Open Access Article
Open Access ArticleOn the environmental competitiveness of sodium-ion batteries under a full life cycle perspective – a cell-chemistry specific modelling approach†
Jens F.
Peters
 *ab,
Manuel
Baumann
*ab,
Manuel
Baumann
 c,
Joachim R.
Binder
d and
Marcel
Weil
ce
c,
Joachim R.
Binder
d and
Marcel
Weil
ce
aUniversity of Alcalá (UAH), Department of Economics, Alcalá de Henares, Madrid, Spain. E-mail: jens.peters@uah.es
bIMDEA Energy, Systems Analysis Unit, Móstoles, Madrid, Spain
cInstitute for Technology Assessment and System Analysis (ITAS), Karlsruhe Institute of Technology (KIT), Germany
dInstitute for Applied Materials (IAM-ESS), Karlsruhe Institute of Technology (KIT), Germany
eHelmholtz Institute Ulm for Electrochemical Energy Storage (HIU), Ulm, Germany
First published on 12th November 2021
Abstract
Sodium-ion batteries (SIB) are among the most promising type of post-lithium batteries, being promoted for environmental friendliness and the avoidance of scarce or critical raw materials. However, the knowledge-base in this regard is weak, and comparatively little is known about the environmental performance of different SIB types in comparison with current lithium-ion batteries (LIB) under consideration of the whole battery life cycle (‘cradle-to-grave’). This work provides a complete and comprehensive update of the state of knowledge in the field of life cycle assessment of SIB. It develops and discloses a specific tool for dimensioning and assessing SIB cells, including a cell-specific model of an advanced hydrometallurgical recycling process. It provides the corresponding inventory data for five different types of SIB and compares their environmental impacts with those of competing LIB, taking into account the full life cycle (cradle-to-grave) and an individual cell dimensioning based on electrochemical considerations. Recycling is found to be highly relevant for minimizing environmental impacts of the batteries, though its benefit depends strongly on the individual cell chemistry. Deep recycling might not be favourable for cathodes based on abundant materials and could even increase impacts. Especially the assessed manganese and nickel–manganese based SIB chemistries show promising results, given that they achieve at least similar lifetimes as their LIB counterparts.
1. Introduction
1.1. Background
While LIB continue their triumphant advance in almost all fields of electricity storage, the next generations of possible post-lithium systems are already being developed.1–3 These hold the promise of overcoming some of the remaining challenges associated with current lithium-ion battery (LIB) technology related with safety, the need for scarce resources and their environmental impacts.4–10 Among them, sodium-ion batteries (SIB) are the currently most promising and most mature post-lithium technology, with good environmental and electrochemical performance, and a lower demand for scarce resources.11–14 In fact, SIB are the only post-LIB technology already being commercialised by several independent providers.15–18 However, while the environmental impacts of LIB are well known and intensively investigated, only a few life cycle assessment (LCA) studies are available on SIB and the knowledge-base in this regard is comparatively weak. The first comprehensive LCA of SIB was published in 2016 and still is the current reference.19 It provided a full SIB model and corresponding inventory data for a layered oxide NaNMMT (Na1.1Ni0.3Mn0.5Mg0.05Ti0.05O2) type SIB cell with a hard carbon anode from carbohydrate precursors (sugar) and found SIB to be potentially competitive with LIB in terms of environmental impacts, but only when achieving similar lifetime. However, the study focused on only one specific cathode material and identified high uncertainties related with the origin of the hard carbon anode material. A follow-up study investigated the impact of different precursor materials for the hard carbon anode materials, showing potential of reducing environmental impacts of SIB cells by sourcing hard carbon materials from organic waste material or fossil carbon material (petroleum coke).20 Other works focused on specific aspects like costs or material demand.11,21 The most recent publication with a specific environmental focus compares a NaNMC (NaNi1/3Mn1/3Co1/3O2) vs. hard carbon SIB cell against a LiNMC (LiNi1/3Mn1/3Co1/3O2) vs. graphite LIB cell, using a physics-based and parametrizable battery cell model for generating own inventory data.22 Unfortunately, the underlying model is not disclosed and can therefore not be verified. The authors obtain greenhouse gas (GHG) emissions significantly lower than those found by prior works,19 attributable mainly to improved battery modelling, advances in energy density and progress made in battery manufacturing technology. However, although the need for considering the whole life cycle for meaningful assessments has repeatedly been pointed out,23,24 none of the available studies considers all the stages of the battery life including use phase and end-of-life (EoL) handling i.e., recycling. This lack of data about SIB recycling can be explained with the early stage of development and the virtual non-existance of returned (spent) SIB that require EoL treatment. Also for LIB the first pioneering LCA focused on the production stage,25–27 and recycling issues started to be considered later.28 Also, while new promising SIB cell chemistries have been developed such as polyanionic or prussian-blue based cathode materials,13,14,29 neither complete environmental assessments nor detailed inventory data on these batteries is yet available.This study aims at closing this gap by providing a comprehensive update of the current state of the art in life cycle assessment (LCA) of SIB under consideration of the whole life cycle. It develops whole new inventory data based on individual dimensioning according to electrochemical considerations and evaluates five different types of SIB. The assessment considers the whole life cycle, including use-phase and recycling. For the latter, a cell-specific process model for an advanced hydrometallurgical recycling is provided, allowing to estimate process inputs and recovered materials as a function of the individual cell composition with an automated spreadsheet calculator. The spreadsheet calculator is disclosed openly for re-use and further development. Due to the high technological similarity of LIB and SIB cells it can be used as a tool for dimensioning both future LIB and SIB cells for follow-up life cycle assessments. Recycling benefits are calculated assuming closed loop recycling i.e., the materials recovered from the recycling process are obtained in the same form and quality as those from virgin sources and therefore substitute the corresponding amount of input material.
2. Methodology
2.1. Assessment framework
Given the still low maturity of SIB and the corresponding lack of inventory data for SIB packs or modules, we limit the assessment to cell level, disregarding additional components of a future battery like packaging, battery management system etc. Being no information available about possible differences in terms of battery pack layout between SIB and LIB, including these based on assumptions would only add uncertainty.The assessment follows a cradle-to-grave approach, considering all stages of the batteries' life cycle i.e., (i) manufacturing including all upstream processes like raw material extraction and -processing, energy generation etc., (ii) use phase (modelled in a simplified way, being the use-phase impacts always dependent on the very specific application case and its performance requirements, and a generic consideration of the use phase therefore difficult),2 and (iii) EoL handling i.e., recycling, which can have a relevant impact on the total environmental performance of LIB and SIB, especially when comparing cells with very different material composition.30 For the EoL stage, a credit for recovered materials is accounted for, assuming a closed loop recycling i.e., the amount of material recovered avoids the impacts of the corresponding amount of input of the equivalent material. This implies that the recovered materials are obtained in the same composition and quality as the precursors obtained from virgin resources and that they can directly replace the metal sulphates, carbonates and hydroxides at the factory gate without further beneficiation, what might be an optimistic assumption.31
Various cathode materials have already been extensively investigated for sodium ion batteries, in particular layered transition metal oxides, Prussian Blue and its analogues (PBAs), as well as polyanionic compounds.32,33 In addition to a ‘baseline’ NaNMMT SIB cell (Na1.1(Ni0.3Mn0.5Mg0.05Ti0.05)O2) according to the most widely cited LCA study (the current reference)19, we consider two further layered oxides in this study, NaMMO (Na2/3(Mn0.95Mg0.05)O2)34 and NaNMC (Na1.05(Ni0.33Mn0.33Co0.33)0.95O2).22,35 On the one hand, we use NaNMC cathodes to compare them with the corresponding lithium variant, and on the other hand NaMMO as an interesting nickel- and cobalt-free layered oxide. As Prussian Blue analogue, (NaPBA; Na2Fe[Fe(CN)6])36 is considered due to its promising electrochemical performance, and, for the same reasons, NaMVP (Na4MnV(PO4)3) as a representative of the polyanionic cathode materials.37,38 More information about the specific characteristics of these materials are provided in Table S2 of the ESI.† Overall, these cathode materials cover roughly the range of the currently most promising cathode materials for SIB and are expected to give a comprehensive picture of their environmental performance and recommendations for improving their environmental performance.
These SIB cells are then compared with their LIB counterparts, taking as reference two common LIB chemistries, LiNMC622 (in the following named LiNMC) and LiFP. For all battery cells, the same prismatic cell housing is assumed (though with varying size according to the cell layout requirements) and the same separator (polyethylene/polypropylene membrane) and electrode binder: CMC (carboxymethyl cellulose) on the anode, and PvDF for the cathode side. Both LIB use LiFP6 in DMC (dimethylcarbonate) as electrolyte, and the SIB NaPF6, correspondingly. For determining the cell layout and inventory data, all battery cells are dimensioned explicitly based on the electrochemical properties of the corresponding active materials, thus providing a stringent modelling approach (described more in detail in the following section and the ESI†). Inventory data for the material synthesis and manufacturing of individual cell components are sourced from literature,26,27,39 and the manufacturing energy demand is updated with the most recent values.10,40 To account for potentially varying manufacturing energy demand without having more detailed data, the electricity and heat demand are scaled according to battery capacity and electrode surface area. More details on the modelling approach are provided in the ESI.†
The functional unit i.e., the common basis of comparison for comparing the manufacturing (and recycling) impacts is 1 kWh of battery cell storage capacity. However, for assessing the battery cells including the use-phase (whole life cycle), the lifetime of the application has to be taken into account, and a second FU is used: 1 kWh of electricity provided by the battery cell over the lifetime of the assumed application.
The environmental assessment is realized in openLCA 1.10.2, using ecoinvent 3.7.1 as background database and the ILCD methodology for impact assessment.41,42 For the sake of comprehensiveness, the considered impact categories are limited to four impact categories: (i) global warming potential (GWP) i.e., GHG emissions, (ii) abiotic resource depletion potential (ADP), (iii) acidification potential (AP), and human toxicity potential (HTP), being these the impact categories most frequently assessed and of specific concern in the field of LCA of LIB and post LIB.28 However, we provide the results for all impact categories in the ESI† and also disclose the complete inventory data in tabulated form and in standardised format (ILCD and JSON-LD) for direct import and re-use in common LCA software, allowing to instantly reproduce and re-use the results. See ESI† for more details.
2.2. Battery cell production
This study provides an update of the current state of the art inventory data for LCA of SIB, using battery cell models relying on the most recent data available on cell composition and cell manufacturing energy. For the dimensioning of the battery cells, a specific spreadsheet calculator is developed based on the BatPac dimensioning tool by Argonne National Laboratories,43 allowing to calculate the layout of different SIB and LIB cells according to their performance targets and the electrochemical characteristics of the employed materials. The excel-based spreadsheet calculator provides inventory data tables for the battery cells and for the corresponding recycling processes, allowing a quicker generation of new inventory data for future battery cells. Inventory data for the individual manufacturing stages are taken from previous literature and updated according to the current state-of the art regarding manufacturing energy demand and upstream processes.40,44,45 The production processes not available in literature (e.g., for the SIB cathode materials) are modelled explicitly by modifying material synthesis process for existing LIB. The corresponding inventory data are provided, together with more details about the modelling approach and the cell dimensioning tool itself in the ESI.† The battery cells are dimensioned according to common performance targets, assuming prismatic cells with a target capacity of 160 W h total capacity (100% depth of discharge (DoD)), or 136 W h useable capacity (85% depth of discharge, being 100% discharge detrimental to cycle stability).43,46 The target power of the cells is 0.8 kW, equivalent to a C-rate of 2 i.e., full discharge of the battery in 0.5 (=1/C) hours. Hard carbon derived from petroleum coke is used as active materials for the anodes, a material that showed a promising environmental performance in previous studies.19,20Table 1 provides the mass balance and key parameters of the assessed battery cells as obtained from the dimensioning tool.| Composition | NaNMMT | NaMMO | NaMVP | NaNMC | NaPBAFCN | LiFP | LiNMC | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (g) | Share | (g) | Share | (g) | Share | (g) | Share | (g) | Share | (g) | Share | (g) | Share | |
| Anode | ||||||||||||||
| Active material | 269.3(HC) | 29.2% | 307.2(HC) | 30.5% | 222.4(HC) | 21.4% | 307.0(HC) | 26.4% | 266.9(HC) | 20.8% | 154.5(Graph) | 19.3% | 136.3(Graph) | 23.4% |
| Cond. Carbon | 8.7(CB) | 0.9% | 9.9(CB) | 1.0% | 7.2(CB) | 0.7% | 9.9(CB) | 0.9% | 8.6(CB) | 0.7% | 0.0(—) | 0.0% | 0.0(—) | 0.0% |
| Binder | 11.6(CMC) | 1.3% | 13.2(CMC) | 1.3% | 9.6(CMC) | 0.9% | 13.2(CMC) | 1.1% | 11.5(CMC) | 0.9% | 3.2(CMC) | 0.4% | 2.8(CMC) | 0.5% |
| Foil | 24.7(Al) | 2.7% | 27.6(Al) | 2.7% | 40.4(Al) | 3.9% | 30.1(Al) | 2.6% | 70.2(Al) | 5.5% | 73.2(Cu) | 9.1% | 40.3(Cu) | 6.9% |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||
| Cathode | ||||||||||||||
| Active material | 333.3(NaNMMT) | 36.2% | 336.1(NaMMO) | 33.4% | 424.0(MVP) | 40.8% | 476.2(NaNMC) | 41.0% | 416.7(FCN) | 32.4% | 325.0(LiFP) | 40.6% | 237.0(LiNMC622) | 40.7% |
| Cond. Carbon | 6.9(CB) | 0.8% | 7.0(CB) | 0.7% | 8.8(CB) | 0.8% | 9.9(CB) | 0.9% | 8.7(CB) | 0.7% | 6.8(CB) | 0.8% | 4.9(CB) | 0.8% |
| Binder | 6.9(PVdF) | 0.8% | 14.0(PVdF) | 1.4% | 8.8(PVdF) | 0.8% | 9.9(PVdF) | 0.9% | 8.7(PVdF) | 0.7% | 6.8(PVdF) | 0.8% | 4.9(PVdF) | 0.8% |
| Foil | 23.1(Al) | 2.5% | 25.8(Al) | 2.6% | 38.2(Al) | 3.7% | 28.2(Al) | 2.4% | 67.0(Al) | 5.2% | 31.2(Al) | 3.9% | 17.0(Al) | 2.9% |
| Electrolyte | 188.9(NaPF6) | 20.5% | 214.3(NaPF6) | 21.3% | 221.9(NaPF6) | 21.3% | 222.5(NaPF6) | 19.2% | 342.5(NaPF6) | 26.7% | 133.0(LiPF6) | 16.6% | 89.7(LiPF6) | 15.4% |
| Separator | 7.4(PE/PP) | 0.8% | 8.3(PE/PP) | 0.8% | 12.3(PE/PP) | 1.2% | 9.1(PE/PP) | 0.8% | 21.7(PE/PP) | 1.7% | 10.0(PE/PP) | 1.2% | 5.4(PE/PP) | 0.9% |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||||||||
| Cell | ||||||||||||||
| Cell housing | 21.7(Al/PE) | 2.4% | 23.9(Al/PE) | 2.4% | 24.8(Al/PE) | 2.4% | 24.6(Al/PE) | 2.1% | 35.3(Al/PE) | 2.7% | 19.1(Al/PE) | 2.4% | 14.2(Al/PE) | 2.4% |
| Cell terminals | 19.1(Al/Al) | 2.1% | 20.5(Al/Al) | 2.0% | 21.0(Al/Al) | 2.0% | 20.9(Al/Al) | 1.8% | 26.8(Al/Al) | 2.1% | 37.6(Cu/Al) | 4.7% | 29.8(Cu/Al) | 5.1% |
| Total | 921.6 | 100% | 1007.7 | 100% | 1039.4 | 100% | 1161.5 | 100% | 1284.5 | 100% | 800.3 | 100% | 582.4 | 100% |
| Capacity | Total | Useable | Total | Useable | Total | Useable | Total | Useable | Total | Useable | Total | Useable | Total | Useable |
| Cell capacity (W h) | 158.4 | 134.7 | 158.3 | 134.5 | 158.5 | 134.7 | 158.3 | 134.6 | 159.4 | 135.5 | 158.0 | 134.3 | 158.5 | 134.7 |
| Eng. dens. (W h kg−1) | 171.9 | 146.1 | 157.1 | 133.5 | 152.5 | 129.6 | 136.3 | 115.9 | 124.1 | 105.5 | 197.4 | 167.8 | 272.1 | 231.3 |
2.3. Use phase
Both the lifetime (calendric and cycles) and the round-trip efficiency of a battery have a strong influence on the environmental impacts related with its use phase.11,47,48 Significant progress has been made in the field of LIB, with LiNMC type LIB typically reaching cycle lives of between 1000 and 4000 cycles, and LiFP-type cells up to over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 equivalent full cycles with a depth of discharge of 80%, 0.2–1C rate at 25 °C until reaching 80% of their initial capacity.46,49–51 Correspondingly, commercial state of the art LIBS can least for up to two decades for stationary storage applications.50,52,53
000 equivalent full cycles with a depth of discharge of 80%, 0.2–1C rate at 25 °C until reaching 80% of their initial capacity.46,49–51 Correspondingly, commercial state of the art LIBS can least for up to two decades for stationary storage applications.50,52,53
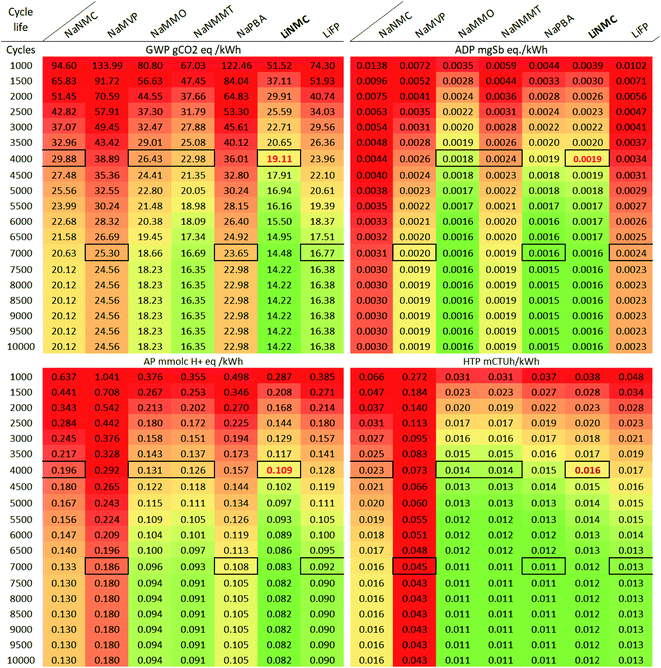
SIB, with a much lower technological maturity, show an even higher variability.53 While cycle lives of experimental laboratory cells are often comparably low, some reports state that SIB achieve between 500 until up to 2000 full cycles at a Depth of Discharge (DOD) of 80%,54 and might reach up to 4000 cycles at 1C until 80% of initial capacity, which is compatible with current state of the art LIB.55 Commercial start-up companies in the field of SIB indicate between 1000 cycles for a retention rate of 70%17 up to 5000 cycles at a remaining capacity of 80%16 or even over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, but without providing any further details.18 SIB with Prussian blue based cathodes seem to be especially promising in terms of cycle stability, with laboratory cells already achieving over 3500 cycles56,57 Polyanionic materials like the NaMVP are also expected to achieve good cycle stability38,55 For the NaPBA and the NaMVP cell, cycle life similar to those of LiFP cells are therefore assumed (Table 2).
000 cycles, but without providing any further details.18 SIB with Prussian blue based cathodes seem to be especially promising in terms of cycle stability, with laboratory cells already achieving over 3500 cycles56,57 Polyanionic materials like the NaMVP are also expected to achieve good cycle stability38,55 For the NaPBA and the NaMVP cell, cycle life similar to those of LiFP cells are therefore assumed (Table 2).
| NaNMC, NaMMO, NaNMMT | NaPBA, NaMVP | LiFP | LiNMC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Base | Max | Min | Base | Max | Min | Base | Max | Min | Base | Max | |
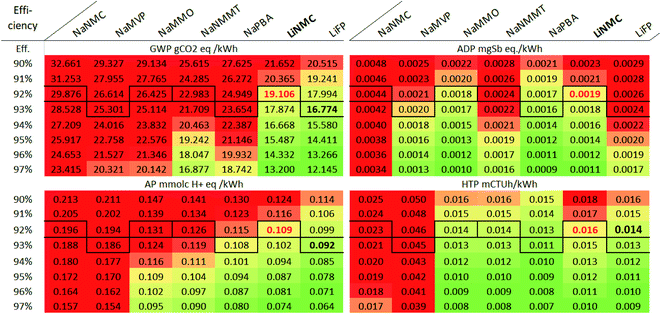
| Efficiency | 90 | 92 | 95 | 90 | 93 | 97 | 90 | 93 | 97 | 90 | 92 | 95 |
| Min SOC% | 10 | 20 | 25 | 5 | 20 | 20 | 5 | 20 | 20 | 10 | 20 | 25 |
| Max SOC% | 95 | 95 | 100 | 95 | 95 | 100 | 95 | 95 | 100 | 95 | 95 | 100 |
| Cycle life | 1000 | 4000 | 9000 | 1000 | 7000 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2500 | 7000 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1000 | 4000 | 9000 |
| Calendric life time | 10 | 15 | 20 | 10 | 15 | 20 | 10 | 15 | 20 | 10 | 15 | 20 |
Similarly, information about the cycling efficiency of sodium ion batteries is scarce.58 However, SIB achieve similar coulombic efficiencies as LIB, and their round trip efficiency can therefore be assumed to be comparable to those of LiFP and LiNMC-type LIB,17,19,46 with values of over 90%. Similar values are reported also for the aqueous SIB alternative.59,60 Current LIB reach efficiencies of up to 95% (LiNMC) and 97% (LiFP),19 and it can be expected that SIB will reach similar levels with increasing technological maturity. However, the scarce information regarding these key parameters makes it difficult to compare the use-phase performance of the different battery types. We therefore use average values based on expert judgement and then vary these in an extended sensitivity analysis for determining target performance values that would need to be reached for environmental competitiveness Table 2 provides the assumptions for the corresponding technical parameters. It has to be stressed that these assumptions represent average values, but are actually highly dependent on the cell type, its maximum DoD, C-rates and operation temperature.
A meaningful assessment of the use-phase requires the definition of an application case, determining the use profile and thus the requirements for the battery.2 Being SIB suited particularly for the stationary sector,53 a 1 MWh stationary system for load shifting services is considered, providing two full cycle equivalents per day on average. The unit of comparison (functional unit) for this purpose is one kWh of electricity provided by the battery system for the defined service over its entire lifetime. Only the internal losses of the battery system (i.e., the electricity dissipated during a charge–discharge cycle due to ohmic and electrochemical losses) and battery replacements are accounted for, which both can have a significant influence on their overall environmental impacts over their lifetime.2,47 The remaining components of a battery system (e.g., pack and module casing, inverter, battery management system, gears and switches) is assumed to be identical for both LIB and SIB systems and are therefore neglected in this assessment, focusing explicitly on the battery cells. The total project lifetime is considered to be 20 years. Herein the cells have to be exchanged in case of insufficient cycle or calendric lifetime. Overview of the assumed use phase data is provided in Table 3. A maximum DoD of 80% is assumed to maintain a reserve to increase battery lifetime. Two different application scenarios are considered in the assessment to highlight the importance of round-trip efficiency: (i) electricity from the German grid and (ii) electricity from PV systems. More details on the application case and the corresponding system parameters are provided in the ESI.†
| Parameter | Value | Unit |
|---|---|---|
| Capacity | 2 | MWh |
| Power | 1 | MW |
| Cycles per day | 2 | — |
| Project life time | 20 | years |
| Cycles over project lifetime | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
Cycles |
| Total energy stored | 14,600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
kWh |
| Total cycles per year | 730 | — |
| Operation rate per year | 17% | 1450 h |
2.4. Recycling
While already for lithium-ion batteries, detailed and high-quality inventory data for recycling processes is scarce, even less information is available for emerging battery systems like SIB. The majority of available works in this regard use data determined for processing a specific cell type (mostly NMC), and assume that these inputs would remain the same independently of the actual feed composition.30,61–63 This limits their applicability to different cell chemistries, being the required amount of chemicals and process inputs dependent on the processed materials, even if the same process chain is used. Also, for automotive-type LiNMC batteries hydrometallurgical recycling facilities achieve notable recovery efficiencies already today, but this is not necessarily the case for lower-value containing batteries like SIB.64–66 In fact, even for current LiFP batteries, recycling is usually limited to recovering the aluminium, copper and steel components obtained from mechanical recycling steps (crushing, shredding and mechanical separation), while the active material fraction, the so-called black mass (which contains majorly lithium., carbon, iron, and phosphorous in the case of LiFP) is usually not further processed but discarded.39,67 To evaluate the individual recycling performance of the considered SIB cells, a cell-specific recycling model is therefore required.The recycling process model relies on a previous work that provided inventory data for different recycling processes.30 There, inventory data were provided in aggregated form and consumables were simply scaled according to the mass of the fed battery cells, resulting in substantial simplification. As a result, the process was found to increase burdens by the deeper hydrometallurgical processing of LiFP and SIB batteries. The underlying model has been updated and incorporated into the excel-calculation tool, with the amount of consumables calculated for the specific cell composition based on stoichiometric calculations and additional information obtained from patents and secondary publications on the recycling process.68,69 The most advanced hydrometallurgical treatment is considered for this purpose, consisting of a mechanical pre-treatment where the battery cells are shredded and ground, directly recovering metal from the cell housings and current collectors vie mechanical separation processes. Plastics from housings, sealings and separators are separated in this stage and disposed of as waste plastics. The electrolyte is also recovered and recycled in this stage. A subsequent deep hydrometallurgical recycling stage processes the remaining black mass, recovering all relevant metals and the carbonaceous anode active material (graphite or hard carbon, respectively).67 More details on the modelling can be found in the ESI.†
3. Results and discussion
3.1. Materials (production phase)
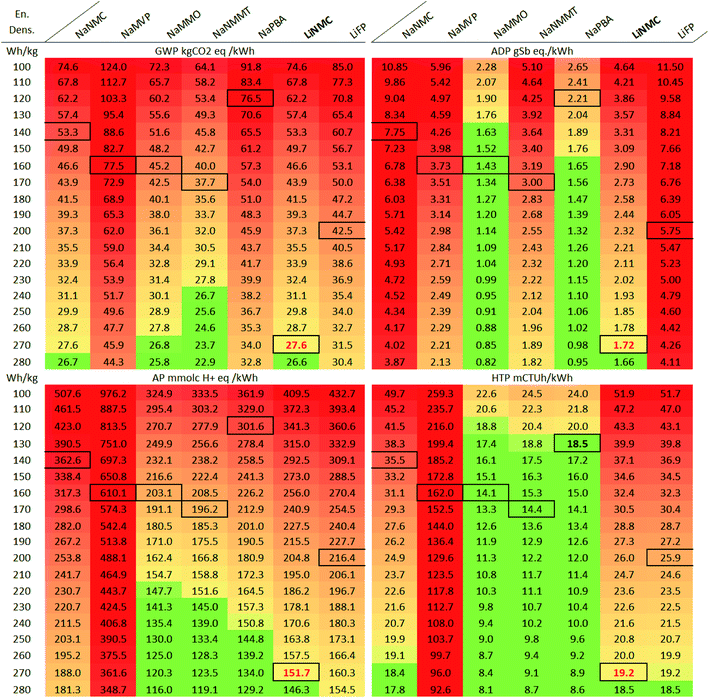
The potential environmental impacts associated with the manufacturing of 1 kWh of battery cell capacity are provided in Fig. 1 for the considered impact categories: GWP (Global warming potential i.e., GHG emissions), ADP (abiotic depletion potential i.e., resource depletion), AP (acidification potential) and HTP (human toxicity potential; considering cancer and non-cancer effects).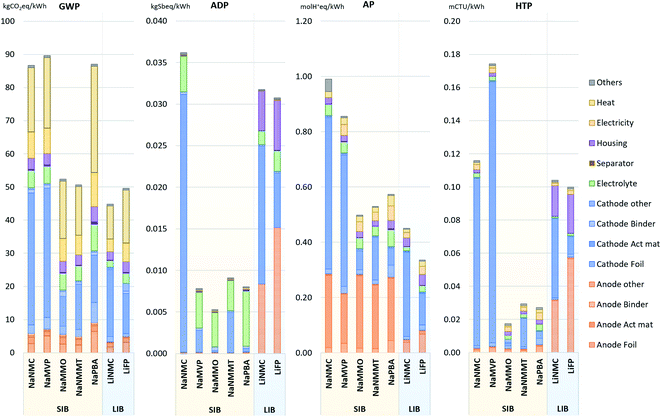 | ||
| Fig. 1 Environmental impacts per kWh of battery cell, manufacturing phase (cradle-to-gate i.e., without use-phase and end-of-life). GWP: global warming potential; ADP: abiotic resource depletion; AP: acidification potential; HTP: human toxicity potential. The underlying numerical values are provided in the ESI.† | ||
In terms of GWP (global warming), two of the assessed SIB chemistries achieve values comparable to those of the LIB, with the NaNMMT and the NaMMO being the best performing SIB (50.6 and 52.3 kg CO2 eq. per kWh, respectively). The NaNMC and NaPBA cells, but also the NaMVP on the other hand show significantly higher GHG emissions (86.7, 87.0 and 89.7 kg CO2 eq. per kWh, respectively) than the LIB (44.8 for LiNMC and 49.6 kg CO2 eq. per kWh for LiFP). This is driven majorly by the lower energy density of the SIB, requiring substantially larger battery cells for the same storage capacity. Manufacturing energy, though adjusted to the latest state of knowledge10,40,44 and noteworthily lower than assumed in previous works,19,39 still is a major source of GHG emissions. This is partially due to the high share of process heat in the total energy demand: Previous studies assumed the electricity demand to make up between 50% to close 100% of the total manufacturing energy demand,39,70–72 while more recent studies indicate lower electricity demand and a higher share (82–94%) of process heat.40,73 Thus, CO2 emissions from natural gas combustion for process heat can be considered as an environmental hotspot, and decarbonizing the process heat generation will be a future task for improving the environmental performance of cell production. Apart from energy demand, other important contributors are the cathode active materials, the electrolyte, and aluminium components (cell housing and collector foils).
ADP (resource depletion) impacts are dominated above all by the amount of copper, cobalt and nickel contained in the cells. The former is used for anode current collectors and anode tab (accounted for under cell housing) in the LIB, which is why the SIB (except the NaNMC), able to employ aluminium also for the anode current collectors, show significantly lower impacts in this category. The other two drivers of ADP impacts, cobalt and nickel, are mainly contained in the NMC cathode active materials (both LiNMC and NaNMC). This, causes the high ADP impacts of both LIB (32 and 31 g Sb eq. per kWh for the LiNMC and LiFP, respectively; driven be the content of copper, nickel and cobalt), and the NaNMC cell (36 g per Sb eq. per kWh; main drivers being nickel and cobalt in the cathode). The NaMMO and NaPBA show, except for the electrolyte, almost negligible impacts from the remaining cell components, being made of only abundant materials (5 and 8 g Sb eq. per kWh, respectively). For the NaNMMT and the NaMVP cells (with 9 and 8 g Sb eq. per kWh), the remaining impacts associated with the cathode active material are driven by their nickel and vanadium content, respectively. All four SIB types obtain ADP values of between 71% (NaNMMT) to 83% (NaMMO) lower than the LiNMC cell. Interestingly, the fluorine content of the electrolyte and the associated mining of fluorspar is the main reason for their ADP, which is why alternative fluorine-free electrolytes including aqueous ones might be promising in this regard.
Under AP (acidification) aspects, a high contribution is obtained for the anode active material (hard carbon) of the SIB. Hard carbon is assumed to be obtained from petroleum coke, and significant SO2 emissions are accounted for its production in the underlying publication.19 If these emissions were abated efficiently, the SIB would achieve results comparable to or even below that of the LiFP, which shows the most favourable results (0.33 mol H+ eq. per kWh). Other major contributors to this category are the mining processes, where SO2 is released during the roasting of sulfidic ores. In consequence, the use of copper (for anode current collectors and anode tabs of LIB), cobalt and nickel (for the active material of NMC cathodes), but also vanadium (for the active cathode material of NaMVP cells) drive up AP impacts. The highest value is therefore obtained for the NaNMC cell (0.99 mol H+ eq. per kWh, due to its cobalt and nickel containing cathode in combination with a comparably low energy density), and the NaMVP cell (0.86 mol H+ eq. per kWh due to SO2 emissions along the vanadium production chain). The remaining SIB show values only slightly higher (between 11 and 27%) than those of the LiNMC cell.
Except for the NaMVP cell, the HTP (human toxicity) impacts show a similar profile to that obtained for ADP. The mining of metals, above all copper (for LIB current collectors), nickel and cobalt (for NMC and, to a lower extent, NaNMMT cathode active materials) are the major cause of toxic impacts. In consequence, the NaMMO, NaPBA and NaNMMT cells obtain the best results (0.017, 0.027 and 0.029 mCTUh, respectively, or 83%, 74% and 72% lower than the LiNMC cell). Again, the NaNMC achieves unfavourable results, combining NMC cathode material with comparably low energy density. Higher impacts are obtained only by the NaMPV (0,17 mCTUh, or 67% higher than the LiNMC), where the vanadium extraction is associated with substantial toxic impacts. However, it has to be considered that the underlying inventory represents a very specific process situated in South Africa,74 and that alternative vanadium extraction routes could lead to significantly lower impacts.
3.2. Battery recycling (EoL)
To obtain an idea of the ‘net impacts’ of battery production, Fig. 2 provides the impacts obtained for the difference cell chemistries when including the EoL phase in the assessment i.e., resting the benefits obtained from cell recycling from the manufacturing impacts. Note that it still does not consider the use-phase, which is subject of the following Section (3.3). The recycling benefit is further broken down to the benefit obtained from the mechanical pre-treatment (recovery of metallic aluminium and copper, and the electrolyte; ‘Mech. Rec.’ in Fig. 2), and the hydrometallurgical treatment of the remaining black mass for recovery of cobalt, nickel, manganese salts and the anode active materials graphite and hard carbon (‘Hydrom. Rec.’ in Fig. 2). Despite the cell-chemistry specific adjustment of the recycling process, still slight positive impacts (i.e., an increase of impacts due to the hydrometallurgical treatment) are obtained from the hydrothermal treatment for some of the battery cells, though much lower than in a previous work.30 This indicates that for these cells a total recovery of all materials is still not associated with environmental benefit in the corresponding impact category. However, since these positive contributions are close to zero and the exclusion of this stage would improve the results only marginally, we consider full recycling for all battery chemistries in the following.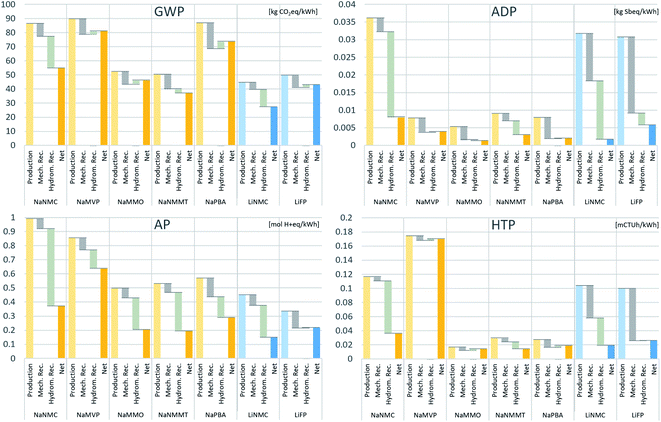 | ||
| Fig. 2 Recycling benefits and net impact when accounting for the recycling benefit. Recycling benefits are broken down to mechanical pre-treatment (including electrolyte recovery) and hydrometallurgical treatment of the black mass. Hydromet. rec. = benefits from hydrometallurgical treatment; mech. recy = benefits from mechanical treatment; net = net impacts when accounting for total recycling benefits. GWP: global warming potential; ADP: abiotic resource depletion; AP: acidification potential; HTP: human toxicity potential. The underlying numerical values are provided in the ESI.† | ||
Under GWP (global warming) aspects, the recycling reduces the impacts from the manufacturing phase noteworthily, more pronounced for the NaNMC and LiNMC and the NaPBA than for the other cell chemistries. However, the overall ranking among the different cell chemistries remains similar after including the recycling benefits. Aluminium, very energy intensive in production, is one of the main drivers for GWP impacts, and the SIB cells with lower energy density and higher aluminium content therefore show higher benefits from the mechanical recycling. This is especially pronounced for the NaPBA cell. On the other hand, LiFP, NaMMO and NaPBA and NaMVP type cells do not benefit from the additional hydrometallurgical processing, which even slightly increases the total impacts.
For ADP (resource depletion), the benefit obtained from cell recycling varies significantly between the assessed cell chemistries. The LiNMC cells, previously with very high ADP impacts, also obtain substantially higher benefits from the recovered materials, resulting in a net impact below that of the SIB, except the NaMMO-type. The NaPBA cell, where the ADP impacts are driven mainly by the electrolyte (Fig. 1), achieves benefits above all from the mechanical recycling (due to the recovery of the electrolyte in the advanced hydrometallurgical treatment, which is beyond the state of the art in current commercial recycling processes), while further processing of the black mass does not bring any further benefits. Still, it shows a very favourable result, outperformed only by the NaNMC.
The results for AP (acidification) are similar to that for GWP, with most favourable net impacts obtained for the NaNMC-type LIB. Recycling reduces impacts for all cell chemistries in both stages (the mechanical and the hydrometallurgical treatment) except the LiFP battery. The reason for this is twofold: Main AP drivers are the hard carbon production, associated with significant SO2 emissions (see Fig. 1), which is recovered in the hydrometallurgical recycling stage, causing a major share of the recycling benefit for the SIB cells. A better SO2 recovery in the hard carbon production process would reduce these impacts but also the benefit of recycling in this category. Second, the mining of cobalt, nickel and copper also are important contributors to AP, and the recovery of these materials correspondingly reduces impacts for the NMC and NMMC cells. Only the LiFP battery, which contains none of these, does not benefit significantly from the hydrometallurgical processing under AP aspects.
For HTP (human toxicity) impacts, the extraction of mineral resources is one of the main drivers. Again, the benefits from recycling are substantially higher for the LIB and the NaNMC battery, driven by the recovery of copper, cobalt and nickel, which in turn are responsible for the major share of toxicity impacts from cell production. Little benefit is obtained for the NaMVP cell, with the recycling process not designed for recovering vanadium, which on the other hand causes high impacts from the mining stage, leading to the by far highest impacts for the NaMVP battery. For the LiFP, NaMMO, NaMVP and NaPBA based batteries, no benefit is obtained from the hydrometallurgical processing of the black mass but rather a slight increase of environmental impacts. Together with the LiNMC cell, the NaMMO, NaPBA and NaNMMT SIB cells achieve the best results in this category.
3.3. Efficiency and battery lifetime (use phase)
The results for the entire life cycle of the batteries are depicted in Fig. 3, each with electricity from a photovoltaic (PV) installation and from the grid used for charging. The impacts are broken down to life cycle stages i.e., the initial production, the cell replacement over the project lifetime and the use-phase impacts (electricity consumption due to internal losses). Manufacturing and replacement impacts are net impacts i.e., after accounting for the recycling credits. However, the results that would be obtained without recycling are shown additionally (yellow dots and red dashed error bars), allowing to perceive the benefits obtained from the recycling. The error bars outline the maximum deviations related to varying cycle life or round-trip efficiency (based on the minimum and maximum values for both according to Table 2). The detailed numeric results are provided in the ESI.†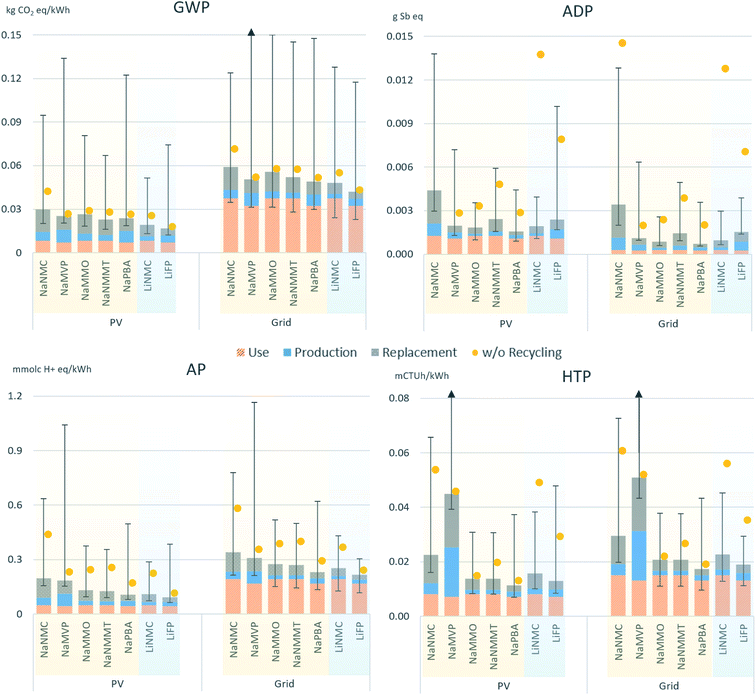 | ||
| Fig. 3 Environmental impacts per kWh of electricity provided by the battery over the whole lifetime of the stationary storage application, broken down into net impacts from battery production (net impacts = including recycling benefits), battery use and replacement during the assumed lifetime of the application. The yellow dots show the corresponding results without accounting for recycling benefits. The whiskers indicate the potential min/max variation according to the parameter range provided in Table 2. PV: results when storing electricity generated purely by photovoltaics; grid: results when storing electricity from the average German grid mix.42 GWP: global warming potential; ADP: abiotic resource depletion; AP: acidification potential; HTP: human toxicity potential. ‘Use’ refers to the impacts associated with electricity lost due to internal inefficiencies. The underlying numerical values are provided in the ESI.† | ||
The relevance of the use-phase becomes evident, contributing a significant share to the total life cycle impacts. Except for ADP, these are higher when grid electricity is charged, basically due to the (still) high share of fossil fuelled power plants such as natural gas- and coal in the European grid mix, contributing significantly to GWP (CO2 emissions from fuel combustion) and AP (emission of NOx and SO2). For ADP on the other hand, photovoltaic (PV) electricity shows higher impacts, requiring the manufacturing of PV panels significant amounts of aluminium, copper and other metals. However, despite its relevance for the overall environmental performance, the efficiency is never decisive for the ranking of the different battery types, principally due to their very similar performance in this regard. Still, the importance of round-trip efficiency for the overall life cycle performance becomes evident when comparing e.g., the two LIB (LiFP and LiNMC), where the assumed efficiency difference of just one percentage point (93% and 92%, respectively) causes a visible difference in the use phase impacts.
The second key parameter, cycle life, is represented by the contribution from cell replacement, requiring, depending on the degradation of the battery cells, more frequent replacements of the batteries and thus additional impacts from manufacturing. These are proportional to the amount of replaced cells and the (net) manufacturing impacts, which is why also the cell chemistries with high lifetime like LiFP, but also NaPBA obtain better results. Overall, the NaNMC chemistry obtains the worst results in all categories, caused by the high impacts from cell manufacturing (cobalt and nickel based cathode) in combination with low energy densities. On the other hand, the LiFP cell, with comparably high impacts from manufacturing (net impacts including recycling benefits), scores well when considering the whole life cycle due to its high efficiency and lifetime, except for ADP, where the impacts from cell manufacturing have a higher relevance.
Noteworthy are the high uncertainties represented by the error bars. These cover the range of values assumed for efficiency, maximum depth-of-discharge and cycle lifetime (Table 2) and are therefore caused by the lack of reliable data on these parameters for SIB. Different sizes of the error bars are consequently caused by the different min–max ranges assumed for the individual cell chemistries. Reliable data for these parameters is scarce even for LIB, and more lifetime tests with cells from different manufacturers and with varying layout would be needed for reducing this uncertainty. In addition, it has to be considered that the obtained impacts for the use-phase are highly dependent on the assumed application and can therefore not be generalized. Changing e.g., the daily full equivalent cycles from two to one would lead to very different results. The results are therefore to be considered as indicative and allow a general comparison of strengths and weaknesses of the different cell chemistries, but cannot support the general recommendation of a specific type of battery. For this purpose, a detailed, case specific study under consideration of the individual requirements of the foreseen application would be required.
3.4. Sensitivity analysis
Under GWP aspects, none of the SIB is able to compete with the LiNMC or even the LiFP battery. Also, it seems little probable that the NaPBA, NaNMC or NaMMO would ever reach this target given their electrochemical limitations. However, they might well catch up with the LiFP and then constitute an alternative for this cell chemistry. Taken into account the much lower technological maturity of SIB compared to LIB, this seems possible. For ADP, the picture is different, and due to the avoidance of scarce materials like cobalt or nickel, SIB (except the NaNMC) are closer to the reference than under GWP aspects. The NaPBA would require an increase by around 33% (120 to 160 W h kg−1) for equalling the LiNMC, while the NaMMO already achieves better values than the LiNMC, and would still do so even with 10% lower energy density. For the NaNMMT and NaNMC, however, the target of outperforming the LiNMC seems far out of reach. AP shows a similar picture as GWP, with the NaNMT, NaNMO and LiFP requiring around 40% higher energy density for equalling the best performing LIB (LiNMC). Under HTP aspects, the SIB (again except the NaNMC and NaMVP) are situated below the LIB already with current energy densities. The two best scoring SIB (NaMMO and NaNMMT), would even outperform the LiNMC in this category still with energy densities around 25% lower. The NaNMC cell would require (like also the LiFP) unrealistically high energy densities similar to that of the LiNMC, while the NaMVP would not even then get close to the benchmark.
In terms of cycle life, 4000 cycles have been assumed as default for all cell chemistries except LiFP and NaPBA with significantly higher cycle lives (7000). In consequence, the LiFP also obtains the best results in three of the assessed impact categories, despite the higher net impacts from manufacturing (see Fig. 2). While not being able to compete under GWP aspects, the low value material based SIB (NaMMO, NaPBA) show good results under ADP, AP and HTP aspects, with results similar or better than the LiNMC already in the baseline (HTP and ADP), or with around 35% higher cycle life (AP). Especially under toxicity aspects, the NaMMO, NaPBA and NaNMMT cells already outperform the LiNMC cell, and would still do so if reaching only 3000 cycles. The NaNMC and NaMVP, on the other hand, are unlikely to achieve high cycle lives required to reach the LiNMC benchmark in any of the assessed categories.
Charge–discharge efficiency, when assuming PV installations as electricity source, is relevant (due to the relatively high impacts of PV electricity on ADP), but not decisive for the ranking of the assessed battery cells. For GWP ad AP, extremely high efficiencies of min. 97% would be required for the SIB in order to equal the LiNMC in terms of environmental impacts. Under ADP aspects the same applies to the NaNMC and NaNMMT cells, while the NaPBA and NaMMO are already competitive with the LiNMC. However, if the final efficiency was lower than that of the LIB (assumed to be 93% for both the NaMMO and LiNMC and 93% for the NaPBA), the LiNMC would be the better choice also under ADP aspects. Finally, for HTP, all battery cells except the NaNMC are very close, and already small variations in efficiency can be decisive for the final ranking.
Considering the technological similarity and the already high efficiency of current LIB, it is questionable whether substantial improvements can be achieved for the SIB, making it little probable to achieve noteworthy changes in the battery rankings due to improved efficiency. However, the efficiency is related with the use-phase and thus highly dependent on the origin of the charged electricity. For grid electricity, the picture is different and efficiency grades gain relevance for GWP and AP impacts (compare Fig. 3 for the use-phase impacts). The results for grid electricity are provided in the ESI.†
3.5. Discussion
As already raised by previous works, the overall energy density of the battery cells is of high importance for their life cycle environmental impacts: the higher the energy density, the lower the amount of materials and other inputs required for producing a given storage capacity.28,47 This is especially relevant for the GWP category (GHG emissions), where the energy-intensive cell manufacturing process is an important contributor to the total environmental impacts. Interestingly (and contrary to the findings from previous works assessing manufacturing impacts),26,39 under a full life-cycle perspective the LiFP cells obtain good results and outperform the LiNMC in three of the assessed categories. This is mainly a result of the more detailed modelling approach and the higher energy density obtained for the LiFP cells compared to previous literature (197.4 W h kg−1 on cell level vs. between 108 and 142 W h kg−1 in previous works).26,30,39,71 In consequence, also GHG emissions associated with cell manufacturing are lower, with 49.6 kg per CO2 eq. per kWh on cell level (LFP), compared to 100.1–168.4 kg CO2 eq. per kWh from previous works.30,39,75 For the LiNMC cell, the corresponding value of 44.9 kg CO2 eq. per kWh on cell level is well in line with other publications (61–106 kg CO2 eq. per kWh on pack level), following the trend towards lower GHG emissions in more recent publications.10 Note that most assessments provide values on pack level, with battery cells making up only around 75% of the total battery pack mass.27,39,44Due to the low number of studies available on SIB, it is difficult to find values for comparison. For NaNMMT cell chemistries, values of between 69–140 kg per CO2 eq. per kWh can be found,19,20,30 though with different assumptions regarding cell housing, hard carbon precursor and manufacturing energy demand, all parameters with relevant influence on the total GHG emissions. This is significantly higher than the 50.6 kg CO2 eq. per kWh obtained by the present work. However, none of the previous works relied on a detailed electrochemical modelling of the cells, which substantially improves the reliability of the outcomes in the present work. Schneider et al.22 assess NaNMC cells based on an undisclosed proprietary electrochemical modelling, and obtain a value of 102 kg per CO2 eq. per kWh for average NaNMC cells (79 for high energy cells, 124 for high power cells), in line with the 86.6 kg per CO2 eq. per kWh for the present high energy NaNMC cell. For the remaining SIB chemistries, no studies have yet been published.
Comparing these values with those obtained for the LIB, it seems difficult for SIB to compete with current LIB under GWP aspects. However, ongoing decarbonisation of the manufacturing process, but also of the upstream process chains will likely reduce GHG emissions associated with the cell production further. The origin of raw materials and the corresponding energy mix used for processing them will play an increasingly important role for the total GWP of future battery cells and might even become more relevant than the exact cell chemistry itself. On the other hand, from a resource depletion (and also toxic emissions) perspective, the SIB based on abundant materials (NaMMO, NaPBA but also NaNMMT) avoid all critical materials in this regard and obtain very positive results, easily outperforming the LIB under a life cycle perspective. For the NaMVP cell, the unfavourable results can partially be attributed to the modelling of the vanadium production, derived from a publication of vanadium redox-flow batteries. This modelled a specific process in South Africa, with significant SO2 emissions along the process chain and a heavy coal-based electricity mix.74 Sourcing vanadium from alternative processes might drastically reduce the associated emissions, and situate also the NaMVP cells in the same range as the remaining SIB. The same applies for the high AP impacts associated with the NaMVP cell, where a significant share is caused by the names SO2 process emissions. Apart from that, all SIB suffer from significantly higher AP impacts associated with the anode active material (hard carbon) than the LIB. The hard carbon is modelled according to a previous work as being sourced from petroleum coke, found to show good environmental results due to a high process efficiency. However, no specific SO2 abatement technology is considered in the underlying process model, and remaining SO2 emissions increase the AP impacts associated with hard carbon production. In any case, the lack of an established hard carbon market impedes to determine a dominating or most probable production pathway. Here, care has to be taken to source hard carbon from processes and precursors with low associated impacts, otherwise the impacts from the anode active material might jeopardise the promising environmental performance of emerging SIB.
Regarding the use-phase, it is very difficult to find robust performance data for efficiency and cycle life for the investigated cell types. A broad range of values has been included here in a parametric study that provides a first idea of which performance is required for SIB to be competitive in relation to LIBs. When assuming that SIB achieve similar lifetimes like current LIB, they can be considered as potentially competitive under environmental aspects, though still lacking behind in terms of GWP. Whether these cycle lives will be reached (or even exceeded) is yet to be seen. Correspondingly, an eco-design must consider all key parameters and their possible interaction, taking into account that materials with minimum environmental impact might jeopardise energy density and/or lifetime and therefore, in sum, even worsen the total environmental performance. In this sense, the present analysis provides some rather general guidelines for eco-optimizing future SIB cells, charting the way towards environmentally friendly alterative to current maximum performance LIB. While not able to compete with those in terms of energy density, SIB do have potential for becoming a green alternative in areas where energy density is not the principal performance parameter.
3.6. Limitations and future work
The present assessment relies on an improved cell-specific recycling model that estimates the input of process chemicals and the output of recycled materials in a cell-specific manner, significantly improving previous modelling approaches. However, while adjusted to the individual cell composition, it still assumes the same hydrometallurgical process pathway (which is essentially designed for LiNMC cells) for all cell chemistries. No alternative pathways for processing black mass from e.g., LiFP cells are yet established, but it can be expected that these require very different approaches better optimised for them. This would also require a reliable separation into cell chemistries prior to recycling.Second, the results obtained for the ADP category (resource depletion) should be taken with care. When looking into the contribution of the individual elementary flows to the total ADP, sulphur, arsenic and copper are among the main contributors. This might be in line with the actual availability of the corresponding elements in earth's crust or the given reserves but seems odd in terms of assessing the impact of the batteries, where cobalt, nickel, lithium and copper are the most relevant constituents.9 This is a direct consequence of the modelling approach taken by the ecoinvent database, where not only process inputs and emissions, but also elementary resource flows are allocated to the mining co-products according to economic criteria and not according to physical relationships. If the elementary resource flows were assigned to the corresponding products (‘subdivision’), the resource depletion results might show a different picture, what would be an interesting question for future work.
Third, charge–discharge efficiency turns out to be an important parameter but is not covered by the cell dimensioning tool developed in this work. Rather, round trip efficiency values are taken from literature, and do not necessarily correspond exactly to the assessed battery cells. Also, efficiencies vary with C-rates and are therefore application-specific. Given the electrochemical similarity between LIB and SIB, differences might be much more driven by layout considerations than varying electrochemistry: Cells designed for high power applications and thus higher currents usually have thicker current collectors and thinner electrodes, reducing ohmic losses and thereby increasing charge–discharge efficiency. Cells designed for maximum energy density will maximise electrode thickness (and with that of active material) and minimise the mass of current collector, with the opposite effect on efficiency, leading to a trade-off between energy density and efficiency. A detailed evaluation of these trade-offs and an improved cell dimensioning tool taking into account ohmic losses would be very relevant in this regard.
Finally, it should be noted that the recycling model provided with this work is based on process modelling, stoichiometric calculations and single previous data from company visits. The advanced hydrometallurgical treatment is the only model considered to be sufficiently reliable for assessment, while the existing data for conventional pyrometallurgical and hydrometallurgical processes are insufficient for a meaningful assessment. However, the recycling processes are designed principally for current LiNMC automotive batteries, while several of the assessed cell chemistries would require specifically tailored processes. Especially for the NaMVP cells, no vanadium recovery is foreseen in the process chain, though vanadium extraction is one of the key drivers of environmental impacts for this cell type. More cell specific recycling processes would be required for unlocking the full recovery potential for these emerging SIB cell chemistries. In fact, assuming a recycling process with a vanadium recovery rate in the same order of magnitude as for other battery materials would drastically reduce the life cycle impacts of the NaMVP cell. The ESI† provides also the process models for these processes for future use, but calls for improving these with first-hand industry data prior to their application. This would advance the current state of the art significantly and also to compare different recycling processes.
4. Conclusions
This work provides new insights into the environmental performance of different sodium-ion battery (SIB) cell chemistries in comparison to current lithium-ion batteries (LIB). For some of the assessed SIB cells (NaMMO and NaNMMT), their environmental performance is getting close to or even better than that of their LIB counterparts, and the remaining progress required for reaching similar values can be considered achievable, considering their still much lower technological maturity. While all stages of the cell's life cycle (manufacturing, material extraction, use phase and end-of-life) contribute significant shares to the total environmental impacts of the assessed cell chemistries, some hotspots can be pointed out. In the production phase, major drivers of environmental impacts (and thus improvement potentials) are the energy demand during cell manufacturing (GHG emissions), the electrolyte salts (resource depletion) and the hard carbon-based anode material (acidification). Interestingly, the NaNMC cell, used as a reference in a previous work, is not able to catch up with current LiNMC, majorly due to its limited energy density. LiFP, though also with lower energy demand and higher impacts from the manufacturing stage, make this up with higher lifetime, and achieve similar or even better results under a full life-cycle perspective. The same applies to the Prussian blue based NaPBA cell. Hence, also for SIB, if unable to achieve higher energy densities, a high cycle life is the key for unlocking their potential and establishing themselves as alternatives to LIB.Efficient recycling is also important for minimizing environmental impacts for all cell chemistries, though much more for LIB and for the nickel, cobalt, or vanadium containing SIB (NaNMC and NaMVP). Essentially, recycling is most beneficial for the cell chemistries that rely on scarce or critical metals and thus are associated with higher impacts from raw materials extraction and -processing. In fact, the high variation of environmental impacts from the manufacturing stage between the different cell chemistries is reduced substantially when accounting for the potential benefit of recovered materials. Assuming that all cells are optimally recycled, the high recycling efficiency and corresponding high recycling benefits especially for LiNMC make these to score best in the majority of the assessed impact categories. However, it also needs to be considered that for all battery cells a complete recycling according to the latest technology and with minimum loss to the environment is assumed. In reality, recovery and recycling rates are lower, and a significant share of batteries never enters the official recovery and recycling streams.76,77 Assuming lower recycling rates uniformly for all cell types, the NaMMO, NaPBA and NaNMMT cells could easily outperform LIB in terms of toxic impacts and resource depletion (see Section 5.3 of the ESI† for a more detailed analysis of this aspect).
Finally, recycling of batteries that are already made up of abundant materials has its limitations. With the applied recycling model, the low-value SIB and LIB (NaMMO, NaPBA and LiFP in the present assessment) show, depending on the considered impact category, only limited environmental benefits or even additional impacts from the hydrometallurgical recycling of the black mass. This indicates that recovering these materials with the assumed process technology might be environmentally not better than mining them from virgin materials. However, considering that the applied recycling model is derived from a high-end process designed for LiNMC cells, it might be inappropriate for processing these low-value chemistries. Here, alternative recycling processes would be needed that either require substantially less process inputs or that recover the active materials ‘as they are’, maintaining their crystal structure (‘direct recycling’).
Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was funded by the European Union's Horizon 2020 Research and Innovation Programme under the Marie Skłodowska-Curie Grant Agreement No. 754382 and by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany's Excellence Strategy – EXC 2154 – Project number 390874152. However, its content does not reflect the official opinion of the European Union. Responsibility for the information and views expressed herein lies entirely with the authors.References
- S. Sripad and V. Viswanathan, J. Electrochem. Soc., 2017, 164, E3635–E3646 CrossRef CAS.
- M. J. Baumann, J. F. Peters, M. Weil and A. Grunwald, Energy Technol., 2017, 5, 1071–1083 CrossRef CAS.
- I. Tsiropoulos, D. Tarvydas and N. Lebedeva, Li-ion batteries for mobility and stationary storage applications, Publications Office of the European Union, Petten, The Netherlands, European Commission, Joint Research Centre, 2018 Search PubMed.
- D. Stephens, P. Shawcross, G. Stout, E. Sullivan, J. Saunders, S. Risser and J. Sayre, Lithium-ion Battery Safety Issues for Electric and Plug-in Hybrid Vehicles, National Highway Traffic Safety Administration., Washington DC, US, 2017 Search PubMed.
- N. Williard, W. He, C. Hendricks and M. Pecht, Energies, 2013, 6, 4682–4695 CrossRef.
- M. Walter, M. V. Kovalenko and K. V. Kravchyk, New J. Chem., 2020, 44, 1677–1683 RSC.
- A. Ponrouch and M. R. Palacín, Philos. Trans. R. Soc., A, 2019, 377, 20180297 CrossRef CAS PubMed.
- M. Weil, J. Peters and M. Baumann, in The Material Basis of Energy Transitions, ed. A. Bleicher and A. Pehlken, Academic Press, 2020, pp. 71–89 Search PubMed.
- J. F. Peters and M. Weil, Resources, 2016, 5, 46 CrossRef.
- E. Emilsson and L. Dahllöf, Lithium-Ion Vehicle Battery Production. Status 2019 on Energy Use, CO2 Emissions, Use of Metals, Products Environmental Footprint, and Recycling, IVL Swedish Environmental Research Institute, Stockholm, Sweden, 2019 Search PubMed.
- C. Vaalma, D. Buchholz, M. Weil and S. Passerini, Nat. Rev. Mater., 2018, 3(18013) Search PubMed.
- J.-Y. Hwang, S.-T. Myung and Y.-K. Sun, Chem. Soc. Rev., 2017, 46, 3529–3614 RSC.
- M. Li, Z. Du, M. A. Khaleel and I. Belharouak, Energy Storage Mater., 2020, 25, 520–536 CrossRef.
- M. Mirzaeian, Q. Abbas, M. R. C. Hunt, A. Galeyeva and R. Raza, in Reference Module in Materials Science and Materials Engineering, Elsevier, 2021 Search PubMed.
- E. E. Onstad, CATL’s new sodium ion battery to help ease lithium shortages, Reuters, 2021 Search PubMed https://www.reuters.com/business/energy/catls-new-sodium-ion-battery-help-ease-lithium-shortages-2021-08-03/ .
- Tiamat, Tiamat - Powerful, fast charging, enduring cells thanks to sodium-ion, http://www.tiamat-energy.com/, accessed September 23, 2021 Search PubMed.
- Faradion Ltd., Sodium Ion Batteries, Faradion Ltd., https://www.faradion.co.uk/, accessed March 24, 2020 Search PubMed.
- Natron Energy, Inc., Natron Energy, https://natron.energy/technology/, accessed April 27, 2021 Search PubMed.
- J. Peters, D. Buchholz, S. Passerini and M. Weil, Energy Environ. Sci., 2016, 9, 1744–1751 RSC.
- J. F. Peters, M. Abdelbaky, M. Baumann and M. Weil, Mater. Technol., 2019, 107, 503 CrossRef CAS.
- J. F. Peters, A. Peña Cruz and M. Weil, Batteries, 2019, 5, 10 CrossRef CAS.
- S. F. Schneider, C. Bauer, P. Novák and E. J. Berg, Sustainable Energy Fuels, 2019, 3, 3061–3070 RSC.
- M. A. Pellow, H. Ambrose, D. Mulvaney, R. Betita and S. Shaw, Sustainable Mater. Technol., 2020, 23, e00120 CrossRef CAS.
- L. A.-W. Ellingsen, C. R. Hung and A. H. Strømman, Transp. Res. D, 2017, 55, 82–90 CrossRef.
- D. A. Notter, M. Gauch, R. Widmer, P. Wäger, A. Stamp, R. Zah and H.-J. Althaus, Environ. Sci. Technol., 2010, 44, 6550–6556 CrossRef CAS PubMed.
- M. Zackrisson, L. Avellán and J. Orlenius, J. Cleaner Prod., 2010, 18, 1519–1529 CrossRef CAS.
- L. A.-W. Ellingsen, G. Majeau-Bettez, B. Singh, A. K. Srivastava, L. O. Valøen and A. H. Strømman, J. Ind. Ecol., 2014, 18, 113–124 CrossRef CAS.
- J. F. Peters, M. J. Baumann, B. Zimmermann, J. Braun and M. Weil, Renewable Sustainable Energy Rev., 2017, 67, 491–506 CrossRef CAS.
- H. S. Hirsh, Y. Li, D. H. S. Tan, M. Zhang, E. Zhao and Y. S. Meng, Adv. Energy Mater., 2020, 10, 2001274 CrossRef CAS.
- M. Mohr, J. F. Peters, M. Baumann and M. Weil, J. Ind. Ecol., 2020, 24(6), 1310–1322 CrossRef CAS.
- M. A. Rajaeifar, M. Raugei, B. Steubing, A. Hartwell, P. A. Anderson and O. Heidrich, J. Ind. Ecol., 2021 DOI:10.1111/jiec.13157.
- M. Chen, Q. Liu, S.-W. Wang, E. Wang, X. Guo and S.-L. Chou, Adv. Energy Mater., 2019, 9, 1803609 CrossRef.
- T. Jin, H. Li, K. Zhu, P.-F. Wang, P. Liu and L. Jiao, Chem. Soc. Rev., 2020, 49, 2342–2377 RSC.
- R. J. Clément, J. Billaud, A. R. Armstrong, G. Singh, T. Rojo, P. G. Bruce and C. P. Grey, Energy Environ. Sci., 2016, 9, 3240–3251 RSC.
- M. Sathiya, K. Hemalatha, K. Ramesha, J.-M. Tarascon and A. S. Prakash, Chem. Mater., 2012, 24, 1846–1853 CrossRef CAS.
- H. Ye, Y. Wang, F. Zhao, W. Huang, N. Han, J. Zhou, M. Zeng and Y. Li, J. Mater. Chem. A, 2016, 4, 1754–1761 RSC.
- J. Song, L. Wang, Y. Lu, J. Liu, B. Guo, P. Xiao, J.-J. Lee, X.-Q. Yang, G. Henkelman and J. B. Goodenough, J. Am. Chem. Soc., 2015, 137, 2658–2664 CrossRef CAS PubMed.
- W. Zhou, L. Xue, X. Lü, H. Gao, Y. Li, S. Xin, G. Fu, Z. Cui, Y. Zhu and J. B. Goodenough, Nano Lett., 2016, 16, 7836–7841 CrossRef CAS PubMed.
- J. F. Peters and M. Weil, J. Cleaner Prod., 2018, 704–713 CrossRef.
- Q. Dai, J. C. Kelly, L. Gaines and M. Wang, Batteries, 2019, 5, 48 CrossRef CAS.
- EC-JRC, ILCD Handbook: Recommendations for Life Cycle Impact Assessment in the European context, European Commission - Joint Research Centre. Institute for Environment and Sustainability, Ispra, Italy, EC-JRC - Institute for Environment and Sustainability, 2011 Search PubMed.
- E. Moreno Ruiz, L. Valsasina, D. Fitzgerald, A. Symeonidis, D. E. Turner, J. Müller, N. Minas, G. Bourgault, C. Vadenbo, D. Ionnidou and G. Wernet, Documentation of changes implemented in the ecoinvent database v3.7 & v3.7.1, Ecoinvent Association, Zürich, Switzerland, 2020 Search PubMed.
- P. A. Nelson, S. Ahmed, K. G. Gallagher and D. W. Dees, Modeling the Performance and Cost of Lithium-Ion Batteries for Electric-Drive Vehicles, Argonne National Lab. (ANL), Argonne, IL (United States), 3rd edn, 2019 Search PubMed.
- E. Crenna, M. Gauch, R. Widmer, P. Wäger and R. Hischier, Resour., Conserv. Recycl., 2021, 170, 105619 CrossRef.
- C. Yuan, Y. Deng, T. Li and F. Yang, CIRP Ann., 2017, 66, 53–56 CrossRef.
- Y. Preger, H. M. Barkholtz, A. Fresquez, D. L. Campbell, B. W. Juba, J. Romàn-Kustas, S. R. Ferreira and B. Chalamala, J. Electrochem. Soc., 2020, 167, 120532 CrossRef CAS.
- J. F. Peters and M. Weil, J. Power Sources, 2017, 364, 258–265 CrossRef CAS.
- T. Le Varlet, O. Schmidt, A. Gambhir, S. Few and I. Staffell, J. Energy Storage, 2020, 28, 101230 CrossRef.
- J. E. Harlow, X. Ma, J. Li, E. Logan, Y. Liu, N. Zhang, L. Ma, S. L. Glazier, M. M. E. Cormier, M. Genovese, S. Buteau, A. Cameron, J. E. Stark and J. R. Dahn, J. Electrochem. Soc., 2019, 166, A3031–A3044 CrossRef CAS.
- CARMEN e.V., Marktübersicht Batteriespeicher, C.A.R.M.E.N. e.V., Centrales Agrar-Rohstoff Marketing- und Energie-Netzwerk, Straubing, DE, 2021 Search PubMed.
- VARTA Element - Technical Datasheet. VARTA Storage GmbH, 2021. https://www.varta-ag.com/fileadmin/varta/consumer/downloads/energy-storage/varta-element/Datasheet_VARTA_element_dach_de_4.pdf Search PubMed.
- D. R. Sørensen, M. Heere, J. Zhu, M. S. D. Darma, S. M. Zimnik, M. J. Mühlbauer, L. Mereacre, V. Baran, A. Senyshyn, M. Knapp and H. Ehrenberg, ACS Appl. Energy Mater., 2020, 3, 6611–6622 CrossRef.
- T. Liu, Y. Zhang, Z. Jiang, X. Zeng, J. Ji, Z. Li, X. Gao, M. Sun, Z. Lin, M. Ling, J. Zheng and C. Liang, Energy Environ. Sci., 2019, 12, 1512–1533 RSC.
- A. Bauer, J. Song, S. Vail, W. Pan, J. Barker and Y. Lu, Adv. Energy Mater., 2018, 8, 1702869 CrossRef.
- T. Broux, F. Fauth, N. Hall, Y. Chatillon, M. Bianchini, T. Bamine, J. Leriche, E. Suard, D. Carlier, Y. Reynier, L. Simonin, C. Masquelier and L. Croguennec, Small Methods, 2019, 3, 1800215 CrossRef CAS.
- C. Yan, A. Zhao, F. Zhong, X. Feng, W. Chen, J. Qian, X. Ai, H. Yang and Y. Cao, Electrochim. Acta, 2020, 332, 135533 CrossRef CAS.
- B. Wang, Y. Han, X. Wang, N. Bahlawane, H. Pan, M. Yan and Y. Jiang, iScience, 2018, 3, 110–133 CrossRef CAS PubMed.
- A. Jana, R. Paul and A. K. Roy, in Carbon Based Nanomaterials for Advanced Thermal and Electrochemical Energy Storage and Conversion, Elsevier, 2019, pp. 25–61 Search PubMed.
- K. Nakamoto, Y. Kano, A. Kitajou and S. Okada, J. Power Sources, 2016, 327, 327–332 CrossRef CAS.
- H. Zhang, X. Liu, H. Li, I. Hasa and S. Passerini, Angew. Chem., 2021, 133, 608–626 CrossRef.
- R. Sommerville, P. Zhu, M. A. Rajaeifar, O. Heidrich, V. Goodship and E. Kendrick, Resour., Conserv. Recycl., 2021, 165, 105219 CrossRef.
- J. B. Dunn, L. Gaines, J. Sullivan and M. Q. Wang, Environ. Sci. Technol., 2012, 46, 12704–12710 CrossRef CAS PubMed.
- Q. Dai, J. Spangenberger, A. Shabbir, L. Gaines, J. C. Kelly and M. Wang, EverBatt: A Closed-loop Battery Recycling Cost and Environmental Impacts Model, Argonne National Laboratories, Energy Systems Division, Argonne, US, 2019 Search PubMed.
- M. Chen, X. Ma, B. Chen, R. Arsenault, P. Karlson, N. Simon and Y. Wang, Joule, 2019, 3, 2622–2646 CrossRef CAS.
- C. M. Costa, J. C. Barbosa, R. Gonçalves, H. Castro, F. J. D. Campo and S. Lanceros-Méndez, Energy Storage Mater., 2021, 37, 433–465 CrossRef.
- Y. Wang, N. An, L. Wen, L. Wang, X. Jiang, F. Hou, Y. Yin and J. Liang, J. Energy Chem., 2021, 55, 391–419 CrossRef.
- M. Mohr, M. Weil, J. Peters and Z. Wang, in Encyclopedia of Electrochemistry, American Cancer Society, 2020, pp. 1–33 Search PubMed.
- C. Hanisch, T. Elwert and L. Brückner, Method for the utilization of lithium batteries, EPO, EP3517641 B1, 2019 Search PubMed.
- T. Or, S. W. D. Gourley, K. Kaliyappan, A. Yu and Z. Chen, Carbon Energy, 2020, 2, 6–43 CrossRef CAS.
- K.-H. Pettinger and W. Dong, J. Electrochem. Soc., 2016, 164, A6274 CrossRef.
- G. Majeau-Bettez, T. R. Hawkins and A. H. Strømman, Environ. Sci. Technol., 2011, 45, 4548–4554 CrossRef CAS PubMed.
- C. M. Lastoskie and Q. Dai, J. Cleaner Prod., 2015, 91, 158–169 CrossRef CAS.
- Q. Dai, J. C. Kelly, J. Dunn and P. T. Benavides, Update of Bill-of-materials and Cathode Materials Production for Lithium-ion Batteries in the GREET Model, Argonne National Laboratories, Argonne, US, 2018 Search PubMed.
- S. Weber, J. Peters, M. J. Baumann and M. Weil, Environ. Sci. Technol., 2018, 52, 10864–10873 CrossRef CAS PubMed.
- H. Hao, Z. Mu, S. Jiang, Z. Liu and F. Zhao, Sustainability, 2017, 9(4), 504 CrossRef.
- J. F. Peters, M. Baumann and M. Weil, Recycling aktueller und zukünftiger Batteriespeicher: Technische, ökonomische und ökologische Implikationen : Ergebnisse des Expertenforums am 6. Juni 2018 in Karlsruhe, Karlsruhe Institute of Technology (KIT), Karlsruhe, Germany, 2018 Search PubMed.
- Eurostat, Waste statistics - recycling of batteries and accumulators, https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Waste_statistics_-_recycling_of_batteries_and_accumulators, accessed May 7, 2021 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional details on the modelling approach, detailed results tables and numerical values used for creating all graphs and figures. The MS-excel based cell dimensioning tool and inventory data (LCI) data for direct import into existing LCA software (JSON-LD and ILCD format) are available for download from Zenodo: https://doi.org/10.5281/zenodo.4742246. See DOI: 10.1039/d1se01292d |
| This journal is © The Royal Society of Chemistry 2021 |