Open Access Article
Open Access ArticleBinding and separation of CO2, SO2 and C2H2 in homo- and hetero-metallic metal–organic framework materials†
Lydia
Briggs‡
a,
Ruth
Newby‡
b,
Xue
Han
a,
Christopher G.
Morris
ac,
Mathew
Savage
 a,
Cristina Perez
Krap
b,
Timothy L.
Easun
a,
Cristina Perez
Krap
b,
Timothy L.
Easun
 d,
Mark D.
Frogley
d,
Mark D.
Frogley
 c,
Gianfelice
Cinque
c,
Gianfelice
Cinque
 c,
Claire A.
Murray
c,
Chiu C.
Tang
c,
Junliang
Sun
c,
Claire A.
Murray
c,
Chiu C.
Tang
c,
Junliang
Sun
 e,
Sihai
Yang
e,
Sihai
Yang
 *a and
Martin
Schröder
*a and
Martin
Schröder
 *a
*a
aSchool of Chemistry, University of Manchester, Oxford Road, Manchester, M13 9PL, UK. E-mail: Sihai.Yang@manchester.ac.uk; M.Schroder@manchester.ac.uk
bSchool of Chemistry, University of Nottingham, University Park, Nottingham, NG7 2RD, UK
cDiamond Light Source, Harwell Science Campus, Oxfordshire OX11 0DE, UK
dSchool of Chemistry, Cardiff University, Cardiff, CF10 3AT, UK
eCollege of Chemistry and Molecular Engineering, Peking University, Beijing, 100871, China
First published on 15th February 2021
Abstract
We report the adsorption of C2H2, CO2 and SO2 in a new, ultra-stable Cr(III)-based MOF, MFM-300(Cr), {[Cr2(OH)2(L)], H4L = biphenyl-3,3′,5,5′-tetracarboxylic acid}. MFM-300(Cr) shows uptakes of 7.37, 7.73 and 8.59 mmol g−1 for CO2, C2H2 and SO2, respectively, at 273 K, 1.0 bar, and shows a higher selectivity for SO2/CO2 compared with the Al(III) analogue MFM-300(Al) (selectivity of 79 vs. 45). In order to monitor the effects of changing metal centre on gas uptake and to integrate the properties of the homometallic analogues, the mixed metal MFM-300(Al0.67Cr0.33), [Al1.34Cr0.66(OH)2(L)] has been synthesised. In situ synchrotron micro-FTIR spectroscopy has identified distinct CO2 binding environments on Al–O(H)–Al, Cr–O(H)–Cr and Al–O(H)–Cr bridges in MFM-300(Al0.67Cr0.33), and we have determined the binding domains for these gases by in situ synchrotron X-ray diffraction in both MFM-300(Cr) and MFM-300(Al0.67Cr0.33). The capability of these materials for gas separation has been confirmed by dynamic breakthrough experiments. The incorporation of Al(III) and Cr(III) within the same framework allows tuning of the host–guest and guest–guest interactions within these functional porous materials.
High porosity, chemical and thermal stability and flexible design are critical features of metal–organic framework (MOF) materials. Design-led incorporation of functional groups such as hydroxyl (–OH),1–3 amine (–NH2)4,5 and halogen (–F, –Cl, –Br)6–8 groups to form supramolecular interactions with guest species is an effective methodology for enhancing gas sorption.9,10 The design of MOFs with open metal sites has been explored widely, but this can often lead to materials that are unstable upon desolvation and/or in contact with moisture.11,12 Variation of metal centres in complex structures is a methodology that may alter or enhance materials properties but does not necessarily introduce significant structural changes.13 We were thus interested to investigate the properties of mixed-metal MOF materials, and chose Al(III) and Cr(III) as target centres to compare within the MFM-300 series.
Cr(III)-Based MOFs tend to be highly stable and have been used in catalysis.14 MIL-101(Cr) has removable terminal water molecules connected to a trinuclear [Cr3(μ3-O)(O2CR)6(F,OH)(H2O)2] building block, leaving two Lewis acidic sites accessible to catalyse a range of reactions such as oxidations and epoxidations.15–17 Its extensive use can be attributed to the stability of MIL-101(Cr) to water, and Cr(III)-based MOFs generally show enhanced chemical stability as a result of the low lability of Cr(III), which can also be exploited for gas sorption.18,19 The Al(III)-tetracarboxylate MOF, MFM-300(Al), shows high adsorption of CO2 and SO2,2 while heterometallic MOFs with multiple metal centres within the same framework can show enhanced gas sorption and catalytic capabilities.20,21 Herein, we report the synthesis and gas adsorption of two new Cr-containing analogues of MFM-300(Al): the homometallic MFM-300(Cr) and the mixed metal analogue MFM-300(Al0.67Cr0.33). Their capability for gas separation has been studied by IAST analysis22 and by dynamic breakthrough experiments. Synchrotron X-ray powder diffraction has been used to determine the preferred binding sites for adsorbed CO2, SO2 and C2H2 within these materials, and synchrotron micro-IR spectroscopy confirms intermolecular interactions of adsorbed gas molecules with M–μ2O(H)–M′ (M and M′ = Al or Cr) functionalities. This study reveals new insights into the effects of partial transmetallation in isostructural MOFs on host–guest interactions and overall gas adsorption capacity.
Results and discussion
MFM-300(Cr) was synthesised by hydrothermal reaction of H4L (biphenyl-3-3′-5-5′-tetracarboxylic acid) and CrCl3·6H2O in acidic (HCl) water, and was isolated as blue, microcrystalline powder (yield = 91%). High resolution synchrotron X-ray diffraction confirms that MFM-300(Cr) possesses extended metal chains of [CrO4(OH)2] bridged by L4− and two cis-μ2-OH groups forming 1D channels that propagate along the c axis. The heterometallic MFM-300(Al1−xCrx) was synthesised via a similar method but using both Al and Cr salts in the reaction to give a light blue microcrystalline powder (yield ∼ 86%). Various ratios of Al and Cr salts were tested for synthesis, and the actual Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cr ratio within the heterometallic product was determined by ICP-OES analysis (see ESI†). Among all the obtained materials with different ratios, MFM-300(Al0.67Cr0.33) shows the best performance in terms of gas sorption. MFM-300(Al0.67Cr0.33) is isostructural to the parent complexes MFM-300(M) (M = Al, Cr) (Table S3†), with M–OH bond distances of 1.930(1) Å,1 1.953(1) Å and 1.868(5) Å in MFM-300(Al), MFM-300(Cr) and MFM-300(Al0.67Cr0.33), respectively. The homogeneity of the distribution of Cr and Al in MFM-300(Al0.67Cr0.33) has been studied by synchrotron FT-IR experiments. Three bands for ν(OH) are observed at 3690, 3672, and 3641 cm−1 assigned to Al–(OH)–Al, Al–(OH)–Cr and Cr–(OH)–Cr moieties, respectively, thus demonstrating a homogeneous distribution of M(III) centres (Fig. 1d). The ratio of Al
Cr ratio within the heterometallic product was determined by ICP-OES analysis (see ESI†). Among all the obtained materials with different ratios, MFM-300(Al0.67Cr0.33) shows the best performance in terms of gas sorption. MFM-300(Al0.67Cr0.33) is isostructural to the parent complexes MFM-300(M) (M = Al, Cr) (Table S3†), with M–OH bond distances of 1.930(1) Å,1 1.953(1) Å and 1.868(5) Å in MFM-300(Al), MFM-300(Cr) and MFM-300(Al0.67Cr0.33), respectively. The homogeneity of the distribution of Cr and Al in MFM-300(Al0.67Cr0.33) has been studied by synchrotron FT-IR experiments. Three bands for ν(OH) are observed at 3690, 3672, and 3641 cm−1 assigned to Al–(OH)–Al, Al–(OH)–Cr and Cr–(OH)–Cr moieties, respectively, thus demonstrating a homogeneous distribution of M(III) centres (Fig. 1d). The ratio of Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cr is further confirmed by TGA (Fig. S1†).
Cr is further confirmed by TGA (Fig. S1†).
Desolvated MFM-300(Cr) and MFM-300(Al0.67Cr0.33) show similar BET surface areas of 1360 and 1305 m2 g−1, respectively, and adsorption isotherms for CO2, C2H2 and SO2 were measured for these materials. At 273 K and 1.0 bar, the total gas uptakes for MFM-300(Al), MFM-300(Cr) and MFM-300(Al0.67Cr0.33) are 7.00, 8.48 and 7.37 mmol g−1 for CO2, 6.89, 8.67 and 7.73 mmol g−1 for C2H2, and 8.1, 10.0 and 8.59 mmol g−1 for SO2, respectively (Fig. 1a–c). Taking the difference in molecular mass per unit cell into account, the number of gas molecules per metal ion in each MOF is given Table S10.† The thermodynamic parameters Qst and ΔS were calculated using the van't Hoff isochore (Fig. S6–S11†). With CO2, C2H2 and SO2 loadings between 0.5–4.0 mmol g−1, the values of Qst are 25.5–28.6, 35.0–42.8 and 39.3–46.0 kJ mol−1 for MFM-300(Cr); and 26.4–29.3, 31.5–32.0 and 43.5–54.6 kJ mol−1 for MFM-300(Al0.67Cr0.33), which are similar to those of MFM-300(Al).1,2 Ten cycles of adsorption/desorption of SO2 were conducted for all three materials at 298 K between 0–0.1 bar, and all maintained their full adsorption capacity, confirming their excellent stability under these conditions (Fig. 2a–c). Throughout the cycles, MFM-300(Al) and MFM-300(Al0.67Cr0.33) were found to retain SO2 upon desorption under dynamic vacuum at 298 K (22% and 5% SO2 retained, respectively), and require elevated temperature to fully remove SO2. In contrast, MFM-300(Cr) shows negligible retention of SO2 (<0.4% or <0.05 mmol g−1) under dynamic vacuum at ambient temperature. Combined with its high structural stability and adsorption capacity, MFM-300(Cr) offers a regenerable platform for SO2 capture of relevance to flue gas desulfurization.23–25 We further explored these MOFs for the separation of SO2 from CO2, as SO2 is an important flue gas impurity in CO2 streams and can lead to numerous operational problems in carbon separation and geological sequestration.26,27 IAST selectivities of SO2/CO2 (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95) were calculated for these MOFs from pure component isotherms between 0–1 bar at 273 K (Fig. 2d). Notably, MFM-300(Cr) shows the highest SO2/CO2 selectivity of 79 at 273 K and 1 bar compared to 56 for MFM-300(Al0.67Cr0.33) and 45 for MFM-300(Al). Dynamic breakthrough experiments with 0.4% SO2 and 16% CO2 in He also confirmed the selective retention of low concentrations of SO2 with MFM-300(Cr) and MFM-300(Al0.67Cr0.33) under flow conditions (Fig. 2e and f). In both cases, CO2 eluted first and saturated rapidly [t = 19 and 21 min g−1 for MFM-300(Al0.67Cr0.33) and MFM-300(Cr), respectively], whereas SO2 starts to elute at t = 56 and 76 min g−1, respectively, and shows a steadier breakthrough curve.
95) were calculated for these MOFs from pure component isotherms between 0–1 bar at 273 K (Fig. 2d). Notably, MFM-300(Cr) shows the highest SO2/CO2 selectivity of 79 at 273 K and 1 bar compared to 56 for MFM-300(Al0.67Cr0.33) and 45 for MFM-300(Al). Dynamic breakthrough experiments with 0.4% SO2 and 16% CO2 in He also confirmed the selective retention of low concentrations of SO2 with MFM-300(Cr) and MFM-300(Al0.67Cr0.33) under flow conditions (Fig. 2e and f). In both cases, CO2 eluted first and saturated rapidly [t = 19 and 21 min g−1 for MFM-300(Al0.67Cr0.33) and MFM-300(Cr), respectively], whereas SO2 starts to elute at t = 56 and 76 min g−1, respectively, and shows a steadier breakthrough curve.
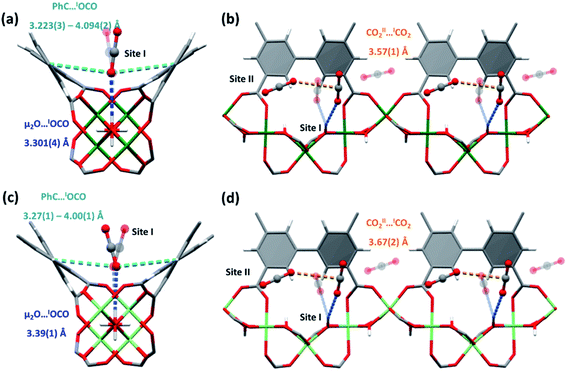
To gain deeper understanding of the host–guest interactions underpinning these processes and to rationalise the observed gas selectivities, in situ high resolution synchrotron PXRD has been used to determine the preferred binding sites for CO2, SO2 and C2H2 molecules within MFM-300(Cr) and MFM-300(Al0.67Cr0.33) via Rietveld refinement (Fig. 3–5; Tables S1 and S2†). For CO2, the primary site of adsorption, CO2I, is bound to the μ2-OH group of the hydroxyl-metal chain, Oμ2-OH⋯OCO2 = 3.301(4), 3.39(1), 3.21(3) Å for MFM-300(Cr), MFM-300(Al0.67Cr0.33) and MFM-300(Al),2 respectively, and is disordered by a mirror plane that dissects the μ2-OH (Fig. 4).28,29 CO2I is further enclosed by aromatic C–H groups of the biphenyl core of the linker and forms additional supramolecular interactions [OCO2I⋯CAromatic = 3.223(3)–4.094(2) Å in MFM-300(Cr) and 3.27(1)–4.00(1) Å in MFM-300(Al0.67Cr0.33)]. The second site, CO2II, is positioned near-perpendicular to CO2I with full occupancy. The CCO2I⋯OCO2II distances in MFM-300(Cr), MFM-300(Al0.67Cr0.33) and MFM-300(Al) are 3.57(1), 3.67(2) and 3.92(1) Å, respectively, suggesting a more compact packing of CO2 in MFM-300(Cr).
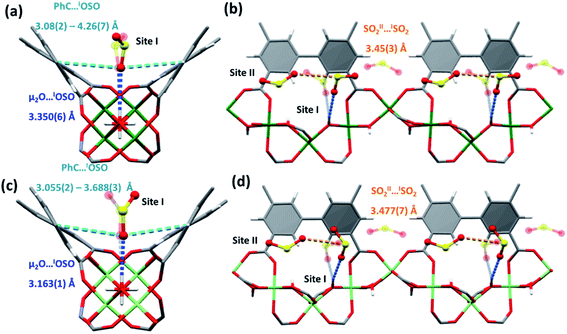
For SO2, two adsorption sites were observed in both MFM-300(Cr) and MFM-300(Al0.67Cr0.33) (Fig. 4). As for CO2, the primary SO2 site, SO2I, is located with an end-on mode to the bridging μ2-OH group, Oμ2-OH⋯OSO2 = 3.350(6), 3.163(1) and 3.201(6) Å in MFM-300(Cr), MFM-300(Al0.67Cr0.33) and MFM-300(Al) respectively. SO2I also interacts with the surrounding linker moieties via van der Waals interactions [OSO2I⋯CAromatic = 3.08(2)–4.26(7) Å in MFM-300(Cr) and 3.055(2)–3.688(3) Å in MFM-300(Al0.67Cr0.33)]. SO2 at site II is located perpendicularly to SO2I with the SSO2I⋯OSO2II distances being 3.45(3), 3.477(7) and 3.34(7) Å for MFM-300(Cr), MFM-300(Al0.67Cr0.33) and MFM-300(Al), respectively. SO2II also shows weak interactions with the phenyl ring of linker with SSO2⋯CAromatic = 3.53(1) and 3.445(3) Å, for MFM-300(Cr) and MFM-300(Al0.67Cr0.33), respectively.
As the bonding distances usually reflect the strength of the host–guest and guest–guest interactions within these system, which often influences the gas selectivities, a summary of bonding distances in CO2 and SO2-loaded MFM-300(Cr), MFM-300(Al0.67Cr0.33) and MFM-300(Al) is shown in Table 1. When comparing the two single-metal MOFs, MFM-300(Cr) shows weaker bonding interaction to both CO2 and SO2 (longer bonding distances) than MFM-300(Al). This reconciles the observed differences in SO2 residues within these two materials upon desorption at ambient temperature. However, the strong interactions with both CO2 and SO2 means that MFM-300(Al) achieves a lower IAST selectivity. The hetero-metallic MFM-300(Al0.67Cr0.33) exhibits the weakest binding to CO2 and the strongest binding to SO2 among these three MOFs. However, the IAST selectivity of SO2/CO2 in MFM-300(Al0.67Cr0.33) falls in between that of the two single-metal MOFs. This strongly suggests that the host–guest bonding distance is not the sole factor to affect the observed selectivity, which will also be influenced by guest–guest interactions. Also, MFM-300(Al0.67Cr0.33) has a slightly enlarged pore diameter compared to MFM-300(Al) due to doping with Cr(III), which also likely contributes to the slightly higher gas uptake. In addition, doping MFM-300(Al) with Cr(III) may well form defects leading to increased porosity and additional binding and interaction sites. CO2 exhibits most compact packing in MFM-300(Cr) (shorter CO2⋯CO2 distance), whereas SO2 packs most tightly in MFM-300(Al).
| Bonding distance (Å) | MFM-300(Cr) | MFM-300(Al0.67Cr0.33) | MFM-300(Al) |
|---|---|---|---|
| Oμ2-OH⋯OCO2 | 3.301(4) | 3.39(1) | 3.21(3) |
| Oμ2-OH⋯OSO2 | 3.350(6) | 3.163(1) | 3.201(6) |
| OCO2I⋯OCO2II | 3.57(1) | 3.67(2) | 3.92(1) |
| OSO2⋯OSO2II | 3.45(3) | 3.477(7) | 3.34(7) |
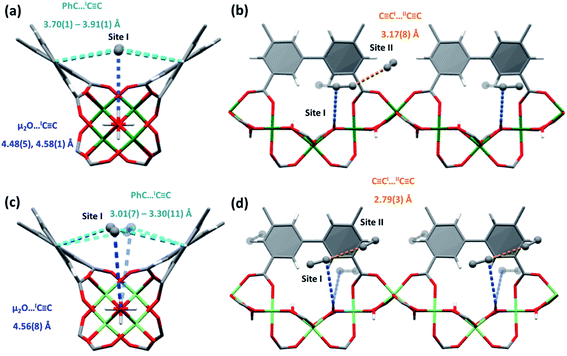
For C2H2, two distinct positions of C2H2 were observed with both MFM-300(Cr) and MFM-300(Al0.67Cr0.33) (Fig. 5). C2H2I interacts with the bridging hydroxyl group of MFM-300(Cr) in a side-on manner, and in MFM-300(Al0.67Cr0.33), C2H2I is slightly off-perpendicular. In both cases, the interactions between the H atom of the hydroxyl to the electron rich π C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C in C2H2 are weaker than that of CO2 and SO2 systems, with longer bonding distances being observed [HO⋯CC2H2I = 4.48(5)–4.58(1) Å and 4.56(8) Å in MFM-300(Cr) and MFM-300(Al0.67Cr0.33), respectively]. C2H2I is further anchored within a pocket through π⋯π stacking interactions with the adjacent phenyl core of the linkers [CC2H2I⋯CAromatic = 3.51(2), 3.73(2), 3.91(1) Å in MFM-300(Cr) and 3.01(7), 3.30(11) Å in MFM-300(Al0.67Cr0.33)]. C2H2II interacts via dipole interactions with C2H2I in a T-shape orientation in MFM-300(Cr) [CC2H2I⋯CC2H2II = 3.17(8) Å], and is more skewed in MFM-300(Al0.67Cr0.33) but with shorter intermolecular distances [CC2H2I⋯CC2H2II = 2.79(3) Å]. The position of C2H2II is further stabilised by weak van der Waals interactions with the phenyl core of the adjacent linker moieties [CC2H2II⋯CAromatic = 3.97(4), 4.35(7) Å and 3.29(13), 3.52(19) Å for MFM-300(Cr) and MFM-300(Al0.67Cr0.33), respectively]. Thus, both MOFs have strong affinity to C2H2 through the combined numerous weak interactions which leads to the efficient packing of C2H2 within the pores and high C2H2 uptake.
C in C2H2 are weaker than that of CO2 and SO2 systems, with longer bonding distances being observed [HO⋯CC2H2I = 4.48(5)–4.58(1) Å and 4.56(8) Å in MFM-300(Cr) and MFM-300(Al0.67Cr0.33), respectively]. C2H2I is further anchored within a pocket through π⋯π stacking interactions with the adjacent phenyl core of the linkers [CC2H2I⋯CAromatic = 3.51(2), 3.73(2), 3.91(1) Å in MFM-300(Cr) and 3.01(7), 3.30(11) Å in MFM-300(Al0.67Cr0.33)]. C2H2II interacts via dipole interactions with C2H2I in a T-shape orientation in MFM-300(Cr) [CC2H2I⋯CC2H2II = 3.17(8) Å], and is more skewed in MFM-300(Al0.67Cr0.33) but with shorter intermolecular distances [CC2H2I⋯CC2H2II = 2.79(3) Å]. The position of C2H2II is further stabilised by weak van der Waals interactions with the phenyl core of the adjacent linker moieties [CC2H2II⋯CAromatic = 3.97(4), 4.35(7) Å and 3.29(13), 3.52(19) Å for MFM-300(Cr) and MFM-300(Al0.67Cr0.33), respectively]. Thus, both MOFs have strong affinity to C2H2 through the combined numerous weak interactions which leads to the efficient packing of C2H2 within the pores and high C2H2 uptake.
To study the dynamics of host–guest binding, in situ synchrotron micro-IR spectroscopy was undertaken on MFM-300(Al), MFM-300(Cr) and MFM-300(Al0.67Cr0.33) as a function of CO2 loading (Fig. 6). Upon activation under a flow of He, bands for the ν(OH) stretching modes were observed at 3690 cm−1 and 3640 cm−1 for MFM-300(Al) and MFM-300(Cr), respectively. In MFM-300(Al), upon increasing the CO2 partial pressures to 1.0 bar, the Al–O(H)–Al band centred at 3690 cm−1 gradually decreases in intensity with a new band growing at 3683 cm−1 which is observable from 60% CO2 loading. This new peak continues to increase in intensity on additional CO2 loading. The redshift of 7 cm−1 is consistent with the μ2-OH site being increasingly occupied by CO2 and is consistent with the crystallographic study. In comparison, the μ2-OH peak observed at 3640 cm−1 in MFM-300(Cr) undergoes a redshift of 2 cm−1 upon increasing CO2 loading and a significant increase in absorbance intensity. For MFM-300(Al0.67Cr0.33), distinct absorbance bands for the different ν(OH) stretching modes were observed at 3692, 3672 and 3644 cm−1 for Al–O(H)–Al, Al–O(H)–Cr and Cr–O(H)–Cr modes, respectively. Upon increasing CO2 partial pressures to 1 bar, the Al–OH–Al band centred at 3692 cm−1 decreases in intensity and a new band at 3683 cm−1 emerges. This indicates that the Al–O(H)–Al band is significantly affected by the presence of CO2, suggesting a partial depletion of Al–O(H)–Al moieties in the material and CO2 binding to this moiety. The same is observed for Al–O(H)–Cr with a redshift of 7 cm−1 to 3665 cm−1. A shift of 2 cm−1 from 3644 to 3642 cm−1 is observed in the Cr–O(H)–Cr mode along with a broadening of the peak. All ν(OH) stretching modes were found to shift to lower frequencies upon increasing CO2 loadings which suggests a weakening of the O–H bond in the metal-hydroxyl moiety consistent with the formation of μ2-OH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O binding site.
O binding site.
 | ||
| Fig. 6 FTIR spectra of the ν(μ2-OH) stretch region of (a) MFM-300(Al), (b) MFM-300(Cr), and (c) MFM-300(Al0.67Cr0.33) upon increasing CO2 loadings from 0 to 100%. | ||
Conclusion
Stable MOFs show increasing promise in the application of capture of toxic gases.23 The binding domains for CO2, SO2 and C2H2 and their host–guest binding dynamics have been studied in a family of three iso-structural MOFs, MFM-300(Al1−xCrx) (x = 0, 0.33, 1) by in situ synchrotron X-ray diffraction and IR micro-spectroscopy. Both MFM-300(Al0.67Cr0.33) and MFM-300(Cr) show enhanced CO2, C2H2 and SO2 adsorption uptake than MFM-300(Al). MFM-300(Al0.67Cr0.33) exhibits the highest number of CO2, C2H2 and SO2 molecules per metal compared with the homo-metallic analogues, which is likely due to the complex distribution of –OH sites within the pores and the formation of defects via doping of MFM-300(Al) with Cr(III) leading to increased porosity and additional binding and interaction sites. MFM-300(Cr) shows the highest SO2/CO2 IAST selectivity, which has also been confirmed by breakthrough experiments. MFM-300(Cr) also promises excellent SO2 regenerability as confirmed by cycling measurements, demonstrating its potential for selective removal of SO2.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
We thank EPSRC (EP/I011870) for support. This project has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement No 742401, NANOCHEM). We thank the Royal Society, Peking University and Universities of Nottingham and Manchester for funding. We thank Diamond Light Source for access to Beamlines B22 and I11.Notes and references
- S. Yang, A. J. Ramirez-Cuesta, R. Newby, V. Garcia-Sakai, P. Manuel, S. K. Callear, S. I. Campbell, C. C. Tang and M. Schröder, Nat. Chem., 2015, 7, 121–129 CrossRef CAS.
- S. Yang, J. Sun, A. J. Ramirez-Cuesta, S. K. Callear, W. I. F. David, D. P. Anderson, R. Newby, A. J. Blake, J. E. Parker, C. C. Tang and M. Schröder, Nat. Chem., 2012, 4, 887–894 CrossRef CAS.
- J. Yang, X. Yan, T. Xue and Y. Liu, RSC Adv., 2016, 6, 55266–55271 RSC.
- G. E. Cmarik, M. Kim, S. M. Cohen and K. S. Walton, Langmuir, 2012, 28, 15606–15613 CrossRef CAS.
- R. Vaidhyanathan, S. S. Iremonger, G. K. H. Shimizu, P. G. Boyd, S. Alavi and T. K. Woo, Science, 2010, 330, 650–653 CrossRef CAS.
- S. Noro and T. Nakamura, NPG Asia Mater., 2017, 9, e433 CrossRef.
- H. Deng, C. J. Doonan, H. Furukawa, R. B. Ferreira, J. Towne, C. B. Knobler, B. Wang and O. M. Yaghi, Science, 2010, 327, 846–850 CrossRef CAS.
- Y. Yuan, J. Li, X. Sun, G. Li, Y. Liu, G. Verma and S. Ma, Chem. Mater., 2019, 31, 1084–1091 CrossRef CAS.
- X. Lin, I. Telepeni, A. J. Blake, A. Dailly, C. M. Brown, J. M. Simmons, M. Zoppi, G. S. Walker, K. M. Thomas, T. J. Mays, P. Hubberstey, N. R. Champness and M. Schröder, J. Am. Chem. Soc., 2009, 131, 2159–2171 CrossRef CAS.
- J. Zhang, S. Yao, S. Liu, B. Liu, X. Sun, B. Zheng, G. Li, Y. Li, Q. Huo and Y. Liu, Cryst. Growth Des., 2017, 17, 2131–2139 CrossRef CAS.
- Y. P. He, Y. X. Tan and J. Zhang, Cryst. Growth Des., 2013, 13, 6–9 CrossRef CAS.
- T. L. Easun, F. Moreau, Y. Yan, S. Yang and M. Schröder, Chem. Soc. Rev., 2017, 46, 239–274 RSC.
- T. Islamoglu, D. Ray, P. Li, M. B. Majewski, I. Akpinar, X. Zhang, C. J. Cramer, L. Gagliardi and O. K. Farha, Inorg. Chem., 2018, 57, 13246–13251 CrossRef CAS.
- Q. Guo, L. Ren, P. Kumar, V. J. Cybulskis, K. A. Mkhoyan, M. E. Davis and M. Tsapatsis, Angew. Chem., Int. Ed., 2018, 57, 4926–4930 CrossRef CAS.
- G. Ferey, C. Mellot-Draznieks, C. Serre and F. Millange, Science, 2005, 309, 2040–2042 CrossRef CAS.
- Y. K. Hwang, D. Y. Hong, J. S. Chang, S. H. Jhung, Y. K. Seo, J. Kim, A. Vimont, M. Daturi, C. Serre and G. Férey, Angew. Chem., Int. Ed., 2008, 47, 4144–4148 CrossRef CAS.
- A. Herbst, A. Khutia and C. Janiak, Inorg. Chem., 2014, 53, 7319–7333 CrossRef CAS.
- X. Lian, D. Feng, Y.-P. Chen and H. Zhou, Chem. Sci., 2015, 6, 7044–7048 RSC.
- I. J. Kang, N. A. Khan, E. Haque and S. H. Jhung, Chem.–Eur. J., 2011, 17, 6437–6442 CrossRef CAS.
- D. Sun, F. Sun, X. Deng and Z. Li, Inorg. Chem., 2015, 54, 8639–8643 CrossRef CAS.
- C. P. Krap, S. Yang, R. Newby, A. Dhakshinamoorthy, H. García, I. Cebula, T. L. Easun, M. Savage, J. E. Eyley, S. Gao, A. J. Blake, W. Lewis, P. H. Beton, M. R. Warren, D. R. Allan, M. D. Frogley, C. C. Tang and M. Schröder, Inorg. Chem., 2016, 55, 1076–1088 CrossRef CAS.
- J. M. P. A. L. Myers, AIChE J., 1964, 11, 121–127 CrossRef.
- X. Han, S. Yang and M. Schröder, Nat. Rev. Chem., 2019, 3, 108–118 CrossRef CAS.
- M. Savage, Y. Cheng, T. L. Easun, J. E. Eyley, S. P. Argent, M. R. Warren, W. Lewis, C. Murray, C. C. Tang, M. D. Frogley, R. T. Murden, M. J. Benham, A. N. Fitch, A. J. Blake, A. J. Ramirez-Cuesta, S. Yang and M. Schröder, Adv. Mater., 2016, 28, 8705–8711 CrossRef CAS.
- D. Z. Yang, M. Q. Hou, H. Ning, J. Ma, X. C. Kang, J. L. Zhang and B. X. Han, ChenSusChem, 2013, 7, 1191–1195 CrossRef.
- J.-Y. Lee, T. C. Keener and Y. J. Yang, J. Air Waste Manage. Assoc., 2009, 59, 725–732 CrossRef CAS.
- J. Yu and P. B. Balbuena, ACS Sustainable Chem. Eng., 2015, 3, 117–124 CrossRef CAS.
- Z. Lu, H. G. W. Godfrey, I. Silva, Y. Cheng, M. Savage, F. Tuna, E. J. L. Mcinnes, S. J. Teat, K. J. Gagnon, M. D. Frogley, P. Manuel, S. Rudic, A. J. Ramirez-Cuesta, S. Yang and M. Schröder, Nat. Commun., 2017, 8, 14212 CrossRef CAS.
- M. Savage, Y. Cheng, T. L. Easun, J. E. Eyley, S. P. Argent, M. R. Warren, W. Lewis, C. Murray, C. C. Tang, M. D. Frogley, R. T. Murden, M. J. Benham, A. N. Fitch, A. J. Blake, A. J. Ramirez-Cuesta, S. Yang and M. Schröder, Adv. Mater., 2016, 28, 8705–8711 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis procedures, characterization, and additional analysis of crystal structures. Structural data of MFM-300(Al1−xCrx) (x = 0, 0.33, 1) derived from powder X-ray diffraction. CCDC 1952013, 1952268, 1952287 and 1952276 for activated MFM-300(Al0.67Cr0.33), CO2-, C2H2- and SO2-loaded structures, respectively, and 1952277, 1952321, 1952320 and 1952280 for activated MFM-300(Cr), CO2-, C2H2-, and SO2-loaded structures, respectively. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1ta00687h |
| ‡ These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2021 |