Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effects of physicochemical properties of polyacrylamide (PAA) and (polydimethylsiloxane) PDMS on cardiac cell behavior
Karim
Daliri
a,
Kurt
Pfannkuche
 *abcd and
Bora
Garipcan
*e
*abcd and
Bora
Garipcan
*e
aInstitute for Neurophysiology, University of Cologne, Medical Faculty, Robert Koch Str. 39, 50931 Cologne, Germany. E-mail: Kurt.Pfannkuche@uni-koeln.de
bDepartment for Pediatric Cardiology, University Hospital Cologne, Cologne, Germany
cMarga-and-Walter-Boll Laboratory for Cardiac Tissue Engineering, University of Cologne, Germany
dCenter for Molecular Medicine, University of Cologne, Germany
eInstitute of Biomedical Engineering, Bogazici University, Cengelkoy, 34684, Istanbul, Turkey. E-mail: bora.garipcan@boun.edu.tr
First published on 7th January 2021
Abstract
In vitro cell culture is commonly applied in laboratories around the world. Cultured cells are either of primary origin or established cell lines. Such transformed cell lines are increasingly replaced by pluripotent stem cell derived organotypic cells with more physiological properties. The quality of the culture conditions and matrix environment is of considerable importance in this regard. In fact, mechanical cues of the extracellular matrix have substantial effects on the cellular physiology. This is especially true if contractile cells such as cardiomyocytes are cultured. Therefore, elastic biomaterials have been introduced as scaffolds in 2D and 3D culture models for different cell types, cardiac cells among them. In this review, key aspects of cell–matrix interaction are highlighted with focus on cardiomyocytes and chemical properties as well as strengths and potential pitfalls in using two commonly applied polymers for soft matrix engineering, polyacrylamide (PAA) and polydimethylsiloxane (PDMS) are discussed.
As in other organs of the human body, the physiological function and organogenesis of the heart are strongly influenced by cellular adhesion, mechanosensing, and signaling, which are mediated by integrins. Members of this superfamily of cell adhesion receptors play key roles in cell adhesion, extracellular matrix (ECM) organization, survival, and proliferation. Twenty-four possible combinations of alpha–beta heterodimers deriving from a minimum of 18 alpha and 8 beta subunits of integrins have been identified in humans to date.1
The simultaneous interaction of many integrin receptors with ECM networks enables cells to acquire chemical and mechanical cues from the surrounding microenvironment. These environmental cues are converted into intracellular signals with a wide range of effects such as differentiation, growth, and energy production. As a result, changes in the microenvironment influence cell phenotype and its fate.2
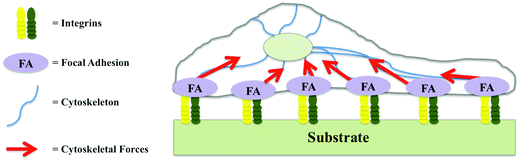
The term integrin adhesion complexes (IACs) is used to describe a range of linker structures connecting different ECM proteins with each cell. These complexes are divided into focal complexes, focal adhesions, and fibrillar adhesions and contain a multitude of highly dynamic components that communicate through hundreds of interactions (Fig. 1).3
Considerable work has focused on the roles of specific constituents of integrin-mediated adhesions, generally termed the “integrin adhesome”, in different pathological conditions.4,5 Mutations in specific components of the adhesome in different human diseases have received special attention, and both experimental as well as in silico methods have provided new perspectives on the molecular mechanisms that cause these pathological conditions, with a view to translation to clinical contexts.6 For example, in the cardiovascular system, antagonists of αIIbβ3 integrin have been used as an anticoagulant drug in millions of patients.7
The main sources of extracellular mechanical signals are other cells, fluid flow, gravity, and polymeric scaffolds in tissue engineering applications.8–10 Cells respond within two minutes to substrates with similar surface topologies but different elastic moduli.11
Integrins act at the molecular level through signaling cascades, which are mediated by integrin-linked kinase (ILK), focal adhesion kinase (FAK), Src kinase, and Rho GTPases (including Rac1, RhoA, and CDC42). These pathways are interconnected; for example, inactivation of RhoA requires Src. As a result, the network output resulting from these signaling pathways can influence the balance between stabilization or remodeling of adherent junctions, and can regulate actin assembly and actomyosin contractility.12–14 Moreover, cellular phenotypes such as cell growth can be influenced by mechanical forces through integrins (Fig. 2).15
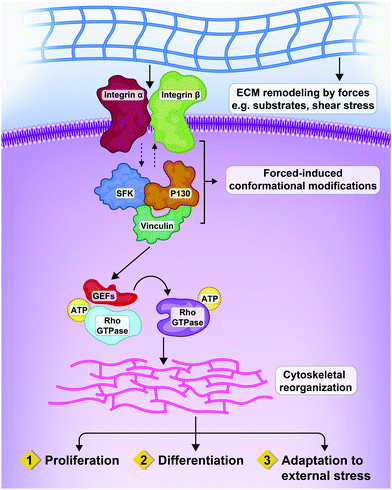 | ||
| Fig. 2 Interactions between extracellular forces, matrix remodeling and integrins resulting in three patterns of cellular response. Mechanical interactions of cells with the ECM by heterodimeric transmembrane receptors called integrins, that are physically connected to cytoskeleton. Mechanical cues from the micro-environment of the cells can impact on cell proliferation, differentiation and adaptation through the Rho GTPases and relevant effectors.16 | ||
Interestingly, most of the intermediate biomolecules noted above, such as GTPase family members and Src, act as molecular crosslinks between integrins and cadherins.17,18 Cadherins mediate cell–cell adhesion by forming adherens junctions, and it has been suggested that cadherins and integrins must communicate effectively to mediate cellular interactions that are necessary for appropriate development.19 For example, during zebrafish development, while integrins are in their inactive conformation status, α5 integrins are physically associated with each other on adjacent cells, and N-cadherin stabilizes the complex of inactive α5 integrins.19,20
Cadherins are a family of mechanosensitive adhesion proteins and expression of specific isoforms of cadherins have an impact on the cellular phenotype.21 β-Catenin is present in adherens junctions (multiprotein scaffolds that couple intercellular contacts with the cytoskeleton) and involved in crosstalk between cadherins, canonical Wnt and transforming growth factor beta-1 (TGF-β1) signaling. Molecular signaling or mechanical forces that disrupt cadherin–cadherin interactions between cells result in release of β-catenin into the cytoplasm where it acts with growth factors of the signaling network.22,23
Integrin-mediated mechanotransduction in cardiac tissue
Cells usually change their structure and function corresponding to mechanical characteristics of the surrounding matrix or cell culture substrates. Therefore, controlling the mechanical features of cell culture substrates crucially depends on cell type and the experimental design. Artificial tissues need to be adapted to the cell- and tissue-specific Young's moduli and potentially viscoelastic properties of the tissue should not be neglected.Cells detect the stiffness of the extracellular environment via integrin-based signalling.24 Integrins play a central role directly as a mechano-transducer to transmit the forces to other related factors or indirectly as a transitional element on pathways triggered by other receptors. It has been widely studied that the effects of mechanical forces on organ physiology are extensive and this is especially important for the cardiovascular system.25
Solid tissues are generally flexible and elastic on a macroscale and small changes can be described by the Young's modulus (E: a measure of the stiffness of an elastic material measured in Pascal). Tissues have a broad range of elasticities i.e. the most flexible is the brain (<1 kPa) and mineralized bone is considered the stiffest.26,27 It should be also pointed out that mechanical properties can differ based upon the resolution with which they are measured. The macroscale elasticity of a tissue reflects its structure on a larger scale and this can be different from the elasticity that a cell faces on a micron scale or smaller.
Numerous ECM proteins can be fabricated on gels with a measurable elasticity. For example, Matrigel which is a combination of secreted proteins (also contains a large amount of growth factors) originated from mouse sarcoma cells. Although these gels can be considered a suitable substrate for investigating of the cell behavior, modifying gels to control stiffness such as density changes and crosslinking can alter the density of the ligands. Indeed, natural materials such as Matrigel have a complex composition that makes it difficult to distinguish between mechanical and biochemical effects.28
Synthetic hydrogels can be simplified with more tangible biological effects. For example, PAA gel coated with a non-fibrillar collagen ligand with a specific density is a commonly used system. And its flexibility can be simply manipulated by varying the crosslinking and/or density of polyacrylamide. Indeed, these systems have been widely used to mimic tissue microenvironments and to investigate cell responses.29
One of the main mechanosensitive components inside muscle cells are called the costamers (Cstms) which are located beneath the sarcolemma (specialized membrane that surrounds striated muscle fiber cells) and is physically attached to the ECM by transmembrane integrins.30 Major function of Cstms is transmission of contractile forces bilaterally from sarcomeres (contractile unit of a striated muscle fiber) to sarcolemma and then to the ECM and finally to neighboring muscle fibers.31
Costamers are composed of three types of multi-protein complexes which are (1) dystrophin–dystroglycan complex, (2) spectrin–ankyrin cytoskeleton complex, and (3) integrin receptor complex.32
ECM–integrin–costameric protein complex is a mechanosensory apparatus. In this complex, the role of the Cstms in addition to attachment is sensing of mechanical forces such as active tension resulting from development of cardiomyocytes – in partnership with focal adhesive complexes – and converting them into biochemical signals leading to sarcomeric assembly leading to gene expression modifications. This form of signaling is known as “outside-in” and represents the major mechanism responsible for adaptive cardiomyocyte growth in response to dynamic loads. It is also noteworthy to mention that stretch-induced deformation of cardiomyocyte integrins initiates the induction and activation of various kinases such as FAK, Src, and Rho to the cytoplasmic side of the focal adhesion complex where they participate in signaling to the nucleus and other organelles.33,34
Integrins are a family of 24 transmembrane αβ heterodimers – at least 18 α and eight β subunits are known in humans – each of them specific to a particular set of ligands in the ECM.35,36 Through a process called integrin activation, which involves conformational changes in the integrin ectodomain, integrin-mediated adhesion commences, causing a low-to-high affinity state shift for ligand binding.37 In addition, responses to substrate stiffness depend heavily on the type of cell. Cardiomyocytes show a strong response, which is reflected as the intact or disrupted assembly of sarcomeres according to the shape of the cells.38
Aberrant integrin expression in the heart is assumed to be linked to severe pathologies. It was found that a specific integrin β1 knockout in ventricular cardiomyocytes resulted in the inability of the murine heart to resist increased hemodynamic loading, accompanied by the development of cardiac fibrosis and dilated cardiomyopathy.39
Recently, Wang and colleagues found that integrin β1 is downregulated in heart tissues of patients with arrhythmogenic right ventricular cardiomyopathy; these authors were able to link low integrin β1 with defective calcium handling by ryanodine receptor type 2 (RyR2).40 In a mouse model (β1D) with integrin-deficient cardiomyocytes, catecholamine-sensitive polymorphic ventricular tachycardia was seen – a finding that further supported the conclusion that impaired integrin signaling can result in cardiac arrhythmia.41 Moreover, altered alpha integrin expression may result in cardiac fibrosis and hypertrophy, as demonstrated in murine models of integrin α11 overexpression and deletion.42
Cardiomyocytes and matrix elasticity
Cardiomyocytes are exposed to different mechanical cues including hemodynamic pressure of the blood, active stretching forces during contraction, and passive elasticity of the ECM. The elastic properties of the matrix have a marked impact on cardiomyocyte physiology. Embryonic cardiomyocytes show stable autonomous contractions on scaffolds with tissue-matched elasticity (11 kPa), but overstrain themselves on substrates with fibrotic scar-like rigidity.38 In neonatal rat cardiomyocytes improved sarcomere alignment, maturation, and force generation were found on gels with a Young's modulus of 10 kPa, whereas on stiffer substrates, stress fibers appeared and sarcomere alignment was reduced.43 Other reports showed evidence of sarcomere fracture in cardiomyocytes grown on gels with a Young's modulus of 30–35 kPa (Fig. 3).44,45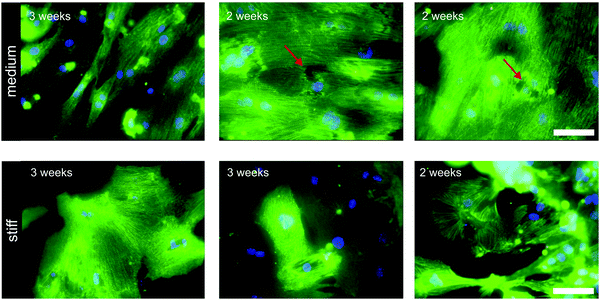 | ||
| Fig. 3 Sarcomeric structure in murine induced pluripotent stem cell derived cardiomyocytes cultured for 2–3 weeks on medium (Young's modulus 35 kPa) and stiff hydrogels (Young's modulus 140 kPa). Immunocytochemical staining of z-discs was performed with antibodies against sarcomeric alpha actinin (green). Red arrows point to cells with sarcomere disruption. Scale bar: 100 μm. Figure with permission from Heras-Bautista CO, N. Mikhael et al.45 | ||
Stem cell-derived cardiomyocytes were successfully cultured on hydrogels with tissue-matched stiffness for up to 7 weeks without loss of quality.46 In contrast, rigid polystyrene (PS) cell culture dishes triggered structural damage and eventually loss of contractility. The fact that long-term cultures of pluripotent stem cell-derived cardiomyocytes have often been obtained on PS can be explained by the observation that the cell layer partially lifts from the substrate during differentiation, and this does not contradict the conclusion that rigid substrates are deleterious to autonomously contracting cells. One recent study deciphered the effect of substrate elasticities matching the properties of fetal, adult, and scarred myocardium on the transcriptome of cardiomyocytes, and found substantial reactivation of ECM genes in cardiomyocytes exposed to a fibrosis-like matrix.45 These examples, and further evidence in the literature, led to the conclusion that matrix elasticity is important to maintain cultured cardiomyocytes in a physiological state.
The mechanisms underlying the ability of cardiomyocytes to sense passive elasticity in the matrix have long remained enigmatic. In 2018, Pandey and colleagues shed light on these mechanisms by showing that cardiomyocytes sense combinations of slow nonmuscular myosin contractions and contractions exerted by fast muscular myosin.47 Intracellularly, mechanical force is converted into cyclic stretching of the adaptor protein talin, which links integrin receptors with the cytoskeleton. Cyclic talin stretching occurs when cells face physiological substrate elasticity, whereas continuous talin stretching is found in cells exposed to stiffer environments. The fact that cardiomyocytes responded to matrix elasticity provides the grounds to reconsider strategies for culturing cells on stiff PS surfaces.
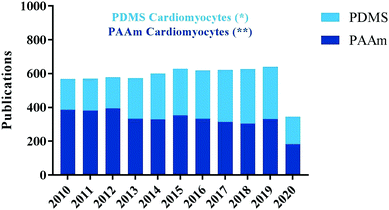
To better investigate the physiology and pathophysiology of cardiomyocytes in vitro, no study is complete without considering chemical factors of the polymers involved and the cellular responses to them. The next part of this review will focus on polyacrylamide (PAA) and polydimethylsiloxane (PDMS), two polymers used frequently for in vitro studies. A recent PubMed search for either “PAA” and “PDMS” with all type of cells ended up with higher number of articles with PAA compared to PDMS. However, when cardiomyocytes used as a keyword articles with PDMS were higher than PAA (Fig. 4).
Chemistry of polyacrylamide and its polymerization
Polyacrylamide [IUPAC poly(2-propenamide), hereafter PAM] is a polymer (–CH2CHCONH2–) formed from acrylamide subunits. Polyacrylamide with only acrylamide monomers is nonionic; other monomers such as acrylate or 2-acrylamido-2-methylpropane sulfonate (AMPS) can be copolymerized at various percentages to form anionic PAM. Among the common co-monomers of cationic PAM are dimethyl diallyl ammonium, ethanaminium (N,N,N-trimethyl-2-((1-oxo-2-propenyl)oxy)) and 1,2-dimethyl-5-vinylpyridinum.48Various methods have been used to synthesize polyacrylamides, such as solution, emulsion, and dispersion polymerization.49,50 However, PAA gels are usually obtained through copolymerization of acrylamide with a bifunctional monomer, or by radical polymerization of acrylamide followed by crosslinking (Fig. 5).51 In general, the three integral components of PAM hydrogel preparations are the monomer, initiator, and crosslinker. Among the factors that influence acrylamide polymerization kinetics, the most important is monomer concentration.52
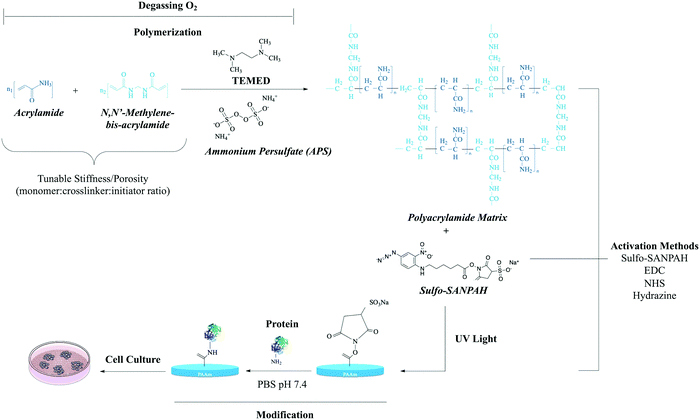 | ||
| Fig. 5 The polymerization reaction of acrylamide.53N,N-Methylene-bis-acrylamide (BIS) acts as a cross-linker between acrylamide monomers. Chemical polymerization initiated by ammonium persulfate (APS) in the presence of tetramethylethylenediamine (TEMED). Stiffness and porosity can be tuned by total concentration of monomer and cross-linker. Sulfo-SANPAH is a heterobifunctional reagent generally used for the covalent conjugation of biomolecules to the PAA structure.29 Carbodiimide (EDC),54 NHS-acrylate29 and hydrazine55 are other conjugation methods to the PAA structure. Abbreviations: sulfo-SANPAH (sulfosuccinimidyl 6-(4-azido-2-nitrophenylamino)hexanoate); EDC(N-(3-dimethylaminopropyl)-N-ethylcarbodiimide hydrochloride); NHS-acrylate (N-hydroxysuccinimidyl acrylate). | ||
To prepare a PAA network structure, the initiator is used as a source of chemical species that reacts with a monomer to create an intermediate compound able to link successively with other monomers.56 The type of initiator has a profound effect on the polymer product. When initiator concentration is increased, the number of active sites available to react with monomers also increases. Consequently, growing oligomers have better contact with monomers, and the possibility of termination reactions increases.57
Chemical polymerization is usually initiated with ammonium persulfate (APS), whereas photochemical polymerization is initiated with riboflavin (or riboflavin-5′-phosphate) as a nontoxic photoinitiator, or with a combination of riboflavin and APS.57 It should be noted that when riboflavin is used, progress of the reaction is easily modified by using different initiator concentrations and light intensities.58 In addition, initiation and polymerization can be catalyzed by tetramethylethylenediamine.59 The free radicals generated from persulfate or riboflavin have oxidation and decomposition potential.58 During copolymerization and crosslinking reactions, monomers are mixed with the multifunctional crosslinking agent, and polymerization is initiated thermally with ultraviolet (UV) irradiation or with a redox initiator system.59
The target gel can be synthesized as a simple linear-chain structure or crosslinked, typically with N,N′-methylenebisacrylamide as a crosslinker.60 In the crosslinked form (the most favorable form) the presence of the monomer is markedly reduced. In other words, a soft insoluble gel forms in a highly water-absorbent milieu by hydration.61 The extent of crosslinking affects the physical and chemical properties of the hydrogel. The parameters altered as a result of crosslinking are mainly elasticity, insolubility, increased glass transition strength and toughness, and transformation of thermoplastics into thermosets. These effects improve the mechanical properties of the hydrogel.62–64
The polymerization of acrylic acid with a water-soluble crosslinker, e.g., 1% N,N′ methylenebisacrylamide in an aqueous solution, is a straightforward process.65 PAM hydrogels can also be crosslinked by gamma irradiation.66
Toxic compounds as crosslinking agents have adverse effects on the environment as well as undesirable reactions with the bioactive substances in the hydrogel matrix. However, these side effects can be prevented by using physical crosslinking, e.g. with radiation or an electron beam. The ability to control the amount of agent through dose manipulation has made radiation methods more suitable in terms of energy efficiency and the absence of undesirable residuals in the products.67,68
Manipulating the surface properties of polyacrylamide
Patterning of polyacrylamide gels
Thin layers of PAM can be used on a rigid substrate such as a cell culture plate or glass coverslip by coating the substrate with aminosilanes to favor adherence and immobilize the PAM hydrogel on the target surface. Because PAM hydrogel is bio-inert, lacking surface receptors or cell interaction sites, conjugating adhesive proteins to its surface is mandatory for cell attachment.69Patterning PAM gels has been suggested as a key approach to optimize the creation of a favorable environment; however, hydrogel characteristics and the aqueous environment are two major obstacles to patterning.69,70 The use of a reducing agent such as hydrazine hydrate to make PAM reactive to oxidized proteins is one suggested option to overcome these obstacles.55
To avoid the use of unstable chemicals such as reactive esters and expensive photoreactive agents such as sulfo-SANPAH for the surface functionalization of PAM hydrogels, a novel PAA hydrogel called hydroxy-polyacrylamide (hydroxy-PAM) has been suggested. Hydroxy-PAM remains stable and active for several weeks, can be mass-produced and easily microprinted, and has high affinity for biomolecules, and so can be used to evaluate the synergistic effects of conjugated ECM proteins on cellular functions. Moreover, hydroxy-PAM hydrogels can be used to quantitatively study the amount of contractile forces exerted by cells on their surrounding microenvironment. However, as in dozens of other microprinting-based methods, the main limitation of hydroxy-PAM hydrogels for microprinting processes is the spatial resolution of protein microfeatures. In other words, on stiff hydrogels (>10 kPa), spatial resolution is on the micrometer scale and is determined by the resolution of the stamp, whereas on “soft” hydrogels, resolution is limited to some extent by surface deformation in the course of protein transfer. Hydroxy-PAM allows the immobilization of any type of ECM protein. Indeed, combining hydroxy-PAM hydrogels with microcontact printing can independently control the shape of single cells, matrix stiffness, and ECM protein density. These features make hydroxy-PAM hydrogels helpful to decipher key mechanisms of complex cellular and tissue processes related to the physicochemical properties of the cell microenvironment, such as wound healing, tissue homeostasis, and the pathogenesis of diseases such as fibrosis and cancer.71,72
One useful method of patterning is soft lithography (comprising a group of techniques), which has been shown to be an efficient approach to patterning for biomaterials. The term “soft lithography” indicates that these techniques fabricate or replicate structures by stamps made from an elastomeric or soft material, most notably PDMS; however, because of its less complicated microfabrication steps and lower oxygen permeability, parylene C is typically used for patterning in PAM hydrogels.73,74
Generally, there are two main strategies for patterning ECM on PAM gels. One is selective activation of the gels for covalent attachment of proteins to activated regions, such as direct surface functionalization by applying UV-reactive, sulfo-SANPAH crosslinkers or polymerizing N-hydroxyacrylamide to the hydrogel surface.72,75,76 The other strategy is copolymerization of ECM proteins in the gels. Fibronectin (FN), laminin (LN) and collagen I are the cell adhesion molecules most commonly used for PAM gel patterning.69 The first approach utilizes expensive functionalization reagents and depends on reagent quality and reaction time. These chemicals are unstable in aqueous media and in the presence of oxygen. However, some functionalization agents can be generated in the laboratory with basic equipment, and this reduces the costs of experiments significantly. A definite advantage of this functionalization approach is its stability for long-term culture experiments. The method of copolymerizing ECM proteins relies on patterning glass coverslips with protein and placing them in direct contact with the hydrogel during polymerization.77 In this method, cells stably adhere to the hydrogel surface and ECM proteins are immobilized, but the mechanisms of protein immobilization, entanglement and chemical binding are not well understood. This approach has been used successfully to functionalize hydrogels with different ECM proteins.78,79
The photoresist lift-off patterning method was reported recently, and makes it possible to control the shape and size of epithelial cells. However, the application of the method is nontrivial.76
Techniques for patterning proteins on PAM are still frequently hampered by the lack of quantitative assessment, which is necessary in order to develop and compare protocols to reliably restrict cells to well-defined shapes. Due to the chemical flexibility of hydrogels and their wide range of applications, the direct patterning of biocompatible gel structures is an approach that merits further investigation.
A final consideration is that the spatial resolution and accuracy of protein patterns directly impact cellular responses, and this is of particular importance in mechanobiological studies.80
Plasma treatment
The formation of polymeric materials under the influence of plasma is called plasma polymerization.81 PAM can be physically adsorbed onto a hydroxylated silicon surface to form a PAM film. These films can also be treated with nitrogen (N2) plasma, and peak intensity is detected at 1214 cm−1 (NH2 stretch vibration), which confirms that N2 can graft to the PA surface in the process of N2 plasma treatment. Furthermore, surface tension is enhanced with increasing plasma grafting time. In the air, surface energy decreases rapidly in the initial phase and eventually reaches equilibrium. This indicates that some of the ions and alkyl radicals adsorbed on the PA surface can rapidly lose their activity. Indeed, the reason for increasing the surface tension of plasma-treated PAM films is to favor covalent bind of the amine groups to the PAM surface.82 Treating the homopolymer surface with plasma can control the solid surface properties in a fast, simple, clean, and non-solvent-dependent manner. In addition, the surface properties of the polymers can be controlled by injecting different types of gases.83,84 The penetration of cold plasma into the top nanometers of the polymer surface results in the formation of a fairly uniform surface with no influence on bulk properties.85In addition, a broad range of compounds can be used as a monomer for plasma polymerization, providing a great diversity of possible surface modifications. Although a number of efforts have resulted in different applications for plasma technology, such as adhesion to composite materials, protective coatings, and plasma printing, polymerization remains a very complex process. Indeed, a number of parameters, including reactor design, power input, monomer flow rate, substrate temperature, chemical composition of the polymers, plasma gases, plasma pressure, and the treatment dose, may limit the application of plasma treatment methods.86–88
Chemistry of polydimethylsiloxane
Polydimethylsiloxane belongs to the group of silicones which comprises silicon, carbon, hydrogen, and oxygen. PDMS is a three-dimensional silicone formed by the crosslinking of linear silicone molecules (Fig. 6). The crosslinking rate and extent of this reaction are significant factors that determine PDMS performance. Non-crosslinked PDMS may be almost liquid or semisolid.89 The chemical formula of PDMS includes a flexible Si–O backbone and a repeating Si(CH3)2O unit. The molecular weight is basically determined by the number of repeating units, and as a result, many of the viscoelastic properties of the material can be defined based on the application of interest. This polymer is also a relatively inexpensive, easy to mold, and very permeable to gases.90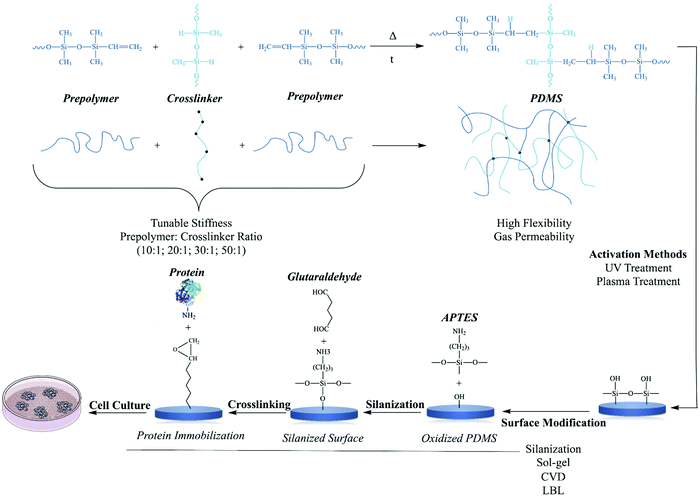 | ||
| Fig. 6 Polymerization reaction between prepolymer and cross-linker.91 UV and plasma treatment are two commonly activation of PDMS surfaces to create hydroxyl (–OH) groups for the modification and the conjugation with molecules and biomolecules.92 Silanization, sol–gel, chemical vapor deposition (CVD) and layer-by-layer are methods for the modification of PDMS polymer for further applications.92 Silanization is given as a model modification of PDMS polymer with proteins for cell culture studies. | ||
Conventional PDMS synthesis usually begins with dichlorosilanes by hydrolysis and condensation, which yields cyclic and linear polymers. This method, however, leads to the synthesis of products with poor molecular weight control which cannot be utilized as model materials for particular purposes. For this reason, in order to better control the molecular parameters, this approach has gradually been replaced by ring opening polymerization of cyclic siloxanes. This process changes a specific cyclic siloxane monomer into a linear siloxane polymer by cleavage of the Si–O–Si bonds in the monomer ring, causing reformation of this bond in the polymer network.93
It should be noted that although PDMS has adjustable stiffness values, it does not promote protein adsorption or cell adhesion due to its hydrophilic nature.
Manipulating the surface properties of polydimethylsiloxane
The initial interaction between biomaterials and cells is determined by chemical composition, surface energy, elastic and viscoelastic properties, and the topography and roughness of biomaterials. Indeed, cells can react to topographical features by changing their morphology and behaviors, such as attachment, migration, and gene expression.94 Examples of topographical features of the target biomaterial that can trigger the cell response at the nano- (less than 100 nm), micro- (100 nm–100 μm), and macro-roughness (100 μm–1 mm) scale are fibers, grooves, ridges, steps, pores, wells, and nodes. A major drawback of PDMS is its hydrophobicity and rapid hydrophobic recovery after surface hydrophilization. Therefore, it is mandatory to improve the surface biocompatibility of PDMS to facilitate long-term cell culture. Because surface wettability is a crucial factor that influences cell adhesion to the substrate, most studies have focused mainly on modifying the PDMS surface to decrease its hydrophobicity. This characteristic of the PDMS surface is easily modified by protein adsorption, plasma oxygenation, or arginylglycylaspartic acid (RGD) peptide conjugation, to create surfaces that promote cellular adhesion and biorecognition.95–98Protein adsorption on the PDMS substrate has been widely used to facilitate cell adhesion because of its intrinsic biocompatibility with proteins, and its molecular recognition properties. However, maintenance of cell adhesions on these surfaces was transitory, and the cells were detached after reaching confluence or else aggregated to form loosely-bound cell clumps.99
Protein adhesion and aggregation during adsorption depends on the interaction between charged domains on the protein and the material surface. These interaction forces (e.g., electrostatic, van der Waals, and hydrophobic) are usually weak and highly susceptible to protein leaching into the medium, so efforts have been made to induce strong, stable covalent linkages between the protein and the material surface.100,101
3-Aminopropyl triethoxy silane (APTES) and glutaraldehyde function as molecular spacers to minimize the direct, weak interactions of proteins with the PDMS surface and to overcome steric hindrance from the environs of the support two essential steps for stronger protein attachment.102 The use of this modified PDMS surface to stabilize cell adhesion and support cell proliferation should study further due to its potential ability to extend the scope of research to include interactions of stable ECM protein-activated surfaces with adherent cells. Reducing PDMS hydrophobicity by oxygen plasma treatment can also improve cell adhesion, although the gains are often short-lived because of hydrophobic recovery.102 Recent evidence showed that plasma treatment of PDMS resulted in consistent surface stiffening at depths of up to 1 μm, whereas stiffness decreased exponentially at depths of 1 mm.103
Another technique to enhance cell adhesion is plasma etching followed by collagen adsorption to convert PDMS from hydrophobic to hydrophilic. The frequently used matrix protein collagen can accumulate on the surface via weak forces (e.g. electrostatic, hydrophobic, and van der Waals), and this can be followed by leaching of the collagen molecules into the solution and nonuniform collagen coating.104
One study used APTES and crosslinker glutaraldehyde (GA) chemistry to immobilize FN and collagen type 1 on PDMS and then evaluate the adhesion and viability of mesenchymal stem cells (MSCs) on the prepared surfaces. Hydrophobicity of the original PDMS was significantly reduced. The adhesion of MSCs was mostly favorable when APTES and glutaraldehyde (APTES + GA) were used. In addition, the spreading area of MSCs was significantly higher on APTES + GA + C1 (collagen type 1) surfaces in comparison to other unmodified or modified PDMS surfaces with C1 adsorption, and there was no significant difference in MSC spreading area on unmodified or modified PDMS surfaces with FN adsorption. These findings indicate that the covalent surface chemical modification of PDMS with APTES + GA protein produced a more biocompatible platform for improved MSC adhesion and proliferation.102
Biocompatibility and cell differentiation on substrate surfaces can also be improved by applying both positively and negatively charged ions. The amount of positive charge on the surface of biomaterials can influence cell behavior. Several studies have shown that cell adhesion and proliferation can be modulated by surface charge density.104,105 For example, as the charge density of hydrogels based on 2-hydroxyethyl methacrylate and 2-methacryloxyethyl trimethyl ammonium chloride copolymer increased, cell adhesion and proliferation improved markedly.105
Studies of polyethylene surfaces with various chargeable functional groups (–COOH, –CH2OH, –CONH2, and –CH2NH2) showed that cell adhesion, growth (in terms of the number of cells attached), and spreading rate were optimized on polar and positively charged surfaces (amine group), whereas on negatively charged surfaces (carboxylic acid group), less growth was observed.106
Cell adhesion can also be modulated through protein adsorption, e.g. integrin binding on negative charged modified surfaces. Several studies have reported that surface charge along with wettability properties influence protein adsorption and cell adhesion.107
Wettability can be considered the main controlling parameter that influences cell behavior on smooth and rough surfaces, compared to the influence of polymer chemistry or the topography of superhydrophobic surfaces. Surface wettability can be easily modified by adjusting roughness.108,109
The molecules of PDMS have low surface energy, with a contact angle of ∼110°. By microstructuring the PDMS surface, contact angles significantly larger than 110° can be obtained. Surface morphology can also be modulated by changing the duration of exposure to UV or ozone radiation. For example, UV irradiation has been used to modify PDMS substrate stiffness from 0.24 MPa to 1.67 MPa or between 0.75 MPa and 2.92 MPa.110,111
Other research found that a micropore size of 1–2 mm is the most suitable for cell adhesion, and that the effect of pore size on cell adhesion is greater than the effect of surface hydrophilicity or hydrophobicity.112 Additionally, PDMS surface roughness can also be easily adjusted by different curing temperatures. Finally, it should be noted that the bulk properties of a biomaterial usually do not change, because surface modification only changes the outermost surface composition.113
Another important note regarding chemical reaction of cells and tissue responses to both PAM and PDMS is that although PAM has adjustable stiffness values, it does not promote protein adsorption or cell adhesion due to its hydrophilic nature. PDMS has shown similar low cell adhesion and proliferation behavior like PAM, however, this time due to its high hydrophobicity.104
Hazard assessment in terms of genotoxicity
A substance with the property of genotoxicity is called a genotoxin, i.e. a substance that has adverse effects on the integrity of the cell's genetic material (DNA, RNA). This may result in various somatic or germline mutations, or chromosomal aberrations all of which imply a substantial risk for developing abnormal phenotypes at the cellular and tissue level, and eventually at the organism level, e.g. apoptosis and cancer.114Rapid progress in the development of new technologies in bioengineering and tissue engineering, especially to obtain scaffolding systems with properties similar to the ECM, is leading to comprehensive evaluations of genetic changes in growing cells in contact with an experimental material intended for use in clinical practice. Recent research has raised concerns about the use of biomaterials because of epigenetic changes. According to its definition, epigenetics means any heritable alteration in genetic expression without changes in the DNA nucleotide sequences. The main alterations include DNA methylation and histone modifications (e.g. methylation and acetylation), and these can result in a broad range of biological processes and diseases. Collectively, these dynamic changes are very specific to tissues and stages of development in response to internal and external stimuli, such as toxicant materials.115,116 These complicated patterns of epigenetic change highlight the importance of epigenomic profiling, with a particular focus on methylation, to answer biological questions.
Currently, most common epigenomic technologies are used to characterize nucleosome-free regions (DNaseI-Seq; MNase-Seq; FAIRE-Seq; ATAC-Seq), protein-mediated DNA interaction sites (Hi-C; 5-C), histone markers and DNA-binding proteins (ChIP-Seq), and DNA methylation (array hybridization, WGBS, MBD-Seq, PacBio, nanopore).117 The use of high-throughput omics technologies to assess potential hazards of materials in tissue engineering is necessary to manage adverse effects on cardiac tissue engineering, especially in light of the scarce literature on this topic. To the best of our knowledge, genotoxicity tests of materials have been limited to traditional molecular techniques such as PCR, while in the era of omics, large-scale massively parallel sequencing, or next-generation sequencing (NGS), have been commercially available for around 10 years and have dramatically changed our understanding of mutagenesis.118
A major challenge in material toxicology is posed by the considerable discrepancies among toxicity studies, owing to different intrinsic properties of materials, cell culture media, and dispersion methods. The latter may have a substantial impact on research results.119 Therefore, a critical step in hazard analysis is the comprehensive assessment of the frequency of somatic mutations in cells after treatment with potentially genotoxic agents or in biopsied tissues from individuals who may have been exposed to such agents. The mutational signatures of genotoxic agents tested in target cells or tissues will help to further elucidate their mechanisms of action.
Sterilization of polyacrylamide and polydimethylsiloxane
Sterilization is a process to inactivate viable microorganisms such as fungi and bacteria, as well as spores and viruses.120 Hydrogels in general, and biodegradable scaffolds specifically, have a high potential for contamination with a variety of living microorganisms, and sterility is a critical condition for the in vitro and in vivo use of scaffold materials. Scaffold sterilization must not alter or damage its biochemical, mechanical or structural properties; ideally sterilization should not affect any of these parameters.121In addition to concerns regarding changes in physicochemical properties caused by different sterilization methods, remaining toxic residues and incomplete elimination of microorganisms are other important concerns in the search for optimal sterilization techniques.122 It is evident that there is no perfect method for sterilization. Therefore, the conditions of different methods should be carefully monitored, and advantages and disadvantages should be considered in light of the specific experimental requirements.
Below we provide an overview of the methods used to sterilize synthetic scaffolds, with emphasis on PDMS and PAM. The classical approach to sterilization is the use of heat, in two key ways: steam (125 °C to 134 °C for up to 20 min) and dry heating (160 °C for 120 min or 180° for 30 min). Although heat is able to remove all types of germs, care should always be taken regarding its side effects, such as alterations in mechanical strength and molecular weight.123 Moreover, dry heat is not suitable for hydrogels or any wet material in general, and heat exposure will result in irreversible denaturation of the matrix proteins used for surface modification. The unmodified polymer itself withstands steam sterilization, as has been shown for PDMS.124
Features that have made irradiation approaches promising candidates for sterilizing biodegradable scaffolds are the low cost, short processing time, and low temperature. Irradiation methods are categorized as ionizing radiation techniques, which include gamma (dosage, 10–30 kGy) and electron beam (dosage, 25–150 kGy) irradiation, and UV (wavelength 200–280 nm), with exposure times ranging from minutes to hours.122,125
A more recent study evaluated the structural modification of PDMS with a molecular weight of 35 kDa by different doses of gamma irradiation. Elastomeric structures with different crosslinked density values were studied as a function of the gamma irradiation dose (250, 300, 350 and 400 kGy). A significant correlation was reported between thermomechanical behavior and the irradiation dose. Thermal stability showed an interesting behavior that indicated a direct correlation between the decomposition temperature and the structure generated by gamma irradiation.126
Gamma irradiation probably induces the formation of reactive species with long-term effects. In polymers, high doses of radiation can cause brittleness and cracks on the surface or promote crosslinking, and even ordinary doses affect the polymer molecular weight, molecular weight distribution, and the physical properties of the treated material.127,128
High energy radiation of commercial PDMS promotes the formation of an infusible and insoluble 3D gel network. Radiation doses beyond the critical gelation dose cause increase in the gel content resulting in the soluble fraction and rubber-like behavior.129
Small-angle neutron scattering and UV-visible spectroscopic techniques were used to study the effects of gamma irradiation on microstructural modifications in PAM hydrogels. This treatment resulted in the presence of nanometer and sub-μm inhomogeneities, and the size of these inhomogeneities was reduced at lower doses.130
Microorganisms show different sensitivities to UV exposure depending on the exposure time and wavelength. The most used wavelength of UV is 260 nm. In the laboratory, UV irradiation is often used to eliminate microorganisms on surfaces. This approach is particularly useful to reduce germs on 2D substrates such as films and foils. For porous hydrogels, UV irradiation is not sufficient and does not eliminate microorganisms inside the scaffold.121 Furthermore, the physicochemical properties of the scaffold may be adversely affected. Because of these undesirable reactions, the optimal UV conditions must be carefully evaluated before full operation. In general, UV irradiation is not the method of choice to sterilize hydrogels.121
Plasma technology, a recent method of scaffold sterilization, involves the use of ions including photons, electrons, positive and negative ions, atoms, free radicals, and nonexcited molecules.131 Low temperature, improved cell interactions, increased wettability of the surface of biodegradable polymers, and straightforward protocols are among the advantages of gas plasma, although drawbacks such as alterations in the chemical and mechanical properties of the polymer and the formation of reactive species should be considered. Bertoldi and colleagues found that the common combination of hydrogen peroxide vapor followed by low-temperature gas plasma is effective in sterilizing polyurethane foams, but reported some degree of material degradation.132 Controlling the exposure time, power, and temperature is important to achieve optimal elimination of a broad range of microorganisms and spores with minimal side effects.133 As plasma sterilization operates under vacuum conditions, it is only suitable for PDMS.
Recently, supercritical carbon dioxide (sCO2) has become popular for the sterilization of delicate materials. The sCO2 method has long been used in the laboratory to dry biological samples for electron microscopy, and its use to eliminate microorganisms from hydrogels is now being explored, with excellent results.134 Supercritical carbon dioxide is able to penetrate deep into the material and reach the inner hydrogel structure. Jiménez et al. have shown that sCO2 efficiently eliminates bacteria while leaving the physicochemical properties of poly(acrylic acid-co-acrylamide) hydrogels almost entirely unaltered.135 The efficiency of this method to reduce pathogens has been investigated in recent years, and sCO2 was shown to substantially reduce germs although it requires additives to reach its full potential.
One additive that can be used for sterilization is peracetic acid (PER). The oxidizing effects of PER increase the effectiveness of sterilization, but may oxidize proteins or peptides used to coat the scaffolds. Although PER bears the risk of oxidizing amino acids, it was found that epidermal growth factor can be sterilized by sCO2 with the addition of PER without losing its biological effect.136 In conclusion, sCO2 with the addition of PER can be considered a powerful yet gentle method to sterilize hydrogels, even with peptide- or protein-based surface modifications.
Cardiovascular applications of other biomaterials
Here, we present a brief overview of other biomaterials especially in terms of cardiovascular applications.The two main classes of scaffolds used in cardiac tissue engineering are synthetic and natural materials. Natural materials readily provide the necessary signals to cells through interactions between intercellular receptors and reacting with matrix molecules. For example, hydrogels derived from natural materials are suitable to apply in tissue engineering applications because of their adjustable mechanical to the natural matrix of the heart.137
The most widely used natural materials are collagen, fibrin, hyaluronic acid (HA), preparations of matrix from Engelbreth–Holm–Swarm-tumor cells (Matrigel) and preparations of native heart matrix.138–143
Biomaterials based on collagen are widely used in cardiac tissue engineering due to its specific physical and chemical properties and rare immunogenicity. Collagen based matrix products are already FDA approved. One important point to consider is the relationship between this natural biomaterial and the target cells, which is of particular importance in iPSC-derived cell populations. Moreover, the purity and batch-to-batch variability are additional critical factors that should be considered.144 Of note, cardiomyocytes do not interact directly with collagen type I and connective tissue cells are required as adaptors.145
Fibrin-based cardiac tissue engineering applications can be a way to solve problems related to cell survival, distribution, delivery of growth factors and revascularization. In addition, the possibility of obtaining it from an autologous source or the possibility of creating a delicate and adjustable structure by changing the conditions of its polymerization are advantageous. It should be noted that new technologies can be used to change its geometric coordinates.146
Hyaluronic acid, which is synthesized by hyaluronan synthases and in normal tissues has different significant roles such as angiogenesis, homeostasis and altered viscoelasticity of extracellular matrix. HA physicochemical properties such as solubility and the availability of reactive functional groups have facilitated its chemical modifications, which has made it an important biocompatible biomaterial for tissue engineering applications. It should also be noted that hyaluronic acid-based biomaterials as well as related bioscaffolds do not cause any allergic reactions or inflammation.147 Yoon et al. reported regenerative effects by using hyaluronic acid-based injectable hydrogels in an ischemic heart model.148
It has been revealed that neonatal rat cardiomyocytes loaded in Matrigel and applied in vivo can be vascularized and mature indicating that immature cells that have received appropriate cues in this scaffold can form tissue with myocardial features.149
Furthermore, the most commonly used synthetic materials are polyesters such as poly(lactic acid), poly(glycolic acid) polylactones, and polyurethanes.150–153 These materials are readily achievable but may be restricted in cellular reactions and therefore often altered by binding to adhesive peptides or releasing biomolecules.
Poly(lactic acid) (PLA) is a synthetic biomaterial-synthesized either by ring opening polymerization or polycondensation – has been shown has a wide range of applications in tissue engineering. It is convenient for medical applications because of degradation into lactic acid which is a metabolite product.154 Many studies have been performed on the use of poly(lactic acid) (PLA) in cardiac tissue engineering. Although there have been barriers such as hydrophobicity and functionalization problems but by combining it with other biomaterials such as poly(glycerol sebacate) these barriers are partially resolved.155
As a biodegradable and biocompatible polyester polyglycolic acid has been approved by the FDA for use in biodegradable structures. Although its use has always been considered in cardiac tissue engineering, the hydrophobicity of its surface has limited cell attachment and cell migration. Further studies are needed to investigate the physicochemical properties and behaviors of derived scaffold composites.156,157
Recent advances in synthetic chemistry have played an important role in the production of hybrid biomaterials with extraordinary conductivity and strength.158 For example, in a study by Shevach et al. gold nanoparticle-decellularized matrix hybrids were developed in terms of cardiac tissue engineering. They showed that cardiac cells engineered within the hybrid scaffolds have elongated and aligned morphology, massive striation, and organized connexin 43 electrical coupling proteins. Moreover, the hybrid patches revealed more suitable function in comparison with pristine patches, such as more powerful contraction force, less excitation threshold, and faster calcium transients.159
In another example carbodiimide-based sequential crosslinking technique was applied to produce aortic valve extracellular matrix (ECM) hybrid scaffolds from collagen type I and HA. The resulting hybrid showed an extensive range of pore size (66–126 μm) which is suitable for valvular tissue regeneration.160
Polyester urethane (PU) also is biocompatible, biodegradable, and flexible with excellent mechanical properties which have introduced it as a major class of elastomers in tissue engineering. The stiffness of the heart muscle at the beginning of diastole varies from 10 kPa to 500 kPa at the end of diastole so an elastomer such as PU that can provide a stiffness in this range may be beneficial in cardiac tissue engineering.161
Insufficient conductivity can be a serious drawback of both synthetic and specially natural biomaterials in the cardiac tissue engineering but recently the use of nanostructured polymers and polymer nanocomposites caused a revolution in the cardiac tissue engineering field and improved electrical and mechanical properties of biomaterial resulting in promoted tissue growth as well.153
There are many studies on the production of conductive hydrogels, which are obtained by combining soft hydrogels and conductive polymers. The controlled electrical properties are useful for the total process of tissue formation.154 For example, adding carbon nanotubes into PDMS can enhance both thermal and electrical properties.155
Furthermore, Hosseinzadeh et al. engineered poly acrylic acid-based hydrogels to create nanofibers using aniline polymerization approach. The resulted composite had a stable electrical conductivity for biological applications.162
Conclusion
The complex relationship between cell networks and hydrogels can potentially influence cardiac-based tissue engineering systems. Hence, a precise understanding is needed of cardiac tissue, especially the components in heterogeneous ECM, as well as detailed knowledge of the structural and chemical properties of biomaterials and how they may react to different sterilization methods. This information can provide ways to control mechanical, compositional, and structural cues in ways the most closely represent the features of the main tissues with minimal hazards. The research reviewed here shows that PDMS and PAM are versatile biomaterials especially suitable for the in vitro study of cardiomyocyte physiology.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
We thank Ms Suzanne Wood for improving the use of English in the manuscript by proofreading.References
- M. Barczyk, S. Carracedo and D. Gullberg, Integrins, Cell Tissue Res., 2010, 339(1), 269 CrossRef CAS.
- N. Alam, H. L. Goel, M. J. Zarif, J. E. Butterfield, H. M. Perkins and B. G. Sansoucy, et al., The integrin—growth factor receptor duet, J. Cell. Physiol., 2007, 213(3), 649–653 CrossRef CAS.
- A. Byron, J. D. Humphries, S. E. Craig, D. Knight and M. J. Humphries, Proteomic analysis of α4β1 integrin adhesion complexes reveals α-subunit-dependent protein recruitment, Proteomics, 2012, 12(13), 2107–2114 CrossRef CAS.
- I. Samaržija, A. Dekanić, J. D. Humphries, M. Paradžik, N. Stojanović and M. J. Humphries, et al., Integrin Crosstalk Contributes to the Complexity of Signalling and Unpredictable Cancer Cell Fates, Cancers, 2020, 12(7), 1910 CrossRef.
- J. Caltagarone, Z. Jing and R. Bowser, Focal adhesions regulate Aβ signaling and cell death in Alzheimer's disease, Biochim. Biophys. Acta, Mol. Basis Dis., 2007, 1772(4), 438–445 CrossRef CAS.
- N. I. Reed, H. Jo, C. Chen, K. Tsujino, T. D. Arnold and W. F. DeGrado, et al., The αvβ1 integrin plays a critical in vivo role in tissue fibrosis, Sci. Transl. Med., 2015, 7(288), 288ra79 CrossRef.
- J. Jing and Y. Sun, An αIIbβ3- and phosphatidylserine (PS)-binding recombinant fusion protein promotes PS-dependent anticoagulation and integrin-dependent antithrombosis, J. Biol. Chem., 2019, 294(17), 6670–6684 CrossRef CAS.
- A. Sarasa-Renedo and M. Chiquet, Mechanical signals regulating extracellular matrix gene expression in fibroblasts, Scand. J. Med. Sci. Sports, 2005, 15(4), 223–230 CrossRef CAS.
- G. Halder, S. Dupont and S. Piccolo, Transduction of mechanical and cytoskeletal cues by YAP and TAZ, Nat. Rev. Mol. Cell Biol., 2012, 13(9), 591 CrossRef CAS.
- T. L. Jenkins and D. Little, Synthetic scaffolds for musculoskeletal tissue engineering: cellular responses to fiber parameters, npj Regener. Med., 2019, 4(1), 15 CrossRef.
- S. F. B. Mennens, M. Bolomini-Vittori, J. Weiden, B. Joosten, A. Cambi and K. van den Dries, Substrate stiffness influences phenotype and function of human antigen-presenting dendritic cells, Sci. Rep., 2017, 7(1), 17511 CrossRef.
- C. D. Lawson and K. Burridge, The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration, Small GTPases, 2014, 5(1), e27958 CrossRef.
- S. K. Mitra and D. D. Schlaepfer, Integrin-regulated FAK–Src signaling in normal and cancer cells, Curr. Opin. Cell Biol., 2006, 18(5), 516–523 CrossRef CAS.
- L. S. Price, J. Leng, M. A. Schwartz and G. M. Bokoch, Activation of Rac and Cdc42 by integrins mediates cell spreading, Mol. Biol. Cell, 1998, 9(7), 1863–1871 CrossRef CAS.
- W. T. Arthur, N. K. Noren and K. Burridge, Regulation of Rho family GTPases by cell-cell and cell-matrix adhesion, Biol. Res., 2002, 35(2), 239–246 CAS.
- Y. H. Bae, K. L. Mui, B. Y. Hsu, S.-L. Liu, A. Cretu and Z. Razinia, et al., A FAK-Cas-Rac-lamellipodin signaling module transduces extracellular matrix stiffness into mechanosensitive cell cycling, Sci. Signaling, 2014, 7(330), ra57 CrossRef.
- W. T. Arthur, L. A. Petch and K. Burridge, Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism, Curr. Biol., 2000, 10(12), 719–722 CrossRef CAS.
- K. A. DeMali, K. Wennerberg and K. Burridge, Integrin signaling to the actin cytoskeleton, Curr. Opin. Cell Biol., 2003, 15(5), 572–582 CrossRef CAS.
- D. Jülich, G. Cobb, A. M. Melo, P. McMillen, A. K. Lawton and S. G. J. Mochrie, et al., Cross-scale integrin regulation organizes ECM and tissue topology, Dev. Cell, 2015, 34(1), 33–44 CrossRef.
- K. L. Mui, C. S. Chen and R. K. Assoian, The mechanical regulation of integrin–cadherin crosstalk organizes cells, signaling and forces, J. Cell Sci., 2016, 129(6), 1093–1100 CrossRef CAS.
- M. Conacci-Sorrell, I. Simcha, T. Ben-Yedidia, J. Blechman, P. Savagner and A. Ben-Ze’ev, Autoregulation of E-cadherin expression by cadherin–cadherin interactions: the roles of β-catenin signaling, Slug, and MAPK, J. Cell Biol., 2003, 163(4), 847–857 CrossRef CAS.
- X. Xu, L. Zheng, Q. Yuan, G. Zhen, J. L. Crane and X. Zhou, et al., Transforming growth factor-β in stem cells and tissue homeostasis, Bone Res., 2018, 6(1), 1–31 CrossRef CAS.
- W. J. Nelson and R. Nusse, Convergence of Wnt, β-catenin, and cadherin pathways, Science, 2004, 303(5663), 1483–1487 CrossRef CAS.
- R. O. Hynes, Integrins: a family of cell surface receptors, Cell, 1987, 48, 549–554 CrossRef CAS.
- T. D. Ross, B. G. Coon, S. Yun, N. Baeyens, K. Tanaka and M. Ouyang, et al., Integrins in mechanotransduction, Curr. Opin. Cell Biol., 2013, 25(5), 613–618 CrossRef CAS.
- P. C. Georges, W. J. Miller, D. F. Meaney, E. S. Sawyer and P. A. Janmey, Matrices with compliance comparable to that of brain tissue select neuronal over glial growth in mixed cortical cultures, Biophys. J., 2006, 90(8), 3012–3018 CrossRef CAS.
- T. S. Keller and D. M. Spengler, Bending strength, stiffness and tissue physical properties of human compact bone, J. Biomech., 1990, 23(7), 741 CrossRef.
- S. Vukicevic, H. K. Kleinman, F. P. Luyten, A. B. Roberts, N. S. Roche and A. H. Reddi, Identification of multiple active growth factors in basement membrane Matrigel suggests caution in interpretation of cellular activity related to extracellular matrix components, Exp. Cell Res., 1992, 202(1), 1–8 CrossRef CAS.
- R. J. Pelham and Y. Wang, Cell locomotion and focal adhesions are regulated by substrate flexibility, Proc. Natl. Acad. Sci. U. S. A., 1997, 94(25), 13661–13665 CrossRef CAS.
- A. M. Samarel, Costameres, focal adhesions, and cardiomyocyte mechanotransduction, Am. J. Physiol. Circ. Physiol., 2005, 289(6), H2291–H2301 CrossRef CAS.
- C. R. Shear and R. J. Bloch, Vinculin in subsarcolemmal densities in chicken skeletal muscle: localization and relationship to intracellular and extracellular structures, J. Cell Biol., 1985, 101(1), 240–256 CrossRef CAS.
- J. M. Ervasti, Costameres: the Achilles’ heel of Herculean muscle, J. Biol. Chem., 2003, 278(16), 13591–13594 CrossRef CAS.
- H. Mansour, P. P. de Tombe, A. M. Samarel and B. Russell, Restoration of resting sarcomere length after uniaxial static strain is regulated by protein kinase Cε and focal adhesion kinase, Circ. Res., 2004, 94(5), 642–649 CrossRef CAS.
- A. S. Torsoni, T. M. Marin, L. A. Velloso and K. G. Franchini, RhoA/ROCK signaling is critical to FAK activation by cyclic stretch in cardiac myocytes, Am. J. Physiol. Circ. Physiol., 2005, 289(4), H1488–H1496 CrossRef CAS.
- R. O. Hynes, Integrins: bidirectional, allosteric signaling machines, Cell, 2002, 110(6), 673–687 CrossRef CAS.
- Y. Takada, X. Ye and S. Simon, The integrins, Genome Biol., 2007, 8(5), 215 CrossRef.
- Y. Su, W. Xia, J. Li, T. Walz, M. J. Humphries and D. Vestweber, et al., Relating conformation to function in integrin α5β1, Proc. Natl. Acad. Sci. U. S. A., 2016, 113(27), E3872–E3881 CrossRef CAS.
- A. J. Engler, C. Carag-Krieger, C. P. Johnson, M. Raab, H.-Y. Tang and D. W. Speicher, et al., Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating, J. Cell Sci., 2008, 121(22), 3794–3802 CrossRef CAS.
- S.-Y. Shai, A. E. Harpf, C. J. Babbitt, M. C. Jordan, M. C. Fishbein and J. Chen, et al., Cardiac myocyte-specific excision of the β1 integrin gene results in myocardial fibrosis and cardiac failure, Circ. Res., 2002, 90(4), 458–464 CrossRef CAS.
- Y. Wang, C. Li, L. Shi, X. Chen, C. Cui and J. Huang, et al., Integrin β1D Deficiency-Mediated RyR2 Dysfunction Contributes to Catecholamine-Sensitive Ventricular Tachycardia in ARVC, Circulation, 2020, 141(18), 1477–1493 CrossRef CAS.
- Y. Wang, C. Li, L. Shi, X. Chen, C. Cui and J. Huang, et al., Integrin β1D Deficiency-Mediated RyR2 Dysfunction Contributes to Catecholamine-Sensitive Ventricular Tachycardia in Arrhythmogenic Right Ventricular Cardiomyopathy, Circulation, 2020, 141(18), 1477–1493 CrossRef CAS.
- A. Romaine, I. W. Sørensen, C. Zeltz, N. Lu, P. M. Erusappan and A. O. Melleby, et al., Overexpression of integrin α11 induces cardiac fibrosis in mice, Acta Physiol., 2018, 222(2), e12932 CrossRef.
- J. G. Jacot, A. D. McCulloch and J. H. Omens, Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes, Biophys. J., 2008, 95(7), 3479–3487 CrossRef CAS.
- A. J. S. Ribeiro, Y.-S. Ang, J.-D. Fu, R. N. Rivas, T. M. A. Mohamed and G. C. Higgs, et al., Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness, Proc. Natl. Acad. Sci. U. S. A., 2015, 112(41), 12705–12710 CrossRef CAS.
- C. O. Heras-Bautista, N. Mikhael, J. Lam, V. Shinde, A. Katsen-Globa and S. Dieluweit, et al., Cardiomyocytes facing fibrotic conditions re-express extracellular matrix transcripts, Acta Biomater., 2019, 89, 180–192 CrossRef CAS.
- C. O. Heras-Bautista, A. Katsen-Globa, N. E. Schloerer, S. Dieluweit, O. M. A. El Aziz and G. Peinkofer, et al., The influence of physiological matrix conditions on permanent culture of induced pluripotent stem cell-derived cardiomyocytes, Biomaterials, 2014, 35(26), 7374–7385 CrossRef CAS.
- P. Pandey, W. Hawkes, J. Hu, W. V. Megone, J. Gautrot and N. Anilkumar, et al., Cardiomyocytes sense matrix rigidity through a combination of muscle and non-muscle myosin contractions, Dev. Cell, 2018, 44(3), 326–336 CrossRef CAS.
- A. Tangri, Polyacrylamide based hydrogels: synthesis, characterization and applications, Int. J. Pharm., Chem. Biol. Sci., 2014, 4(4), 951–959 Search PubMed.
- H. Jamshidi and A. Rabiee, Synthesis and characterization of acrylamide-based anionic copolymer and investigation of solution properties, Adv. Mater. Sci. Eng., 2014, 2014, 728675 Search PubMed.
- J. Lu, B. Peng, M. Li, M. Lin and Z. Dong, Dispersion polymerization of anionic polyacrylamide in an aqueous salt medium, Pet. Sci., 2010, 7(3), 410–415 CrossRef CAS.
- M. A. Mohsin and N. F. Attia, Inverse emulsion polymerization for the synthesis of high molecular weight polyacrylamide and its application as sand stabilizer, Int. J. Polym. Sci., 2015, 2015, 436583 Search PubMed.
- D. T. Valente and A. P. de Aguiar, Synthesis of polyacrylamide in aqueous solution: solubility, Orbital: Electron. J. Chem., 2012, 4(1), 84–85 Search PubMed.
- C. E. Kandow, P. C. Georges, P. A. Janmey and K. A. Beningo, Polyacrylamide hydrogels for cell mechanics: steps toward optimization and alternative uses, Methods Cell Biol., 2007, 83, 29–46 CAS.
- K. A. Beningo and Y. Wang, Fc-receptor-mediated phagocytosis is regulated by mechanical properties of the target, J. Cell Sci., 2002, 115(4), 849–856 CAS.
- V. Damljanovic, B. Christoffer Lagerholm and K. Jacobson, Bulk and micropatterned conjugation of extracellular matrix proteins to characterized polyacrylamide substrates for cell mechanotransduction assays, Biotechniques, 2005, 39(6), 847–851 CrossRef CAS.
- F. Yan, C. Zheng, X. Zhai and D. Zhao, Preparation and characterization of polyacrylamide in cationic microemulsion, J. Appl. Polym. Sci., 1998, 67(4), 747–754 CAS.
- S. Yamamoto, F. Okada, M. Kinoshita and S. Suzuki, On-line microchip electrophoresis-mediated preconcentration of cationic compounds utilizing cationic polyacrylamide gels fabricated by in situ photopolymerization, Analyst, 2018, 143(18), 4429–4435 RSC.
- R. R. Batchelor, G. Kwandou, P. T. Spicer and M. H. Stenzel, (−)-Riboflavin (vitamin B2) in the thiol–ene polymerisation of PEG-based hydrogels, Polym. Chem., 2017, 8(6), 980–984 RSC.
- D. Benda, J. Šňupárek and V. Čermák, Inverse emulsion polymerization of acrylamide and salts of acrylic acid, Eur. Polym. J., 1997, 33(8), 1345–1352 CrossRef CAS.
- J. Bartoň, S. Kawamoto, K. Fujimoto, H. Kawaguchi and I. Capek, Preparation of partly hydrophobized, crosslinked polyacrylamide particles by terpolymerization of acrylamide/N,N-methylenebisacrylamide/styrene in inverse microemulsion, Polym. Int., 2000, 49(4), 358–366 CrossRef.
- T. K. Mudiyanselage and D. C. Neckers, Highly absorbing superabsorbent polymer, J. Polym. Sci., Part A: Polym. Chem., 2008, 46(4), 1357–1364 CrossRef CAS.
- K. Studer, C. Decker, E. Beck, R. Schwalm and N. Gruber, Redox and photoinitiated crosslinking polymerization: I. Dual-cure isocyanate-acrylate system, Prog. Org. Coat., 2005, 53(2), 126–133 CrossRef CAS.
- S. K. Singh, A. Dhyani and D. Juyal, Hydrogel: Preparation, Characterization and Applications, Pharma Innov., 2017, 6(6, Part A), 25 CAS.
- J. Maitra and V. K. Shukla, Cross-linking in hydrogels-a review, Am. J. Polym. Sci., 2014, 4(2), 25–31 Search PubMed.
- W. Liu, X. Zhu, X. Yang, K. Li and Z. Yang, Preparation of highly cross-linked hydrophilic porous microspheres poly(N,N′-methylenebisacrylamide) and poly(N,N′-methylenebisacrylamide-co-acrylic acid) with an application on the removal of cadmium, Polym. Adv. Technol., 2018, 29(11), 2724–2734 CrossRef CAS.
- T. Ogawa, Gamma ray-induced crosslinking of polyacrylamide in the solid state, J. Polym. Sci., Part B: Polym. Lett., 1983, 21(8), 615–620 Search PubMed.
- E. Jabari and S. Nouzari, Synthesis of acrylic acid hydrogel by gamma-irradiation cross-linking of polyacrylic acid in aqueous solution, Iran. Polym. J., 1999, 8(4), 263–270 Search PubMed.
- F. Jiang, X. Wang, C. He, S. Saricilar and H. Wang, Mechanical properties of tough hydrogels synthesized with a facile simultaneous radiation polymerization and cross-linking method, Radiat. Phys. Chem., 2015, 106, 7–15 CrossRef CAS.
- X. Tang, M. Y. Ali and M. T. A. Saif, A novel technique for micro-patterning proteins and cells on polyacrylamide gels, Soft Matter, 2012, 8(27), 7197–7206 RSC.
- F. Di Benedetto, A. Biasco, D. Pisignano and R. Cingolani, Patterning polyacrylamide hydrogels by soft lithography, Nanotechnology, 2005, 16(5), S165 CrossRef CAS.
- T. Grevesse, M. Versaevel and S. Gabriele, Preparation of hydroxy-PAAm hydrogels for decoupling the effects of mechanotransduction cues, J. Visualized Exp., 2014, 90, e51010 Search PubMed.
- T. Grevesse, M. Versaevel, G. Circelli, S. Desprez and S. Gabriele, A simple route to functionalize polyacrylamide hydrogels for the independent tuning of mechanotransduction cues, Lab Chip, 2013, 13(5), 777–780 RSC.
- I. Sanzari, M. Callisti, A. De Grazia, D. J. Evans, T. Polcar and T. Prodromakis, Parylene C topographic micropattern as a template for patterning PDMS and Polyacrylamide hydrogel, Sci. Rep., 2017, 7(1), 5764 CrossRef . Available from: http://www.nature.com/articles/s41598-017-05434-6.
- C. Rao, T. Prodromakis, L. Kolker, U. A. R. Chaudhry, T. Trantidou and A. Sridhar, et al., The effect of microgrooved culture substrates on calcium cycling of cardiac myocytes derived from human induced pluripotent stem cells, Biomaterials, 2013, 34(10), 2399–2411 CrossRef CAS . Available from: http://www.ncbi.nlm.nih.gov/pubmed/23261219.
- J. R. Tse and A. J. Engler, Preparation of hydrogel substrates with tunable mechanical properties, Curr. Protoc. Cell Biol., 2010, 47(1), 10–16 Search PubMed.
- J. Moeller, A. K. Denisin, J. Y. Sim, R. E. Wilson, A. J. S. Ribeiro and B. L. Pruitt, Controlling cell shape on hydrogels using lift-off protein patterning, PLoS One, 2018, 13(1), e0189901 CrossRef.
- T. Vignaud, H. Ennomani and M. Théry, Polyacrylamide hydrogel micropatterning, Methods in cell biology, Elsevier, 2014, pp. 93–116 Search PubMed.
- J. M. S. Garcia, A. Panitch and S. Calve, N-terminal Specific Conjugation of Extracellular Matrix Proteins to 2-Pyridinecarboxaldehyde Functionalized Polyacrylamide Hydrogels, Acta Biomater., 2019, 84, 169–179 CrossRef.
- J. I. MacDonald, H. K. Munch, T. Moore and M. B. Francis, One-step site-specific modification of native proteins with 2-pyridinecarboxyaldehydes, Nat. Chem. Biol., 2015, 11(5), 326–331 CrossRef CAS.
- J. Y. Sim, J. Moeller, K. C. Hart, D. Ramallo, V. Vogel and A. R. Dunn, et al., Spatial distribution of cell–cell and cell–ECM adhesions regulates force balance while maintaining E-cadherin molecular tension in cell pairs, Mol. Biol. Cell, 2015, 26(13), 2456–2465 CrossRef.
- H. K. Yasuda, Plasma polymerization, Academic Press, 2012 Search PubMed.
- Z. Chen, X. Lu, C.-M. Chan and Y. Mi, Manipulating the surface properties of polyacrylamide with nitrogen plasma, Eur. Polym. J., 2006, 42(11), 2914–2920 CrossRef CAS.
- R. W. Paynter, XPS studies of the ageing of plasma-treated polymer surfaces, Surf. Interface Anal., 2000, 29(1), 56–64 CrossRef CAS.
- B. Olander, A. Wirsén and A.-C. Albertsson, Argon microwave plasma treatment and subsequent hydrosilylation grafting as a way to obtain silicone biomaterials with well-defined surface structures, Biomacromolecules, 2002, 3(3), 505–510 CrossRef CAS.
- C.-M. Chan, T.-M. Ko and H. Hiraoka, Polymer surface modification by plasmas and photons, Surf. Sci. Rep., 1996, 24(1–2), 1–54 CrossRef CAS.
- N. Hasirci, T. Endogan, E. Vardar, A. Kiziltay and V. Hasirci, Effect of oxygen plasma on surface properties and biocompatibility of PLGA films, Surf. Interface Anal., 2010, 42(6–7), 486–491 CrossRef CAS.
- T. Jacobs, R. Morent, N. De Geyter, P. Dubruel and C. Leys, Plasma surface modification of biomedical polymers: influence on cell-material interaction, Plasma Chem. Plasma Process., 2012, 32(5), 1039–1073 CrossRef CAS.
- M. R. Wertheimer, L. Martinu and E. M. Liston, Plasma surface modification of polymers for improved adhesion: a critical review, J. Adhes. Sci. Technol., 1993, 7(10), 1091–1127 CrossRef.
- T. R. E. Simpson, B. Parbhoo and J. L. Keddie, The dependence of the rate of crosslinking in poly (dimethyl siloxane) on the thickness of coatings, Polymer, 2003, 44(17), 4829–4838 CrossRef CAS.
- A. Izuka, H. H. Winter and T. Hashimoto, Molecular weight dependence of viscoelasticity of polycaprolactone critical gels, Macromolecules, 1992, 25(9), 2422–2428 CrossRef CAS.
- N. Tucher, Analysis of photonic structures for silicon solar cells, Albert-Ludwigs-Universität Freiburg, 2016 Search PubMed.
- J. Zhou, A. V. Ellis and N. H. Voelcker, Recent developments in PDMS surface modification for microfluidic devices, Electrophoresis, 2010, 31(1), 2–16 CrossRef CAS.
- J. Chojnowski, Ring opening polymerization of cyclosiloxanes, in Silicon Compd Silanes Silicones, ed. A. Larson, Gelest, 2004, pp. 389–405 Search PubMed.
- M. Ferrari and F. Cirisano, Mammalian Cell Behavior on Hydrophobic Substrates: Influence of Surface Properties, Colloids Interfaces, 2019, 3(2), 48 CrossRef CAS.
- A. Klymov, L. Prodanov, E. Lamers, J. A. Jansen and X. F. Walboomers, Understanding the role of nano-topography on the surface of a bone-implant, Biomater. Sci., 2013, 1(2), 135–151 RSC.
- Y. J. Chuah, S. Kuddannaya, M. H. A. Lee, Y. Zhang and Y. Kang, The effects of poly (dimethylsiloxane) surface silanization on the mesenchymal stem cell fate, Biomater. Sci., 2015, 3(2), 383–390 RSC.
- M. Nishikawa, T. Yamamoto, N. Kojima, K. Kikuo, T. Fujii and Y. Sakai, Stable immobilization of rat hepatocytes as hemispheroids onto collagen-conjugated poly-dimethylsiloxane (PDMS) surfaces: importance of direct oxygenation through PDMS for both formation and function, Biotechnol. Bioeng., 2008, 99(6), 1472–1481 CrossRef CAS.
- B. Li, J. Chen and J. H. Wang, RGD peptide-conjugated poly (dimethylsiloxane) promotes adhesion, proliferation, and collagen secretion of human fibroblasts, J. Biomed. Mater. Res., Part A, 2006, 79(4), 989–998 CrossRef.
- K. Y. Chumbimuni-Torres, R. E. Coronado, A. M. Mfuh, C. Castro-Guerrero, M. F. Silva and G. R. Negrete, et al., Adsorption of proteins to thin-films of PDMS and its effect on the adhesion of human endothelial cells, RSC Adv., 2011, 1(4), 706–714 RSC.
- A. Gökaltun, Y. B. A. Kang, M. L. Yarmush, O. B. Usta and A. Asatekin, Simple Surface Modification of Poly (dimethylsiloxane) via Surface Segregating Smart Polymers for Biomicrofluidics, Sci. Rep., 2019, 9(1), 1–14 CrossRef.
- A. Gokaltun, M. L. Yarmush, A. Asatekin and O. B. Usta, Recent advances in nonbiofouling PDMS surface modification strategies applicable to microfluidic technology, Technology, 2017, 5(01), 1–12 CrossRef.
- S. Kuddannaya, Y. J. Chuah, M. H. A. Lee, N. V. Menon, Y. Kang and Y. Zhang, Surface chemical modification of poly (dimethylsiloxane) for the enhanced adhesion and proliferation of mesenchymal stem cells, ACS Appl. Mater. Interfaces, 2013, 5(19), 9777–9784 CrossRef CAS.
- G. Bartalena, Y. Loosli, T. Zambelli and J. G. Snedeker, Biomaterial surface modifications can dominate cell–substrate mechanics: the impact of PDMS plasma treatment on a quantitative assay of cell stiffness, Soft Matter, 2012, 8(3), 673–681 RSC.
- Y. J. Chuah, Y. T. Koh, K. Lim, N. V. Menon, Y. Wu and Y. Kang, Simple surface engineering of polydimethylsiloxane with polydopamine for stabilized mesenchymal stem cell adhesion and multipotency, Sci. Rep., 2015, 5(1), 1–12 Search PubMed.
- I. De Luca, A. Di Salle, N. Alessio, S. Margarucci, M. Simeone and U. Galderisi, et al., Positively charged polymers modulate the fate of human mesenchymal stromal cells via ephrinB2/EphB4 signaling, Stem Cell Res., 2016, 17(2), 248–255 CrossRef CAS.
- J. H. Lee, H. W. Jung, I.-K. Kang and H. B. Lee, Cell behaviour on polymer surfaces with different functional groups, Biomaterials, 1994, 15(9), 705–711 CrossRef CAS.
- J. H. Lee, J. W. Lee, G. Khang and H. B. Lee, Interaction of cells on chargeable functional group gradient surfaces, Biomaterials, 1997, 18(4), 351–358 CrossRef CAS.
- S. Guo, X. Zhu, M. Li, L. Shi, J. L. T. Ong and D. Jańczewski, et al., Parallel control over surface charge and wettability using polyelectrolyte architecture: effect on protein adsorption and cell adhesion, ACS Appl. Mater. Interfaces, 2016, 8(44), 30552–30563 CrossRef CAS.
- B. N. Lourenço, G. Marchioli, W. Song, R. L. Reis, C. A. van Blitterswijk and M. Karperien, et al., Wettability influences cell behavior on superhydrophobic surfaces with different topographies, Biointerphases, 2012, 7(1), 46 CrossRef.
- S. Prauzner-Bechcicki, J. Raczkowska, E. Madej, J. Pabijan, J. Lukes and J. Sepitka, et al., PDMS substrate stiffness affects the morphology and growth profiles of cancerous prostate and melanoma cells, J. Mech. Behav. Biomed. Mater., 2015, 41, 13–22 CrossRef.
- J. Raczkowska, S. Prauzner-Bechcicki, J. Lukes, J. Sepitka, A. Bernasik and K. Awsiuk, et al., Physico-chemical properties of PDMS surfaces suitable as substrates for cell cultures, Appl. Surf. Sci., 2016, 389, 247–254 CrossRef CAS.
- C. M. Murphy, M. G. Haugh and F. J. O’brien, The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering, Biomaterials, 2010, 31(3), 461–466 CrossRef CAS.
- J. Cabrera, M. Ruiz, M. Fascio, N. D’Accorso, R. Mincheva and P. Dubois, et al., Increased surface roughness in polydimethylsiloxane films by physical and chemical methods, Polymers, 2017, 9(8), 331 CrossRef.
- R. P. Araldi, T. C. de Melo, T. B. Mendes, P. L. de Sá Júnior, B. H. N. Nozima and E. T. Ito, et al., Using the comet and micronucleus assays for genotoxicity studies: a review, Biomed. Pharmacother., 2015, 72, 74–82 CrossRef CAS.
- M. Szyf, Nongenetic inheritance and transgenerational epigenetics, Trends Mol. Med., 2015, 21(2), 134–144 CrossRef.
- B. Smolkova, M. Dusinska and A. Gabelova, Nanomedicine and epigenome. Possible health risks, Food Chem. Toxicol., 2017, 109, 780–796 CrossRef CAS.
- L. T. Kagohara, G. L. Stein-O’Brien, D. Kelley, E. Flam, H. C. Wick and L. V. Danilova, et al., Epigenetic regulation of gene expression in cancer: techniques, resources and analysis, Briefings Funct. Genomics, 2017, 17(1), 49–63 CrossRef.
- A. Y. Maslov, W. Quispe-Tintaya, T. Gorbacheva, R. R. White and J. Vijg, High-throughput sequencing in mutation detection: a new generation of genotoxicity tests?, Mutat. Res., Fundam. Mol. Mech. Mutagen., 2015, 776, 136–143 CrossRef CAS.
- A. Marucco, F. Catalano, I. Fenoglio, F. Turci, G. Martra and B. Fubini, Possible chemical source of discrepancy between in vitro and in vivo tests in nanotoxicology caused by strong adsorption of buffer components, Chem. Res. Toxicol., 2015, 28(1), 87–91 Search PubMed.
- W. A. Rutala and D. J. Weber, Disinfection, sterilization, and antisepsis: An overview, Am. J. Infect. Control, 2016, 44(5), e1–e6 CrossRef CAS.
- C. Fischbach, J. Tessmar, A. Lucke, E. Schnell, G. Schmeer and T. Blunk, et al., Does UV irradiation affect polymer properties relevant to tissue engineering?, Surf. Sci., 2001, 491(3), 333–345 CrossRef CAS.
- Z. Dai, J. Ronholm, Y. Tian, B. Sethi and X. Cao, Sterilization techniques for biodegradable scaffolds in tissue engineering applications, J. Tissue Eng., 2016, 7 DOI:10.1177/2041731416648810.
- S. Gogolewski and P. Mainil-Varlet, Effect of thermal treatment on sterility, molecular and mechanical properties of various polylactides: 2. Poly (l/d-lactide) and poly (l/dl-lactide), Biomaterials, 1997, 18(3), 251–255 CrossRef CAS.
- C. Yavuz, S. N. B. Oliaei, B. Cetin and O. Yesil-Celiktas, Sterilization of PMMA microfluidic chips by various techniques and investigation of material characteristics, J. Supercrit. Fluids, 2016, 107, 114–121 CrossRef CAS.
- R. Navarro, G. Burillo, E. Adem and A. Marcos-Fernández, Effect of ionizing radiation on the chemical structure and the physical properties of polycaprolactones of different molecular weight, Polymers, 2018, 10(4), 397 CrossRef.
- M. Meléndez-Zamudio, A. Villegas, J. A. González-Calderón, R. Meléndrez, M. Meléndez-Lira and J. Cervantes, Study of a Polydimethylsiloxane (PDMS) Elastomer Generated by γ Irradiation: Correlation Between Properties (Thermal and Mechanical) and Structure (Crosslink Density Value), J. Inorg. Organomet. Polym. Mater., 2017, 27(3), 622–632 CrossRef.
- A. A. Miller, Radiation chemistry of polydimethylsiloxane. 1 I. Crosslinking and gas yields, J. Am. Chem. Soc., 1960, 82(14), 3519–3523 CrossRef CAS.
- I. R. Williams, M. B. Mayor and J. P. Collier, The impact of sterilization method on wear in knee arthroplasty, Clin. Orthop. Relat. Res., 1998, 356, 170–180 CrossRef.
- A. J. Satti, N. A. Andreucetti, J. A. Ressia, M. F. Vallat, C. Sarmoria and E. M. Vallés, Modelling molecular weight changes induced in polydimethylsiloxane by gamma and electron beam irradiation, Eur. Polym. J., 2008, 44(5), 1548–1555 CrossRef CAS.
- M. Sivanantham, B. V. R. Tata and V. K. Aswal, Structural investigation on gamma-irradiated polyacrylamide hydrogels using small-angle neutron scattering and ultraviolet–visible spectroscopy, Pramana, 2016, 86(3), 609–615 CrossRef CAS.
- S. G. Wise, A. Waterhouse, A. Kondyurin, M. M. Bilek and A. S. Weiss, Plasma-based biofunctionalization of vascular implants, Nanomedicine, 2012, 7(12), 1907–1916 CrossRef CAS.
- S. Bertoldi, S. Fare, H. J. Haugen and M. C. Tanzi, Exploiting novel sterilization techniques for porous polyurethane scaffolds, J. Mater. Sci.: Mater. Med., 2015, 26(5), 182 CrossRef.
- C. E. Holy, C. Cheng, J. E. Davies and M. S. Shoichet, Optimizing the sterilization of PLGA scaffolds for use in tissue engineering, Biomaterials, 2000, 22(1), 25–31 CrossRef.
- A. Bernhardt, M. Wehrl, B. Paul, T. Hochmuth, M. Schumacher and K. Schütz, et al., Improved sterilization of sensitive biomaterials with supercritical carbon dioxide at low temperature, PLoS One, 2015, 10(6), e0129205 CrossRef.
- A. Jimenez, J. Zhang and M. A. Matthews, Evaluation of CO2-based cold sterilization of a model hydrogel, Biotechnol. Bioeng., 2008, 101(6), 1344–1352 CrossRef CAS.
- P. Du, W. Liu, H. Cao, H. Zhao and C.-H. Huang, Sterilization of epidermal growth factor with supercritical carbon dioxide and peracetic acid; analysis of changes at the amino acid and protein level, Water Res. X, 2018, 1, 100002 CrossRef.
- A. Marsano, R. Maidhof, L. Q. Wan, Y. Wang, J. Gao and N. Tandon, et al., Scaffold stiffness affects the contractile function of three-dimensional engineered cardiac constructs, Biotechnol. Prog., 2010, 26(5), 1382–1390 CrossRef CAS.
- W.-H. Zimmermann, I. Melnychenko, G. Wasmeier, M. Didié, H. Naito and U. Nixdorff, et al., Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts, Nat. Med., 2006, 12(4), 452–458 CrossRef CAS.
- K. L. Christman, A. J. Vardanian, Q. Fang, R. E. Sievers, H. H. Fok and R. J. Lee, Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium, J. Am. Coll. Cardiol., 2004, 44(3), 654–660 CrossRef CAS.
- A. Khademhosseini, G. Eng, J. Yeh, P. A. Kucharczyk, R. Langer and G. Vunjak-Novakovic, et al., Microfluidic patterning for fabrication of contractile cardiac organoids, Biomed. Microdevices, 2007, 9(2), 149–157 CrossRef.
- M. A. Laflamme, K. Y. Chen, A. V. Naumova, V. Muskheli, J. A. Fugate and S. K. Dupras, et al., Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts, Nat. Biotechnol., 2007, 25(9), 1015–1024 CrossRef CAS.
- J. M. Singelyn, P. Sundaramurthy, T. D. Johnson, P. J. Schup-Magoffin, D. P. Hu and D. M. Faulk, et al., Catheter-deliverable hydrogel derived from decellularized ventricular extracellular matrix increases endogenous cardiomyocytes and preserves cardiac function post-myocardial infarction, J. Am. Coll. Cardiol., 2012, 59(8), 751–763 CrossRef CAS.
- K. L. Christman, J. M. Singelyn, M. Salvatore, P. J. Schup-Magoffin, D. P. Hu and T. Johnson, et al., Catheter-Deliverable Hydrogel Derived from Decellularized Ventricular Extracellular Matrix Increases Cardiomyocyte Survival and Preserves Cardiac Function Post-Myocardial Infarction, J. Am. Coll. Cardiol., 2011, 57(14S), E2017 CrossRef.
- N. J. Kaiser, R. J. Kant, A. J. Minor and K. L. K. Coulombe, Optimizing blended collagen-fibrin hydrogels for cardiac tissue engineering with human iPSC-derived cardiomyocytes, ACS Biomater. Sci. Eng., 2018, 5(2), 887–899 CrossRef.
- K. Pfannkuche, S. Neuss, F. Pillekamp, L. P. Frenzel, W. Attia and T. Hannes, et al., Fibroblasts facilitate the engraftment of embryonic stem cell-derived cardiomyocytes on three-dimensional collagen matrices and aggregation in hanging drops, Stem Cells Dev., 2010, 19(10), 1589–1599 CrossRef CAS.
- M. C. Barsotti, F. Felice, A. Balbarini and R. Di Stefano, Fibrin as a scaffold for cardiac tissue engineering, Biotechnol. Appl. Biochem., 2011, 58(5), 301–310 CrossRef CAS.
- M. Hemshekhar, R. M. Thushara, S. Chandranayaka, L. S. Sherman, K. Kemparaju and K. S. Girish, Emerging roles of hyaluronic acid bioscaffolds in tissue engineering and regenerative medicine, Int. J. Biol. Macromol., 2016, 86, 917–928 CrossRef CAS.
- S. J. Yoon, Y. H. Fang, C. H. Lim, B. S. Kim, H. S. Son and Y. Park, et al., Regeneration of ischemic heart using hyaluronic acid-based injectable hydrogel, J. Biomed. Mater. Res., Part B, 2009, 91(1), 163–171 CrossRef.
- A. N. Morritt, S. K. Bortolotto, R. J. Dilley, X. Han, A. R. Kompa and D. McCombe, et al., Cardiac tissue engineering in an in vivo vascularized chamber, Circulation, 2007, 115(3), 353–360 CrossRef.
- R. L. Carrier, M. Papadaki, M. Rupnick, F. J. Schoen, N. Bursac and R. Langer, et al., Cardiac tissue engineering: cell seeding, cultivation parameters, and tissue construct characterization, Biotechnol. Bioeng., 1999, 64(5), 580–589 CrossRef CAS.
- O. Ishii, M. Shin, T. Sueda and J. P. Vacanti, In vitro tissue engineering of a cardiac graft using a degradable scaffold with an extracellular matrix–like topography, J. Thorac. Cardiovasc. Surg., 2005, 130(5), 1358–1363 CrossRef.
- T. C. McDevitt, M. A. Laflamme and C. E. Murry, Proliferation of cardiomyocytes derived from human embryonic stem cells is mediated via the IGF/PI 3-kinase/Akt signaling pathway, J. Mol. Cell. Cardiol., 2005, 39(6), 865–873 CrossRef CAS.
- A. Marsano, R. Maidhof, J. Luo, K. Fujikara, E. E. Konofagou and A. Banfi, et al., The effect of controlled expression of VEGF by transduced myoblasts in a cardiac patch on vascularization in a mouse model of myocardial infarction, Biomaterials, 2013, 34(2), 393–401 CrossRef CAS.
- M. S. Lopes, A. L. Jardini and R. Maciel Filho, Poly (lactic acid) production for tissue engineering applications, Procedia Eng., 2012, 42, 1402–1413 CrossRef.
- F. Flaig, H. Ragot, A. Simon, G. Revet, M. Kitsara and L. Kitasato, et al., Design of functional electrospun scaffolds based on poly (glycerol sebacate) elastomer and poly (lactic acid) for cardiac tissue engineering, ACS Biomater. Sci. Eng., 2020, 6(4), 2388–2400 CrossRef CAS.
- H. Park, M. Radisic, J. O. Lim, B. H. Chang and G. Vunjak-Novakovic, A novel composite scaffold for cardiac tissue engineering, In Vitro Cell. Dev. Biol., 2005, 41(7), 188–196 CrossRef CAS.
- M. Generali, D. Kehl, A. K. Capulli, K. K. Parker, S. P. Hoerstrup and B. Weber, Comparative analysis of poly-glycolic acid-based hybrid polymer starter matrices for in vitro tissue engineering, Colloids Surf., B, 2017, 158, 203–212 CrossRef CAS.
- D. Li, T. Liu, X. Yu, D. Wu and Z. Su, Fabrication of graphene–biomacromolecule hybrid materials for tissue engineering application, Polym. Chem., 2017, 8(30), 4309–4321 RSC.
- M. Shevach, S. Fleischer, A. Shapira and T. Dvir, Gold nanoparticle-decellularized matrix hybrids for cardiac tissue engineering, Nano Lett., 2014, 14(10), 5792–5796 CrossRef CAS.
- R. Nazir, A. Bruyneel, C. Carr and J. Czernuszka, Collagen type I and hyaluronic acid based hybrid scaffolds for heart valve tissue engineering, Biopolymers, 2019, 110(8), e23278 CrossRef.
- G. R. da Silva, A. da Silva-Cunha Jr, F. Behar-Cohen, E. Ayres and R. L. Oréfice, Biodegradation of polyurethanes and nanocomposites to non-cytotoxic degradation products, Polym. Degrad. Stab., 2010, 95(4), 491–499 CrossRef.
- S. Hosseinzadeh, S. M. Rezayat, E. Vashegani-Farahani, M. Mahmoudifard, S. Zamanlui and M. Soleimani, Nanofibrous hydrogel with stable electrical conductivity for biological applications, Polymer, 2016, 97, 205–216 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2021 |