Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Interfacial behavior of the decane + brine + surfactant system in the presence of carbon dioxide, methane, and their mixture†
Nilesh
Choudhary
 ,
Arun Kumar
Narayanan Nair
,
Arun Kumar
Narayanan Nair
 * and
Shuyu
Sun
*
* and
Shuyu
Sun
*
Physical Science and Engineering Division (PSE), Computational Transport Phenomena Laboratory, King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900, Saudi Arabia. E-mail: arun.narayanannair@kaust.edu.sa; shuyu.sun@kaust.edu.sa
First published on 4th November 2021
Abstract
Molecular dynamics simulations are carried out to get insights into the interfacial behavior of the decane + brine + surfactant + CH4 + CO2 system at reservoir conditions. Our results show that the addition of CH4, CO2, and sodium dodecyl sulfate (SDS) surfactant at the interface reduces the IFTs of the decane + water and decane + brine (NaCl) systems. Here the influence of methane was found to be less pronounced than that of carbon dioxide. As expected, the addition of salt increases the IFTs of the decane + water + surfactant and decane + water + surfactant + CH4/CO2 systems. The IFTs of these surfactant-containing systems decrease with temperature and the influence of pressure is found to be less pronounced. The atomic density profiles show that the sulfate head groups of the SDS molecules penetrate the water-rich phase and their alkyl tails are stretched into the decane-rich phase. The sodium counterions of the surfactant molecules are located very close to their head groups. Furthermore, the density profiles of water and salt ions are hardly affected by the presence of the SDS molecules. However, the interfacial thickness between water and decane/CH4/CO2 molecules increases with increasing surfactant concentration. An important result is that the enrichment of CH4 and/or CO2 in the interfacial region decreases with increasing surfactant concentration. These results may be useful in the context of the water-alternating-gas approach that has been utilized during CO2-enhanced oil recovery operations.
1 Introduction
The emission of anthropogenic CO2 is one of the major causes of global climatic changes.1–4 Carbon capture and storage technology might be beneficial for mitigating these emissions. Various adsorbents (e.g., carbon nanotubes and clays)5–14 have been extensively utilized for carbon dioxide capture. In enhanced oil recovery (EOR) operations, the oil recovery could also be combined with the carbon dioxide storage.15–21 The water-alternating-gas (WAG) approach has been utilized for mobility control during CO2-EOR operations.16,18,19,21 The WAG cycles consist of injecting water (or surfactant) and CO2 alternatively into the reservoirs. Lowering the interfacial tension (IFT) of the oil + water system leads to an increase in the capillary number,17,20 which may help to recover more oil. In general, the presence of surfactant/CO2 decreased this IFT. In addition, the captured CO2 contains impurities (e.g., CH4)7,22–24 that may have an important influence on the EOR operations.Experiments,25–37 theory,32–34,36–39 and simulations38–45 have been successfully employed to understand the bulk and interfacial properties of alkane + water + CH4/CO2 systems. These studies have reported the occurrence of, for example, a two-phase region at high pressures. The IFT of the alkane + water + CH4/CO2 two-phase systems was more similar to that of the corresponding alkane + water system alone.25,35,36,38–43,45 The IFTs of the alkane + water + CH4/CO2 and alkane + brine + CH4/CO2 systems increased with decreasing xCH4/xCO2 (xCH4 and xCO2 are the mole fractions of CH4 and CO2 in the alkane-rich phase, respectively). This can be attributed to the fact that the interface was enriched with CH4 and CO2 molecules.38–43 The IFTs of the alkane + brine and alkane + brine + CH4/CO2 systems were reported to increase with increasing salt concentration.26,27,29,39,44,45 It is also known that the addition of surfactants such as sodium dodecyl sulfate (SDS) generally decreases the IFT.46–58 Molecular simulations showed that, in the water + surfactant system, water molecules and sodium counterions are relatively near the SDS headgroups.49,51–58 Bruce et al. found distortions in the water–water hydrogen bonding network because of the SDS–water hydrogen bond formations.49 Lin et al. found that, in the water + surfactant + CH4 system, the solubility of CH4 in water is not affected by the presence of SDS molecules at the interface.55 da Rocha et al. observed favorable CO2-fluorinated surfactant tail interactions at the interface in the water + surfactant + CO2 system.50 The high density of CO2 in the bulk enabled strong interaction between CO2 and the hydrocarbon tail of SDS at the interface.56,57 However, the interfacial behavior of the decane + brine + surfactant + CH4 + CO2 system has not been studied yet.
Molecular simulations have emerged as important tools for accurately predicting the bulk and interfacial properties.9,12–14,38,59–62 Here, we perform molecular dynamics (MD) simulations to get insights into the interfacial behavior of the decane + brine + surfactant system in the presence of CH4 and CO2 at reservoir conditions.
2 Simulation details
MD simulations of decane + water + surfactant and decane + brine + surfactant two-phase systems in the presence of CH4 and CO2 at 323 and 443 K, and pressure up to 100 MPa were carried out using the GROMACS package.63 The salt (NaCl) concentration is 2.7 mol kg−1 and the amounts of surfactant adsorbed at the interface are 0.008 and 0.016 SDS per Å2. The method is similar to that used previously by us.39,64,65 In short, the TraPPE force field was used to model normal decane, methane, and carbon dioxide.66–68 Water is represented by the TIP4P/2005 model69 and the Na+ and Cl− ions are described using the Smith and Dang70 parameters. As in the case of, e.g., decane, the hydrocarbon tail of SDS (C12) was also modeled using the TraPPE united atom force field. The sulfate head group of SDS was modeled using the all-atom CHARMM36 forcefield.71 The Lennard-Jones energy (ε) and distance (σ) parameters, and the charges (q) of the SDS molecules are given in Table 1. The number of each species employed in our simulations is given in Table 2. For example, all the systems had 2048 water and 200 decane molecules. Also, all the systems had the dimensions of 36 × 36 Å parallel to the interfaces (Fig. 1). The cell size in the z-direction (perpendicular to the interfaces) Lz was about three times this value. The system sizes used here ensure that the finite-size effects are negligible.38,40–43,72–74 Each system was equilibrated for 5 ns in the NPT ensemble (only Lz varied) and we ran a 5 ns production under NVE conditions. The temperature was controlled using the Nosé–Hoover thermostat and pressure using the Parrinello–Rahman barostat.| System | Water no. | Decane no. | CH4 no. | CO2 no. | Na+/Cl− no. | SDS no. |
|---|---|---|---|---|---|---|
| Water + decane + SDS | 2048 | 200 | 20–40 | |||
| Brine + decane + SDS | 2048 | 200 | 100 | 20–40 | ||
| Water + 50%decane + 50%CH4 + SDS | 2048 | 200 | 200 | 20–40 | ||
| Brine + 50%decane + 50%CH4 + SDS | 2048 | 200 | 200 | 100 | 20–40 | |
| Water + 50%decane + 50%CO2 + SDS | 2048 | 200 | 200 | 20–40 | ||
| Brine + 50%decane + 50%CO2 + SDS | 2048 | 200 | 200 | 100 | 20–40 | |
| Water + 50%decane + 25%CH4 + 25%CO2 + SDS | 2048 | 200 | 100 | 100 | 20–40 | |
| Brine + 50%decane + 25%CH4 + 25%CO2 + SDS | 2048 | 200 | 100 | 100 | 100 | 20–40 |
The IFT was estimated from the below equation:38,39,64,65,72–75
 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 76,77). Note, however, that higher surfactant concentrations might be considered by using coarse-grained models.78
76,77). Note, however, that higher surfactant concentrations might be considered by using coarse-grained models.78
 | ||
| Fig. 2 IFT dependence on the surface concentration of surfactant for the (a) water + surfactant system at 298 K and (b) water + surfactant + CH4 system at 273.2 K and 7.2 MPa. | ||
3 Results
3.1 Interfacial tension
The simulated IFTs of the decane + water + surfactant and decane + brine + surfactant systems in the presence of CH4 (xCH4 = 0.5), CO2 (xCO2 = 0.5), and their equimolar mixture (xCH4 = xCO2 = 0.25) at 443 K are provided in Fig. 3. The salt (NaCl) concentration was 2.7 mol kg−1 and the amounts of surfactant adsorbed at the interface were 0.008 and 0.016 SDS per Å2. The corresponding simulation results at 323 K are provided in Fig. S1 (ESI†). Note that our previous results of the simulated IFTs of the decane + water38 and decane + brine (NaCl)39 systems in the presence of CH4, CO2, and their mixture compared well with the corresponding experimental25,35 and density gradient theory (DGT)38,39 results. Past studies have shown that the IFT of the alkane + water + CH4/CO2 systems is more similar to that of the corresponding alkane + water system alone.25,35,36,38–43,45 Also, the IFT of the decane + brine + CH4/CO2 systems was more similar to that of the corresponding decane + brine system alone.26,29,39,44,45 The IFTs of all these systems decreased with temperature. For instance, the simulation value of the IFT of the decane + water system (20 MPa) decreased from about 47.8 mN m−1 at 323 K to about 36.5 mN m−1 at 443 K.39 It was found that the IFTs of these systems generally increased with pressure. The influence of pressure was, however, found to be less pronounced at lower temperatures and higher values of xCH4/xCO2. Note that the IFT of the water + CH4/CO2 and brine + CH4/CO2 systems depends nonmonotonically on pressure.72–75,79–88 Furthermore, the IFTs of the alkane + water + CH4/CO2 and alkane + brine + CH4/CO2 systems increased with decreasing xCH4/xCO2. Here, the influence of methane was found to be less pronounced than that of carbon dioxide. This can be attributed to the fact that the interface was highly enriched with CO2 molecules than with methane molecules. It was found that the IFTs of the alkane + brine and alkane + brine + CH4/CO2 systems increase with salt concentration.26,27,29,39,44,45 Here, the linear slope in the IFT versus salt (NaCl) concentration plot was about 2 mN (m mol kg−1)−1 under all conditions. This value was similar to that reported for the brine + CH4/CO2 systems.72,73,79 However, higher slopes were reported for the corresponding systems containing divalent ions.73,79Previous studies47,55 have also shown that the IFTs decreased with increasing SDS concentration, e.g., at the methane/water interface (see Fig. 2). Our current results show that the addition of the SDS surfactant reduces the IFTs of the decane + water and decane + water + CH4/CO2 systems. Similar behavior is also observed for the decane + brine and decane + brine + CH4/CO2 systems. For example, the IFT of the decane + water + surfactant (0.016 SDS per Å2) system is about 16.5 mN m−1 at 20 MPa and 443 K. As in the case of, for example, the alkane + water system, the IFTs of these surfactant containing systems decrease with temperature and the influence of pressure is found to be less pronounced. We see that the IFTs of the decane + water + surfactant + CH4/CO2 and decane + brine + surfactant + CH4/CO2 systems decrease with increasing xCH4/xCO2. Again the influence of methane is found to be less pronounced than that of carbon dioxide. For example, the IFTs of the decane + water + surfactant (0.016 SDS per Å2) system in the presence of CH4 (xCH4 = 0.5) and CO2 (xCO2 = 0.5) were about 13.7 and 11.5 mN m−1, respectively, at 20 MPa and 443 K. It can be seen that the IFT of, for instance, the decane + water + surfactant + CH4 + CO2 system is more similar to that of the corresponding decane + water + surfactant + CO2 system. Furthermore, the addition of salt increased the IFTs of the decane + water + surfactant and decane + water + surfactant + CH4/CO2 systems. For example, the IFT of the decane + brine + surfactant (0.016 SDS per Å2) system is about 25.2 mN m−1 at 20 MPa and 443 K.
3.2 Atomic density profiles
The atomic density profiles may provide insights into the bulk and interfacial properties of the studied systems. The simulated density profiles for the decane + brine + surfactant + CH4 + CO2 (xCH4 = xCO2 = 0.25) system at 443 K and 20 MPa are provided in Fig. 4. These profiles at other conditions are provided in Fig. S2–S4 (ESI†). Note that our simulation results of the atomic density profiles for the decane + water38 and decane + brine (NaCl)39 systems in the presence of CH4, CO2, and their mixture compared well with the corresponding DGT38,39 results. Previous studies of the alkane + water + CH4/CO2 and alkane + brine + CH4/CO2 systems36,38–43,45 have shown that the presence of CH4 and CO2 hardly affects the density profiles of water, alkane, and salt. It was found that, in general, the density profiles of water and alkane vary monotonically across the interfacial region. These profiles might be approximated by a hyperbolic tangent function.89 The salt ions were excluded from the interfacial region and distributed homogenously within the H2O-rich phase. However, water is enriched at the interfacial region with the addition of salt.39,73,86,90 This can be explained by the enhanced ionic desorption from the interfacial region at high salt concentrations. Also, at high xCH4, the interfacial region was enriched with decane in the alkane + water + CH4 system.38 It is worth noting that the simulated distributions of alkane exhibited artificial oscillations in the interfacial region due to finite-size effects.89,91 The interfacial region was enriched with CH4 and CO2 molecules for the alkane + water + CH4/CO2 and alkane + brine + CH4/CO2 systems.36,38–43,45 This enrichment was found to decrease with temperature and increase with pressure. Also, in general, this enrichment increased with increasing xCH4/xCO2. Here, the enrichment of methane was found to be less pronounced than that of carbon dioxide. The presence of salt had no significant effect on the CH4/CO2 enrichment.39 At low pressures, however, the enrichment of the interfacial region with CO2 (CH4) depends nonmonotonically on xCO2 (xCH4).38,40 Details of the interfacial behavior of the water + CH4/CO2 and brine + CH4/CO2 systems have been described by us and others.72–75,80–84,86–88 Here the enrichment of the interfacial region with CH4 and CO2 depends nonmonotonically on pressure.Note that the behavior of the IFT can be further understood by means of the Gibbs adsorption equation:
 | (2) |
Our current results show that the sulfate head groups (see, e.g., sulfur atoms) of the SDS surfactant molecules penetrate the water-rich phase and their alkyl tails are stretched into the decane-rich phase. The Na+ counterions of the SDS surfactant molecules are located very close to their head groups (see, e.g., Fig. S2, ESI†). Furthermore, the distributions of water and Cl− ions are independent of the surfactant concentration for the studied systems (see also Fig. S5, ESI†). However, the interfacial thickness between water and decane/CH4/CO2 molecules increases with increasing surfactant concentration. We estimated the interfacial thickness between water and decane by applying the “90–90” interfacial thickness criterion (distance between positions where densities of decane and H2O were 90% of their own bulk densities).51 The results are provided in Fig. S6–S9 (ESI†). The interfacial thickness between water and decane is in the range of about 1.1–1.7 nm for the studied systems. This interfacial thickness shows an opposite trend to that seen for the IFT. For example, the interfacial thickness decreases with the addition of salt and increases with the addition of CH4/CO2. Interestingly, the enrichment of CH4 and/or CO2 in the interfacial region decreases with increasing surfactant concentration. It seems that the enrichment is followed by a minimum in the density profile of CO2 at high surfactant concentrations. This minimum is found near the location of the surfactant tails.
We calculated the surface excess38,39,64,65,73,74,84,85 by using these density profiles. Our results show that the surface excess of CH4, CO2, and decane decreases with increasing surfactant concentration (Fig. 5). The surface excess of CH4/CO2 changes sign from positive to negative as surfactant concentration increases. The addition of SDS surfactants has a more pronounced effect on the surface excess of decane. For example, at 443K and 20 MPa, the surface excess of decane in the decane + brine + CH4 + CO239 and decane + brine + surfactant + CH4 + CO2 (0.016 SDS per Å2) systems is about −0.47 × 10−6 mol m−2 and −2.7 × 10−6 mol m−2, respectively. The corresponding surface excess of CH4 is about 0.19 × 10−6 mol m−2 and − 0.73 × 10−6 mol m−2, respectively, and that of CO2 is about 0.57 × 10−6 mol m−2 and −0.03 × 10−6 mol m−2, respectively. Here the effects of pressure, temperature, and mole fraction on the surface excess are similar to those observed for the decane + water + CH4/CO2 and decane + brine + CH4/CO2 systems.38,39 For example in all cases, we see that the surface excess of decane decreases with pressure, whereas the surface excess of CH4 and CO2 increases with pressure.
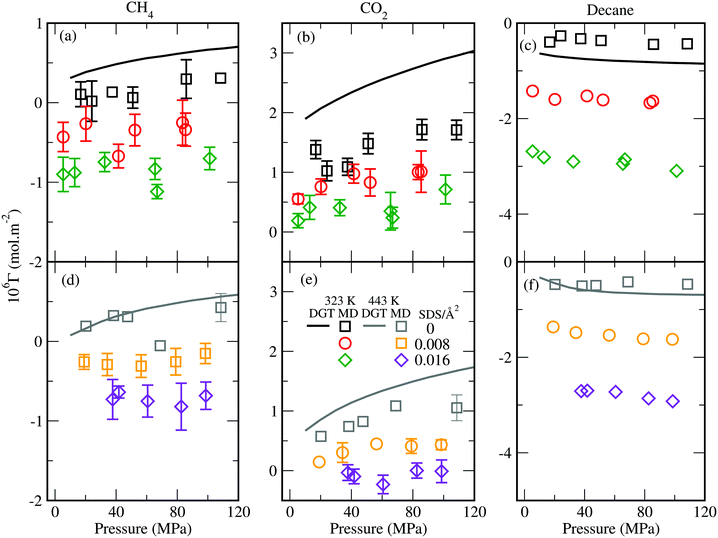 | ||
| Fig. 5 Simulated surface excess (symbols) of (a) CH4, (b) CO2, and (c) decane for the decane + brine + surfactant + CH4 + CO2 (xCH4 = xCO2 = 0.25) system at 323 K and NaCl concentration of 2.7 mol kg−1. The corresponding surface excess at 443 K is shown in (d), (e), and (f), respectively. The lines denote the DGT results.39 | ||
3.3 Radial distribution functions
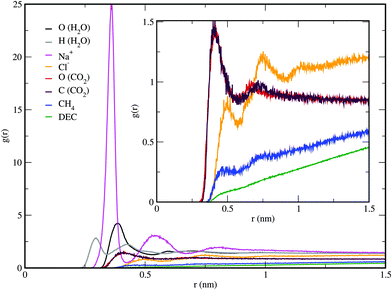
The RDFs may give further insights into the interfacial properties of the surfactant-containing systems. The simulated RDFs for the decane + brine + surfactant + CH4 + CO2 system at 443 K and 20 MPa are provided in Fig. 6. We see that water molecules and counterions are nearest to the SDS headgroups. The first peak in the RDF of S–H (water), S–Na+, and S–O (water) is around 0.29, 0.36, and 0.38 nm, respectively. These peak positions obtained here are consistent with the previous simulation results.52–54,58 The presence of water molecules near the SDS headgroups is possibly due to the hydrogen bonding between SDS headgroups and water molecules.49 Whereas, CO2, CH4, decane, and Cl− are further away from the SDS headgroups. The first peak in the RDF of S–O (CO2), S–CH4 site, and S–Cl− is around 0.40, 0.46, and 0.49 nm, respectively. The RDFs show that the interactions of the surfactant headgroups with methane are weaker than those with CO2. This is expected due to the quadrupole moment of CO2 molecule.94 Similar positions of these peaks were found in all studied systems. It is worth mentioning that here the RDFs may not go to unity at large distances due to the inhomogeneous nature of the system.52–54,58 In comparison, the alkyl tails of SDS interact strongly with decane molecules leading to peaks, for example, at 0.55 nm (Fig. S10, ESI†). Furthermore, it is found that the alkyl tails of SDS interact similarly with CH4 and CO2. For both these interactions the first peak in the RDF plot is around 0.47 nm and its magnitude is about 1.7. A similar result is obtained for the interactions between alkane and CH4/CO2.64,65We calculated the end-to-end distance and radius of gyration62,64,65 of the alkyl tails of SDS molecules for the decane + water + surfactant system (Table 3). Here the end-to-end distance is in the range of about 1.12 to 1.18 nm. This end-to-end distance decreases with temperature and increases with the amount of surfactant. We found that pressure has no effect on the end-to-end distance. Similar trends were observed for the radius of gyration. Furthermore, these sizes were not affected by the presence of salt/CH4/CO2 under the studied conditions.
| No. of SDS per Å2 | End to end distance/radius of gyration (nm) | |
|---|---|---|
| 323 K | 443 K | |
| 0.008 | 1.16/0.394 | 1.12/0.383 |
| 0.016 | 1.18/0.396 | 1.14/0.385 |
Regarding the SDS force field (see Table 1), it was shown that the difference among the force fields does not have much effect on the overall structure of small aggregates of surfactant.54 Among different water models, the surface tension simulated using TIP4P/2005 model was close to the experimental data.72 Moreover, our simulated RDF peaks for SDS–water spatial correlations are consistent with previous studies.52–54,58 It will be challenging to obtain the relevant activity coefficients of, e.g., salt from molecular simulations for these multi-component systems.95 However, for decane + brine + CH4/CO2 systems, theoretical analysis showed that changing salt concentration does not have much effect on the chemical potential of decane, CH4, and CO2.38,39 This explains the fact that the IFTs increased with salt concentration because of the negative surface excess of salt ions (see eqn (2)).38,39 Note that we did not consider any SDS in the bulk and the salting-out of SDS to the interface.48 Therefore, further studies are necessary to fully understand the effects of surfactants on the decane + brine + CH4/CO2 systems.
4 Conclusions
The interfacial behavior of the decane + brine + surfactant + CH4 + CO2 two-phase system was studied using MD simulations at 323 and 443 K, and pressure up to 100 MPa. Note that our previous results38,39 of the simulated IFTs of decane + brine + CH4/CO2 systems compared well with the corresponding experimental25,35 and DGT38,39 results. Our current results show that the addition of CH4, CO2, and the presence of SDS surfactant at the interface reduced the IFTs of the decane + water and decane + brine (NaCl) systems. Notably, the influence of methane was observed to be less pronounced than that of carbon dioxide. For example, the IFT of the decane + water + SDS + CH4 + CO2 (xCH4 = xCO2= 0.25) system is more similar to that of the corresponding decane + water + SDS + CO2 (xCO2= 0.5) system. This may be attributed to the fact that the interface was highly enriched with CO2 molecules than with CH4 molecules. As expected, at a fixed surface concentration of SDS, the addition of salt increases the IFTs of the decane + water + SDS and decane + water + SDS + CH4/CO2 systems. The IFTs of these surfactant-containing systems decreased with temperature and the influence of pressure was found to be less pronounced.The atomic density profiles show that the sulfate head groups of the SDS molecules penetrate the water-rich phase and their alkyl tails are stretched into the decane-rich phase. The Na+ counterions of the surfactant molecules are positioned very close to their head groups. Furthermore, the density profiles of water and salt ions are independent of the surfactant concentration. However, the interfacial thickness between water and decane/CH4/CO2 molecules increases with increasing surfactant concentration. For instance, the interfacial thickness between water and decane is in the range of about 1.1–1.7 nm for the studied systems. This interfacial thickness decreased with the addition of salt and increased with the addition of CH4/CO2. An important finding is that the enrichment of CH4 and/or CO2 in the interfacial region decreases with increasing surfactant concentration. The calculated RDFs show that the interactions of the surfactant headgroups with methane are weaker than those with CO2.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the support of this work by the King Abdullah University of Science and Technology (KAUST) under Award No. OSR-2019-CRG8-4074. We also thank the computational support from KAUST.References
- E. Houghton, Climate change 1995: The science of climate change: contribution of working group I to the second assessment report of the Intergovernmental Panel on Climate Change, Cambridge University Press, 1996, vol. 2 Search PubMed.
- P. M. Cox, R. A. Betts, C. D. Jones, S. A. Spall and I. J. Totterdell, Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model, Nature, 2000, 408, 184 CrossRef CAS PubMed.
- F. Birol, CO2 Emissions From Fuel Combustion Highlights, 2016 Search PubMed.
- C. J. Rhodes, The 2015 Paris climate change conference: COP21, Sci. Progr., 2016, 99, 97–104 CrossRef PubMed.
- S. Kim, J. R. Jinschek, H. Chen, D. S. Sholl and E. Marand, Scalable fabrication of carbon nanotube/polymer nanocomposite membranes for high flux gas transport, Nano Lett., 2007, 7, 2806–2811 CrossRef CAS.
- H. Yang, Z. Xu, M. Fan, R. Gupta, R. B. Slimane, A. E. Bland and I. Wright, Progress in carbon dioxide separation and capture: A review, J. Environ. Sci., 2008, 20, 14–27 CrossRef CAS.
- D. M. D'Alessandro, B. Smit and J. R. Long, Carbon dioxide capture: Prospects for new materials, Angew. Chem., Int. Ed., 2010, 49, 6058–6082 CrossRef PubMed.
- N. Rangnekar, N. Mittal, B. Elyassi, J. Caro and M. Tsapatsis, Zeolite membranes – A review and comparison with MOFs, Chem. Soc. Rev., 2015, 44, 7128–7154 RSC.
- A. Kadoura, A. K. Narayanan Nair and S. Sun, Molecular simulation study of montmorillonite in contact with variably wet supercritical carbon dioxide, J. Phys. Chem. C, 2017, 121, 6199–6208 CrossRef CAS.
- G. Kupgan, L. J. Abbott, K. E. Hart and C. M. Colina, Modeling amorphous microporous polymers for CO2 capture and separations, Chem. Rev., 2018, 118, 5488–5538 CrossRef CAS PubMed.
- V. Romanov, Greenhouse Gases and Clay Minerals: Enlightening Down-to-Earth Road Map to Basic Science of Clay-Greenhouse Gas Interfaces, Springer, 2018 Search PubMed.
- Y. Yang, A. K. Narayanan Nair and S. Sun, Adsorption and diffusion of methane and carbon dioxide in amorphous regions of cross-linked polyethylene: A molecular simulation study, Ind. Eng. Chem. Res., 2019, 58, 8426–8436 CrossRef CAS.
- Y. Yang, A. K. Narayanan Nair and S. Sun, Adsorption and diffusion of carbon dioxide, methane, and their mixture in carbon nanotubes in the presence of water, J. Phys. Chem. C, 2020, 124, 16478–16487 CrossRef CAS.
- Y. Yang, A. K. Narayanan Nair and S. Sun, Sorption and diffusion of methane and carbon dioxide in amorphous poly(alkyl acrylates): A molecular simulation study, J. Phys. Chem. B, 2020, 124, 1301–1310 CrossRef CAS PubMed.
- M. Langston S. Hoadley D. Young et al., Definitive CO2 flooding response in the SACROC unit, in SPE Enhanced Oil Recovery Symposium, 1988 Search PubMed.
- R. Farajzadeh, A. Andrianov and P. Zitha, Investigation of immiscible and miscible foam for enhancing oil recovery, Ind. Eng. Chem. Res., 2010, 49, 1910–1919 CrossRef CAS.
- C. Zhang, M. Oostrom, T. W. Wietsma, J. W. Grate and M. G. Warner, Influence of viscous and capillary forces on immiscible fluid displacement: Pore-scale experimental study in a water-wet micromodel demonstrating viscous and capillary fingering, Energy Fuels, 2011, 25, 3493–3505 CrossRef CAS.
- R. M. Enick and D. K. Olsen, Mobility and conformance control for carbon dioxide enhanced oil recovery (CO2-EOR) via thickeners, foams, and gels – A detailed literature review of 40 years of research. Contract DE-FE0004003, Activity, 2012, 4003, 01 Search PubMed.
- Z. Dai, H. Viswanathan, R. Middleton, F. Pan, W. Ampomah, C. Yang, W. Jia, T. Xiao, S.-Y. Lee and B. McPherson, et al., CO2 accounting and risk analysis for CO2 sequestration at enhanced oil recovery sites, Environ. Sci. Technol., 2016, 50, 7546–7554 CrossRef CAS PubMed.
- H. Zhang, T. Ramakrishnan, A. Nikolov and D. Wasan, Enhanced oil recovery driven by nanofilm structural disjoining pressure: Flooding experiments and microvisualization, Energy Fuels, 2016, 30, 2771–2779 CrossRef CAS.
- G. Jian, L. Zhang, C. Da, M. Puerto, K. P. Johnston, S. L. Biswal and G. J. Hirasaki, Evaluating the transport behavior of CO2 foam in the presence of crude oil under high-temperature and high-salinity conditions for carbonate reservoirs, Energy Fuels, 2019, 33, 6038–6047 CrossRef CAS.
- A. A. Olajire, CO2 capture and separation technologies for end-of-pipe applications – A review, Energy, 2010, 35, 2610–2628 CrossRef CAS.
- H. Li, J. P. Jakobsen, Ø. Wilhelmsen and J. Yan, PVTxy properties of CO2 mixtures relevant for CO2 capture, transport and storage: Review of available experimental data and theoretical models, Appl. Energy, 2011, 88, 3567–3579 CrossRef CAS.
- S. T. Blanco, C. Rivas, J. Fernandez, M. Artal and I. Velasco, Influence of methane in CO2 transport and storage for CCS technology, Environ. Sci. Technol., 2012, 46, 13016–13023 CrossRef CAS.
- H. Y. Jennings and G. H. Newman, The effect of temperature and pressure on the interfacial tension of water against methane-normal decane mixtures, Soc. Petrol. Eng. J., 1971, 11, 171–175 CrossRef CAS.
- R. Aveyard and S. M. Saleem, Interfacial tensions at alkane–aqueous electrolyte interfaces, J. Chem. Soc., Faraday Trans. 1, 1976, 1609–1617 RSC.
- N. Ikeda, M. Aratono and K. Motomura, Thermodynamic study on the adsorption of sodium chloride at the water/hexane interface, J. Colloid Interface Sci., 1992, 149, 208–215 CrossRef CAS.
- G. Brunner, J. Teich and R. Dohrn, Phase equilibria in systems containing hydrogen, carbon dioxide, water and hydrocarbons, Fluid Phase Equilib., 1994, 100, 253–268 CrossRef CAS.
- B.-Y. Cai, J.-T. Yang and T.-M. Guo, Interfacial tension of hydrocarbon + water/brine systems under high pressure, J. Chem. Eng. Data, 1996, 41, 493–496 CrossRef CAS.
- C.-Y. Sun and G.-J. Chen, Measurement of interfacial tension for the CO2 injected crude oil+ reservoir water system, J. Chem. Eng. Data, 2005, 50, 936–938 CrossRef CAS.
- D. Yang, P. Tontiwachwuthikul and Y. Gu, Interfacial tensions of the crude oil + reservoir brine + CO2 systems at pressures up to 31 MPa and temperatures of 27 °C and 58 °C, J. Chem. Eng. Data, 2005, 50, 1242–1249 CrossRef CAS.
- L. Gil, S. Avila, P. Garca-Giménez, S. Blanco, C. Berro, S. Otin and I. Velasco, Dew points of binary propane or n-butane + carbon dioxide, ternary propane or n-butane + carbon dioxide + water, and quaternary propane or n-butane + carbon dioxide + water + methanol mixtures: Measurement and modeling, Ind. Eng. Chem. Res., 2006, 45, 3974–3980 CrossRef CAS.
- A. Bahramian, A. Danesh, F. Gozalpour, B. Tohidi and A. Todd, Vapour–liquid interfacial tension of water and hydrocarbon mixture at high pressure and high temperature conditions, Fluid Phase Equilib., 2007, 252, 66–73 CrossRef CAS.
- E. Forte, A. Galindo and J. M. Trusler, Experimental and molecular modeling study of the three-phase behavior of (n-decane + carbon dioxide + water) at reservoir conditions, J. Phys. Chem. B, 2011, 115, 14591–14609 CrossRef CAS.
- A. Georgiadis, G. Maitland, J. M. Trusler and A. Bismarck, Interfacial tension measurements of the (H2O + n-decane + CO2) ternary system at elevated pressures and temperatures, J. Chem. Eng. Data, 2011, 56, 4900–4908 CrossRef CAS.
- L. M. C. Pereira A. Chapoy B. Tohidi et al., Vapor-liquid and liquid–liquid interfacial tension of water and hydrocarbon systems at representative reservoir conditions: Experimental and modelling results, in SPE Annual Technical Conference and Exhibition, 2014 Search PubMed.
- S. Z. Al Ghafri, E. Forte, A. Galindo, G. C. Maitland and J. M. Trusler, Experimental and modeling study of the phase behavior of (heptane + carbon dioxide + water) mixtures, J. Chem. Eng. Data, 2015, 60, 3670–3681 CrossRef CAS.
- Y. Yang, A. K. Narayanan Nair, M. F. Anwari Che Ruslan and S. Sun, Bulk and interfacial properties of the decane + water system in the presence of methane, carbon dioxide, and their mixture, J. Phys. Chem. B, 2020, 124, 9556–9569 CrossRef CAS.
- N. Choudhary, M. F. A. Che Ruslan, A. K. Narayanan Nair, R. Qiao and S. Sun, Bulk and interfacial properties of the decane + brine system in the presence of carbon dioxide, methane, and their mixture, Ind. Eng. Chem. Res., 2021, 60, 11525–11534 CrossRef CAS.
- L. Zhao, L. Tao and S. Lin, Molecular dynamics characterizations of the supercritical CO2-mediated hexane-brine interface, Ind. Eng. Chem. Res., 2015, 54, 2489–2496 CrossRef CAS.
- B. Liu, J. Shi, M. Wang, J. Zhang, B. Sun, Y. Shen and X. Sun, Reduction in interfacial tension of water–oil interface by supercritical CO2 in enhanced oil recovery processes studied with molecular dynamics simulation, J. Supercrit. Fluids, 2016, 111, 171–178 CrossRef CAS.
- J. Zhang, Z. Dong, Y. Zhang, M. Wang and Y. Yan, Effects of the methane content on the water–oil interface: Insights from the molecular level, Energy Fuels, 2017, 31, 7026–7032 CrossRef CAS.
- S. Mohammed and G. A. Mansoori, The role of supercritical/dense CO2 gas in altering aqueous/oil interfacial properties: A molecular dynamics study, Energy Fuels, 2018, 32, 2095–2103 CrossRef CAS.
- T. R. Underwood and H. C. Greenwell, The water–alkane interface at various NaCl salt concentrations: A molecular dynamics study of the readily available force fields, Sci. Rep., 2018, 8, 1–11 CAS.
- A. Aminian and B. ZareNezhad, Molecular dynamics simulations study on the shear viscosity, density, and equilibrium interfacial tensions of CO2 + brines and brines + CO2 + n-decane systems, J. Phys. Chem. B, 2021, 125, 2707–2718 CrossRef CAS PubMed.
- T. Sasaki, M. Hattori, J. Sasaki and K. Nukina, Studies of aqueous sodium dodecyl sulfate solutions by activity measurements, Bull. Chem. Soc. Jpn., 1975, 48, 1397–1403 CrossRef CAS.
- C.-Y. Sun, G.-J. Chen and L.-Y. Yang, Interfacial tension of methane+ water with surfactant near the hydrate formation conditions, J. Chem. Eng. Data, 2004, 49, 1023–1025 CrossRef CAS.
- T. D. Gurkov, D. T. Dimitrova, K. G. Marinova, C. Bilke-Crause, C. Gerber and I. B. Ivanov, Ionic surfactants on fluid interfaces: Determination of the adsorption; role of the salt and the type of the hydrophobic phase, Colloids Surf., A, 2005, 261, 29–38 CrossRef CAS.
- C. D. Bruce, S. Senapati, M. L. Berkowitz, L. Perera and M. D. Forbes, Molecular dynamics simulations of sodium dodecyl sulfate micelle in water: The behavior of water, J. Phys. Chem. B, 2002, 106, 10902–10907 CrossRef CAS.
- S. R. da Rocha, K. P. Johnston and P. J. Rossky, Surfactant-modified CO2-water interface: A molecular view, J. Phys. Chem. B, 2002, 106, 13250–13261 CrossRef CAS.
- S. S. Jang, S.-T. Lin, P. K. Maiti, M. Blanco, W. A. Goddard, P. Shuler and Y. Tang, Molecular dynamics study of a surfactant-mediated decane–water interface: Effect of molecular architecture of alkyl benzene sulfonate, J. Phys. Chem. B, 2004, 108, 12130–12140 CrossRef CAS.
- H. Domnguez, Self-aggregation of the SDS surfactant at a solid–liquid interface, J. Phys. Chem. B, 2007, 111, 4054–4059 CrossRef PubMed.
- F. Palazzesi, M. Calvaresi and F. Zerbetto, A molecular dynamics investigation of structure and dynamics of SDS and SDBS micelles, Soft Matter, 2011, 7, 9148–9156 RSC.
- X. Tang, P. H. Koenig and R. G. Larson, molecular dynamics simulations of sodium dodecyl sulfate micelles in water – the effect of the force field, J. Phys. Chem. B, 2014, 118, 3864–3880 CrossRef CAS PubMed.
- Z.-Y. Lin, D. T. Wu and S.-T. Lin, Equilibrium and transport properties of methane at the methane/water interface with the presence of SDS, J. Phys. Chem. C, 2018, 122, 29259–29267 CrossRef CAS.
- J. G. Parra, H. Domnguez, Y. Aray, P. Iza, X. Zarate and E. Schott, Structural and interfacial properties of the CO2-in-water foams prepared with sodium dodecyl sulfate (SDS): A molecular dynamics simulation study, Colloids Surf., A, 2019, 578, 123615 CrossRef CAS.
- C. Fan, J. Jia, B. Peng, Y. Liang, J. Li and S. Liu, Molecular dynamics study on CO2 foam films with sodium dodecyl sulfate: Effects of surfactant concentration, temperature, and pressure on the interfacial tension, Energy Fuels, 2020, 34, 8562–8574 CrossRef CAS.
- Y. Nan, W. Li and Z. Jin, Ion valency and concentration effect on the structural and thermodynamic properties of brine–decane interfaces with anionic surfactant (SDS), J. Phys. Chem. B, 2021, 125, 9610–9620 CrossRef CAS PubMed.
- N. A. Kumar and C. Seidel, Polyelectrolyte brushes with added salt, Macromolecules, 2005, 38, 9341–9350 CrossRef CAS.
- N. A. Kumar and V. Ganesan, Communication: Self-assembly of semiflexible-flexible block copolymers, 2012 Search PubMed.
- E. B. Stukalin, L.-H. Cai, N. A. Kumar, L. Leibler and M. Rubinstein, Self-healing of unentangled polymer networks with reversible bonds, Macromolecules, 2013, 46, 7525–7541 CrossRef CAS PubMed.
- A. K. Narayanan Nair, A. Martinez Jimenez and S. Sun, Complexation behavior of polyelectrolytes and polyampholytes, J. Phys. Chem. B, 2017, 121, 7987–7998 CrossRef CAS PubMed.
- M. J. Abraham, T. Murtola, R. Schulz, S. Páll, J. C. Smith, B. Hess and E. Lindahl, GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers, SoftwareX, 2015, 1, 19–25 CrossRef.
- N. Choudhary, A. K. N. Nair, M. F. A. C. Ruslan and S. Sun, Bulk and interfacial properties of decane in the presence of carbon dioxide, methane, and their mixture, Sci. Rep., 2019, 9, 1–10 CAS.
- N. Choudhary, M. F. A. Che Ruslan, A. K. Narayanan Nair and S. Sun, Bulk and interfacial properties of alkanes in the presence of carbon dioxide, methane, and their mixture, Ind. Eng. Chem. Res., 2021, 60, 729–738 CrossRef CAS.
- M. G. Martin and J. I. Siepmann, Transferable potentials for phase equilibria. 1. United-atom description of n-alkanes, J. Phys. Chem. B, 1998, 102, 2569–2577 CrossRef CAS.
- J. J. Potoff and J. I. Siepmann, Vapor–liquid equilibria of mixtures containing alkanes, carbon dioxide, and nitrogen, AIChE J., 2001, 47, 1676–1682 CrossRef CAS.
- M. Sedghi L. Goual et al., Molecular Dynamics Simulations of Asphaltene Dispersion by Limonene and PVAc Polymer During CO2 Flooding, in SPE International Conference and Exhibition on Formation Damage Control, 2016 Search PubMed.
- J. L. Abascal and C. Vega, A general purpose model for the condensed phases of water: TIP4P/2005, J. Chem. Phys., 2005, 123, 234505 CrossRef CAS.
- D. E. Smith and L. X. Dang, Computer simulations of NaCl association in polarizable water, J. Chem. Phys., 1994, 100, 3757–3766 CrossRef CAS.
- J. Huang, S. Rauscher, G. Nawrocki, T. Ran, M. Feig, B. L. De Groot, H. Grubmüller and A. D. MacKerell, CHARMM36m: An improved force field for folded and intrinsically disordered proteins, Nat. Methods, 2017, 14, 71–73 CrossRef CAS.
- Y. Yang, A. K. Narayanan Nair and S. Sun, Molecular dynamics simulation study of carbon dioxide, methane, and their mixture in the presence of brine, J. Phys. Chem. B, 2017, 121, 9688–9698 CrossRef CAS PubMed.
- Y. Yang, M. F. A. Che Ruslan, A. K. Narayanan Nair and S. Sun, Effect of ion valency on the properties of the carbon dioxide–methane–brine system, J. Phys. Chem. B, 2019, 123, 2719–2727 CrossRef CAS PubMed.
- L. C. Nielsen, I. C. Bourg and G. Sposito, Predicting CO2–water interfacial tension under pressure and temperature conditions of geologic CO2 storage, Geochim. Cosmochim. Acta, 2012, 81, 28–38 CrossRef CAS.
- J. M. Garrido, H. Quinteros-Lama, J. M. Miguez, F. J. Blas and M. M. Piñeiro, On the physical insight into the barotropic effect in the interfacial behavior for the H2O + CO2 mixture, J. Phys. Chem. C, 2019, 123, 28123–28130 CrossRef CAS.
- K. Tajima, M. Muramatsu and T. Sasaki, Radiotracer studies on adsorption of surface active substance at aqueous surface. I. Accurate measurement of adsorption of tritiated sodium dodecylsulfate, Bull. Chem. Soc. Jpn., 1970, 43, 1991–1998 CrossRef CAS.
- J. R. Lu, A. Marrocco, T. J. Su, R. K. Thomas and J. Penfold, Adsorption of dodecyl sulfate surfactants with monovalent metal counterions at the air-water interface studied by neutron reflection and surface tension, J. Colloid Interface Sci., 1993, 158, 303–316 CrossRef CAS.
- A. Ghoufi, P. Malfreyt and D. J. Tildesley, Computer modelling of the surface tension of the gas–liquid and liquid–liquid interface, Chem. Soc. Rev., 2016, 45, 1387–1409 RSC.
- L. Zhao, J. Ji, L. Tao and S. Lin, Ionic effects on supercritical CO2–brine interfacial tensions: Molecular dynamics simulations and a universal correlation with ionic strength, temperature, and pressure, Langmuir, 2016, 32, 9188–9196 CrossRef CAS PubMed.
- L. M. Pereira, A. Chapoy, R. Burgass, M. B. Oliveira, J. A. Coutinho and B. Tohidi, Study of the impact of high temperatures and pressures on the equilibrium densities and interfacial tension of the carbon dioxide/water system, J. Chem. Thermodyn., 2016, 93, 404–415 CrossRef CAS.
- L. M. Pereira, A. Chapoy, R. Burgass and B. Tohidi, Interfacial tension of CO2+ brine systems: Experiments and predictive modelling, Adv. Water Resour., 2017, 103, 64–75 CrossRef CAS.
- C. Chalbaud, M. Robin, J. Lombard, F. Martin, P. Egermann and H. Bertin, Interfacial tension measurements and wettability evaluation for geological CO2 storage, Adv. Water Resour., 2009, 32, 98–109 CrossRef CAS.
- X. Li, D. A. Ross, J. M. Trusler, G. C. Maitland and E. S. Boek, Molecular dynamics simulations of CO2 and brine interfacial tension at high temperatures and pressures, J. Phys. Chem. B, 2013, 117, 5647–5652 CrossRef CAS PubMed.
- K. Kashefi, L. M. Pereira, A. Chapoy, R. Burgass and B. Tohidi, Measurement and modelling of interfacial tension in methane/water and methane/brine systems at reservoir conditions, Fluid Phase Equilib., 2016, 409, 301–311 CrossRef CAS.
- W. Li and Z. Jin, Molecular dynamics simulations of natural gas-water interfacial tensions over wide range of pressures, Fuel, 2019, 236, 480–492 CrossRef CAS.
- W. Li and Z. Jin, Effect of ion concentration and multivalence on methane–brine interfacial tension and phenomena from molecular perspectives, Fuel, 2019, 254, 115657 CrossRef CAS.
- P. Naeiji, T. K. Woo, S. Alavi and R. Ohmura, Molecular dynamics simulations of interfacial properties of the CO2–water and CO2–CH4–water systems, J. Chem. Phys., 2020, 153, 044701 CrossRef CAS PubMed.
- S. Blazquez, I. Zeron, M. Conde, J. Abascal and C. Vega, Scaled charges at work: Salting out and interfacial tension of methane with electrolyte solutions from computer simulations, Fluid Phase Equilib., 2020, 513, 112548 CrossRef CAS.
- C. D. Wick, T.-M. Chang, J. A. Slocum and O. T. Cummings, Computational investigation of the n-alkane/water interface with many-body potentials: The effect of chain length and ion distributions, J. Phys. Chem. C, 2012, 116, 783–790 CrossRef CAS.
- H. Peng and M. Firouzi, Evaluation of interfacial properties of concentrated KCl solutions by molecular dynamics simulation, Colloids Surf., A, 2018, 538, 703–710 CrossRef CAS.
- O. T. Cummings and C. D. Wick, Computational study on the effect of alkyl chain length on alkane–water interfacial width, Chem. Phys. Lett., 2013, 556, 65–69 CrossRef CAS.
- B. Xue, D. B. Harwood, J. L. Chen and J. I. Siepmann, Monte Carlo simulations of fluid phase equilibria and interfacial properties for water/alkane mixtures: An assessment of nonpolarizable water models and of departures from the Lorentz–Berthelot combining rules, J. Chem. Eng. Data, 2018, 63, 4256–4268 CrossRef CAS.
- A. M
![[a with combining cedilla]](https://www.rsc.org/images/entities/char_0061_0327.gif) czyński, B. Wiśniewska-Gocłowska and M. Góral, Recommended liquid–liquid equilibrium data. Part 1. Binary alkane–water systems, J. Phys. Chem. Ref. Data, 2004, 33, 549–577 CrossRef.
czyński, B. Wiśniewska-Gocłowska and M. Góral, Recommended liquid–liquid equilibrium data. Part 1. Binary alkane–water systems, J. Phys. Chem. Ref. Data, 2004, 33, 549–577 CrossRef. - A. Buckingham and R.-L. Disch, The quadrupole moment of the carbon dioxide molecule, Proc. R. Soc. London, Ser. A, 1963, 273, 275–289 CAS.
- Z. Mester and A. Z. Panagiotopoulos, Mean ionic activity coefficients in aqueous NaCl solutions from molecular dynamics simulations, J. Chem. Phys., 2015, 142, 044507 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional details of simulation analysis. See DOI: 10.1039/d1sm01267c |
| This journal is © The Royal Society of Chemistry 2021 |