Magnetic nanoparticle-based amplification of microRNA detection in body fluids for early disease diagnosis
Isabel
Gessner
 a,
Jochen W. U.
Fries
a,
Jochen W. U.
Fries
 *b,
Veronika
Brune
a and
Sanjay
Mathur
*b,
Veronika
Brune
a and
Sanjay
Mathur
 *a
*a
aInstitute of Inorganic Chemistry, University of Cologne, Greinstr. 6, 50939 Cologne, Germany. E-mail: sanjay.mathur@uni-koeln.de
bInstitute of Pathology, University Hospital of Cologne, Kerpener Str. 62, 50924 Cologne, Germany
First published on 6th November 2020
Abstract
Circulating biomarkers such as microRNAs (miRNAs), short noncoding RNA strands, represent prognostic and diagnostic indicators for a variety of physiological disorders making their detection and quantification an attractive approach for minimally invasive early disease diagnosis. However, highly sensitive and selective detection methods are required given the generally low abundance of miRNAs in body fluids together with the presence of large amounts of other potentially interfering biomolecules. Although a variety of miRNA isolation and detection methods have been established in clinics, they usually require trained personnel and often constitute labor-, time- and cost-intensive approaches. During the last years, nanoparticle-based biosensors have received increasing attention due to their superior detection efficiency even in very low concentration regimes. This is based on their unique physicochemical properties in combination with their high surface area that allows for the immobilization of multiple recognition sites resulting in fast and effective recognition of analytes. Among various materials, magnetic nanoparticles have been identified as useful tools for the separation, concentration, and detection of miRNAs. Here, we review state-of-the-art technology with regard to magnetic particle-based miRNA detection from body fluids, critically discussing challenges and future perspective of such biosensors while comparing their handling, sensitivity as well as selectivity against the established miRNA isolation and detection methods.
1. Introduction
A large portion of the human genome is considered to be potentially noncoding due to missing sequence homologies to known proteins, the absence of open reading frames, and frequent codon substitutions. They produce small, conserved ribonucleic acids, microRNAs (miRNAs), to control intracellular signaling,1 and are involved in numerous mechanisms in health as well as disease.2 MiRNAs are involved in development, cell differentiation, regulation of cell cycle,3 and consequently have been described in pathologic diseases of every major organ and condition e.g., cancer,4–6 senescence,7 heart,8 neurodegenerative diseases,9,10 gastrointestinal system,11 and kidney/transplantation.12MiRNAs have been used as diagnostic molecules in multiple studies, as noninvasive biomarkers either alone or in combination with other known disease predictors. However, as reviewed by Witwer,13 the reproducibility of biomarker studies is almost not existent, largely attributable to the non-standard terms of the studies, for instance, information on the procedure of detection is missing. Also, the standardization of assays in terms of detection sensitivity and specificity, expression levels, as well as compatibility between methods demands larger data sets as most of the published results involve small sample sizes. The availability of this information will then enable investigators to reliably compare profiles across various patient cohorts and demonstrate long-term outcome data and implications. Moreover, the detection of miRNAs from biological samples remains challenging due to their innately low concentrations and extensive interferences existing in body fluids. Nevertheless, given the possibility of magnetically concentrating samples, magnetic particles have demonstrated huge potential to overcome scalability issues with regard to extracting and quantifying disease-relevant, circulating biomarkers including antibodies,14 circulating tumor cells15 and nucleic acids.16,17
This review highlights the difficulties arising from the diversity of methods currently used for miRNA isolation and detection. It presents the advantages in using surface-functionalized nanoparticles for enhancing the biorecognition event between miRNA and target ligands that results in an inherent local enrichment in point-of-care devices. Moreover, an easy separation of magnetic nanoparticles-miRNA conjugates by using an external magnetic field from serum, plasma or cell lysates enables superior detection capacity by reducing the interference with other biomolecules, such as proteins.18
2. MiRNAs
2.1. MiRNA biogenesis
MicroRNAs (miRNAs) are typically 19–22 nucleotide long, noncoding RNAs.19,20 A primary miRNA (pri-miRNA) transcript is made through transcription by the RNA Polymerase II. By interaction with the so-called microprocessors, Drosha and DGCR8, a miRNA hairpin is generated, and exported to the cytoplasm via a complex of exportin-5 and GTP-bound Ran (RanGTP). After the nuclear export RNase III Dicer, in a complex with TAR RNA binding protein (TRBP) in a Dicer complex, processes this hairpin to result in a mature double-stranded miRNA. One strand in association with the Argonaute protein 2 forms the so-called miRNA induced silencing complex (miRISC). The remaining miRNA strand, miRNA*, also called passenger, is degraded. Within the RISC, the miRNA binds the target mRNA, based on the degree of complementarity. The complementarity of sequences between the miRNA and its target is preferentially located at the 5′ end of the miRNA, termed the seed, consisting of nucleotides 2–8, counting from the miRNA 5′ end.21 It leads to full mRNA degradation or blocking of the ribosomal machinery, both result in gene silencing.22,23 MiRNA can bind at the 3′-untranslated region (3′-UTR), coding sequences (CDS) and 5′-untranslated region (5′-UTR), leading to the estimate of more than 60% of human mRNAs being regulated by miRNAs.21,24 Binding of miRNA has been categorized into 5 classes, depending upon whether the seed sequence was used as well as on the complementarity of the nucleotides.252.2. Body fluids as sources for miRNA detection
Recent studies have shown that “cell-free” miRNAs exist in all bodily fluids, which explains the growing interest in exploring their potential as disease markers.26,27 Initially, miRNA expression was studied using different tissues (either fresh or paraffinized) or cell culture to determine functional and diagnostic roles of miRNAs.28–30 However, bodily fluids are more readily available, less invasive than biopsies, and can be repeatedly taken for follow-up studies with reduced risks. Currently, body fluids from all different sources have been used.31–36 Principally, they can be divided into 2 different types, depending on whether they can be obtained without invasive means (sputum, ejaculate, vaginal secret, menstruation blood, breast milk, colostrum, sweat, tears, urine, and feces) or only with invasive means (serum, plasma, cerebrospinal fluid, pleural fluid, peritoneal fluid, amniotic fluid, follicular fluid, synovial fluid, pancreatic juice, bile, gastric juice, and others) invasive means.20,37 MiRNAs are secreted by cells through exosomes and extracellular vesicles.38 It has been shown that the secreted miRNAs remain stable in bodily fluids.39 Nevertheless, correlation of miRNAs in different body fluids under pathological conditions is still elusive and presumably not only dependent on the disease type but also on its stage (early or advanced) as well as patient-related aspects such as age, diet, medication, etc. For instance, in our previous study, Löser et al. demonstrated that levels of miRNA15a can significantly differ in blood/serum and urine. They showed that in proteinuric diseases high levels of miRNA15a could be detected in the urine, while the respective levels in serum were about 300 times lower.402.3. Isolation methods
Isolation and accurate quantification of non-coding RNAs in body fluid samples is challenging due to the small effective concentration of small miRNAs and the coexistence of contaminating proteins and inhibitors, which may interfere with the isolation and detection of miRNAs.41 The most traditional approach is the use of a phenol-chloroform extraction followed by RNA precipitation.42 Lu and Rothenberg43 described the loss of short structured RNAs (miRNAs, some pre-miRNAs, small interfering RNA (siRNA) duplexes, and transfer RNAs (tRNAs)) with low guanine–cytosine content (GC) due to the inefficiency of precipitating small versus long nucleic acid RNA as well as their lack of stability due to a stable secondary structure.44 For these reasons, commercially available extraction kits45–47 are used, which are more specialized in the miRNA extraction from different sample types. Even in currently available and applied bioanalytical procedures, lipoproteins and RNA–protein complexes may interfere with isolation, while co-isolation may falsify quantification affecting further applications.48,49 Moreover, the hemolysis and cell remnants especially in blood samples may considerably change the miRNA yield.50,51 In addition, available RNA quantification methods such as Qubit miRNA assay,52 the NanoDrop,53 and the Bioanalyzer54,55 vary in their quality of detection of low abundance targets, in comparative replicate extractions, and in the overall miRNA quantity measured.412.4. Current detection methods for miRNAs
Currently employed detection methods depend on the purpose of identifying miRNAs. All of these methods have in common that miRNA gene expression profiles are analyzed and compared between different disease and healthy states. Principally, one can differentiate between two types of detection approaches: (i) a screening assay to newly identify a broad range of simultaneously up-/down-regulated miRNAs (a miRNA microarray). These are done using either microarray assays or next generation sequencing. According to a very detailed study by Mestdagh et al., microarray assays often show low specificity, low concordance of differential expression, problems with sensitivity, specificity and compatibility, poor reproducibility, technology- vs. platform-related problems, and particularly exhibit a strong and significant inverse correlation between sensitivity and specificity.56 Next generation sequencing is used as a screening tool because of its high throughput capabilities, however it needs highly specialized equipment to analyze complex data, which makes it more time consuming. Both methods need confirmation, for which quantitative real-time polymerase chain reaction (qRT-PCR) is chosen. (ii) The second type of detection focuses on individually expressed and regulated, already known miRNAs. There has been a multitude of techniques described as potential detection methods,34e.g., the northern blot analysis with radiolabeled or biotinylated probes that involves size-selective differentiation to separate mature from pre-miRNA. The in situ hybridization is suitable for highly abundant miRNAs and is generally inconsistent for miRNAs in low abundance. Other alternative techniques involve bioluminescence miRNA detection as competitive solid-phase hybridization-based method,57 surface-enhanced Raman spectroscopy method,58 surface plasmon resonance imaging (SPRI)-based,59 and quantitative real-time PCR (qRT-PCR).60,61 For a detailed comparison of current miRNA detection methods with other parallel approaches, including comprehensive overviews on their advantages and disadvantages, we refer to other specialized reports and reviews.62–64Over the last 10 years, the most commonly used assay technique has been the qRT-PCR analysis. According to Lu and Rothenberg,43 three different approaches can be chosen:
(i) Reverse transcription with miRNA specific stem-loop primer, followed by qPCR using a miRNA specific probe. This is the more expensive variant, but it can differentiate between pri-, pre- and mature miRNA. In addition, stem loop primers prevent the biding of double-stranded genomic DNA molecules as well as enhancing the thermal stability of RNA–DNA heteroduplexes.65 If the material quantity is small, pooled stem-loop primers may provide advantages,66 while pre-amplification may help if accuracy is the primary question.67
(ii) Universal reverse transcription by adding polyA tails to 3′ end of miRNA, followed by reverse transcription with a poly T primer with a universal sequence (Tag) appended at the 3′ end.
(iii) Universal reverse transcription by adding a 5′ adapter sequence and polyA tails to 3′ end of miRNA, followed by reverse transcription with a poly T primer and a universal sequence (Tag) appended at the 3′ end. Adding a polyA tail is cost-efficient, and useful if different miRNAs have to be analyzed in a small amount of starting material but it does not allow differentiation between precursor miRNAs.
The current setbacks of this method are at least threefold: (i) extraction of miRNA is necessary (high vs. low abundance being an issue); (ii) varying composition in the SYBR Green solution; and (iii) the detection device for qRT-PCR itself. There is currently no comparative analysis available for any of these three potential problems, so that qRT-PCR may yield different mRNA levels even if the identical material source is used. According to the current state-of-the-art, such variations cannot be tolerated in a reliable and meaningful diagnostic test.
In a recent review, Fauth et al.68 have outlined basic criteria for the validation of miRNA quantification assay using qRT-PCR. Several criteria have to be met as outlined below:
(1) Linearity of quantification within the calibration range of measurement, including the smallest accurately measurable concentration standard (lower limit of quantification, LLOQ), being measured on three different days.69
(2) Sensitivity by determining the LLOQ.
(3) Recovery: samples being spiked with known miRNA concentrations before and after RNA isolation followed by qRT-PCR.70
(4) Precision being defined as repeatability (intraday-precision) and reproducibility (interday-precision) by three independent runs.71 According to the literature, variations of 25% for intraday-precision and 35% for interday-precision are acceptable.69,70
(5) Accuracy means the deviation of the measured concentration from the nominal concentration, ±25% being acceptable for the detection of nucleic acids.72
(6) Matrix effect was determined by spiking water and plasma with miRNA at three different concentrations, where quantification cycle (Cq)-values of water were compared with those in the matrix.
(7) Stability was analyzed in plasma versus RNA-isolate in DNAse-free water stored up to one month (−80 °C), and complementary DNA (cDNA) at −20 °C for one month.
Normalization with reference genes73 has to regard the tissue specificity of miRNAs from where it originates, which should be assessed in the tissue of interest before start of the analyses.74 Selection of the most stably expressed miRNAs seem to provide the best non-functional reference genes independent of disease stage.75 Run-to-run variation is composed of instrument related variation (PCR block, lamp, filters, etc.), data analysis settings (baseline correction and threshold), reagents (polymerase, fluorophores, etc.) and the optical properties of plastics. The amount of input RNA required varies substantially between platforms and could be a limiting factor when choosing a platform. Ultimately, the RNA quantity and quality may dictate the platform used to obtain the extracellular miRNA profile. In the end, detection rate is a combination of sensitivity, detection cutoff, and platform content (number of assays or probes available on the platform).
3. Nanomaterial-based miRNA biosensors
The possibility of selectively sensing and analyzing single molecules or cells provides rich information on the quantity and presence of rare and aberrant species that would otherwise remain unseen given the complex composition of biological samples and the co-existence of several biomolecules.76 MiRNAs are intrinsically involved in all biological processes and omnipresent in body fluids, which makes their selective capture and detection critically important for enhancing the diagnostic and prognostic capabilities of bio-analytical assays. However, the visualization of potential heterogeneities in biological species, including miRNAs, are of critical importance for the diagnosis and progression monitoring of diseases at very early stages. Therefore, while much progress has been made in the development of biosensing platforms capable of delivering a signal specific to only one type of species, other analyte signals remain nonspecific and thus do not interfere with the bioassays. In the presence of the target analyte, a biorecognition event takes place that is transferred to a transducer for a quantifiable signal.77 The choice of the biorecognition element and the transducer is, hence, crucial to obtaining biosensors that possess high selectivity, sensitivity, reproducibility and the reusability needed for potential point-of-care analysis of biomarkers.Nanomaterials possess promising potential for the detection of analytes due to their intrinsic electrical, magnetic, or optical properties and, more importantly, due to their high surface area that can be exploited for the immobilization of target ligands with high sensitivity towards target biomolecules.78 Multiple approaches for the sensing of oligonucleotides have been reported based on metal or metal oxide nanostructured films.79,80 Compared to thin films or particles immobilized on a solid surface, where diffusion of analytes and contact to recognition sites on the sensor surface can be very tedious, dispersible sensor particles can provide much faster detection times and higher efficiencies due to enhanced probability of ligand-receptor collisions. In fact, biosensors based on dispersible nanoparticles have shown 1000 times superior response time and detection limit.76 The latter aspect can be attributed to the availability of the large number of recognition sites on the nanoparticle surface, which results in higher sensitivities due to increased density of biomolecule capturing events.
Nanoparticles generally demonstrate ease of functionalization though the abundance of surface-active chemical groups, e.g., surface hydroxyl groups in terms of metal oxide nanoparticles. Immobilization of target-specific ligands to nanoparticles through surface groups can occur either covalently or non-covalently, e.g., through electrostatic interactions, π–π stackings or van der Waals forces.81 Although unspecific physisorption can result in higher numbers of attached ligands, the number and orientation of surface-bound molecules can be precisely controlled during chemical conjugation reactions.82 Most cases of miRNA sensors rely on the attachment of single-strand DNA or RNA, complementary to the analyte sequence, to nanoparticle surfaces. Conjugation strategies therefore often involve carbodiimide or click reactions. Nevertheless, most approaches especially those based on magnetic nanoparticles involve the immobilization of streptavidin, which demonstrates an exceptional high binding affinity (dissociation constant Kd ∼ 10−15 M) to biotin, which thus represents one of the strongest non-covalent interactions known in nature.83 The selectivity of miRNA biosensors is usually tested by employing single-nucleotide mismatches.
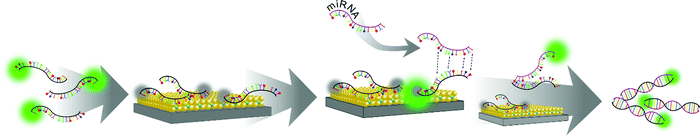
Much effort has been devoted to the controlled engineering of materials at the nanoscale to obtain efficient miRNA sensor platforms that could be used in future point-of-care diagnostic devices. As a result, various nanomaterials including magnetic particles and composites thereof have been incorporated into sensing applications and devices used for the detection of miRNAs (Fig. 1), providing enhanced sensitivities and reduced detection times compared to traditional detection kits.84,85
 | ||
| Fig. 1 Overview of functional properties of magnetic nanoparticles and their composites with other nanomaterials as used for miRNA sensing applications. | ||
3.1. Magnetic nanomaterials in miRNA sensors
Given the generally low concentration of miRNAs in body fluids, potential strategies for the enrichment of miRNAs in the samples, e.g., through use of magnetic extraction represents a powerful tool for the detection of biomarkers and is of significant importance for reliable and early diagnosis of various diseases.85 Besides the material composition, the employed particle size is of critical importance in the context of their efficient employment in extraction and biosensing devices. In fact, most magnetic separation studies rely on (sub)micron particles used for their high magnetic moment that allows for rapid extraction of analytes.86 However, besides being less prone to sedimentation, smaller (<100 nm) nanoparticles offer a higher surface area that enables attachment of large amounts of analyte recognition sites leading to high sensitivities. Nevertheless, if employed particles are below a certain critical size range (usually below 20 nm), the viscous drag, predominantly present in body fluids, dominates the magnetophoretic force and hampers their diffusion, which leads to unwanted prolongation of the separation process.87 The use of magnetic particles in miRNA sensors therefore also provides the possibility to concentrate larger sample volumes, which is beneficial compared to commonly used small volumes in the μl range as it allows for a simple handling and is much more representative of the bulk population.76During the past decade, magnetic nanoparticles have been efficiently used in numerous miRNA sensors. In many cases, optical or electrochemical readout techniques are employed providing ease of handling. Therefore, combining the beneficial properties of two or more material types has been proven very advantageous for biosensing applications.
3.2. Plasmonic noble metal nanoparticles, quantum dots and their composites with magnetic particles in miRNA sensors
Noble metal nanoparticles such as gold and silver nanoparticles have been exhaustively used for the sensing of various biological analytes mainly due to their size- and shape-dependent surface plasmon resonance (SPR), the collective oscillations of metal conduction band electrons with visible light photons.88,89 Gold nanoparticles represent a highly versatile tool in biosensors for the detection of numerous disease-related miRNAs18 from body fluids including blood,90 urine,91 and saliva.92 Besides simple metal nanoparticles, composite structures have demonstrated high efficiency in miRNA detection, e.g., using Ag/Pt nanoclusters for miRNA-21 sensing in human urine samples.93 A recently developed biosensor for miRNA-133a, an acute myocardial infarction-related miRNA, employed hollow Au/Ag nanospheres using surface-enhanced Raman scattering (SERS) as a highly sensitive detection method.94 Moreover, during the last few years fluorescent nanobiosensors for miRNAs have been developed based on the tunable optical properties of quantum dots, e.g., made of carbon95 or CdTe.96A broad variety of composite structures with magnetic nanoparticles has been reported for miRNA sensing, e.g., using assemblies of nano-γ-Fe2O3 particles in combination with Pt nanoparticles for the detection of miRNA-21,97 polydopamine-coated Fe3O4 nanoparticles and carbon dots for sensing of miRNA-167,98 or carboxyl-terminated Fe3O4 nanoparticles in microfluidic concentrator devices to quantify miRNA-200a-3p with silica embedded Au nanoparticles that function as Raman tags.99 Moreover, Fe3O4@Ag core–shell nanoparticles have been employed for miRNA let-7b capture and quantification with sensitivities in the lower fM range using SERS as a detection method.100 Even higher sensitivities with detection limits of miRNAs in the aM range could be recently obtained using Fe3O4@Au nanomaterials employing a DNA four-way junction-based electrochemical detection of target miRNA.101 The selectivity of the used system was employed using six different mismatched miRNAs and demonstrated similar results to qRT-PCR on healthy and breast cancer patient samples (Fig. 3).
Moreover, a multicomponent sensor consisting of magnetic nanoparticles for magnetic extraction of analytes, silver nanoparticles for chemical amplification and CdTe quantum dots as luminescent signals were reported for the detection of miR-141 in the femtomolar concentration range.102
3.3. Two-dimensional nanostructures and their composites with magnetic particles in miRNA sensors
Few layer graphene oxide (GO) that characterizes highly dispersed and stable two-dimensional (2D) carbon material, represents one of the most commonly employed 2D structures in medicinal applications including miRNA biosensors, based on its unique optical properties, extremely high specific surface area and π-conjugated structure.103,104 Hizir and co-workers demonstrated the versatility of graphene oxide for miRNA sensing applications and reported on the efficient simultaneous detection of exogenous miRNA-21 and miRNA-141 from human body fluids including blood, urine, and saliva.105 Additionally, two-dimensional transition metal dichalcogenides (TMDCs; e.g., MoS2, WS2, etc.) have shown great potential for the sensing of miRNAs owing to the high affinity of sulfur that allows simple surface functionalization, e.g., through non-covalent self-assembly of thiolated compounds including miRNAs on its surfaces (Fig. 2). For instance, Xi and co-workers employed WS2 nanosheets for the detection of miRNA-21 through fluorescence quenching,106 while Cai et al. reported on the suitability of MoS2 for sensing miRNA-21 from serum samples.107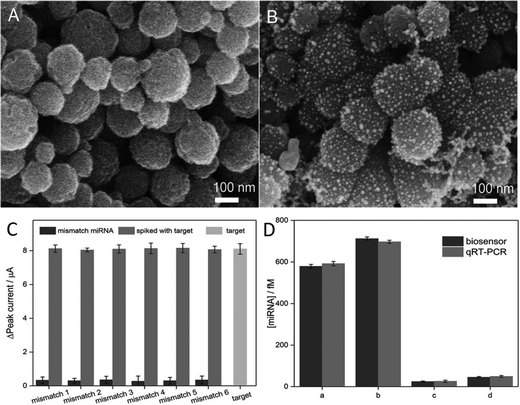 | ||
| Fig. 2 SEM images of (A) Fe3O4 and (B) Fe3O4@Au. (C) Comparison of peak current variations for the detection of mismatch miRNAs with and without spiking target miRNA. (D) Quantification of miRNA concentrations in serum samples from (a and b) breast cancer patients and (c and d) healthy individuals. Reprinted from Yu et al.101 Copyright (2020) with permission from Elsevier. | ||
Magnetic composites with 2D materials have been mainly reported for graphene-based miRNA sensors, although studies employing TMDCs have been described, even in body fluids (see Section 4.2). For instance, GO-loaded superparamagnetic iron oxide nanoparticles have been employed for the electrocatalytic detection of cancer-related miRNA-21 with low detection limit of 1.0 fM.108 Another approach used GO-magnetic microparticle hybrids employing chemiluminescence as detection method for miRNA-122 sensing in the lower picomolar range.109 A complex sensor design employing reduced GO as sensing platform in combination with Au nanoparticles and CdSe@CdS quantum dots-contained magnetic nanocomposites was presented by Daneshpour and coworkers for dual-signal simultaneous detection of miRNA-106a and let-7a.110
4. Magnetic particle-based miRNA isolation and detection from body fluids
Even though the efficiency of magnetic nanoparticles and its composite structures for miRNA sensing applications has been demonstrated, most experiments were performed in the absence of potentially interfering biomolecules, and thus only few studies have been performed in natural body fluids. Still, the fast, sensitive, and selective extraction of analytes in biologically complex media is the key challenge to receive highly efficient biosensing platforms that enable their simple handling and short processing times.Here, we highlight some of the recent approaches that used magnetic particles for the detection, separation and quantification of circulating miRNAs out of body fluids including serum, whole blood and urine (Table 1). The chosen sensing platforms all incorporate magnetic structures or their composites in the nano to lower micron range.
| Employed material | Target miRNA(s) | Tested body fluid(s) | Signal readout | Limit of detection | Linear range | Ref. |
|---|---|---|---|---|---|---|
| Streptavidin-coated magnetic microparticles | miRNA-141 | Serum | Fluorescence | 46 pM | 50 pM–5 nM | 111 |
| Fe2O3@Au composite nanoparticles | miRNA-21 | Serum | Colorimetric | 1 aM | 1 aM–5 pM | 112 |
| Streptavidin-coated magnetic microparticles | miRNA-21 | Serum | Mass spectrometry (LC-ESI-MS/MS) | 60 fM | 0.2 pM–0.25 nM | 113 |
| Fe3O4@SiO2 microsphere–rGO composite | miRNA-21 | Serum | Fluorescence | 0.098 nM | 0.2–20 nM | 116 |
| Two-dimensional Fe3O4/DNA network structures | miRNA let-7a | Serum | Colorimetric | 13 aM | 0.05 fM–12 nM | 117 |
| Multilayer core–satellite magnetic superstructures | miRNA let-7b | Serum | Optomagnetic | 4.8 fM | 10 fM–10 nM | 118 |
| Fe3O4@Au composite nanoparticles | miRNA-21 and miRNA-155 | Serum | Electrochemical | 1.5 fM; 1.8 fM | 5 fM–2 nM | 120 |
| Streptavidin-coated magnetic microparticles | miRNA-122, miRNA-155 and miRNA-21 | Serum | HPLC-fluorescence | 0.39 fM; 0.30 fM; 0.26 fM | 1 fM–100 pM | 121 |
| Quantum dot decorated magnetic beads | miRNA-16 and miRNA-296 | Serum | Colorimetric | 0.5 pM | 0.5 pM to 10 pM | 122 |
| Fe3O4@Au composite nanoparticles | miRNA-21 | Whole blood | Electrochemical | 10 aM | 10 aM–1 nM | 123 |
| Magnetic beads coupled with gold nanoparticles | miRNA-141 | Whole blood | Photoelectrochemical | 0.5 fM | 1 fM–10 pM | 124 |
| MoS2 sheets modified with CuFe2O4 and streptavidin-coated magnetic beads | miRNA-205, miRNA-92, miRNA-7857 and miRNA-378 | Blood | Electrochemical | 0.48 pM | 1 pM–1.5 nM | 125 |
| Streptavidin-coated magnetic beads | miRNA-21 | Urine | Enzymatic conversion of sucrose to glucose | 1.8 pM | 10–200 pM | 130 |
| Carboxylic acid modified magnetic microparticles | miRNA-21 | Urine | Magnetic relaxation switch | 5 fM | 5 fM–0.5 nM | 131 |
| Fe3O4 nanoparticles in combination with a capped gold nanoslit | miRNA-16-5p | Urine | SPR | 17 fM | 1 pM–1 nM | 132 |
4.1. Detection from serum
The detection of small amounts of miRNAs from serum samples usually requires amplification of analytes and corresponding signals, similar to qRT-PCR, which is the current gold standard, for miRNA expression profiling analysis. The required concentrations in a typical serum analysis usually range between 10−16–10−12 M but can be even lower.76 Besides using magnetic concentration as an amplification approach, proportional and exponential amplification strategies based on circulatory systems have been developed in miRNA biosensing devices. DNA-based cycles usually rely on the modification of nanoparticles with specific DNA sequences that are displaced in parts as soon as the analyte is present and attached. Released molecules are then detected, e.g., based on fluorescence measurements as described for the detection of miRNA-141 out of serum samples using DNA modified magnetic beads.111 Here, binding of the target DNA to modified magnetic beads resulted in a toehold-mediated DNA displacement reaction, creating an intermediate toehold structure. The addition of a fuel DNA then triggered the release of miRNA-141 and a dye labeled DNA, which could be fluorescently detected while the target miRNA could undergo the same proportional DNA cycle leading to signal amplification (Fig. 4). If a trigger DNA with the identical sequence to miRNA-141 is additionally employed, signal and target amplification can be exponential. Direct comparison between proportional and exponential amplification revealed a higher sensitivity and shorter processing times in terms of the exponential amplification, although a higher noise fluorescence signal was observed. | ||
| Fig. 4 Schematic illustration of the principles of DNA circuit systems based on (a) proportional and (b) exponential amplification strategies. Reprinted from ref. 111 (https://pubs.acs.org/doi/full/10.1021/acsomega.7b01866) with permission from ACS. Further permissions related to the material excerpted should be directed to the ACS. | ||
A few exponential amplification strategies were also employed on composite structures using gold decorated Fe2O3 nanoparticles as detection platforms.112 Using this approach, attomolar detection limits of miRNA-21 in serum samples were achieved based on a highly sensitive calorimetric approach. Alternatively, target amplification can be performed enzymatically through duplex specific nuclease (DSN) addition. DSN demonstrates strong cleavage preference for DNA in double-stranded nucleic acids (e.g., DNA/RNA heteroduplexes), while being able to differentiate perfectly matching duplexes from imperfect matches. Therefore, DSN will selectively cleave duplexes formed upon hybridization of target miRNA with DNA modified magnetic beads. The released target miRNA can be recycled and undergo the same process several times to amplify the analyte signal. This approach was recently demonstrated for the quantification of miRNA-21 out of serum samples using liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) and DNA modified magnetic beads as platform.113 Magnetic separation of DNA fragments that were formed through the addition of DSN enabled their concentration followed by acid hydrolyzation into nucleobases before LC-ESI-MS/MS quantification. The authors chose miRNA-21 as target analyte which represents one of the earliest identified cancer-promoting ‘oncomiRs’, oncogenic miRNAs that are usually overexpressed in cancers,114 is related with multiple cancer types, and thus constitutes an important diagnostic and prognostic biomarker.115 Sensing of this target was also demonstrated employing a composite structure of silica (SiO2) coated magnetic Fe3O4 particles and reduced graphene oxide (rGO) using the fluorescence quenching ability of rGO as read-out.116 Although high selectivity compared to other miRNAs could be demonstrated, obtained detection limits remained in the lower nM range.
Attomolar concentrations of miRNA let-7a, which is part of the lethal-7 (let-7) family and is suppressed in tissues of gastric cancer patients, could be detected using two-dimensional DNA/Fe3O4 networks displaying triple amplification via synergy of the amplification events hybridization chain reaction, networking and catalytic reaction.117 Validation of this sensor was additionally approved in serum samples of gastric cancer patients and healthy people demonstrating its practicability in real body samples. An optomagnetic readout for sensing let-7b was described using ‘core–satellite’ magnetic superstructures that comprise multiple layers of differently sized magnetic beads.118 The presence of the target miRNA triggers release of satellite magnetic particles though addition of DSN, which can be quantified using an optomagnetic signal. This simple approach provided high sensitivities in the lower femtomolar range and could be used in serum samples. Chan and coworkers just recently presented a novel biosensor based on silica coated magnetite nanoparticles employing a ligase-mediated amplification for the detection of miRNA-149 in human serum samples.119 Since miRNA-149 has been reported to be involved in multiple cancer types, the proposed sensor was evaluated in both healthy and cancer patient's serums reliably quantifying circulating miRNA-149 with a detection limit of 314 fM.
While the above-mentioned approaches demonstrate the feasibility of single miRNA detection with high sensitivity and selectivity in serum samples, the coincident detection of multiple target analytes still represents a major challenge, also considering particle pre-modification. Nevertheless, Shen and co-workers just recently presented an electrochemical biosensor based on magnetic Fe3O4@Au composite nanoparticles capable of simultaneously detecting miRNA-21 and miRNA-155, which are both overexpressed in the serum of patients with breast cancer.120 For this purpose, electrochemical tag-labeled DNAs were immobilized on the surface of nanoparticles via a hyperbranched hybridization chain reaction. Upon attachment of target analytes, both miRNAs were magnetically concentrated and quantified up to detection limits of 1.5 fM (miRNA-21) and 1.8 fM (miRNA-155), respectively, in serum samples. By use of high-performance liquid chromatography (HPLC)-fluorescence as detection method, Qi and coworkers, reported on the simultaneous detection of miRNA-122, miRNA-155, and miRNA-21 in serum samples from healthy persons and cervical cancer patients.121 High sensitivities in the lower fM range were obtained using a DSN-assisted target recycling amplification. Therefore, magnetic beads were functionalized with oligonucleotide sequences complementary to target miRNAs and upon hybridization, DSN was added to selectively cleave heteroduplexes and release the target miRNAs which were separated and quantified by HPLC-fluorescence. Their results indicated that miRNA-155 and miRNA-21 were significantly overexpressed in cancer patients compared to those of healthy persons, which was consistent with qRT-PCR amplification experiments. Moreover, Wang and coworkers recently presented the employment of magnetic beads that were surface decorated with quantum dots for the simultaneous detection of miR-296 and miR-16. Detection of both miRNAs with femtomolar sensitivity and single-base mismatch specificity in cells and in diluted serum samples provides a novel approach for early stage diagnosis of hand, foot, and mouth disease.122
4.2. Detection from whole blood
Tavallaie et al. were the first to describe a nanoparticle-based biosensor that was able to detect miRNAs in a range of 10 aM to 1 nM in unprocessed blood samples.123 Detection of target miRNA was performed using gold-coated magnetic nanoparticles (Au@MNPs) as platform by surface modifying them via an Au–S bond with a short DNA sequence complementary to the sequence of target miRNA. Moreover, a methylene blue redox label at the end of the DNA served as a detecting agent upon miRNA–DNA hybridization and subsequent magnetic concentration of conjugates on the surface of a gold microelectrode measuring square-wave voltammetry.Based on the fact that circulating miRNA-141 in blood samples of patients has been recognized as potential biomarkers for prostate carcinoma, Zhang and co-workers developed a biosensor based on magnetic beads and gold nanoparticles capable of quantifying the target miRNA in unprocessed blood samples.124 In the presence of the target molecule, selective binding to magnetic beads that were surface decorated with DNA modified gold nanoparticles occurred. Magnetic separation was carried out followed by the addition of a sufficient fuel strand that led to the release of miRNA-141 as well as DNA-modified gold nanoparticles. The latter bound with complementary sequences on a photoactive polymer dots/indium tin oxide (ITO) electrode allowing to measure a decrease in photocurrent caused by the energy transfer between gold nanoparticles and polymer beads which directly correlated to the amount of target miRNA present in tested blood samples.
Chand and co-workers reported the use of MoS2 nanosheets modified with CuFe2O4 nanoparticles, which functioned as electrochemical amplifiers, in combination with streptavidin functionalized magnetic beads for miRNA detection from body fluids.125 The sensor was designed to detect paratuberculosis, a bacterial infection of the intestinal tract of dairy cattle, by detecting four different miRNAs (miRNA-205, miRNA-92, miRNA-7857, and miRNA-378) simultaneously. While a detection limit of 0.48 pM could be obtained in spiked serum samples, their functionality was additionally proven in bovine blood samples although not a complete, albeit good, agreement with ELISA and qRT-PCR results could be obtained.
4.3. Detection from urine
Besides the fact that a variety of studies have demonstrated that miRNAs released via urine can provide a wealth of information useful to diagnose multiple diseases like urinary system cancer, kidney injury, and diabetes,126–128 only few miRNA sensing systems based on magnetic nanomaterials have been developed so far to extract and detect miRNAs from urine samples. Although commercial kits such as urine microRNA purification kit (Norgen Biotek) or miRNeasy kit (Qiagen) exist, Xu and co-workers recently demonstrated that the use of magnetic nanoparticles led to 25–30% improved miRNA yields besides saving reagents and time.129 Three types of urinary disease biomarkers, namely miRNA-10a, miRNA-16, and miRNA-30d, were extracted from rat urine upon enrichment using carboxyl-coated magnetic nanoparticles. Since most miRNAs in urine remain protected from RNAses either enclosed in microvesicles such as exosomes or complexed in Argonaute 2 (Ago2)-containing protein complexes, the enrichment was based on a protein corona formation on the surface of magnetic nanoparticles. A more specific miRNA detection approach from urine samples was demonstrated by Huang and co-workers, who developed biosensors based on magnetic nanoparticles for the detection of miRNA-21 from mouse urine using glucose meters.130 In this study, streptavidin coated magnetic nanoparticles were functionalized with a biotinylated DNA strand to which invertase was linked. Upon binding of target miRNA-21 via DNA/RNA hybridization, DSN was applied, resulting in the release of invertase which efficiently converts sucrose to glucose. After magnetic separation, and addition of sucrose to the supernatant, measured glucose levels thus directly correlated with miRNA-21 levels with a detection limit of 1.8 pM. Besides the high sensitivity and high sequence selectivity, this approach demonstrates a convenient and low-cost strategy using a simple glucose meter as signal readout device. The magnetic properties of employed nanoparticles are not only very beneficial for analyte enrichment purposes but can also be used for the detection itself. In this regard, Lu et al. recently employed magnetic microparticles, combining magnetic separation with visualization of magnetic relaxation changes that enabled rapid and efficient sensing of miRNA-21 in urine samples.131 The significant change of transverse relaxation time (ΔT2) that was observed upon binding of the target miRNA to the particle surface functioned as signal readout to quantify the oligonucleotide at concentrations of 5 fM.A microfluidic system comprised of magnetic nanoparticles and gold surfaces which enabled magnetic separation followed by detection of analytes via surface plasmon resonance (SPR) was demonstrated by Mousavi et al. who focused on the detection and quantification of miRNA-16-5p, a prognostic indicator for acute kidney injury, in human urine samples (Fig. 5).132 For this purpose, magnetite nanoparticles were surface modified with an oligonucleotide sequence complementary to a unique region of the target miRNA-16-5p sequence via a sulfhydryl-reactive cross-linker. Upon capturing of the target molecule, hybridization occurred with a second complementary strand that was linked to a gold surface and allowed for the visualization and quantification of attached miRNA by SPR.
 | ||
| Fig. 5 A schematic of the double hybridization method to detect the nucleic acid target. (a) First step: isolation of the target molecule using magnetic nanoparticles. (b) Second step: hybridizing the target molecule isolated on the magnetic nanoparticles with probe II on the capped gold nanoslit. Republished from Mousavi et al.132 with permission from The Royal Society of Chemistry, Copyright (2015). | ||
5. Conclusion and future perspectives
The detection and quantification of specific miRNAs as circulating biomarkers from body fluids carries enormous potential as diagnostic biomarkers for a variety of disorders including the early diagnosis life-threatening disease such as cancers and/or ailments with hardly predictable course. However, their small size, high sequence homology among family members, and low abundance in body fluids bedevils their detection in short time and with high accuracies that poses a challenge on miRNA-based bioanalytical approaches. In order to overcome cumbersome and tedious steps of traditional extraction processes, biosensors based on nanomaterials have been developed that offer enhanced miRNA yields and reduced costs. Especially magnetic materials have received increasing attention due to the possibility of concentrating the analyte through specific surface conjugation that has served as the basis for a number of highly efficient miRNA sensing devices. Nevertheless, the current challenge lies in the demonstration of the miRNA capture and detection strategies in real biological samples, which is a prerequisite for the development of point-of-care testing systems. While most sensors demonstrate high sensitivities and selectivity in simple water-based model assays, the presence of other interfering biomolecules in conjunction with the low number of testing analyte, increasing viscosity of the sample and possible influence due to pH changes might affect many detecting systems. Some of the approaches highlighted in this work display the efforts that have been made in capturing and quantifying biomarker miRNAs from (unprocessed) biologically complex body fluids with very low detection limits down to fM and even aM levels and fast processing times, e.g., using electrochemical or magnetic relaxation readouts. In view of the biochemical complexity of many diseases, simultaneous detection of multiple miRNAs, potentially even obtained from different body fluids, would be of high interest to enable an accurate disease diagnosis. In this context, since current researches focuses on the correlation of miRNA expression in different body fluids, it might be of particular interest to develop magnetic amplification-based miRNA sensors that go beyond the tested fluids such as serum, blood, and urine.Although some sensors still rely on specific analytical equipment that requires trained personnel, first integrations into low-cost signal readout devices, such as glucose meters demonstrate a convenient alternative for patient-centric point-of-care devices. These methods now have to be optimized towards their collection, processing efficiency and reproducibility to be suitable for reliable disease diagnosis (Fig. 6).
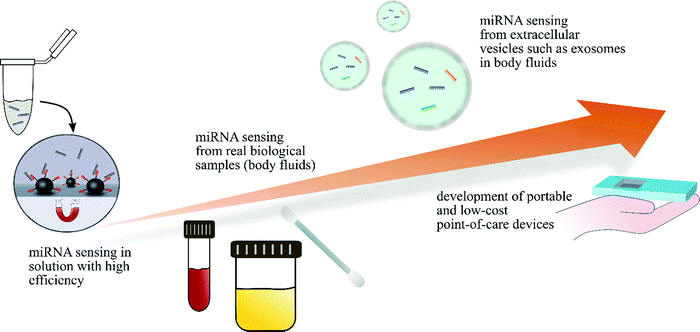 | ||
| Fig. 6 Future steps on the way to efficient and portable miRNA sensing devices based on magnetic nanoparticles. | ||
Moreover, while some miRNAs are free-floating in biological fluids, many are contained in extracellular vesicles, such as exosomes. Therefore, current research points towards the development of biosensors capable of capturing those microvesicles and detecting the encapsulated oligonucleotide which is expected to provide new and highly promising opportunities regarding disease diagnosis.133,134
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Authors are thankful to the University of Cologne for the infrastructural and financial support. Dr Isabel Gessner is thankful to the German Science Foundation (DFG) for the award of a Walter Benjamin Fellowship (2020#x2013;21) to her.References
- R. P. Alexander, G. Fang, J. Rozowsky, M. Snyder and M. B. Gerstein, Nat. Rev. Genet., 2010, 11, 559–571 CrossRef CAS.
- D. Trzybulska, E. Vergadi and C. Tsatsanis, eJIFCC, 2018, 29, 221–226 CAS.
- D. P. Bartel, Cell, 2009, 136, 215–233 CrossRef CAS.
- Y. Tutar, Curr. Pharm. Biotechnol., 2014, 15, 429 CAS.
- M. Cui, H. Wang, X. Yao, D. Zhang, Y. Xie, R. Cui and X. Zhang, Front. Genet., 2019, 10, 626 CrossRef CAS.
- X. Zhou, Z. Zhang and X. Liang, Cell J., 2020, 21, 459–466 Search PubMed.
- R. Munk, A. C. Panda, I. Grammatikakis, M. Gorospe and K. Abdelmohsen, Int. Rev. Cell Mol. Biol., 2017, 334, 177–205 CAS.
- A. Wojciechowska, A. Braniewska and K. Kozar-Kamińska, Adv. Clin. Exp. Med., 2017, 26, 865–874 Search PubMed.
- M. Ferrante and G. O. Conti, MicroRNA, 2017, 6, 157–165 CrossRef CAS.
- Z.-G. Liu, Y. Li, J.-H. Jiao, H. Long, Z.-Y. Xin and X.-Y. Yang, Neural Regener. Res., 2020, 15, 2123–2130 CrossRef.
- R. Nedaeinia, M. Manian, M. H. Jazayeri, M. Ranjbar, R. Salehi, M. Sharifi, F. Mohaghegh, M. Goli, S. H. Jahednia, A. Avan and M. Ghayour-Mobarhan, Cancer Gene Ther., 2017, 24, 48–56 CrossRef CAS.
- K. J. Ledeganck, E. M. Gielis, D. Abramowicz, P. Stenvinkel, P. G. Shiels and A. H. Van Craenenbroeck, Clin. J. Am. Soc. Nephrol., 2019, 14, 454–468 CrossRef CAS.
- K. W. Witwer, Clin. Chem., 2015, 61, 56–63 CrossRef CAS.
- S. Yadav, M. K. Masud, M. N. Islam, V. Gopalan, A. K. Y. Lam, S. Tanaka, N. T. Nguyen, M. S. Al Hossain, C. Li, M. Y. Yamauchi and M. J. A. Shiddiky, Nanoscale, 2017, 9, 8805–8814 RSC.
- S. Bhana, Y. Wang and X. Huang, Nanomedicine, 2015, 10, 1973–1990 CrossRef CAS.
- N. Ali, R. D. C. P. Rampazzo, A. D. T. Costa and M. A. Krieger, BioMed Res. Int., 2017, 2017, 9306564 Search PubMed.
- I. Gessner, X. Yu, C. Jüngst, A. Klimpel, L. Wang, T. Fischer, I. Neundorf, A. C. Schauss, M. Odenthal and S. Mathur, Sci. Rep., 2019, 9, 1–10 CrossRef CAS.
- C. Coutinho and Á. Somoza, Anal. Bioanal. Chem., 2019, 411, 1807–1824 CrossRef CAS.
- O. J. Snove and J. J. Rossi, Genome Biol., 2006, 7, 231 CrossRef.
- M. von Brandenstein, C. Richter and J. W. U. Fries, Life Sci., 2012, 91, 475–489 CrossRef CAS.
- T. M. Witkos, E. Koscianska and W. J. Krzyzosiak, Curr. Mol. Med., 2011, 11, 93–109 CrossRef CAS.
- K. Sun and E. C. Lai, Nat. Rev. Genet., 2013, 14, 535–548 CrossRef CAS.
- A. Vishnoi and S. Rani, Methods Mol. Biol., 2017, 1509, 1–10 CrossRef CAS.
- P. Maziere and A. J. Enright, Drug Discovery Today, 2007, 12, 452–458 CrossRef CAS.
- A. M. Mohr and J. L. Mott, Semin. Liver Dis., 2015, 35, 3–11 CrossRef CAS.
- S. Junqueira-Neto, I. A. Batista, J. L. Costa and S. A. Melo, Acta Cytol., 2019, 63, 479–488 CrossRef CAS.
- S. Kumar, M. Vijayan, J. S. Bhatti and P. H. Reddy, Prog. Mol. Biol. Transl. Sci., 2017, 146, 47–94 CAS.
- E. Azzalini, E. De Martino, P. Fattorini, V. Canzonieri, G. Stanta and S. Bonin, Int. J. Mol. Sci., 2019, 20, 4819 CrossRef CAS.
- M. I. K. Anindo and A. Yaqinuddin, Int. J. Surg., 2012, 10, 443–449 CrossRef.
- A. M. L. Coenen-Stass, M. J. Pauwels, B. Hanson, C. Martin Perez, M. Conceição, M. J. A. Wood, I. Mäger and T. C. Roberts, RNA Biol., 2019, 16, 696–706 CrossRef.
- G. Tzimagiorgis, E. Z. Michailidou, A. Kritis, A. K. Markopoulos and S. Kouidou, Cancer Epidemiol., 2011, 35, 580–589 CrossRef CAS.
- A. Baldassarre, C. Felli, G. Prantera and A. Masotti, Genes, 2017, 8, 234 CrossRef.
- J. Shen, S. A. Stass and F. Jiang, Cancer Lett., 2013, 329, 125–136 CrossRef CAS.
- M. de Planell-Saguer and M. C. Rodicio, Anal. Chim. Acta, 2011, 699, 134–152 CrossRef CAS.
- G. Cheng, Adv. Drug Delivery Rev., 2015, 81, 75–93 CrossRef CAS.
- M. A. Javidi, A. H. Ahmadi, B. Bakhshinejad, N. Nouraee, S. Babashah and M. Sadeghizadeh, Med. Oncol., 2014, 31, 295 CrossRef.
- J. A. Weber, D. H. Baxter, S. Zhang, D. Y. Huang, K. H. Huang, M. J. Lee, D. J. Galas and K. Wang, Clin. Chem., 2010, 56, 1733–1741 CrossRef CAS.
- C. Thery, F1000 Biol. Rep., 2011, 3, 15 Search PubMed.
- X. Chen, Y. Ba, L. Ma, X. Cai, Y. Yin, K. Wang, J. Guo, Y. Zhang, J. Chen, X. Guo, Q. Li, X. Li, W. Wang, Y. Zhang, J. Wang, X. Jiang, Y. Xiang, C. Xu, P. Zheng, J. Zhang, R. Li, H. Zhang, X. Shang, T. Gong, G. Ning, J. Wang, K. Zen, J. Zhang and C.-Y. Zhang, Cell Res., 2008, 18, 997–1006 CrossRef CAS.
- H. Loeser, M. von Brandenstein, V. Burst, C. Richter, R. Buettner, C. Kurschat, B. Hoppe and J. W. U. Fries, J. Mol. Med. Clin. Appl., 2018, 2, 1–10 Search PubMed.
- K. Wright, K. de Silva, A. C. Purdie and K. M. Plain, Sci. Rep., 2020, 10, 825 CrossRef CAS.
- Y.-K. Kim, J. Yeo, B. Kim, M. Ha and V. N. Kim, Mol. Cell, 2012, 46, 893–895 CrossRef CAS.
- T. X. Lu and M. E. Rothenberg, J. Allergy Clin. Immunol., 2018, 141, 1202–1207 CrossRef CAS.
- F. Marabita, P. de Candia, A. Torri, J. Tegner, S. Abrignani and R. L. Rossi, Briefings Bioinf., 2016, 17, 204–212 CrossRef.
- R. A. M. Brown, M. R. Epis, J. L. Horsham, T. D. Kabir, K. L. Richardson and P. J. Leedman, BMC Biotechnol., 2018, 18, 16 CrossRef.
- A. Brunet-Vega, C. Pericay, M. E. Quílez, M. J. Ramírez-Lázaro, X. Calvet and S. Lario, Anal. Biochem., 2015, 488, 28–35 CrossRef CAS.
- M. Monleau, S. Bonnel, T. Gostan, D. Blanchard, V. Courgnaud and C.-H. Lecellier, BMC Genomics, 2014, 15, 395 CrossRef.
- J. D. Arroyo, J. R. Chevillet, E. M. Kroh, I. K. Ruf, C. C. Pritchard, D. F. Gibson, P. S. Mitchell, C. F. Bennett, E. L. Pogosova-Agadjanyan, D. L. Stirewalt, J. F. Tait and M. Tewari, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 5003–5008 CrossRef CAS.
- K. C. Vickers, B. T. Palmisano, B. M. Shoucri, R. D. Shamburek and A. T. Remaley, Nat. Cell Biol., 2011, 13, 423–433 CrossRef CAS.
- M. B. Kirschner, S. C. Kao, J. J. Edelman, N. J. Armstrong, M. P. Vallely, N. van Zandwijk and G. Reid, PLoS One, 2011, 6, e24145 CrossRef CAS.
- J. S. McDonald, D. Milosevic, H. V. Reddi, S. K. Grebe and A. Algeciras-Schimnich, Clin. Chem., 2011, 57, 833–840 CrossRef CAS.
- A. Garcia-Elias, L. Alloza, E. Puigdecanet, L. Nonell, M. Tajes, J. Curado, C. Enjuanes, O. Díaz, J. Bruguera, J. Martí-Almor, J. Comín-Colet and B. Benito, Sci. Rep., 2017, 7, 7725 CrossRef.
- I. Moret, D. Sánchez-Izquierdo, M. Iborra, L. Tortosa, A. Navarro-Puche, P. Nos, J. Cervera and B. Beltrán, PLoS One, 2013, 8, e82753 CrossRef.
- C. Becker, A. Hammerle-Fickinger, I. Riedmaier and M. W. Pfaffl, Methods, 2010, 50, 237–243 CrossRef CAS.
- M. Mraz, K. Malinova, J. Mayer and S. Pospisilova, Biochem. Biophys. Res. Commun., 2009, 390, 1–4 CrossRef CAS.
- P. Mestdagh, N. Hartmann, L. Baeriswyl, D. Andreasen, N. Bernard, C. Chen, D. Cheo, P. D’Andrade, M. DeMayo, L. Dennis, S. Derveaux, Y. Feng, S. Fulmer-Smentek, B. Gerstmayer, J. Gouffon, C. Grimley, E. Lader, K. Y. Lee, S. Luo, P. Mouritzen, A. Narayanan, S. Patel, S. Peiffer, S. Ruberg, G. Schroth, D. Schuster, J. M. Shaffer, E. J. Shelton, S. Silveria, U. Ulmanella, V. Veeramachaneni, F. Staedtler, T. Peters, T. Guettouche, L. Wong and J. Vandesompele, Nat. Methods, 2014, 11, 809–815 CrossRef CAS.
- H. Ravan, S. Kashanian, N. Sanadgol, A. Badoei-Dalfard and Z. Karami, Anal. Biochem., 2014, 444, 41–46 CrossRef CAS.
- W. Zhou, Y.-F. Tian, B.-C. Yin and B.-C. Ye, Anal. Chem., 2017, 89, 6120–6128 CrossRef CAS.
- F. Hu, J. Xu and Y. Chen, Anal. Chem., 2017, 89, 10071–10077 CrossRef CAS.
- D. Zubakov, A. W. M. Boersma, Y. Choi, P. F. van Kuijk, E. A. C. Wiemer and M. Kayser, Int. J. Leg. Med., 2010, 124, 217–226 CrossRef.
- Y. Chen, J. A. L. Gelfond, L. M. McManus and P. K. Shireman, BMC Genomics, 2009, 10, 407 CrossRef.
- K. A. Cissell, S. Shrestha and S. K. Deo, Anal. Chem., 2007, 79, 4754–4761 CrossRef CAS.
- T. Tian, J. Wang and X. Zhou, Org. Biomol. Chem., 2015, 13, 2226–2238 RSC.
- I. Krepelkova, T. Mrackova, J. Izakova, B. Dvorakova, L. Chalupova, R. Mikulik, O. Slaby, M. Bartos and V. Ruzicka, Biotechniques, 2019, 66, 277–284 CrossRef CAS.
- C. Chen, D. A. Ridzon, A. J. Broomer, Z. Zhou, D. H. Lee, J. T. Nguyen, M. Barbisin, N. L. Xu, V. R. Mahuvakar, M. R. Andersen, K. Q. Lao, K. J. Livak and K. J. Guegler, Nucleic Acids Res., 2005, 33, e179 CrossRef.
- K. Lao, N. L. Xu, V. Yeung, C. Chen, K. J. Livak and N. A. Straus, Biochem. Biophys. Res. Commun., 2006, 343, 85–89 CrossRef CAS.
- P. Mestdagh, T. Feys, N. Bernard, S. Guenther, C. Chen, F. Speleman and J. Vandesompele, Nucleic Acids Res., 2008, 36, e143 CrossRef.
- M. Fauth, A. B. Hegewald, L. Schmitz, D. J. Krone and M. J. Saul, FASEB BioAdv., 2019, 1, 481–492 CrossRef CAS.
- Codex Committee on Methods of Analysis and Sampling, CAC/GL 74-2010. Guidelines on performance criteria and validation of methods for detection, identification and quantification of specific DNA sequences and specific proteins in foods, 2010.
- European Network of GMO Laboratories, JRC Technical Report: Definition of Minimum Performance Requirements for Analytical Methods of GMO Testing, 2015.
- S. A. Bustin, V. Benes, J. A. Garson, J. Hellemans, J. Huggett, M. Kubista, R. Mueller, T. Nolan, M. W. Pfaffl, G. L. Shipley, J. Vandesompele and C. T. Wittwer, Clin. Chem., 2009, 55, 611–622 CrossRef CAS.
- S. Broeders, I. Huber, L. Grohmann, G. Berben, I. Taverniers, M. Mazzara, N. Roosens and D. Morisset, Trends Food Sci. Technol., 2014, 37, 115–126 CrossRef CAS.
- J. R. Dijkstra, L. J. M. Mekenkamp, S. Teerenstra, I. De Krijger and I. D. Nagtegaal, J. Cell. Mol. Med., 2012, 16, 683–690 CrossRef CAS.
- H. J. Peltier and G. J. Latham, RNA, 2008, 14, 844–852 CrossRef CAS.
- K. H. Chang, P. Mestdagh, J. Vandesompele, M. J. Kerin and N. Miller, BMC Cancer, 2010, 10, 173 CrossRef CAS.
- J. J. Gooding and K. Gaus, Angew. Chem., Int. Ed., 2016, 55, 11354–11366 CrossRef CAS.
- M. A. Morales and J. M. Halpern, Bioconjugate Chem., 2018, 29, 3231–3239 CrossRef CAS.
- X. Zhang, Q. Guo and D. Cui, Sensors, 2009, 9, 1033–1053 CrossRef CAS.
- B. Lu, L. Liu, J. Wang, Y. Chen, Z. Li, S. C. B. Gopinath, T. Lakshmipriya and Z. Huo, Nanoscale Res. Lett., 2020, 15, 105 CrossRef CAS.
- S. Mathur, A. Erdem, C. Cavelius, S. Barth and J. Altmayer, Sens. Actuators, B, 2009, 136, 432–437 CrossRef CAS.
- W. Putzbach and N. J. Ronkainen, Sensors, 2013, 13, 4811–4840 CrossRef CAS.
- I. Gessner and S. Mathur, in Nanotechnologies in Preventive and Regenerative Medicine, ed. V. Uskovic, Elsevier Ltd, Oxford, 1st edn, 2018, pp. 260–297 Search PubMed.
- N. M. Green, Biochem. J., 1963, 89, 599–609 CrossRef CAS.
- F. Degliangeli, P. P. Pompa and R. Fiammengo, Chem. – Eur. J., 2014, 20, 9476–9492 CrossRef CAS.
- K. M. Koo, N. Soda and M. J. A. Shiddiky, Curr. Opin. Electrochem., 2020, 100645 Search PubMed.
- S. Berensmeier, Appl. Microbiol. Biotechnol., 2006, 73, 495–504 CrossRef CAS.
- S. S. Leong, S. P. Yeap and J. K. Lim, Interface Focus, 2016, 6, 20160048 CrossRef.
- J. H. Choi, J. H. Lee, J. Son and J. W. Choi, Sensors, 2020, 20, 1003 CrossRef CAS.
- J. N. Anker, W. P. Hall, O. Lyandres, N. C. Shah, J. Zhao and R. P. Van Duyne, Nanosci. Technol. A Collect. Rev. from Nat. Journals, 2009, 7, 308–319 CrossRef.
- L. Shi, Y. Sun, L. Mi and T. Li, ACS Sens., 2019, 4, 3219–3226 CrossRef CAS.
- J. Ki, H. Y. Lee, H. Y. Son, Y. M. Huh and S. Haam, ACS Appl. Mater. Interfaces, 2019, 11, 18923–18929 CrossRef CAS.
- S. Shin Low, Y. Pan, D. Ji, Y. Li, Y. Lu, Y. He, Q. Chen and Q. Liu, Sens. Actuators, B, 2020, 308, 127718 CrossRef CAS.
- N. Fakhri, S. Abarghoei, M. Dadmehr, M. Hosseini, H. Sabahi and M. R. Ganjali, Spectrochim. Acta, Part A, 2020, 227, 117529 CrossRef CAS.
- Y. Sun and T. Li, Anal. Chem., 2018, 90, 11614–11621 CrossRef CAS.
- Z. Wang, Z. Xue, X. Hao, C. Miao, J. Zhang, Y. Zheng, Z. Zheng, X. Lin and S. Weng, Anal. Chim. Acta, 2020, 1103, 212–219 CrossRef CAS.
- Y. S. Borghei, M. Hosseini and M. R. Ganjali, J. Photochem. Photobiol., A, 2020, 391, 112351 CrossRef CAS.
- Y. Y. Bai, Z. Wu, C. M. Xu, L. Zhang, J. Feng, D. W. Pang and Z. L. Zhang, Anal. Chem., 2020, 92, 853–959 CrossRef CAS.
- X. Cao, K. Zhang, W. Yan, Z. Xia, S. He, X. Xu, Y. Ye, Z. Wei and S. Liu, Microchim. Acta, 2020, 187, 212 CrossRef CAS.
- W. Y. Chen, C. H. Wang, K. H. Wang, Y. L. Chen, L. K. Chau and S. C. Wang, Biomicrofluidics, 2020, 14, 014102 CrossRef CAS.
- Y. Pang, C. Wang, J. Wang, Z. Sun, R. Xiao and S. Wang, Biosens. Bioelectron., 2016, 79, 574–580 CrossRef CAS.
- L. Yu, P. He, Y. Xu, X. Kou, Z. Yu, X. Xie and P. Miao, Sens. Actuators, B, 2020, 313, 128015 CrossRef CAS.
- P. Chen, X. Jiang, K. Huang, P. Hu, X. Li, L. Wei, W. Liu, L. Wei, C. Tao, B. Ying, X. Wei and J. Geng, ACS Appl. Mater. Interfaces, 2019, 11, 36476–36484 CrossRef CAS.
- K. Yang, L. Feng, X. Shi and Z. Liu, Chem. Soc. Rev., 2013, 42, 530–547 RSC.
- C. Zhang, P. Miao, M. Sun, M. Yan and H. Liu, Small, 2019, 15, 1–23 Search PubMed.
- M. S. Hizir, M. Balcioglu, M. Rana, N. M. Robertson and M. V. Yigit, ACS Appl. Mater. Interfaces, 2014, 6, 14772–14778 CrossRef CAS.
- Q. Xi, D. M. Zhou, Y. Y. Kan, J. Ge, Z. K. Wu, R. Q. Yu and J. H. Jiang, Anal. Chem., 2014, 86, 1361–1365 CrossRef CAS.
- B. Cai, S. Guo and Y. Li, Anal. Methods, 2018, 10, 230–236 RSC.
- M. N. Islam, L. Gorgannezhad, M. K. Masud, S. Tanaka, M. S. A. Hossain, Y. Yamauchi, N.-T. Nguyen and M. J. A. Shiddiky, ChemElectroChem, 2018, 5, 2488–2495 CrossRef CAS.
- S. Bi, M. Chen, X. Jia and Y. Dong, Nanoscale, 2015, 7, 3745–3753 RSC.
- M. Daneshpour, B. Karimi and K. Omidfar, Biosens. Bioelectron., 2018, 109, 197–205 CrossRef CAS.
- M. Oishi, ACS Omega, 2018, 3, 3321–3329 CrossRef CAS.
- E. Hosseinzadeh, H. Ravan, A. Mohammadi and R. Mohammad-rezaei, Biosens. Bioelectron., 2018, 117, 567–574 CrossRef CAS.
- X. Li, J. Zhao, R. Xu, L. Pan and Y. M. Liu, Analyst, 2020, 145, 1783–1788 RSC.
- A. A. Svoronos, D. M. Engelman and F. J. Slack, Cancer Res., 2016, 76, 3666–3670 CrossRef CAS.
- Y. H. Feng and C. J. Tsao, Biomed. Rep., 2016, 5, 395–402 CrossRef CAS.
- S. Li, K. He, R. Liao, C. Chen, X. Chen and C. Cai, Talanta, 2017, 174, 679–683 CrossRef CAS.
- S. Tang, Y. Li, A. Zhu, Y. Yao, J. Sun, F. Zheng, Z. Lin and W. Shen, Chem. Commun., 2019, 55, 8386–8389 RSC.
- B. Tian, J. Ma, Z. Qiu, T. Zardán Gómez de la Torre, M. Donolato, M. F. Hansen, P. Svedlindh and M. Strömberg, ACS Nano, 2017, 11, 1798–1806 CrossRef CAS.
- H. Chan, S. Ho, D. He and H. Li, Talanta, 2020, 206, 120217 CrossRef CAS.
- Z. Shen, L. He, W. Wang, L. Tan and N. Gan, Biosens. Bioelectron., 2020, 148, 111831 CrossRef CAS.
- T. Qi, C. Song, J. He, W. Shen, D. Kong, H. Shi, L. Tan, R. Pan, S. Tang and H. K. Lee, Anal. Chem., 2020, 92, 5033–5040 CrossRef CAS.
- J. J. Wang, C. Zheng, Y. Z. Jiang, Z. Zheng, M. Lin, Y. Lin, Z. L. Zhang, H. Wang and D. W. Pang, Anal. Chem., 2020, 92, 830–837 CrossRef CAS.
- R. Tavallaie, J. McCarroll, M. Le Grand, N. Ariotti, W. Schuhmann, E. Bakker, R. D. Tilley, D. B. Hibbert, M. Kavallaris and J. J. Gooding, Nat. Nanotechnol., 2018, 13, 1066–1071 CrossRef CAS.
- N. Zhang, X. M. Shi, H. Q. Guo, X. Z. Zhao, W. W. Zhao, J. J. Xu and H. Y. Chen, Anal. Chem., 2018, 90, 11892–11898 CrossRef CAS.
- R. Chand, S. Ramalingam and S. Neethirajan, Nanoscale, 2018, 10, 8217–8225 RSC.
- C. Mall, D. M. Rocke, B. Durbin-Johnson and R. H. Weiss, Biomarkers Med., 2013, 7, 623–631 CrossRef CAS.
- S. Pospisilova, E. Pazourkova, A. Horinek, A. Brisuda, I. Svobodova, V. Soukup, J. Hrbacek, O. Capoun, T. Hanus, J. Mares, M. Korabecena and M. Babjuk, Neoplasma, 2016, 63, 799–808 CrossRef CAS.
- J. W. U. Fries, Biol. Cell., 2019, 111, 169–186 CrossRef CAS.
- S. Xu, S. Hossaini Nasr, D. Chen, X. Zhang, L. Sun, X. Huang and C. Qian, ACS Biomater. Sci. Eng., 2018, 4, 654–662 CrossRef CAS.
- X. Huang, Z. Xu, J. H. Liu, B. Y. Yu and J. Tian, RSC Adv., 2020, 10, 11257–11262 RSC.
- W. Lu, Y. Chen, Z. Liu, W. Tang, Q. Feng, J. Sun and X. Jiang, ACS Nano, 2016, 10, 6685–6692 CrossRef CAS.
- M. Z. Mousavi, H. Y. Chen, K. L. Lee, H. Lin, H. H. Chen, Y. F. Lin, C. S. Wong, H. F. Li, P. K. Wei and J. Y. Cheng, Analyst, 2015, 140, 4097–4104 RSC.
- N. Soda, B. H. A. Rehm, P. Sonar, N. T. Nguyen and M. J. A. Shiddiky, J. Mater. Chem. B, 2019, 7, 6670–6704 RSC.
- J. Lim, M. Choi, H. Lee, Y. H. Kim, J. Y. Han, E. S. Lee and Y. Cho, J. Nanobiotechnol., 2019, 17, 1–12 CrossRef.
| This journal is © The Royal Society of Chemistry 2021 |