2+, a variety of other divalent ions including Cu2+, Zn2+ and Co2+ also assist in the reaction.13–15 His-tag (6His in particular) is thus the most attractive protein marker, and is frequently used in studies for the intracellular signalling pathway as well as drug screening.12 An intrinsic difficulty for NTA-based fluorescent probes, however, is their poor ability to penetrate the cell membrane,1 which severely limits their use to study intracellular proteins, and much less in subcellular organelles such as the nucleus.
Peptide supramolecular nanofibers are of great utility in biomedical applications including drug delivery and modulation of immune responses.16–18 Short peptides can be catalysed by ALP on the cell membrane to expose hydrophobic residues resulting in self-assembling of nanofibers that readily enter the cell by endocytosis.19,20 Fluorescence-generating or affinity-enhancing probes may be attached to short peptides to improve the applications in living cells.
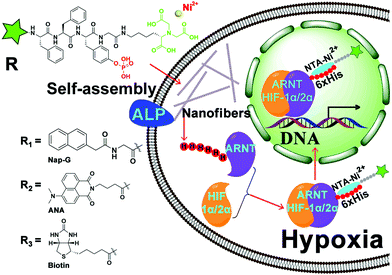
In this study, we developed a nanofiber probe consisting of an NTA group, a self-assembling peptide FFpY,21 and a hydrophobic end-capped group (Nap-G/ANA/Biotin). We show that in living cells the ANA-FFpYGK-NTA(Ni2+) probe can be used to specifically track His-tagged proteins, and the Biotin-FFpYGK-NTA(Ni2+) probe is used to enrich proteins that interact with His-tagged proteins. The self-assembled NTA probes are thus fit for spatio-temporal studies on protein functions and flexible drug screening.
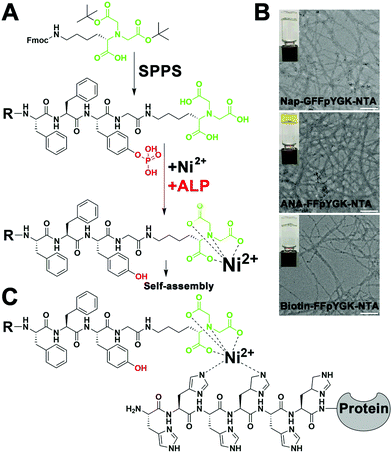
As shown in Scheme 1, the structure of the NTA probes consists of (i) an NTA(Ni2+) group, (ii) a self-assembling peptide FFpY, and (iii) a hydrophobic group (2-naphthylacetyl, biotin or ANA fluorophore) at the N-terminus to enhance the aromatic–aromatic interaction. The synthetic route of Nap-G/Biotin/ANA-FFpYGK-NTA is shown in Fig. 1A and Fig. S1 (ESI†). The chemical synthesis route of the ALP inhibitor (DQB) is shown in Fig. S2 (ESI†). After purification by high performance liquid chromatography (HPLC), NMR spectroscopy and HR-MS analysis confirmed the structure of the designed molecules (Fig. S3A–S8B, ESI†). The NTA group is responsible for forming a stable complex with His-tagged proteins in the presence of Ni2+ (Fig. 1C).22
 | ||
| Scheme 1 Chemical structure of Nap-G/Biotin/ANA-FFpYGK-NTA(Ni2+) and the mechanism of interaction between the probes and His-tagged protein in living cells. | ||
The self-assembling ability of the NTA probes was tested under the action of ALP and Ni2+. At 10 mmol L−1 in PBS (pH = 7.4), the Nap-G/Biotin/ANA-FFpYGK-NTA forms a clear solution (Fig. S9A, ESI†), as the phosphate group and the carboxylic acid group were present as negative ions, significantly increasing the solubility. We mixed the Nap-G/Biotin/ANA-FFpYGK-NTA (10 mmol L−1) and Ni2+ (10 mmol L−1) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio for 30 minutes at room temperature and then added 2.0 U mL−1 ALP to trigger the self-assembly at 37 °C. Images from transmission electron microscopy showed that all the three types of NTA probes formed nanofibers with a diameter of about 10 nm (Fig. 1B). Additionally, we found that ALP also catalyzed the self-assembly of the probes with other common divalent metal ions such as Cu2+, Zn2+ or Co2+ (Fig. S9B, ESI†), indicative of the flexibility in application scenarios of the probes.
1 ratio for 30 minutes at room temperature and then added 2.0 U mL−1 ALP to trigger the self-assembly at 37 °C. Images from transmission electron microscopy showed that all the three types of NTA probes formed nanofibers with a diameter of about 10 nm (Fig. 1B). Additionally, we found that ALP also catalyzed the self-assembly of the probes with other common divalent metal ions such as Cu2+, Zn2+ or Co2+ (Fig. S9B, ESI†), indicative of the flexibility in application scenarios of the probes.
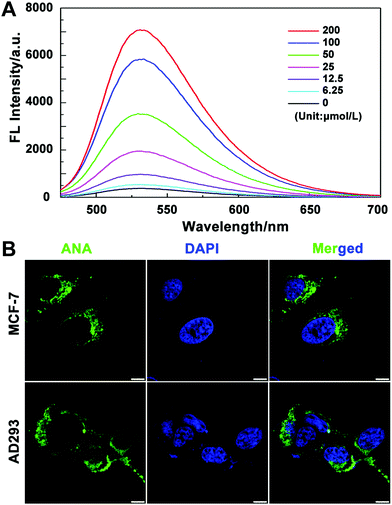
We then tested the absorption efficiency of the fluorescent probes in living cells. After culturing MCF-7 cells with a series of concentrations of ANA-FFpYGK-NTA(Ni2+) for 6 h, we collected and washed the cells with PBS and then lysed and measured the fluorescence intensity using a fluorescence spectrophotometer. As shown in Fig. 2A, the intracellular fluorescence intensity is positively correlated with the probe concentration, showing good cell penetration ability. We also observed the distribution of the probes in MCF-7 and AD-293 cells. Laser confocal microscopy photos confirmed that the probes successfully entered the cytoplasm, but not in the nucleus (Fig. 2B). The efficiency of ANA-FFpYGK-NTA(Ni2+) to enter the cell is significantly reduced when the ALP inhibitor DQB was added (Fig. S10, ESI†). We treated the cells with various concentrations of the probes for 48 h and observed less than 20% inhibition of cell proliferation at the highest concentration (200 μmol L−1) (Fig. S11, ESI†), indicating reasonable cytocompatibility of the probes.
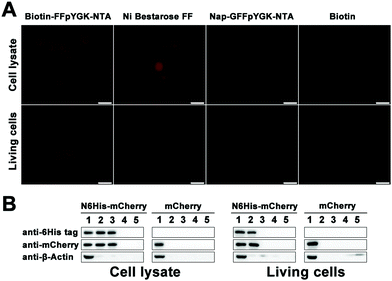
We put the coding sequence of His-tagged mCherry downstream of the simian virus 40 (SV40) enhancer and the early promoter, and transfected both AD-293 and MCF-7 cells using a polyetherimide (PEI)-based protocol. Successful expression of the mCherry protein within 48 h was confirmed by confocal microscopy (Fig. S12A and C, ESI†) and western blotting (Fig. S12B and D, ESI†). We then homogenized the cells and incubated the solutions first with Biotin-FFpYGK-NTA(Ni2+), Nap-GFFpYGK-NTA(Ni2+) or biotin alone (100 μmol L−1) at 4 °C for 4 h, and then with Streptavidin agarose resin 6FF. We added either Biotin-FFpYGK-NTA(Ni2+), Nap-GFFpYGK-NTA(Ni2+), Ni Bestarose FF or biotin (100 μmol L−1) to the culture media, homogenized the cells with RIPA buffer, and incubated the supernatants with Streptavidin agarose resin 6FF for 12 h. Fluorescence microscopy analysis of the resin beads showed that the red fluorescence of mCherry was associated with Biotin-FFpYGK-NTA(Ni2+) and Ni-Bestarose FF, but not with either Nap-GFFpYGK-NTA(Ni2+) or biotin alone in the cell lysates (Fig. 3A). Fluorescence of the resins revealed that the red fluorescence was extracted only by the Biotin-FFpYGK-NTA(Ni2+) from the living cells (Fig. 3A). These data suggest that Biotin-FFpYGK-NTA(Ni2+) was able to enter the cell and form a complex with 6His-mCherry. Ni-Bestarose FF, a reagent widely used to extract His-tagged proteins from solutions and cannot be taken up by the cells, was used as control. We then boiled these samples and carried out western blot analysis and confirmed the association of the Biotin-FFpYGK-NTA(Ni2+) probe with 6His-mCherry (Fig. 3B). Similar results were obtained when we replaced Ni2+ with other divalent metal ions (Cu2+, Zn2+ or Co2+) and repeated the experiment under otherwise identical conditions (Fig. S13 and S14, ESI†). However, Biotin-FFpYGK-NTA could not bind to the 6His-mCherry protein in the cell lysate when there was no divalent metal ion (Ni2+, Cu2+, Zn2+ or Co2+) (Fig. S15, ESI†). Compared with Biotin-FFpYGK-NTA(Ni2+), Biotin-NTA(Ni2+), which has no enzymatic self-assembly properties, has poor cell permeability and cannot effectively recognize 6His-mCherry protein in living cells (Fig. S16, ESI†).
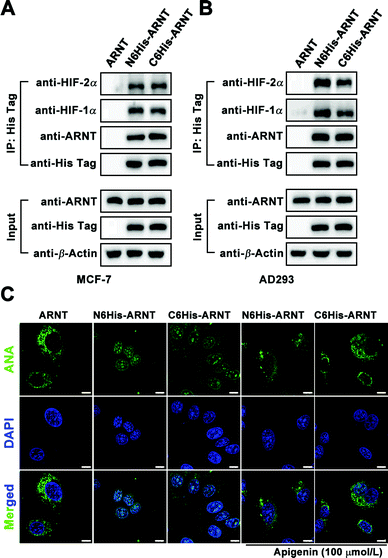
For application of Biotin/ANA-FFpYGK-NTA(Ni2+) probes to study protein–protein interactions in living cells, we applied the Biotin-FFpYGK-NTA(Ni2+) probe to study a well-known intracellular signaling system involving multiple proteins. Hypoxia-inducible factor (HIF) is a mammalian transcription factor that consists of two subunits of α and β (also known as ARNT). The two subunits under hypoxic conditions form a heterodimer, which then translocates into the nucleus to initiate the transcription of a host of genes.23 We modified ARNT with a 6His-tag at either the N- or C-terminus and expressed the protein in MCF-7 or AD-293 cells. We then added the Biotin-FFpYGK-NTA(Ni2+) probe (100 μmol L−1) in the culture media and incubated under hypoxic conditions (1% O2, 5% CO2 and 94% N2) for 6 h. The cells were homogenized, the supernatants were incubated with Streptomycin agarose resin, and the resins were subjected to western blot analysis. We found that both the C6His-ARNT and the N6His-ARNT proteins were readily extracted together with HIF-1α or HIF-2α (Fig. 4A and B). These results demonstrate the versatility of the Biotin-FFpYGK-NTA(Ni2+) probe to extract the known or unknown target proteins that interact with His-tagged protein bait in living cells.
We evaluated the ability of the fluorescent ANA-FFpYGK-NTA(Ni2+) probe to track subcellular translocation of His-tagged proteins. After transfection of MCF-7 and AD-293 cells with vectors expressing C6His-ARNT, N6His-ARNT, or wild-type ARNT, the cells were incubated under normoxic conditions for 48 h, and the ANA-FFpYGK-NTA(Ni2+) probe (100 μmol L−1) was added to the culture media, and then the cell cultures in the experimental group were incubated in hypoxia for 6 h, while the cells in the control group were continued to be cultured under normoxic conditions. Confocal microscopy analysis indicated that under normoxic conditions the green fluorescent probe remained in the cytoplasm (Fig. 4C and Fig. S18, ESI†); in sharp contrast, the cells cultured under hypoxic conditions exhibited a marked accumulation of the fluorescent probe in the nucleus in the 6His-ARNT groups (both N- and C-tagged) (Fig. 4C and Fig. S18, ESI†), indicating the succesful formation of a heterodimer by the α subunit of HIF (HIF-1α or HIF-2α) and ARNT and translocation into the nucleus. It is noticeable that the ANA-FFpYGK-NTA(Ni2+) probe did not identify the HIF heterodimer generated by wild type ARNT because the probe was specific for His-tagged proteins.
We used apigenin to determine whether the ANA-FFpYGK-NTA(Ni2+) probe is responsive to the changes of the target protein levels in the cells since it was reported that treatment of cells with apigenin leads to reduced levels of HIF-1α under hypoxic conditions.24 The cells were cultured for 6 h under 1% O2, treated with or without apigenin (100 μmol L−1), and then treated with the ANA-FFpYGK-NTA(Ni2+) probe as above. Western blot analysis of the cell homogenates showed that the expression of both HIF-1α and HIF-2α markedly decreased as a result of apigenin treatment (Fig. S17, ESI†). Confocal microscopy images of the cell cultures indicated the declined level of the fluorescence intensity in the nucleus in the apigenin-treated cells compared with the controls without apigenin treatment (Fig. 4C and Fig. S18, ESI†). These findings indicate that the self-assembling ANA-FFpYGK-NTA(Ni2+) probe is suitable for studying the quantitative changes of the intracellular levels of His-tagged proteins.
In summary, in this study, we designed and synthesized a self-assembling nanofiber bio-probe that contains an NTA group, a FFpY peptide and a biotin or a fluorescent ANA group. The self-assembling characteristics of the FFpY peptide and the presence of the phosphate group not only increase the solubility of the NTA probe, but also lead to an improvement of the intracellular delivery of target-tracking probes in living mammalian cells. The nanofiber probe enables real-time tracking of His-tagged target protein and other proteins or intracellular components that interact with the target protein. The nanofibers also provide a convenient tool to enrich and isolate the target protein, potentially together with its interacting proteins, for identification of the signaling pathway. These utilities may also have applications in setting up quantitative assays or drug-screening operations in cellular as well as in animal models. Furthermore, a combination of these probes with other protein expression-modifying techniques such as exogenous or endogenous gene-editing may help provide insights into the behaviors of a protein target regarding its spatiotemporal relationships with other intracellular components.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (NSFC) projects (Grant no. 81672740, 81972687 and 81874167).Notes and references
- R. Wieneke, N. Laboria, M. Rajan, A. Kollmannsperger, F. Natale, M. C. Cardoso and R. Tampe, J. Am. Chem. Soc., 2014, 136, 13975–13978 CrossRef CAS.
- Y. Zeng, T. N. Ramya, A. Dirksen, P. E. Dawson and J. C. Paulson, Nat. Methods, 2009, 6, 207–209 CrossRef CAS.
- K. L. Kim, G. Sung, J. Sim, J. Murray, M. Li, A. Lee, A. Shrinidhi, K. M. Park and K. Kim, Nat. Commun., 2018, 9, 1712 CrossRef.
- Z. L. Chang, M. H. Lorenzini, X. Chen, U. Tran, N. J. Bangayan and Y. Y. Chen, Nat. Chem. Biol., 2018, 14, 317–324 CrossRef CAS.
- T. Tamura and I. Hamachi, J. Am. Chem. Soc., 2019, 141, 2782–2799 CrossRef CAS.
- G. Mann, G. Satish, R. Meledin, G. B. Vamisetti and A. Brik, Angew. Chem., 2019, 58, 13540–13549 CrossRef CAS.
- S. R. Adusumalli, D. G. Rawale, U. Singh, P. Tripathi, R. Paul, N. Kalra, R. K. Mishra, S. Shukla and V. Rai, J. Am. Chem. Soc., 2018, 140, 15114–15123 CrossRef CAS.
- J. Liu, M. Spulber, D. Wu, R. M. Talom, C. G. Palivan and W. Meier, J. Am. Chem. Soc., 2014, 136, 12607–12614 CrossRef CAS.
- B. A. Liu, M. Ogiue-Ikeda and K. Machida, Methods Mol. Biol., 2017, 1555, 117–162 CrossRef CAS.
- S. Shao, J. Geng, H. Ah Yi, S. Gogia, S. Neelamegham, A. Jacobs and J. F. Lovell, Nat. Chem., 2015, 7, 438–446 CrossRef CAS.
- W. C. Huang, B. Deng, C. Lin, K. A. Carter, J. Geng, A. Razi, X. He, U. Chitgupi, J. Federizon, B. Sun, C. A. Long, J. Ortega, S. Dutta, C. R. King, K. Miura, S. M. Lee and J. F. Lovell, Nat. Nanotechnol., 2018, 13, 1174–1181 CrossRef CAS.
- K. Gatterdam, E. F. Joest, V. Gatterdam and R. Tampe, Angew. Chem., 2018, 57, 12395–12399 CrossRef CAS.
- M. Singh, M. Holzinger, M. Tabrizian, S. Winters, N. C. Berner, S. Cosnier and G. S. Duesberg, J. Am. Chem. Soc., 2015, 137, 2800–2803 CrossRef CAS.
- T. H. Seefeld, A. R. Halpern and R. M. Corn, J. Am. Chem. Soc., 2012, 134, 12358–12361 CrossRef CAS.
- J. C. Joyner, L. Hocharoen and J. A. Cowan, J. Am. Chem. Soc., 2012, 134, 3396–3410 CrossRef CAS.
- C. Tomasini and N. Castellucci, Chem. Soc. Rev., 2013, 42, 156–172 RSC.
- X. Miao, W. Cao, W. Zheng, J. Wang, X. Zhang, J. Gao, C. Yang, D. Kong, H. Xu, L. Wang and Z. Yang, Angew. Chem., 2013, 52, 7781–7785 CrossRef CAS.
- R. V. Ulijn and A. M. Smith, Chem. Soc. Rev., 2008, 37, 664–675 RSC.
- H. Wang, Z. Feng, Y. Wang, R. Zhou, Z. Yang and B. Xu, J. Am. Chem. Soc., 2016, 138, 16046–16055 CrossRef CAS.
- D. Zheng, Y. Chen, S. Ai, R. Zhang, Z. Gao, C. Liang, L. Cao, Y. Chen, Z. Hong, Y. Shi, L. Wang, X. Li and Z. Yang, Research, 2019, 4803624 CAS.
- J. Gao, J. Zhan and Z. Yang, Adv. Mater., 2020, 32, e1805798 CrossRef.
- S. V. Wegner and J. P. Spatz, Angew. Chem., 2013, 52, 7593–7596 CrossRef CAS.
- D. Wu, N. Potluri, J. Lu, Y. Kim and F. Rastinejad, Nature, 2015, 524, 303–308 CrossRef CAS.
- G. N. Masoud and W. Li, Acta Pharm. Sin. B, 2015, 5, 378–389 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0tb02302g |
| This journal is © The Royal Society of Chemistry 2021 |