Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Rational synthesis of novel biocompatible thermoresponsive block copolymer worm gels†
Deborah L.
Beattie
,
Oleksandr O.
Mykhaylyk
 ,
Anthony J.
Ryan
and
Steven P.
Armes
,
Anthony J.
Ryan
and
Steven P.
Armes
 *
*
Dainton Building, Department of Chemistry, University of Sheffield, Brook Hill, Sheffield, South Yorkshire S3 7HF, UK. E-mail: s.p.armes@shef.ac.uk
First published on 17th May 2021
Abstract
It is well known that reversible addition–fragmentation chain transfer (RAFT) aqueous dispersion polymerization of 2-hydroxypropyl methacrylate (HPMA) enables the rational design of diblock copolymer worm gels. Moreover, such hydrogels can undergo degelation on cooling below ambient temperature as a result of a worm-to-sphere transition. However, only a subset of such block copolymer worms exhibit thermoresponsive behavior. For example, PMPC26–PHPMA280 worm gels prepared using a poly(2-(methacryloyloxy)ethyl phosphorylcholine) (PMPC26) precursor do not undergo degelation on cooling to 6 °C (see S. Sugihara et al., J. Am. Chem. Soc., 2011, 133, 15707–15713). Informed by our recent studies (N. J. Warren et al., Macromolecules, 2018, 51, 8357–8371), we decided to reduce the mean degrees of polymerization of both the PMPC steric stabilizer block and the structure-directing PHPMA block when targeting a pure worm morphology. This rational approach reduces the hydrophobic character of the PHPMA block and hence introduces the desired thermoresponsive character, as evidenced by the worm-to-sphere transition (and concomitant degelation) that occurs on cooling a PMPC15–PHPMA150 worm gel from 40 °C to 6 °C. Moreover, worms are reconstituted on returning to 40 °C and the original gel modulus is restored. This augurs well for potential biomedical applications, which will be examined in due course. Finally, small-angle X-ray scattering studies indicated a scaling law exponent of 0.67 (≈2/3) for the relationship between the worm core cross-sectional diameter and the PHPMA DP for a series of PHPMA-based worms prepared using a range of steric stabilizer blocks, which is consistent with the strong segregation regime for such systems.
Introduction
Polymerization-induced self-assembly (PISA) enables the highly efficient and convenient preparation of block copolymer nano-objects in the form of concentrated dispersions.1,2 In view of its remarkable versatility and tolerance of monomer functionality, reversible addition–fragmentation chain transfer (RAFT) polymerization3–6 has emerged as the most popular synthetic technique for PISA syntheses.7,8 Thus, if the target diblock composition and other synthesis parameters are optimized, then spherical,9,10 worm-like11–13 or vesicular14,15 morphologies can be produced in various solvents using a wide range of vinyl monomers.16–22 In particular, PISA via RAFT aqueous dispersion polymerization23–26 is ideally suited for the preparation of nano-objects with potential biomedical applications. Such formulations involve the chain extension of a water-soluble precursor using a water-miscible monomer. The growing second block becomes water-insoluble at some critical degree of polymerization (DP), thus driving in situ self-assembly to form sterically-stabilized nanoparticles comprising amphiphilic diblock copolymer chains.One of the most extensively researched RAFT aqueous dispersion polymerization formulations employs poly(glycerol monomethacrylate) (PGMA) as the hydrophilic steric stabilizer and poly(2-hydroxypropyl methacrylate) (PHPMA) as the hydrophobic core-forming block.27 PGMA–PHPMA worm gels are of particular interest because they form soft physical gels via multiple inter-worm contacts.27 Such gels are highly biocompatible and exhibit thermoresponsive behavior,28 undergoing a reversible worm-to-sphere transition on cooling from 20 °C to 4 °C.29 At a sufficiently high copolymer concentration, this change in morphology results in degelation to produce a free-flowing dispersion at sub-ambient temperature, enabling facile sterilization via cold ultrafiltration.30 Both the critical gelation temperature (CGT) and the worm gel strength can be tuned by statistical incorporation of suitable methacrylic comonomers or appropriate reactive functional groups (e.g. disulfide bonds) into the core or stabilizer blocks, respectively.31–34 Thus, PGMA–PHPMA worm gels induce human pluripotent stem cell colonies to undergo reversible stasis at 37 °C35 and can be used in conjunction with poly(vinyl alcohol) for the cryopreservation of red blood cells,36 while closely-related disulfide-functionalized worm gels provide a convenient 3D cell culture medium for up to ten days.37 In each case, thermoreversible (de)gelation is a critical feature for the intended biomedical application.
Poly(2-(methacryloyloxy)ethyl phosphorylcholine) (PMPC) is a well-known highly biocompatible zwitterionic polymer38–45 that has been used to manufacture low-irritation soft contact lenses, coatings for drug-eluting stents and other biomedical devices.46–51 In 2011 Sugihara et al.15 conducted the RAFT aqueous dispersion polymerization of 2-hydroxypropyl methacrylate (HPMA) using a relatively short PMPC steric stabilizer and obtained well-defined spheres, worms, or vesicles depending on the precise PISA formulation. A pseudo-phase diagram was constructed for a series of PMPC25–PHPMAx diblock copolymers but pure worms and vesicles were only obtained when targeting relatively long PHPMA blocks (x > 220). Unfortunately, such higher order nano-objects did not exhibit thermoresponsive behavior, which precludes many potential biomedical applications.
Recently, Warren et al.52 compared the aqueous rheological properties of PGMA37–PHPMA80, PGMA54–PHPMA140 and PGMA71–PHPMA200 worms and found that their thermoresponsive behavior was strongly dependent on the diblock copolymer composition. More specifically, PGMA37–PHPMA80 worms proved to be unstable with respect to dilution, while a 10% w/w aqueous dispersion of PGMA71–PHPMA200 worms merely exhibited irreversible degelation on cooling; only the PGMA54–PHPMA140 worms exhibited the desired thermoreversible (de)gelation at this copolymer concentration. This indicates that such behavior requires careful optimization of the mean DPs of both blocks. Thus, if the DP of the core-forming PHPMA block is too high, then these chains become too hydrophobic to exhibit thermoreversible (de)gelation behavior. Moreover, if the hydrophilic PGMA block is too long, the multiple sphere-sphere fusion that is required for regelation via a sphere-to-worm transition is unlikely to occur within a useful experimental timescale (minutes).
In the present study, we revisit the PMPC25–PHPMAx formulation reported by Sugihara et al.15 and target a significantly lower DP of 15 for the hydrophilic PMPC stabilizer block. In principle, this strategy should ensure that the DP required for the hydrophobic PHPMA block to produce a pure worm phase is sufficiently short to produce the desired thermoreversible (de)gelation behavior. The veracity of this rational approach is demonstrated by direct comparison of the aqueous solution properties of such worms with those exhibited by higher molecular weight non-thermoresponsive PMPC26–PHPMA280 worms (i.e. similar to the PMPC25–PHPMAx worm examples previously reported by Sugihara et al.).15 The longer-term aim of this fundamental study is to examine whether such phosphorylcholine-based thermoresponsive worm gels offer potential biomedical applications as new wholly synthetic media for either cell culture35,36 or cell storage.37,53
Experimental
Materials
2-(Methacryloyloxy)ethyl phosphorylcholine (MPC) was purchased from NOF Corporation (Japan) and was used as received. 2-Cyano-2-propyl benzodithioate (CPDB) was purchased from Sigma-Aldrich (Dorset, UK) and 2,2′-azobis(2-methylpropionitrile) (AIBN) was purchased from Molekula (UK). HPMA monomer was kindly provided by GEO Specialty Chemicals (Hythe, UK) and was used as received. 2,2′-Azobis[2-(2-imidazolin-2-yl)propane]dihydrochloride (VA-044) initiator was purchased from Fluorochem (Glossop, UK). Deionized water was used in all experiments and was obtained from an Elgastat Option 3A water purification unit. HPLC-grade chloroform, methanol and ethanol were obtained from VWR Chemicals (UK). Deuterated methanol (CD3OD) was purchased from Cambridge Isotope Laboratories (UK).Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v acetone/methanol mixture. Residual solvent was removed under reduced pressure, then the purified PMPC was dissolved in deionized water and freeze-dried overnight to produce a glassy pink solid. 1H NMR spectroscopy analysis indicated a mean degree of polymerization of 15. GPC analysis [refractive index detector, 3
1 v/v acetone/methanol mixture. Residual solvent was removed under reduced pressure, then the purified PMPC was dissolved in deionized water and freeze-dried overnight to produce a glassy pink solid. 1H NMR spectroscopy analysis indicated a mean degree of polymerization of 15. GPC analysis [refractive index detector, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chloroform/methanol eluent, poly(methyl methacrylate) (PMMA) calibration standards] indicated an Mn of 6300 g mol−1 and an Mw/Mn of 1.12.
1 chloroform/methanol eluent, poly(methyl methacrylate) (PMMA) calibration standards] indicated an Mn of 6300 g mol−1 and an Mw/Mn of 1.12.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chloroform/methanol eluent, PMMA calibration standards) without further purification (Mn = 29
1 chloroform/methanol eluent, PMMA calibration standards) without further purification (Mn = 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 g mol−1; Mw/Mn = 1.30). The PMPC15–PHPMA150 worms were characterized as a function of temperature using DLS, TEM, SAXS, NMR, rheology and SIPLI.
600 g mol−1; Mw/Mn = 1.30). The PMPC15–PHPMA150 worms were characterized as a function of temperature using DLS, TEM, SAXS, NMR, rheology and SIPLI.
Characterisation methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v chloroform/methanol eluent containing 2 mM LiBr at a flow rate of 1.0 mL min−1. The instrument set-up comprised an Agilent 1260 GPC system, two Agilent PL gel 5 μm Mixed-C columns connected in series with a guard column, a refractive index detector and a variable wavelength UV detector set to 308 nm. Calibration was achieved using a series of ten near-monodisperse PMMA standards with Mp values ranging from 625 to 618
1 v/v chloroform/methanol eluent containing 2 mM LiBr at a flow rate of 1.0 mL min−1. The instrument set-up comprised an Agilent 1260 GPC system, two Agilent PL gel 5 μm Mixed-C columns connected in series with a guard column, a refractive index detector and a variable wavelength UV detector set to 308 nm. Calibration was achieved using a series of ten near-monodisperse PMMA standards with Mp values ranging from 625 to 618![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1.
000 g mol−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/λ and θ is one-half of the scattering angle). Measurements were conducted on 1.0% w/w aqueous copolymer dispersions placed within 2.0 mm diameter capillary sample holders. The resulting two-dimensional scattering patterns were calibrated and reduced to one-dimensional curves using Irena SAS macro for Igor Pro.54
θ/λ and θ is one-half of the scattering angle). Measurements were conducted on 1.0% w/w aqueous copolymer dispersions placed within 2.0 mm diameter capillary sample holders. The resulting two-dimensional scattering patterns were calibrated and reduced to one-dimensional curves using Irena SAS macro for Igor Pro.54
Results and discussion
The two-step aqueous PISA synthesis of the two types of PMPC–PHPMA worms examined in this study is outlined in Scheme 1. A dithiobenzoate-based RAFT agent (CPDB) and AIBN initiator were employed for the initial RAFT solution polymerization of MPC in ethanol at 70 °C. After isolation and purification, 1H NMR spectra recorded for the two PMPC precursors in CD3OD indicated mean DPs of 15 and 26, respectively. More specifically, the integrated signal assigned to the five aromatic dithiobenzoate protons between 7.4 and 8.0 ppm was compared with those corresponding to the six protons of the three oxymethylene proton signals [i.e. –COO–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2, C
2, C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2–O–P and (H3C)3N+–CH2–C
2–O–P and (H3C)3N+–CH2–C![[H with combining low line]](https://www.rsc.org/images/entities/char_0048_0332.gif) 2] in the MPC repeat units between 4.0 and 4.5 ppm (see Fig. S1, ESI†). GPC analysis (refractive index detector, 3
2] in the MPC repeat units between 4.0 and 4.5 ppm (see Fig. S1, ESI†). GPC analysis (refractive index detector, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chloroform/methanol eluent) gave low final dispersities (Mw/Mn < 1.12), indicating good RAFT control (see Fig. S2, ESI†).
1 chloroform/methanol eluent) gave low final dispersities (Mw/Mn < 1.12), indicating good RAFT control (see Fig. S2, ESI†).
These two relatively well-defined PMPC homopolymer precursors were then chain-extended in turn via RAFT aqueous dispersion polymerization of HPMA at 50 °C to afford the desired PMPC15–PHPMA150 (or PMPC26–PHPMA280) worms at 25% w/w solids. 1H NMR studies confirmed that the HPMA polymerization proceeded to more than 99% conversion in each case (based on the almost complete disappearance of the vinyl proton signals at 5.7 and 6.2 ppm, see Fig. S1, ESI†). Moreover, high blocking efficiencies were achieved in both cases, with final dispersities of 1.30 and 1.36 being observed for PMPC15–PHPMA150 and PMPC26–PHPMA280, respectively. According to a prior study by Li and Armes,26 the high molecular weight shoulders observed in these chromatograms are attributed to a relatively low level (<0.30 mol%) of dimethacrylate impurity in the HPMA monomer, which inevitably leads to light branching when targeting higher degrees of polymerization for the PHPMA block.57 As reported by Sugihara et al.15 and Warren et al.,52 the mean cross-sectional diameter of the worms depends on the mean degree of polymerization of the structure-directing hydrophobic PHPMA block. Thus, the PMPC15–PHPMA150 worms are schematically depicted as being somewhat thinner than the PMPC26–PHPMA280 worms, see Scheme 1B.
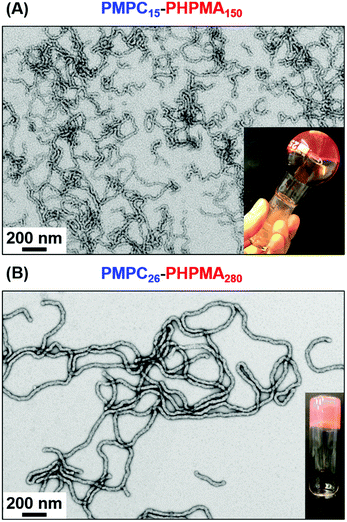
It is perhaps worth emphasizing that it is normally rather difficult to access pure worms via PISA because this copolymer morphology typically occupies relatively narrow phase space.15,25,58 However, the two diblock copolymer compositions were chosen based on our prior knowledge of aqueous PISA formulations. Thus, the PMPC25–PHPMAx phase diagram reported by Sugihara et al.15 was used as a starting point to identify an appropriate target PHPMA DP to afford pure worms at 25% w/w solids. According to TEM analysis (see Fig. 1B), the lowest PHPMA DP to afford a pure worm phase was 280, with a 25% w/w aqueous dispersion of PMPC26–PHPMA280 worms forming a turbid free-standing gel at 25 °C. According to the master phase diagram reported for the PGMA–PHPMA PISA formulation by Warren et al.,52 it should be possible to use this PHPMA/PMPC molar ratio of 10.8 (i.e. 280/26) to estimate the likely diblock copolymer composition that should correspond to pure worms when using shorter PMPC stabilizer blocks. More specifically, for the PMPC15 precursor employed in the current study, targeting a mean PHPMA DP of around 162 should produce a pure worm phase. Indeed, this rational approach proved to be fruitful, with well-defined worms being obtained when targeting PMPC15–PHPMA150. The resulting 25% w/w aqueous dispersion formed a transparent free-standing gel at 25 °C, with the distinctive pink color ascribed to the dithiobenzoate chain-ends (see Fig. 1A). It is perhaps worth mentioning that the thicker PMPC26–PHPMA280 worms scatter light more strongly, which explains why this 25% w/w aqueous dispersion is relatively turbid, rather than transparent (see Fig. 1B). The formation of soft, free-standing hydrogels in both cases is believed to be the result of multiple inter-worm contacts, as reported by Lovett and co-workers for PGMA–PHPMA worms.27
Visual inspection of as-synthesized 25% w/w worm gels stored at (sub-)ambient temperature suggested that neither PMPC15–PHPMA150 nor PMPC26–PHPMA280 were thermoresponsive at this relatively high copolymer concentration. However, serial dilution of these gels led to divergent behavior. Thus, an 18% w/w aqueous dispersion of PMPC15–PHPMA150 worms formed a transparent free-standing gel at 25 °C but underwent degelation on cooling to 6 °C to afford a free-flowing fluid (Fig. S3, ESI†). According to Blanazs et al., this suggests that a worm-to-sphere morphology transition has occurred.30 On returning to 25 °C, a tube inversion test confirmed that regelation had occurred (see Fig. S3, ESI†). Dilution of the 25% w/w aqueous dispersion of PMPC26–PHPMA280 worms to 10% w/w produced a rather soft free-standing, turbid gel at 25 °C. However, in this case no thermoresponsive behavior was observed: degelation did not occur on cooling this gel to 6 °C, even after leaving it to stand for several days at this temperature. This is consistent with observations made by Warren et al., who found that a 10% w/w aqueous PGMA71–PHPMA200 worm gel underwent degelation on cooling but did not reform a gel on the timescale of the oscillatory rheology experiments.52 Clearly, PHPMA chains become significantly more hydrophobic as higher DPs are targeted, which is consistent with the thermoresponsive behavior observed by Lovett and co-workers for a series of PGMA43–PHPMA175–225 vesicles.34
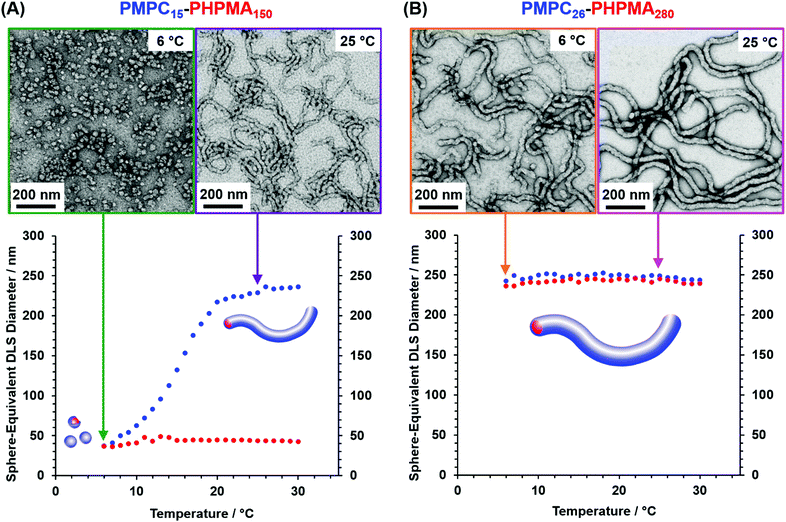
Next, variable temperature DLS studies were conducted between 6 and 30 °C on 0.10% w/w aqueous dispersions of PMPC15–PHPMA150 and PMPC26–PHPMA280 worms (Fig. 2). DLS is well-suited to characterize isotropic nanoparticles, since the Stokes–Einstein equation assumes a spherical morphology.59,60 However, useful information can also be obtained for highly anisotropic worms, as reported by Fielding and co-workers.61 In the present study, the particle size reported by DLS refers to neither the worm length nor the worm cross-sectional diameter (or worm width). Instead, a so-called ‘sphere-equivalent’ diameter is obtained. Thus, if the worms undergo a morphological transition to form spheres, this can be monitored as a substantial reduction in the ‘sphere-equivalent’ diameter, even though this parameter only becomes physically meaningful for the final spheres. It is also worth noting that a concomitant reduction in the DLS polydispersity (PDI) would be expected for such a worm-to-sphere transition because the initial worms are relatively polydisperse in length while the final spheres should be more uniform in size.61 On the other hand, if the initial worms do not undergo a worm-to-sphere transition on cooling, then no change in their sphere-equivalent diameter (or PDI) would be expected.
According to Fig. 2A, the PMPC15–PHPMA150 worms exhibit a ‘sphere-equivalent’ diameter of approximately 235 nm (PDI = 0.30) at 30 °C. On cooling to 20 °C, there is an initial gradual reduction to around 220 nm diameter (PDI = 0.30), prior to a much more rapid reduction in apparent size to 37 nm (PDI = 0.21) at 6 °C. These DLS data indicate that a worm-to-sphere transition occurs on cooling. This interpretation is confirmed by TEM analyses of dilute dispersions dried at 25 °C and 6 °C, respectively. Well-defined worms are present at 25 °C while only much smaller spheres are observed at 6 °C. Moreover, on returning to 30 °C, the ‘sphere-equivalent’ diameter increased only marginally up to 43 nm (PDI = 0.09), indicating that the original worms are not reformed. This is because the 1D stochastic fusion of multiple spheres is very inefficient at the relatively low copolymer concentration required for these DLS experiments, which leads to kinetically-trapped spheres. Similar observations were made by Warren et al. for both PGMA54–PHPMA140 and PGMA71–PHPMA200 worms.52 Thus, sufficiently dilute dispersions of worms are characterized by irreversible thermoresponsive behavior even if the DP of the PHPMA block has been optimized.
In striking contrast, the ‘sphere-equivalent’ diameter of the PMPC26–PHPMA280 worms remains essentially constant at 240–250 nm during a thermal cycle between 6 °C and 30 °C (Fig. 2B). These observations are consistent with TEM images recorded after drying at either 25 °C or 6 °C, which confirm that the original worm morphology remains intact over this temperature range.
DLS studies confirmed that worm reconstitution did not occur at 0.10% w/w copolymer concentration (see Fig. 2A). Accordingly, it was assumed that the nano-objects formed on cooling would become kinetically trapped when diluted to 1.0% w/w at 6 °C, thus allowing SAXS studies to be performed at 25 °C. Thus, PMPC15–PHPMA150 worms were studied by SAXS at 25 °C after sequential dilution of an 18% w/w aqueous dispersion before, during and after a thermal cycle. More specifically, SAXS patterns were recorded for 1.0% w/w aqueous dispersions of (i) the original PMPC15–PHPMA150 worms at 25 °C, (ii) the PMPC15–PHPMA150 spheres formed on cooling to 6 °C and (iii) the reconstituted worms obtained on returning the concentrated dispersion to 25 °C, as indicated in the I(q) vs. q plots shown in Fig. 3. Each copolymer dispersion was equilibrated at the relevant temperature overnight prior to sequential dilution at that temperature. A gradient of approximately −1 is observed for the initial (red) and the final (green) patterns in the low q region, which is consistent with the presence of highly anisotropic worms.52 In contrast, the low q gradient is close to zero for the nano-objects diluted at 6 °C, which is consistent with the formation of spheres that become kinetically trapped after dilution. Moreover, these SAXS patterns can be satisfactorily fitted using appropriate scattering models for worm-like micelles (Fig. 3, 25 °C) and spherical micelles (Fig. 3, 6 °C).52,62,63 Thus, these SAXS data combined with TEM studies confirm that the PMPC15–PHPMA150 worms exhibit thermoreversible behaviour at 18% w/w (see Fig. S3, ESI†). This is because the stochastic 1D fusion of multiple spheres to form worms is much more efficient at this higher copolymer concentration.
To achieve a satisfactory fit to the SAXS patterns recorded for the original and reconstituted PMPC15–PHPMA150 worms present at 25 °C, it was necessary to make the Kuhn length (bw) equal to the worm contour length (Lw) when using the worm-like micelle model. This implies that the worms behave as rigid rods in dilute aqueous solution. Modeling also indicated that the worm core radius (Rw) remained approximately constant before and after being subjected to a thermal cycle (see Table 1). However, a slight increase in the standard deviation for Rw was observed for the reconstituted worms. Furthermore, modeling indicated that the reconstituted worms exhibited slightly shorter Lw values. Unfortunately, there is relatively high experimental uncertainty in the worm contour length. Nevertheless, the contour length obtained from the SAXS fitting is reasonably consistent with the worm length observed in TEM images (see Fig. 1). The number-average core cross-sectional diameter estimated for the original PMPC15–PHPMA150 worms from TEM analysis (see Fig. 1) was 24.1 ± 1.9 nm, which exceeds the volume-average core cross-sectional diameter (2Rw) of 18.8 nm determined by SAXS analysis prior to the thermal cycle.
| Thermal history | TEM | SAXS | ||||
|---|---|---|---|---|---|---|
| Morphology assignment | R s/nm | R w/nm | σ R/nm | L w/nm | b w /nm | |
| a To achieve a satisfactory data fit, it was necessary to assume that bw was equal to Lw, i.e. that the worms are relatively inflexible. | ||||||
| 25 °C (initial) | Worms | — | 9.4 | 0.8 | 283 | 283 |
| 6 °C | Spheres | 12.4 | — | 1.7 | — | — |
| 25 °C (final) | Worms | — | 9.5 | 1.1 | 247 | 247 |
In principle, TEM should undersize relative to SAXS but the former technique suffers from relatively poor sampling statistics. Modeling of the SAXS pattern recorded for the 1.0% w/w aqueous dispersion of PMPC15–PHPMA150 nano-objects diluted at 6 °C indicated a somewhat larger sphere core radius (Rs) of 12.4 ± 1.7 nm. In principle, this parameter should slightly exceed the worm core radius owing to the subtle change in geometry when multiple spheres fuse together to form cylindrical worms (see Fig. S4, ESI†). Under such circumstances, the worm core radius divided by the sphere core radius should be equal to  or ≈0.82. However, this value should be regarded as an upper limit because the overall surface area is constrained when fusing multiple spheres to form each worm. Thus, reasonably good agreement is observed with the radius ratio of ∼0.76 (see Table 1). DLS reports an overall hydrodynamic z-average diameter of 37 nm at 6 °C. Using the manufacturer's software, we calculate a corresponding volume-average diameter of 32 nm. Bearing in mind the thickness of the hydrated PMPC stabilizer layer (2Rg = 2.46 nm), the volume-average spherical core diameter (2Rs) of 24.8 nm calculated using SAXS is consistent with the latter DLS diameter (since 2Rs + 4Rg = 29.7 nm). Furthermore, the increase in the standard deviation of the mean core radius for the spheres formed at 6 °C compared to that of the original worms at 25 °C is consistent with the corresponding TEM images (see Table 1 and Fig. 2).
or ≈0.82. However, this value should be regarded as an upper limit because the overall surface area is constrained when fusing multiple spheres to form each worm. Thus, reasonably good agreement is observed with the radius ratio of ∼0.76 (see Table 1). DLS reports an overall hydrodynamic z-average diameter of 37 nm at 6 °C. Using the manufacturer's software, we calculate a corresponding volume-average diameter of 32 nm. Bearing in mind the thickness of the hydrated PMPC stabilizer layer (2Rg = 2.46 nm), the volume-average spherical core diameter (2Rs) of 24.8 nm calculated using SAXS is consistent with the latter DLS diameter (since 2Rs + 4Rg = 29.7 nm). Furthermore, the increase in the standard deviation of the mean core radius for the spheres formed at 6 °C compared to that of the original worms at 25 °C is consistent with the corresponding TEM images (see Table 1 and Fig. 2).
As predicted by theory,64 amphiphilic diblock copolymers self-assemble to form nano-objects in selective solvents with core diameters (d) that scale according to the mean DP (N) for the insoluble block.65–68 These two parameters obey a power law of the form d = kNα, where k is a constant related to the Flory–Huggins parameter and α is an exponent that depends on the extent of chain stretching within the nano-object cores.68 In the context of the present study, such a power law is expected between the worm core diameter (2Rw) determined via SAXS analysis and the DP of the hydrophobic PHPMA block. Fig. 4 shows the relationship between these two parameters determined for a series of aqueous dispersions of PHPMA–core worms prepared using a non-ionic PGMA stabilizer,52 various binary mixtures of poly(methacrylic acid) (PMAA) and PGMA,69 a non-ionic poly[N-(2-hydroxypropyl)methacrylamide] (PHPMAC) stabilizer70 and the two zwitterionic PMPC stabilizers reported herein (see Fig. 3, Table 1, Fig. S5 and Table S1, ESI†). The data fit to this power law returned an exponent of 0.67 (≈2/3), which corresponds to that expected for the strong segregation regime. This indicates that the PHPMA chains located within such worm cores lie between fully stretched (α = 1) and unperturbed random (Gaussian) coils (α = 1/2).68 Moreover, α = 0.67 is in fairly good agreement with the exponent of 0.70 calculated by Warren et al. for three examples of PGMAx–PHPMAy worms.52 However, it is not consistent with the α exponent of unity determined for a series of four PMPC25–PHPMAy worms reported by Sugihara and co-workers.15 This discrepancy is most likely because TEM was used to estimate the mean worm core diameter rather than SAXS,15 with the former parameter being much less statistically robust and also prone to drying artifacts. It is perhaps worth mentioning that increasingly weak thermoresponsive character is observed for PHPMA-based worms as the DP of this structure-directing block exceeds 150. This suggests that there is a maximum core diameter for thermoreversible PHPMA-based worms, although further studies would be required to confirm this hypothesis.
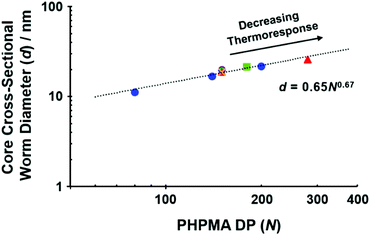 | ||
| Fig. 4 Scaling relationship between the core cross-sectional diameter, d, of a series of aqueous PHPMA-based worms and the mean DP of the hydrophobic PHPMA block, N. Data were fitted using a power law of the form d = kNα, where k is a constant and α is the scaling exponent. From the data fit, α is determined to be 0.67 or ≈2/3. Data reported herein for PMPC15–PHPMA150 and PMPC26–PHPMA280 worms (closed red triangles) are plotted with additional data collated from multiple studies: e.g. PGMA37–PHPMA80, PGMA54–PHPMA140 and PGMA71–PHPMA200 (closed blue circles),52 [0.05PMAA85 + 0.95PGMA62]–PHPMA150 (yellow cross),69 [0.2PMAA85 + 0.8PGMA62]–PHPMA150 (open green diamond),69 [0.2PMAA37 + 0.8PGMA68]–PHPMA150 (open purple circle)69 and PHPMAC41–PHPMA180 (closed green square).70 | ||
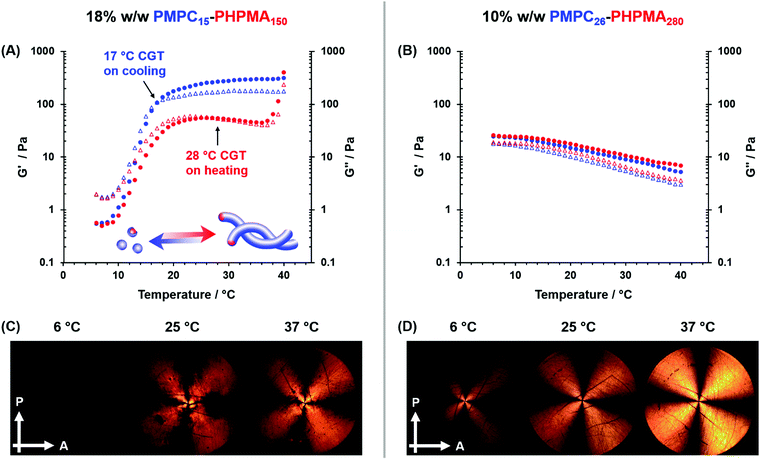
The thermoresponsive behavior of the 18% w/w aqueous dispersion of PMPC15–PHPMA150 worms was subsequently investigated by oscillatory rheology during a 40 °C to 6 °C to 40 °C thermal cycle (Fig. 5A). Preliminary studies enabled the linear viscoelastic regime to be identified (see Fig. S6 and S7, ESI†). G′ of the initial worm gel was determined to be approximately 300 Pa at 37 °C. During the cooling cycle, G′′ becomes equal to G′ at around 17 °C, which corresponds to the CGT. At 6 °C, a free-flowing fluid is obtained (G′ < 1 Pa). On heating, there is a monotonic increase in G′ (albeit with some hysteresis) and a CGT is observed at around 28 °C, with the original bulk modulus eventually being restored at around 40 °C. Similar observations have been reported by Verber et al. when subjecting a PGMA54–PHPMA150 worm gel to a thermal cycle.29
The oscillatory rheology data are consistent with TEM and SAXS analysis recorded at lower copolymer concentrations, with both techniques indicating the presence of worms at 25 °C and spheres at 6 °C (see Fig. 3 and Fig. S3, ESI†). Moreover, both TEM and SAXS studies confirm that the worms are reformed on returning to 25 °C, which suggests that the much higher copolymer concentration required for these rheology studies leads to far more efficient 1D sphere-sphere fusion. This is essential for achieving regelation within normal experimental timescales (i.e. minutes/hours) because worm reconstitution from spheres is a highly cooperative associative process. In contrast, converting worms into spheres is simply a dissociative process that can proceed rapidly without any impediment. Nevertheless, high copolymer concentrations can retard this transition, as reported by Warren et al. for oscillatory rheology studies of 20% w/w aqueous dispersions of PGMA54–PHPMA140 and PGMA71–PHPMA200 worms.52 Based on prior studies by Fielding et al., this latter transition most likely involves a ‘budding’ mechanism whereby spheres emerge from the worm ends, rather than random worm scission.61 It is perhaps worth emphasizing that thermoreversible (de)gelation behavior is critical for potential biomedical applications because it enables facile sterilization of such worm gels via cold ultrafiltration.30
SIPLI studies were conducted at 6, 25 and 37 °C to provide further evidence for the thermally-induced change in copolymer morphology (Fig. 5C). As previously reported by Mykhaylyk et al.,55,56 this relatively new technique enables the presence of isotropic or anisotropic particles to be assessed under shear. After equilibration for 10 min at 6 °C, a dark image was observed for the 18% w/w aqueous dispersion of PMPC15–PHPMA150 when subjected to an applied shear rate of 1.0 s−1, which indicates the presence of non-birefringent isotropic spheres.55 At 25 °C, a characteristic Maltese cross pattern is observed; this results from the birefringence produced by in situ shear alignment of the highly anisotropic worms. This Maltese cross is retained on further heating to 37 °C, indicating that the worms remain intact at this temperature. These data are fully consistent with the TEM analysis and oscillatory rheology studies conducted on these PMPC15–PHPMA150 nano-objects. The distorted nature of the Maltese cross observed at 25 and 37 °C in Fig. 5C is attributed to worm entanglements at the relatively high copolymer concentration (18% w/w) used for these SIPLI experiments. A more traditional Maltese cross motif can be obtained by conducting such studies at 10% w/w (see Fig. S8, ESI†).
Variable temperature oscillatory rheology studies were also conducted using a 10% w/w aqueous dispersion of PMPC26–PHPMA280 worms (see Fig. 5B). On cooling this dispersion from 40 °C to 6 °C, no degelation occurred. In fact, a modest increase in gel modulus was observed, with the original gel modulus being restored on returning to 40 °C. Furthermore, SIPLI studies conducted on the same copolymer dispersion revealed a characteristic Maltese cross from 6 °C to 37 °C, suggesting the permanent presence of anisotropic worms (see Fig. 5D). These rheological data are consistent with tube inversion tests, DLS data and TEM analysis, which confirm that PMPC26–PHPMA280 worms do not exhibit any thermoresponsive behavior over this temperature range.
Serial dilution enabled determination of the CGC at 25 °C. For PMPC15–PHPMA150 worms, a free-standing transparent gel was obtained at copolymer concentrations of ≥15% w/w, whereas a slightly turbid viscous liquid was formed at 12% w/w (see Fig. S9A, ESI†). Gel moduli determined over time indicated that G′ was only marginally greater than G′′ at approximately 15% w/w. Similar experiments for PMPC26–PHPMA280 worms indicated a much lower CGC of around 2.5 to 4.0% w/w. However, the latter gels only became free-standing at approximately 10% w/w as judged by the tube inversion test; this is most likely owing to their relatively low gel moduli (<10 Pa, see Fig. S9B, ESI†). The CGC is an important parameter in the context of potential biomedical applications, such as 3D cell culture media or long-term cell storage media.35,37,71 This is because high copolymer concentrations can adversely affect biocompatibility, leading to a reduction in cell viability. Thus, the relatively high CGC observed for the PMPC15–PHPMA150 worms is not ideal in this context.
However, such cell biology studies require worm gels to be prepared using PBS buffer or a commercial cell culture medium (e.g. Nutristem), rather than deionized water. Recently, Sponchioni and co-workers reported substantially different rheological behavior for PEG57–PHPMAx worm gels in Nutristem compared to deionized water.71 Indeed, to achieve the required thermoresponsive degelation behavior, Sponchioni and co-workers found that the diblock copolymer composition had to be adjusted from PEG57–PHPMA120 in water to PEG57–PHPMA65 in Nutristem.71 If similar optimization were required for the PMPC15–PHPMA150 worms to produce the desired rheological performance, reducing the mean DP of the PHPMA block might lead to a significantly lower CGC. Moreover, Sponchioni and co-workers demonstrated that the chemical functionality of the steric stabilizer block is important in determining the fate of naïve embryonic human stem cells.71 Thus, using a PEG stabilizer block led to cell proliferation, whereas a PGMA stabilizer block induced cell stasis. In this context, it would be fascinating to examine how the PMPC stabilizer block influences stem cell behavior. However, such experiments are likely to require further optimization of the rheological properties of the PMPC15–PHPMA150 worms reported herein.
Conclusions
Informed by our recent studies of thermoresponsive PGMA–PHPMA diblock copolymer worm gels, which undergo degelation on cooling as a result of a worm-to-sphere transition, we decided to revisit an earlier PMPC–PHPMA worm gel formulation that did not exhibit any thermoresponsive behavior. A rational approach was adopted based on our prior knowledge of various aqueous PISA formulations. More specifically, by targeting a shorter mean degree of polymerization for the structure-directing PHPMA block, we were able to reduce its hydrophobic character and hence introduce the desired thermoresponsive degelation behavior via a worm-to-sphere transition when cooling from 25 °C to 6 °C. Moreover, this change in copolymer morphology is reversible: worms are reconstituted on returning to ambient temperature as confirmed by TEM and SAXS studies and the original gel modulus is restored. This should enable facile sterilization via cold ultrafiltration and augurs well for potential biomedical applications of such PMPC–PHPMA worm gels, which will be examined in due course. Finally, combining SAXS data previously reported for various PHPMA–core worms with the two types of PMPC–PHPMA worms studied herein enabled examination of the scaling relationship between the worm cross-sectional core diameter and the PHPMA DP. An exponent of 0.67 was observed regardless of the nature of the steric stabilizer block, which is consistent with the strong segregation regime.Conflicts of interest
There are no conflicts to declare.Acknowledgements
EPSRC is thanked for a DTA PhD studentship to support D. L. B. and for a four-year EPSRC Established Career Particle Technology Fellowship (EP/003009) for S. P. A. EPSRC is also acknowledged for a capital equipment grant to purchase the Xenocs-Excillum SAXS laboratory beamline (EP/M028437/1).References
- B. Charleux, G. Delaittre, J. Rieger and F. D’Agosto, Macromolecules, 2012, 45, 6753–6765 CrossRef CAS.
- N. J. W. Penfold, J. Yeow, C. Boyer and S. P. Armes, ACS Macro Lett., 2019, 8, 1029–1054 CrossRef CAS.
- J. Chiefari, Y. K. B. Chong, F. Ercole, J. Krstina, J. Jeffery, T. P. T. Le, R. T. A. Mayadunne, G. F. Meijs, C. L. Moad, G. Moad, E. Rizzardo, S. H. Thang and C. South, Macromolecules, 1998, 31, 5559–5562 CrossRef CAS.
- G. Moad, E. Rizzardo and S. H. Thang, Polymer, 2008, 49, 1079–1131 CrossRef CAS.
- G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2012, 65, 985–1076 CrossRef CAS.
- S. Perrier, Macromolecules, 2017, 50, 7433–7447 CrossRef CAS.
- S. L. Canning, G. N. Smith and S. P. Armes, Macromolecules, 2016, 49, 1985–2001 CrossRef CAS PubMed.
- F. D’Agosto, J. Rieger and M. Lansalot, Angew. Chem., Int. Ed., 2020, 59, 8368–8392 CrossRef PubMed.
- S. Sugihara, M. Sudo and Y. Maeda, Langmuir, 2019, 35, 1346–1356 CrossRef CAS PubMed.
- B. Zhang, X. Lv and Z. An, ACS Macro Lett., 2017, 6, 224–228 CrossRef CAS.
- G. Mellot, J. M. Guigner, L. Bouteiller, F. Stoffelbach and J. Rieger, Angew. Chem., Int. Ed., 2019, 58, 3173–3177 CrossRef CAS PubMed.
- J. Tan, Y. Bai, X. Zhang and L. Zhang, Polym. Chem., 2016, 7, 2372–2380 RSC.
- O. Nahi, O. J. Cayre, Y. Y. Kim, A. J. Smith, N. J. Warren and F. C. Meldrum, Chem. Commun., 2020, 56, 7463–7466 RSC.
- J. Tan, D. Liu, X. Zhang, C. Huang, J. He, Q. Xu, X. Li and L. Zhang, RSC Adv., 2017, 7, 23114–23121 RSC.
- S. Sugihara, A. Blanazs, S. P. Armes, A. J. Ryan and A. L. Lewis, J. Am. Chem. Soc., 2011, 133, 15707–15713 CrossRef CAS PubMed.
- V. Ladmiral, A. Charlot, M. Semsarilar and S. P. Armes, Polym. Chem., 2015, 6, 1805–1816 RSC.
- M. Chen, J. W. Li, W. J. Zhang, C. Y. Hong and C. Y. Pan, Macromolecules, 2019, 52, 1140–1149 CrossRef.
- E. R. Jones, M. Semsarilar, P. Wyman, M. Boerakker and S. P. Armes, Polym. Chem., 2016, 7, 851–859 RSC.
- D. Zhou, R. P. Kuchel, S. Dong, F. P. Lucien, S. Perrier and P. B. Zetterlund, Macromol. Rapid Commun., 2019, 40, 1800335 CrossRef PubMed.
- M. J. Rymaruk, C. T. O’Brien, S. L. Brown, C. N. Williams and S. P. Armes, Macromolecules, 2020, 53, 1785–1794 CrossRef CAS.
- M. J. Derry, L. A. Fielding and S. P. Armes, Polym. Chem., 2015, 6, 3054–3062 RSC.
- Q. Zhang and S. Zhu, ACS Macro Lett., 2015, 4, 755–758 CrossRef CAS.
- N. J. Warren and S. P. Armes, J. Am. Chem. Soc., 2014, 136, 10174–10185 CrossRef CAS PubMed.
- J. Rieger, Macromol. Rapid Commun., 2015, 36, 1458–1471 CrossRef CAS PubMed.
- A. Blanazs, A. J. Ryan and S. P. Armes, Macromolecules, 2012, 45, 5099–5107 CrossRef CAS.
- Y. Li and S. P. Armes, Angew. Chem., Int. Ed., 2010, 49, 4042–4046 CrossRef CAS PubMed.
- J. R. Lovett, M. J. Derry, P. Yang, F. L. Hatton, N. J. Warren, P. W. Fowler and S. P. Armes, Chem. Sci., 2018, 9, 7138–7144 RSC.
- Y. Pei, A. B. Lowe and P. J. Roth, Macromol. Rapid Commun., 2017, 38, 1600528 CrossRef PubMed.
- R. Verber, A. Blanazs and S. P. Armes, Soft Matter, 2012, 8, 9915–9922 RSC.
- A. Blanazs, R. Verber, O. O. Mykhaylyk, A. J. Ryan, J. Z. Heath, C. W. I. Douglas and S. P. Armes, J. Am. Chem. Soc., 2012, 134, 9741–9748 CrossRef CAS PubMed.
- V. J. Cunningham, L. P. D. Ratcliffe, A. Blanazs, N. J. Warren, A. J. Smith, O. O. Mykhaylyk and S. P. Armes, Polym. Chem., 2014, 5, 6307–6317 RSC.
- N. J. Warren, J. Rosselgong, J. Madsen and S. P. Armes, Biomacromolecules, 2015, 16, 2514–2521 CrossRef CAS PubMed.
- L. P. D. Ratcliffe, K. J. Bentley, R. Wehr, N. J. Warren, B. R. Saunders and S. P. Armes, Polym. Chem., 2017, 8, 5962–5971 RSC.
- J. R. Lovett, N. J. Warren, S. P. Armes, M. J. Smallridge and R. B. Cracknell, Macromolecules, 2016, 49, 1016–1025 CrossRef CAS PubMed.
- I. Canton, N. J. Warren, A. Chahal, K. Amps, A. Wood, R. Weightman, E. Wang, H. Moore and S. P. Armes, ACS Cent. Sci., 2016, 2, 65–74 CrossRef CAS PubMed.
- D. E. Mitchell, J. R. Lovett, S. P. Armes and M. I. Gibson, Angew. Chem., Int. Ed., 2016, 55, 2801–2804 CrossRef CAS PubMed.
- K. A. Simon, N. J. Warren, B. Mosadegh, M. R. Mohammady, G. M. Whitesides and S. P. Armes, Biomacromolecules, 2015, 16, 3952–3958 CrossRef CAS PubMed.
- S. I. Yusa, K. Fukuda, T. Yamamoto, K. Ishihara and Y. Morishima, Biomacromolecules, 2005, 6, 663–670 CrossRef CAS PubMed.
- T. Ueda, H. Oshida, K. Kurita, K. Ishihara and N. Nakabayashi, Polym. J., 1992, 24, 1259–1269 CrossRef CAS.
- K. Ishihara, H. Nomura, T. Mihara, K. Kurita, Y. Iwasaki and N. Nakabayashi, J. Biomed. Mater. Res., 1998, 39, 323–330 CrossRef CAS.
- S. Monge, B. Canniccioni, A. Graillot and J. J. Robin, Biomacromolecules, 2011, 12, 1973–1982 CrossRef CAS PubMed.
- K. Ishihara, J. Biomed. Mater. Res., Part A, 2019, 107, 933–943 CrossRef CAS PubMed.
- A. L. Lewis, Colloids Surf., B, 2000, 18, 261–275 CrossRef CAS PubMed.
- Y. Iwasaki and K. Ishihara, Anal. Bioanal. Chem., 2005, 381, 534–546 CrossRef CAS PubMed.
- H. Kitano, M. Imai, T. Mori, M. Gemmei-Ide, Y. Yokoyama and K. Ishihara, Langmuir, 2003, 19, 10260–10266 CrossRef CAS.
- T. Goda and K. Ishihara, Expert Rev. Med. Devices, 2006, 3, 167–174 CrossRef CAS PubMed.
- A. L. Lewis, T. A. Vick, A. C. M. Collias, L. G. Hughes, R. R. Palmer, S. W. Leppard, J. D. Furze, A. S. Taylor and P. W. Stratford, J. Mater. Sci.: Mater. Med., 2001, 12, 865–870 CrossRef CAS PubMed.
- K. Ishihara, Sci. Technol. Adv. Mater., 2000, 1, 131–138 CrossRef CAS.
- T. A. Snyder, H. Tsukui, S. Kihara, T. Akimoto, K. N. Litwak, M. V. Kameneva, K. Yamazaki and W. R. Wagner, J. Biomed. Mater. Res., Part A, 2007, 81, 85–92 CrossRef PubMed.
- T. Moro, Y. Takatori, K. Ishihara, T. Konno, Y. Takigawa, T. Matsushita, U. I. L. Chung, K. Nakamura and H. Kawaguchi, Nat. Mater., 2004, 3, 829–836 CrossRef CAS PubMed.
- Y. Iwasaki and K. Ishihara, Sci. Technol. Adv. Mater., 2012, 13, 064101 CrossRef CAS PubMed.
- N. J. Warren, M. J. Derry, O. O. Mykhaylyk, J. R. Lovett, L. P. D. Ratcliffe, V. Ladmiral, A. Blanazs, L. A. Fielding and S. P. Armes, Macromolecules, 2018, 51, 8357–8371 CrossRef CAS PubMed.
- R. Spelat, F. Ferro, P. Contessotto, N. J. Warren, G. Marsico, S. P. Armes and A. Pandit, Mater. Today Bio, 2020, 5, 100040 CrossRef PubMed.
- J. Ilavsky and P. R. Jemian, J. Appl. Crystallogr., 2009, 42, 347–353 CrossRef CAS.
- O. O. Mykhaylyk, N. J. Warren, A. J. Parnell, G. Pfeifer and J. Laeuger, J. Polym. Sci., Part B: Polym. Phys., 2016, 54, 2151–2170 CrossRef CAS.
- O. O. Mykhaylyk, Soft Matter, 2010, 6, 4430–4440 RSC.
- I. Bannister, N. C. Billingham, S. P. Armes, S. P. Rannard and P. Findlay, Macromolecules, 2006, 39, 7483–7492 CrossRef CAS.
- J. Tan, D. Liu, Y. Bai, C. Huang, X. Li, J. He, Q. Xu, X. Zhang and L. Zhang, Polym. Chem., 2017, 8, 1315–1327 RSC.
- C. M. Maguire, M. Rösslein, P. Wick and A. Prina-Mello, Sci. Technol. Adv. Mater., 2018, 19, 732–745 CrossRef CAS PubMed.
- S. E. Harding, D. B. Sattelle and V. A. Bloomfield, Laser light scattering in biochemistry, The Royal Society of Chemistry, Cambridge, 1992 Search PubMed.
- L. A. Fielding, J. A. Lane, M. J. Derry, O. O. Mykhaylyk and S. P. Armes, J. Am. Chem. Soc., 2014, 136, 5790–5798 CrossRef CAS PubMed.
- J. S. Pedersen, J. Appl. Crystallogr., 2000, 33, 637–640 CrossRef CAS.
- J. S. Pedersen and P. Schurtenberger, Macromolecules, 1996, 29, 7602–7612 CrossRef CAS.
- F. S. Bates and G. H. Fredrickson, Annu. Rev. Phys. Chem., 1990, 41, 525–557 CrossRef CAS PubMed.
- J. Noolandi and K. M. Hong, Macromolecules, 1983, 16, 1443–1448 CrossRef CAS.
- S. Förster, M. Zisenis, E. Wenz and M. Antonietti, J. Chem. Phys., 1996, 104, 9956–9970 CrossRef.
- P. Dalhaimer, H. Bermudez and D. E. Discher, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 168–176 CrossRef CAS.
- G. Battaglia and A. J. Ryan, J. Am. Chem. Soc., 2005, 127, 8757–8764 CrossRef CAS PubMed.
- L. A. Fielding, C. T. Hendley, E. Asenath-Smith, L. A. Estroff and S. P. Armes, Polym. Chem., 2019, 10, 5131–5141 RSC.
- L. P. D. Ratcliffe, M. J. Derry, A. Ianiro, R. Tuinier and S. P. Armes, Angew. Chem., Int. Ed., 2019, 58, 18964–18970 CrossRef CAS PubMed.
- M. Sponchioni, C. T. O’Brien, C. Borchers, E. Wang, M. N. Rivolta, N. J. W. Penfold, I. Canton and S. P. Armes, Chem. Sci., 2020, 11, 232–240 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Assigned 1H NMR spectra; GPC curves; TEM images for PMPC15–PHPMA150 nano-objects subject to temperature cycling; relationship between sphere and worm core radii; fitted SAXS pattern of PMPC26–PHPMA280 worms and associated structural parameters; oscillatory rheology strain, angular frequency and time sweeps and X-ray scattering model details. See DOI: 10.1039/d1sm00460c |
| This journal is © The Royal Society of Chemistry 2021 |