Open Access Article
Open Access ArticleChains of cubic colloids at fluid–fluid interfaces†
Carmine
Anzivino
 *a,
Giuseppe
Soligno
*a,
Giuseppe
Soligno
 b,
René
van Roij
b,
René
van Roij
 c and
Marjolein
Dijkstra
c and
Marjolein
Dijkstra
 a
a
aSoft Condensed Matter, Debye Institute for Nanomaterials Science, Utrecht University, Princetonplein 1, 3584 CC Utrecht, The Netherlands. E-mail: m.dijkstra@uu.nl; c.anzivino@uu.nl
bCondensed Matter and Interfaces, Debye Institute for Nanomaterials Science, Utrecht University, Princetonplein 1, 3584 CC Utrecht, The Netherlands
cInstitute for Theoretical Physics, Utrecht University, Princetonplein 5, 3584 CC Utrecht, The Netherlands
First published on 26th November 2020
Abstract
Inspired by recent experimental observations of spontaneous chain formation of cubic particles adsorbed at a fluid–fluid interface, we theoretically investigate whether capillary interactions can be responsible for this self-assembly process. We calculate adsorption energies, equilibrium particle orientations, and interfacial deformations, not only for a variety of contact angles but also for single cubes as well as an infinite 2D lattice of cubes at the interface. This allows us to construct a ground-state phase diagram as a function of areal density for several contact angles, and upon combining the capillary energy of a 2D lattice with a simple expression for the entropy of a 2D fluid we also construct temperature–density or size–density phase diagrams that exhibit large two-phase regions and triple points. We identify several regimes with stable chainlike structures, in line with the experimental observations.
I. Introduction
Capillary-driven self-assembly of colloidal particles trapped at a fluid–fluid interface has recently gained a great deal of attention in the soft-matter community.1–3 Indeed, by controlling the strength and the directionality of capillary interactions, quasi-2D ordered structures at interfaces can be obtained, which have interesting applications ranging from the stabilization of foams and Pickering emulsions4 to the development of new materials.5 Several strategies to control capillarity have been elaborated in recent years. These include the use of particles with surface chemical heterogeneities, i.e. patchy and Janus particles,6–10 the application of external electric or magnetic fields,11 and the employment of interfaces with a finite curvature.12 Another popular method to control capillarity consists of considering particles with anisotropic shapes. Indeed, because of the constraint imposed by Young's law,13 nonspherical colloidal particles adsorbed at a fluid–fluid interface induce interfacial deformations that determine their orientations and generate mutual capillary interactions. Different anisotropic shapes induce different deformation fields such that the particles shape plays a key role in regulating the self-assembly of colloids trapped at an interface.Since a large variety of colloidal particles is nowadays available, with tunable shapes and surface functionalizations,14,15 the behavior at interfaces of nm- and μm-sized particles with several anisotropic shapes, e.g. rods, cylinders, cubes, dumbbells and discs16–22 has been widely studied in the past few years. Among the several shapes studied so far, cubic particles have been proven to form 2D structures with striking features. In recent experiments23,24 for example, polystyrene cubes with 30 μm edge length have been reported to self-assemble at a water–air interface into three distinct structures, namely square close-packed, linear, and hexagonal structures. Furthermore, the relative stability of these three phases has been shown to strongly depend on their wettability, which is specified by the so-called Young contact angle θ.13 While hydrophilic cubes with a small θ ∼ 0 give rise to square close-packed aggregates, by increasing θ first linear aggregates and finally hexagonal aggregates are observed. Cases of coexistence between linear and hexagonal aggregates have also been reported.23,24
By noting that the cubes display different single-particle configurations in the different observed structures, i.e. a “face up” configuration in the square close-packed structure, an “edge up” configuration in the linear structure, and a “vertex up” configuration in the hexagonal structure, the authors of ref. 23 and 24 have argued that the formation of each 2D structure could be caused by a different kind of deformation field induced by the cubes at the interface. In particular these authors have proposed that cubes adsorbed in the “face up”, “edge up”, and “vertex up” configurations would induce monopolar, quadrupolar and hexapolar deformation fields, respectively.
The scenario experimentally found in ref. 23 and 24 represents, at the same time, a confirmation and an extension of the theoretical predictions previously presented in ref. 25 and 26. Here it was shown that cubic colloidal particles with a Young contact angle θ = π/2 indeed adsorb at a fluid–fluid interface with one vertex pointing upward with respect to the interface plane (“vertex up” configuration), and induce a hexapolar deformation field in the interface height profile which causes nm-sized cubes to self-assemble into thermodynamically-stable “vertex up” honeycomb, square, and hexagonal lattices. Crucial for these findings was the development of a numerical method27 that takes into account interfacial deformations. This method allows to go beyond the so-called Pieranski approximation28 which assumes the fluid–fluid interface to be flat even when a particle is adsorbed. Although often used in the literature,29–33 Pieranski's approximation can lead to erroneous predictions, and in the case of cubes it cannot predict the equilibrium hexapolar-generating “vertex up” configuration when θ = π/2.25
Despite the importance that linear aggregates of cubes trapped at a fluid–fluid interface appear to have in technological applications,23,24 no theoretical investigation on this kind of aggregates has been performed so far. To fill this gap, we extend in this paper the theoretical calculations of ref. 25 and 26 in order to understand under what conditions linear chains of cubes could be more stable than other 2D structures. To this aim we first study the deformation field induced by a single adsorbed cube as a function of the Young contact angle θ and of the particle configuration. We find that, below θ = π/2, a regime of contact angles exists in which the speculation of ref. 23 and 24 concerning a quadrupolar deformation field induced by a particle adsorbed in the “edge up” configuration is correct. Then, by computing the capillary interactions acting among a 2D lattice of N → ∞ cubes, we find that in this regime the quadrupolar deformation field leads to the formation of stable linear aggregates which replace the hexagonal patterns observed at θ = π/2. Besides the linear chains we also find stable “edge up” square structures. However, for θ below a certain threshold, we find that both the linear and “edge up” square structures become metastable with respect to close-packed square aggregates, in line with the experimental findings. Nevertheless, in contrast to speculations in ref. 23 and 24, the stabilization of the close-packed square aggregates appears to be driven by packing entropy rather than by a monopolar deformation field. Finally, we introduce a simple free-energy model to estimate the interplay between capillary interactions and particle entropy. In this way we aim to include in our analysis the case of nm-sized particles for which, in contrast with μm-sized particles, entropic effects cannot be neglected. We summarize our results in a series of temperature–density or size–density phase diagrams.
The paper is organized as follows: after briefly reviewing our numerical method in Section II, we report our results in Section III, and present our conclusions and a discussion in Section IV.
II. Numerical method
In this section, we briefly review the numerical method developed in ref. 27. We refer to ref. 34 for more details, and to ref. 25, 26 and 35–38 for other examples of its application to colloidal particles at fluid–fluid interfaces and to a droplet in contact with heterogeneous surfaces.We consider two immiscible, homogeneous, and incompressible fluids, separated by a fluid–fluid interface of surface tension γ. We introduce a Cartesian coordinate system x, y, z, and by convention call fluid 1 and fluid 2 the fluid at z → +∞ and z → −∞, respectively. We assume the fluid–fluid interface as a 2D, possibly curved surface with zero thickness, coinciding with a plane parallel to z = 0 when no particles are adsorbed. In the case that colloidal particles are adsorbed, the equilibrium shape of the fluid–fluid interface is given by the Young–Laplace equation, with Young's law as a boundary condition for the Young contact angle θ.27 This is the angle formed by the fluid–fluid interface along the three-phase contact line, i.e. where the fluid–fluid interface encounters the solid surface of the adsorbed particles. Calling γ1 and γ2 the solid–fluid surface tension of the particles with fluid 1 and fluid 2, respectively, Young's law states that θ (measured inside fluid 2) is given by cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = (γ1 − γ2)/γ.
θ = (γ1 − γ2)/γ.
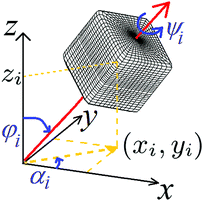
In our formalism we rewrite the force balance described by the Young–Laplace equation as a minimum condition of a suitable (free) energy, and perform the minimization numerically. To this aim we represent the fluid–fluid interface as a grid of points, and the fixed position and orientation of N adsorbed particles by a 6N-dimensional vector Ω = (Ω1,…ΩN), with Ωi = (xi, yi, zi, φi, ψi, αi) defining the configuration of the i-th particle (i = 1,…N). As shown in Fig. 1, we consider smooth-edge cubic particles in this paper. The in-plane Cartesian coordinates of the center of mass of the i-th particle is denoted by xi and yi, zi denotes the difference between the Cartesian coordinate z of the center of mass and the interface height far away from the particle, φi the polar angle of the vertical axis with respect to the far-field interface normal, ψi the angle of the particle around its vertical axis, and αi is the azimuthal angle of the vertical axis in the interface plane. Moreover we write the free energy EN of a system of N colloidal particles adsorbed at a fluid–fluid interface as27
EN(Ω) = γ(S(Ω) − A + W(Ω)cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ), θ), | (1) |
We now adopt the following protocol: first we fix the contact angle θ. Then, by varying Ω to minimize EN, we obtain the equilibrium particle configuration and the corresponding shape of the fluid–fluid interface, at fixed contact angle θ. We repeat this protocol for several θ in order to investigate different particle wettabilities. However, it is important to note that in this paper we are interested in the deformation fields induced by equilibrium configurations as well as by (metastable) non-equilibrium ones.
III. Results
In this section we investigate whether we can find conditions for colloidal cubes to assemble into stable linear chains rather than into other 2D structures, like square, hexagonal, or honeycomb lattices. We first study the deformation field induced by a single adsorbed cube as a function of the Young contact angle θ and of the particle configuration. Then we numerically compute the capillary interactions acting among a 2D lattice of N → ∞ cubes adsorbed at an interface and perform a stability analysis among the several phases that the cubes can form. Finally, by using a simple free-energy model, we summarize our results in a series of density–temperature phase diagrams. Here the temperature is actually the dimensionless combination kBT/(γΣ), which in practise is seen as an inverse particle size.Following ref. 25 and 26 we will refer to the “vertex up”, “edge up”, and “face up” configurations, as the {111}, {110}, and {100} configurations, respectively.
A. Hexapolar and quadrupolar deformation fields
As stated in the introduction, in ref. 23 and 24 it has been suggested that the self-assembly of micronscale cubes trapped at a fluid–fluid interface into hexagonal aggregates and linear ones would be caused by two different kinds of deformation fields induced by the adsorbed particles. More specifically, the stabilization of the hexagonal phase would be caused by a hexapolar deformation field generated by cubes in the {111} configuration while the stabilization of the linear phase would be caused by a quadrupolar deformation field generated by cubes in the {110} configuration. While these suggestions agree with the theoretical calculations performed so far in the former case, they disagree in the latter case. Indeed, in ref. 25 and 26 it has been theoretically predicted that the deformation field induced by a cube adsorbed in the {111} configuration is dominated by the hexapolar mode, but it has also been found that the same cube adsorbed in the {110} configuration does not induce any interfacial deformation. However, these calculations have been performed only for cubes with contact angle θ = π/2. Since the wettability has been experimentally proven to play a decisive role in the stabilization of the various phases at the interface, we here extend the calculations performed in ref. 25 and 26 of the deformation field induced by a single cube adsorbed at a fluid–fluid interface, from the case θ = π/2 to smaller values of θ.As explained in Section II, the configuration of a single adsorbed cube at the interface, is given by Ω = (x, y, z, φ, ψ, α), where the subscript “1” can be omitted since we consider only a single particle (i.e. N = 1), and the equilibrium shape of the fluid–fluid interface is the one that minimizes the free energy E1 (eqn (1)), for fixed values of Ω and θ. We note that symmetry considerations in the case of a single particle show that the energy E1 is independent of x, y, and α, such that the particle configuration is specified by only φ, ψ, and z. Furthermore the equilibrium height z of the cube at the interface is automatically found by our numerical method, for each fixed φ, ψ, and θ, by allowing the two fluids to exchange volume while minimizing E1.38 The {111} configuration is specified by ψ{111} = π/4, φ{111} = π/3.29, while the {110} configuration by ψ{110} = 0, φ{110} = π/4. Note that we consider φ{111} = π/3.29 rather than φ{111} = π/4 since the former configuration has a (slightly) lower energy than the latter one.25,26 In the following we will indicate the energies of a single cube adsorbed in the {111} and {110} configurations with E{111}1 and E{110}1, respectively.
The results of the minimization procedure for cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ ∈ {0, 0.2, 0.4, 0.6} are shown in Fig. 2 and 3. We find that the deformation field induced by the adsorption process strongly depends on the contact angle θ. In agreement with ref. 25 and 26, we find that for cos
θ ∈ {0, 0.2, 0.4, 0.6} are shown in Fig. 2 and 3. We find that the deformation field induced by the adsorption process strongly depends on the contact angle θ. In agreement with ref. 25 and 26, we find that for cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 0 a cube adsorbed in the {111} configuration generates a hexapolar deformation field consisting of three rises and three depressions, exhibiting a 3-fold symmetry around the particle (see Fig. 2(a) and 3(a)), while the same particle adsorbed in the {110} configuration does not induce any deformation (see Fig. 2(e) and 3(e)). The situation changes by changing the contact angle. By decreasing θ, the intensity of the hexapolar deformation field decreases until it essentially vanishes for cos
θ = 0 a cube adsorbed in the {111} configuration generates a hexapolar deformation field consisting of three rises and three depressions, exhibiting a 3-fold symmetry around the particle (see Fig. 2(a) and 3(a)), while the same particle adsorbed in the {110} configuration does not induce any deformation (see Fig. 2(e) and 3(e)). The situation changes by changing the contact angle. By decreasing θ, the intensity of the hexapolar deformation field decreases until it essentially vanishes for cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 0.6 (see Fig. 2(d) and 3(d)). In this case the fluid–fluid interface becomes actually flat. By contrast, the flat interface observed for the {110} configuration at cos
θ = 0.6 (see Fig. 2(d) and 3(d)). In this case the fluid–fluid interface becomes actually flat. By contrast, the flat interface observed for the {110} configuration at cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 0 evolves into a quadrupolar deformation field consisting of two rises and two depressions, showing a 2-fold symmetry around the particle, for larger cos
θ = 0 evolves into a quadrupolar deformation field consisting of two rises and two depressions, showing a 2-fold symmetry around the particle, for larger cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ. In particular the intensity of the quadrupole increases with increasing cos
θ. In particular the intensity of the quadrupole increases with increasing cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ. Note that the quadrupolar deformation field for cos
θ. Note that the quadrupolar deformation field for cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 0.6 has two depressions that are more spread and less intense than the two rises. As a consequence the depressions can hardly be appreciated in the contour plot of Fig. 3(h). Nevertheless the presence of the quadrupole at cos
θ = 0.6 has two depressions that are more spread and less intense than the two rises. As a consequence the depressions can hardly be appreciated in the contour plot of Fig. 3(h). Nevertheless the presence of the quadrupole at cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 0.6 can clearly be seen in the 3D plot of Fig. S1 of the ESI.†
θ = 0.6 can clearly be seen in the 3D plot of Fig. S1 of the ESI.†
 | ||
| Fig. 3 Contour plot of the deformation field induced by a single cube of side length L adsorbed at a fluid–fluid interface in the “vertex up” {111} and “edge up” {110} configuration, respectively, for the same Young contact angles θ as in Fig. 2. | ||
In addition to the deformation fields induced by a single cube adsorbed in the {111} and {110} configurations, we employ our numerical method to compute the deformation field induced by a single cube adsorbed in the {100} configuration (ψ{100} = 0, φ{100} = 0), for several values of the contact angle θ. We indicate the energy of a single cube adsorbed in this configuration with E{100}1. As shown in Fig. S2 of the ESI,† we find that in these cases the cube never deforms the interface. This finding disagrees with the speculation of ref. 23 and 24, where it was suggested that cubes with 30 μm edge length and a very small contact angle θ ∼ 0 would induce a monopolar deformation field at the interface, when adsorbed in the {100} configuration. This does not appear to be the case. Indeed, by considering typical values for the mass density difference between the particle and the fluids δ ∼ 103 kg m−3, the fluid–fluid surface tension γ ∼ 10−2 N m−1, and (the modulus of) the gravitational acceleration g on planet Earth, we obtain a Bond number  for a cubic particle with side length L ≪ 1 mm. It follows that a monopolar mode is not expected for a sub-mm sized particle at its equilibrium adsorption configuration, unless non-gravitational external forces act on the particle.
for a cubic particle with side length L ≪ 1 mm. It follows that a monopolar mode is not expected for a sub-mm sized particle at its equilibrium adsorption configuration, unless non-gravitational external forces act on the particle.
We plot E{111}1, E{110}1, and E{100}1 as a function of the contact angle θ in Fig. 4. We observe that the particle configurations discussed above are not always equilibrium configurations. Indeed, the minimal single-particle adsorption energy is E{111}1 for cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ ≤ 0.25 and E{100}1 for cos
θ ≤ 0.25 and E{100}1 for cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ > 0.25. It follows that the equilibrium (minimal (free) energy) configuration of a single adsorbed cube is the {111} in the former case, and the {100} in the latter case. One could therefore naively think that the linear-chain phase will never be stable at an interface since the quadrupolar-generating {110} single-particle configuration is always metastable with respect to the {111} and the {100} ones. However, this is not necessarily true because when more particles are adsorbed at an interface, capillary interactions have to be considered such that an equilibrium phase of many particles can be stabilized even though the corresponding single-particle configuration is not the stable one. In particular we will see that a regime exists for cos
θ > 0.25. It follows that the equilibrium (minimal (free) energy) configuration of a single adsorbed cube is the {111} in the former case, and the {100} in the latter case. One could therefore naively think that the linear-chain phase will never be stable at an interface since the quadrupolar-generating {110} single-particle configuration is always metastable with respect to the {111} and the {100} ones. However, this is not necessarily true because when more particles are adsorbed at an interface, capillary interactions have to be considered such that an equilibrium phase of many particles can be stabilized even though the corresponding single-particle configuration is not the stable one. In particular we will see that a regime exists for cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ ≥ 0.35 in which the quadrupolar and the hexapolar deformation fields induced by cubes in the {110} and {111} configurations, respectively, have comparable intensities. As we will show in the next subsection it is in this regime that the linear-chain phase of {110} particles is stable, even though the single-particle equilibrium configuration is the {100} one in this regime of contact angles θ.
θ ≥ 0.35 in which the quadrupolar and the hexapolar deformation fields induced by cubes in the {110} and {111} configurations, respectively, have comparable intensities. As we will show in the next subsection it is in this regime that the linear-chain phase of {110} particles is stable, even though the single-particle equilibrium configuration is the {100} one in this regime of contact angles θ.
Furthermore we observe that for cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ ≥ 0.55, E{100}1 ≪ E{110}1 < E{111}1, with E{110}1 and E{111}1 approaching zero. In other words the adsorption energies of cubes in the {111} and {110} configuration are very weak in this regime of contact angles and, as we will show, this results in weak capillary interactions.
θ ≥ 0.55, E{100}1 ≪ E{110}1 < E{111}1, with E{110}1 and E{111}1 approaching zero. In other words the adsorption energies of cubes in the {111} and {110} configuration are very weak in this regime of contact angles and, as we will show, this results in weak capillary interactions.
B. Stable chains of cubes at a fluid–fluid interface
Two or more cubes adsorbed in the hexapolar-generating {111} and in the quadrupolar-generating {110} configurations give rise to different kinds of energetically-favorable bonds. This can be understood by considering that a pair of cubes (N = 2) adsorbed at a fluid–fluid interface can attract or repel each other, depending on their relative azimuthal orientation |α1 − α2|. Attractive relative orientations are those that allow overlap of interfacial deformations with the same sign, i.e. rises with other rises and depressions with other depressions. In this case the fluid–fluid surface area, and hence the energy E2 (eqn (1)), decrease as the particles approach each other. Repulsive relative orientations, instead, are those in which interfacial deformations with the opposite sign, i.e. rises with depressions, overlap. In this case, the fluid–fluid surface area, and hence the energy E2 (eqn (1)), increase as the particles approach each other.As already explained in ref. 25 and 26 and as shown in Fig. 5, it follows that N = 2 cubes adsorbed in the {111} configuration can form two different kinds of energetically-favorable bonds: a dipole–dipole bond, when a rise-depression dipole of one hexapole overlaps with a rise-depression dipole of the other hexapole (see Fig. 5(a)), and a tripole–tripole bond, when either a rise-depression-rise or a depression-rise–depression tripole of one hexapole overlaps with, respectively, a rise-depression-rise or a depression-rise–depression tripole of the other hexapole (see Fig. 5(b)). By contrast, two cubes adsorbed in the {110} configuration can only form a monopole–monopole bond, when a rise monopole of one quadrupole overlaps with a rise monopole of the other quadrupole (see Fig. 5(c)), or when a depression monopole of one quadrupole overlaps with a depression monopole of the other quadrupole (see Fig. 5(d)).
The different energetically-favorable bonds induced by cubes in the {111} and {110} configuration, respectively, lead in turn to the formation of different phases at the interface, as illustrated in Fig. 6. In particular, a regular lattice of N → ∞ cubes adsorbed in the {111} configuration can lead to the formation of three distinct 2D phases, namely hexagonal (x), honeycomb (h), and “vertex up” square (sv) phases. In the x phase each particle forms a dipole–dipole bond with each of its six nearest neighbors (see Fig. 6(a)), in the h phase each particle forms a tripole–tripole bond with each of its three nearest neighbors (see Fig. 6(b)), in the sv phase each particle forms a dipole–dipole bond with two opposite nearest neighbors and a tripole–tripole bond with the two remaining neighbors (see Fig. 6(c)). By constrast, N → ∞ cubes adsorbed in the {110} configuration can lead to the formation of two distinct phases, namely chains (c), and “edge up” square (se) phases. In the c phase each particle forms a monopole–monopole bond with only two neighbors, along a single linear “preferred” direction (see Fig. 6(d)), where the particles do not interact along the direction perpendicular to the principal one. In the se phase, each particle forms a monopole–monopole bond with four nearest neighbors, i.e. the cubes not only interact along a “preferred” direction as in the case of the chains, but also perpendicularly to this (see Fig. 6(e)).
We find that in principle two different chain phases exist. In the first case the chains are obtained by overlapping rises with rises, in the second case by overlapping depressions with depressions. As shown in Fig. S3 of the ESI,† we find that the pair interaction acting between two cubes with overlapping rises is much stronger than the pair interaction acting between two cubes with overlapping depressions. As a consequence it is more favorable for cubes to assemble into chains with overlapping rises rather than overlapping depressions, and we will only consider the former case throughout the paper.
Finally no capillary interactions act among N → ∞ cubes adsorbed in the {100} configuration, since cubes in this configuration do not deform the interface. The formation of a “face up” square phase sf (see Fig. 6(f)) is then entropy-driven at sufficiently high areal densities.
The intensity of the (capillary) interaction energy EN acting among a 2D lattice of N → ∞ cubes in the several phases indicated above changes with the contact angle θ, and consequently different phases stabilize at different θ. To show this, we compute EN for the various phases as a function of θ. To this aim we apply the numerical method described in section II to a lattice unit cell, and impose periodic boundary conditions (PBCs) to the interface height profile. The size of the cell determines the particle packing fraction η in the lattice, while the shape of the unit cell and the PBCs specify the particular phase under considerations. Details on the lattice unit cells and the PBCs used to define the several phases sketched in Fig. 6 can be found in Fig. S4–S6 of the ESI.†
We observe that while the x, h, sv, se and sf phases are 2D phases, the c phase is essentially a 1D phase. It follows that the definition of the 2D packing fraction η is rather arbitrary in this latter case. In order to overcome this problem, we describe the c phase as a “gas” of chains that do not interact laterally (see Fig. 6(d)). As explained in Fig. S4 of the ESI,† to define the c phase we choose one side of the unit lattice cell such that the lateral distance DL among the chains corresponds to the smallest distance  for which two cubes in the {110} configuration do not interact. The case
for which two cubes in the {110} configuration do not interact. The case  corresponds to laterally interacting chains, i.e. the se phase. On the other hand, as shown in Fig. S7 of the ESI,† considering
corresponds to laterally interacting chains, i.e. the se phase. On the other hand, as shown in Fig. S7 of the ESI,† considering 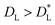 does not significantly affect the η dependence of the capillary interaction energy per unit area ηEN in the c phase, at sufficiently low η.
does not significantly affect the η dependence of the capillary interaction energy per unit area ηEN in the c phase, at sufficiently low η.
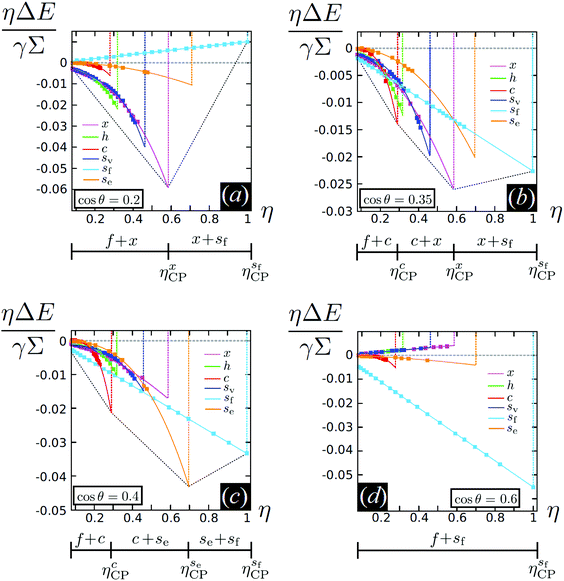
We present our results for cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ ∈ {0.2, 0.35, 0.4, 0.6} in Fig. 7. The horizontal axis represents the dimensionless areal density η = NL2/A, which we will call packing fraction for convenience below, even though L2 is only (close to) the areal coverage of a cube in the {100} configuration. We indicate the closest-packing of a specific phase with ηξCP, where ξ = x, h, sv, c, se, sf. As explained in Fig. S4 of the ESI† these correspond to ηxCP = 0.59, ηhCP = 0.32,
θ ∈ {0.2, 0.35, 0.4, 0.6} in Fig. 7. The horizontal axis represents the dimensionless areal density η = NL2/A, which we will call packing fraction for convenience below, even though L2 is only (close to) the areal coverage of a cube in the {100} configuration. We indicate the closest-packing of a specific phase with ηξCP, where ξ = x, h, sv, c, se, sf. As explained in Fig. S4 of the ESI† these correspond to ηxCP = 0.59, ηhCP = 0.32,  , ηcCP = 0.28,
, ηcCP = 0.28,  , and
, and  . Note that the sf phase is the densest-packed state. The vertical axis of Fig. 7 represents the dimensionless energy per unit area ηΔE/(γΣ), where ΔE ≡ EN/N − E{110}1 is the capillary interaction energy per particle minus a conveniently but arbitrary constant that we set to the adsorption energy of a single particle in the {110} configuration, for each of the phases described above. In Fig. 7 we employ the common tangent construction as represented by the convex envelope (dashed black line), to determine the ground state (T = 0) phase diagram.
. Note that the sf phase is the densest-packed state. The vertical axis of Fig. 7 represents the dimensionless energy per unit area ηΔE/(γΣ), where ΔE ≡ EN/N − E{110}1 is the capillary interaction energy per particle minus a conveniently but arbitrary constant that we set to the adsorption energy of a single particle in the {110} configuration, for each of the phases described above. In Fig. 7 we employ the common tangent construction as represented by the convex envelope (dashed black line), to determine the ground state (T = 0) phase diagram.
We find several scenarios by changing θ. At cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 0.2 (Fig. 7(a)) the capillary interaction energy among cubes adsorbed in the {111} configuration, i.e. cubes in the x, h, and sv phases, is much stronger (more negative) than the capillary interaction energy among cubes adsorbed in the {110} configuration, i.e. cubes in the c and se phases. As a consequence, we find that for η < ηxCP an extremely dilute disordered fluid phase f coexists with the close-packed x phase, while for η > ηxCP the close-packed x phase coexists with the close-packed sf phase. By increasing cos
θ = 0.2 (Fig. 7(a)) the capillary interaction energy among cubes adsorbed in the {111} configuration, i.e. cubes in the x, h, and sv phases, is much stronger (more negative) than the capillary interaction energy among cubes adsorbed in the {110} configuration, i.e. cubes in the c and se phases. As a consequence, we find that for η < ηxCP an extremely dilute disordered fluid phase f coexists with the close-packed x phase, while for η > ηxCP the close-packed x phase coexists with the close-packed sf phase. By increasing cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ, instead, the intensity of the capillary interaction energy among cubes in the {110} configuration becomes stronger while the capillary interaction energy among cubes in the {111} configuration simultaneously becomes weaker. This leads to the scenarios observed at cos
θ, instead, the intensity of the capillary interaction energy among cubes in the {110} configuration becomes stronger while the capillary interaction energy among cubes in the {111} configuration simultaneously becomes weaker. This leads to the scenarios observed at cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 0.35 and cos
θ = 0.35 and cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 0.4. At cos
θ = 0.4. At cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 0.35 (Fig. 7(b)), for η < ηcCP the f phase coexists with the close-packed c phase, for ηcCP < η < ηxCP the close-packed c phase coexists with the close-packed x phase, while for η > ηxCP the x phase coexists with the close-packed sf phase. Instead, at cos
θ = 0.35 (Fig. 7(b)), for η < ηcCP the f phase coexists with the close-packed c phase, for ηcCP < η < ηxCP the close-packed c phase coexists with the close-packed x phase, while for η > ηxCP the x phase coexists with the close-packed sf phase. Instead, at cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 0.4 (Fig. 7(c)) for η < ηcCP the f phase coexists with the close-packed c phase, for
θ = 0.4 (Fig. 7(c)) for η < ηcCP the f phase coexists with the close-packed c phase, for  the close-packed c phase coexists with the close-packed se phase, while for
the close-packed c phase coexists with the close-packed se phase, while for  the se phase coexists with the close-packed sf phase. Finally, at cos
the se phase coexists with the close-packed sf phase. Finally, at cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 0.6 (Fig. 7(d)) the sf phase has the lowest energy per unit area ηΔE for each η, and the capillary interactions among cubes in the {111} as well as in the {110} configuration are very weak. It follows that at this contact angle the sf phase is the only stable phase, besides the f phase. Since in the sf phase no capillary interactions act among the cubes, its stabilization is completely driven by packing entropy.
θ = 0.6 (Fig. 7(d)) the sf phase has the lowest energy per unit area ηΔE for each η, and the capillary interactions among cubes in the {111} as well as in the {110} configuration are very weak. It follows that at this contact angle the sf phase is the only stable phase, besides the f phase. Since in the sf phase no capillary interactions act among the cubes, its stabilization is completely driven by packing entropy.
We note that the cubes display different orientations in the dilute fluid phase f, as a function of the Young contact angle θ. They are indeed adsorbed in the minimum energy configuration at the θ of interest: the {111} configuration at cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 0, the {100} configuration at the other contact angles.
θ = 0, the {100} configuration at the other contact angles.
Finally, we summarize the results of Fig. 7 in the ground state (T = 0) phase diagram of Fig. 8. In this diagram we report the packing density η on the horizontal axis, and the cosine of the contact angle θ on the vertical axis. We note that three regimes of θ exist, in which different phases stabilize by varying η. In the regime of large contact angles (cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ < 0.35) the f, x, and sf phases stabilize, in the regime of intermediate contact angles (0.35 ≤ cos
θ < 0.35) the f, x, and sf phases stabilize, in the regime of intermediate contact angles (0.35 ≤ cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ ≤ 0.4) the f, x, c, se and sf phases stabilize, in the regime of small contact angles (cos
θ ≤ 0.4) the f, x, c, se and sf phases stabilize, in the regime of small contact angles (cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ > 0.4) the f, and sf phases are stable. We note that in Fig. 8 we included the case of cos
θ > 0.4) the f, and sf phases are stable. We note that in Fig. 8 we included the case of cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 0.0, for which a graph similar to those of Fig. 7 is reported in Fig. S8 of the ESI.†
θ = 0.0, for which a graph similar to those of Fig. 7 is reported in Fig. S8 of the ESI.†
In the analysis performed so far we only have considered the effect of capillary interactions while we have completely neglected entropic contributions. Although this approximation is correct when studying the phase behaviour of μm-sized particles as those of ref. 23 and 24, it is less accurate when smaller particles are considered. We now extend the analysis to the case of nm-sized particles and introduce an approximate model to estimate the interplay between capillary interactions and particle entropy. We write the (Helmholtz) free energy Fξ of a system of N colloidal particles adsorbed at a fluid–fluid interface of area A and temperature T, in a particular phase ξ, as
 | (2) |
By considering kBT/(γΣ) ≠ 0 in eqn (2), we map out the phase diagrams depicted in Fig. 9 for cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ ∈ {0.2, 0.35, 0.4}. Here we represent the particle density η on the horizontal axis, and the dimensionless temperature kBT/(γΣ) on the left vertical axis. The right vertical axis represents the corresponding value of the particle size L at room temperature (T = 300 K) using a typical surface tension γ = 0.01 N m−1. We find that our results reproduce remarkably well the experimental observations in ref. 23 and 24. Indeed for μm-sized L we find that, by decreasing θ, first hexagonal x structures and then linear chains c stabilize. In agreement with experiments we also predict regions in which the x and the c phases coexist, as well as the stabilization of “face up: square sf patterns at very small θ ∼ 0. Furthermore we see that for cos
θ ∈ {0.2, 0.35, 0.4}. Here we represent the particle density η on the horizontal axis, and the dimensionless temperature kBT/(γΣ) on the left vertical axis. The right vertical axis represents the corresponding value of the particle size L at room temperature (T = 300 K) using a typical surface tension γ = 0.01 N m−1. We find that our results reproduce remarkably well the experimental observations in ref. 23 and 24. Indeed for μm-sized L we find that, by decreasing θ, first hexagonal x structures and then linear chains c stabilize. In agreement with experiments we also predict regions in which the x and the c phases coexist, as well as the stabilization of “face up: square sf patterns at very small θ ∼ 0. Furthermore we see that for cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 0.4 regions exist in which “edge up” square se aggregates stabilize in coexistence with either f, c, or sf phases. For cubes with nm-sized L we also find the presence of f–c–x, and f–c–se triple-points. Note that in the limit of high temperature T our model for the free energy becomes less accurate and only the phase f survives. For kBT/(γΣ) = 0, instead, the scenario depicted in Fig. 9 coincides with the ground states depicted in Fig. 7 and 8.
θ = 0.4 regions exist in which “edge up” square se aggregates stabilize in coexistence with either f, c, or sf phases. For cubes with nm-sized L we also find the presence of f–c–x, and f–c–se triple-points. Note that in the limit of high temperature T our model for the free energy becomes less accurate and only the phase f survives. For kBT/(γΣ) = 0, instead, the scenario depicted in Fig. 9 coincides with the ground states depicted in Fig. 7 and 8.
As a final remark we observe that we cannot build a proper phase diagram in the case of cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = 0.6, where the capillary interactions become so weak that the phase behaviour is governed by packing entropy among cubes in the adsorbed “face up” {100} configuration. A more accurate theoretical model of the free energy that takes into account the particle shape and excluded volume is then required to describe the entropy-driven phase transitions.
θ = 0.6, where the capillary interactions become so weak that the phase behaviour is governed by packing entropy among cubes in the adsorbed “face up” {100} configuration. A more accurate theoretical model of the free energy that takes into account the particle shape and excluded volume is then required to describe the entropy-driven phase transitions.
IV. Conclusions and discussion
In this paper, we numerically studied the adsorption and self-assembly of cubic colloidal particles at a fluid–fluid interface, as a function of the Young contact angle θ. For values of θ close to π/2, we confirmed the scenario already found in a series of previous papers: a single cube prefers to adsorb with one vertex pointing upward with respect to the interface plane (“vertex up” configuration) and induces a hexapolar deformation field in the interface height profile which causes a lattice of N → ∞ adsorbed cubes to assemble into “vertex up” hexagonal structures. At the same time, a cube with θ = π/2 adsorbed with one edge pointing upward (“edge up” configuration) or with one face pointing upward (“face up” configuration), does not induce any deformation in the interface height profile. By decreasing θ, however, we found that the intensity of the hexapolar deformation field induced by a cube in the “vertex up” configuration decreases, while a cube in the “edge up” configuration induces a finite quadrupolar deformation field. The intensity of this quadrupole increases with decreasing θ, and a regime exists in which it causes a lattice of N → ∞ adsorbed cubes to assemble into stable “edge up” chains and square aggregates. Finally we observed that, by decreasing θ even further, the cubes give rise to “face up” square patterns, whose stabilization is driven by packing entropy as the capillary interactions become very weak.Our results are in agreement with the findings of recent experiments performed on μm-sized polystyrene cubes. In particular they give strong theoretical support to the idea of a capillary-driven stabilization of chains of cubic colloids at fluid–fluid interfaces, in addition to the stabilization of other, already found, 2D structures. Our analysis also explains the linear aggregates of “edge up” cubes observed in the case of the cubic nanocrystals (of roughly 5-to-10 nm size) studied in ref. 40–42. Nevertheless for these experiments the true underlying mechanism (capillarity, van der Waals forces, steric interactions due to capping ligands,…) that drives the self-assembly is still under investigation.
We note that chains of cubic colloids have also been observed in systems of magnetic cubes with a fixed magnetic dipole moment and in suspensions of colloidal cubes in an electric field,43–48 inducing dipolar interactions between the cubes. As the physical mechanism behind the chain formation is very different from the capillary-induced chain formation of colloidal cubes at an interface, the structure of the chains can also be very different. Depending on the orientation of the fixed dipole moment of the magnetic cubes, the cubes within the chains can have different orientations, and in the case of dipolar cubes in an electric field, the cubes can even freely rotate along the field axis.48
Finally several extensions of the work presented in this paper could be considered. First it could be interesting to investigate the effects of slightly tuning the shape of the cubic particles (i.e. considering cubes with edges truncated at different levels) on the self-assembly at the interface, as a function of the Young contact angle θ. In addition, the simple model used here for the free energy could be improved to take into account, for instance the orientational entropy of the cubes in each phase. In ref. 25 and 26 it was shown that considering this contribution can lead to stable honeycomb and “vertex up” square patterns coexisting with hexagonal ones, for nm-sized cubes with θ = π/2. For smaller θ it could reveal the presence of coexistence regions between the chains with honeycomb and “vertex up” square patterns. We plan to address these issues in future work.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support by the “Nederlandse Organisatie voor Wetenschappelijk Onderzoek” (NWO) TOPPUNT. This work is part of the D-ITP consortium, a program of the NWO that is funded by the Dutch Ministry of Education, Culture and Science (OCW).References
- E. M. Furst, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 20853 CrossRef CAS , ISSN 0027-8424, https://www.pnas.org/content/108/52/20853.
- R. McGorty, J. Fung, D. Kaz and V. N. Manoharan, Mater. Today, 2010, 13, 34 CrossRef CAS , ISSN 1369-7021, http://www.sciencedirect.com/science/article/pii/S1369702110701073.
- A. Böker, J. He, T. Emrick and T. P. Russell, Soft Matter, 2007, 3, 1231, 10.1039/B706609K.
- S. Sacanna, W. K. Kegel and A. P. Philipse, Phys. Rev. Lett., 2007, 98, 13 CrossRef , ISSN 00319007.
- K. Stratford, R. Adhikari, I. Pagonabarraga, J.-C. Desplat and M. E. Cates, Science, 2005, 309, 2198 CrossRef CAS , ISSN 0036-8075, https://science.sciencemag.org/content/309/5744/2198.
- B. J. Park and D. Lee, Soft Matter, 2012, 8, 7690, 10.1039/C2SM25775K.
- B. J. Park and D. Lee, ACS Nano, 2012, 6, 782, DOI:10.1021/nn204261w.
- L. C. Bradley, W.-H. Chen, K. J. Stebe and D. Lee, Curr. Opin. Colloid Interface Sci., 2017, 30, 25 CrossRef CAS , ISSN 1359-0294, http://www.sciencedirect.com/science/article/pii/S1359029417300146.
- A. Kumar, B. J. Park, F. Tu and D. Lee, Soft Matter, 2013, 9, 6604, 10.1039/C3SM50239B.
- Q. Xie, G. B. Davies, F. Günther and J. Harting, Soft Matter, 2015, 11, 3581 RSC , ISSN 17446848, 1501.07707.
- M. Grzelczak, J. Vermant, E. M. Furst and L. M. Liz-Marzán, ACS Nano, 2010, 4, 3591, DOI:10.1021/nn100869j , pMID: 20568710.
- D. Ershov, J. Sprakel, J. Appel, M. A. Cohen Stuart and J. van der Gucht, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 9220 CrossRef CAS , ISSN 0027-8424, https://www.pnas.org/content/110/23/9220.
- P. G. de Gennes, Rev. Mod. Phys., 1985, 57, 827, DOI:10.1103/RevModPhys.57.827.
- S. C. Glotzer and M. J. Solomon, Nat. Mater., 2007, 6, 557, DOI:10.1038/nmat1949 , ISSN 1476-4660.
- S. Sacanna and D. J. Pine, Curr. Opin. Colloid Interface Sci., 2011, 16, 96 CrossRef CAS , ISSN 1359-0294, http://www.sciencedirect.com/science/article/pii/S1359029411000069.
- E. P. Lewandowski, J. A. Bernate, A. Tseng, P. C. Searson and K. J. Stebe, Soft Matter, 2009, 5, 886, 10.1039/B812257A.
- E. P. Lewandowski, M. Cavallaro, L. Botto, J. C. Bernate, V. Garbin and K. J. Stebe, Langmuir, 2010, 26, 15142, DOI:10.1021/la1012632.
- L. Botto, E. P. Lewandowski, M. Cavallaro and K. J. Stebe, Soft Matter, 2012, 8, 9957, 10.1039/C2SM25929J.
- I. B. Liu, N. Sharifi-Mood and K. J. Stebe, Annu. Rev. Condens. Matter Phys., 2018, 9, 283, DOI:10.1146/annurev-conmatphys-031016-025514.
- B. Madivala, J. Fransaer and J. Vermant, Langmuir, 2009, 25, 2718, DOI:10.1021/la803554u.
- S. Dasgupta, M. Katava, M. Faraj, T. Auth and G. Gompper, Langmuir, 2014, 30, 11873, DOI:10.1021/la502627h , ISSN 0743-7463.
- S. Dasgupta, T. Auth and G. Gompper, J. Phys.: Condens. Matter, 2017, 29, 373003, DOI:10.1088/1361-648x/aa7933.
- Q. Song, M. Steuber, S. I. Druzhinin and H. Schönherr, Angew. Chem., Int. Ed., 2019, 58, 5246, DOI:10.1002/anie.201814076.
- Q. Song and H. Schönherr, Langmuir, 2019, 35, 6742, DOI:10.1021/acs.langmuir.9b00792 , ISSN 0743-7463.
- G. Soligno, M. Dijkstra and R. van Roij, Phys. Rev. Lett., 2016, 116, 258001, DOI:10.1103/PhysRevLett.116.258001.
- G. Soligno, M. Dijkstra and R. van Roij, Soft Matter, 2018, 14, 42, 10.1039/C7SM01946G.
- G. Soligno, M. Dijkstra and R. van Roij, J. Chem. Phys., 2014, 141, 244702, DOI:10.1063/1.4904391.
- P. Pieranski, Phys. Rev. Lett., 1980, 45, 569, DOI:10.1103/PhysRevLett.45.569.
- J. de Graaf, M. Dijkstra and R. van Roij, J. Chem. Phys., 2010, 132, 164902, DOI:10.1063/1.3389481.
- A. R. Morgan, N. Ballard, L. A. Rochford, G. Nurumbetov, T. S. Skelhon and S. A. F. Bon, Soft Matter, 2013, 9, 487, 10.1039/C2SM26556G.
- W. van der Stam, A. P. Gantapara, Q. A. Akkerman, G. Soligno, J. D. Meeldijk, R. van Roij, M. Dijkstra and C. de Mello Donega, Nano Lett., 2014, 14, 1032, DOI:10.1021/nl4046069.
- B. Peng, G. Soligno, M. Kamp, B. de Nijs, J. de Graaf, M. Dijkstra, R. van Roij, A. van Blaaderen and A. Imhof, Soft Matter, 2014, 10, 9644, 10.1039/C4SM01989J.
- N. Ballard and S. A. F. Bon, J. Colloid Interface Sci., 2015, 448, 533 CrossRef CAS , ISSN 0021-9797, http://www.sciencedirect.com/science/article/pii/S0021979715002453.
- G. Soligno, Droplets, Capillary Interactions and Self-assembly, 2017, ISBN 9789463321242 Search PubMed.
- M. Vis, J. Opdam, I. S. J. van't Oor, G. Soligno, R. van Roij, R. H. Tromp and B. H. Erné, ACS Macro Lett., 2015, 4, 965, DOI:10.1021/acsmacrolett.5b00480.
- I. Dević, G. Soligno, M. Dijkstra, R. van Roij, X. Zhang and D. Lohse, Langmuir, 2017, 33, 2744, DOI:10.1021/acs.langmuir.7b00002.
- M. Kamp, G. Soligno, F. Hagemans, B. Peng, A. Imhof, R. van Roij and A. van Blaaderen, J. Phys. Chem. C, 2017, 121, 19989, DOI:10.1021/acs.jpcc.7b04188.
- C. Anzivino, F. Chang, G. Soligno, R. van Roij, W. K. Kegel and M. Dijkstra, Soft Matter, 2019, 15, 2638, 10.1039/C8SM02361A.
- P. M. Chaikin and T. C. Lubensky, Principles of Condensed Matter Physics, Cambridge University Press, 1995, ISBN 9780521432245, https://www.cambridge.org/core/product/identifier/9780511813467/type/book Search PubMed.
- W. H. Evers, B. Goris, S. Bals, M. Casavola, J. de Graaf, R. van Roij, M. Dijkstra and D. Vanmaekelbergh, Nano Lett., 2013, 13, 2317, DOI:10.1021/nl303322k , ISSN 1530-6984.
- M. P. Boneschanscher, W. H. Evers, J. J. Geuchies, T. Altantzis, B. Goris, F. T. Rabouw, S. A. P. van Rossum, H. S. J. van der Zant, L. D. A. Siebbeles and G. Van Tendeloo, et al. , Science, 2014, 344, 1377 CrossRef CAS , ISSN 0036-8075, https://science.sciencemag.org/content/344/6190/1377.
- J. J. Geuchies, C. van Overbeek, W. H. Evers, B. Goris, A. de Backer, A. P. Gantapara, F. T. Rabouw, J. Hilhorst, J. L. Peters and O. Konovalov, et al. , Nat. Mater., 2016, 15, 1248, DOI:10.1038/nmat4746 , ISSN 1476-4660.
- J. G. Donaldson and S. S. Kantorovich, Nanoscale, 2015, 7, 3217, 10.1039/C4NR07101H.
- J. G. Donaldson, P. Linse and S. S. Kantorovich, Nanoscale, 2017, 9, 6448, 10.1039/C7NR01245D.
- P. Linse, Soft Matter, 2015, 11, 3900, 10.1039/C5SM00338E.
- L. Rossi, J. G. Donaldson, J. M. Meijer, A. V. Petukhov, D. Kleckner, S. S. Kantorovich, W. T. Irvine, A. P. Philipse and S. Sacanna, Soft Matter, 2018, 14, 1080 RSC , ISSN 17446848.
- L. Abelmann, T. A. G. Hageman, P. A. Löthman, M. Mastrangeli and M. C. Elwenspoek, Sci. Adv., 2020, 6, eaba2007 CrossRef CAS.
- H. R. Vutukuri, F. Smallenburg, S. Badaire, A. Imhof, M. Dijkstra and A. Van Blaaderen, Soft Matter, 2014, 10, 9110 RSC , ISSN 17446848.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0sm01815e |
| This journal is © The Royal Society of Chemistry 2021 |