Open Access Article
Open Access Article15N-Azides as practical and effective tags for developing long-lived hyperpolarized agents†
Junu
Bae‡
a,
Guannan
Zhang‡
a,
Hyejin
Park
a,
Warren S.
Warren
*abc and
Qiu
Wang
 *a
*a
aDepartment of Chemistry, Duke University, Durham, North Carolina 27708, USA. E-mail: warren.warren@duke.edu; qiu.wang@duke.edu
bDepartment of Physics, Duke University, Durham, North Carolina 27708, USA
cDepartment of Radiology and Biomedical Engineering, Duke University, Durham, North Carolina 27708, USA
First published on 12th October 2021
Abstract
Azide moieties, unique linear species containing three nitrogen atoms, represent an attractive class of molecular tag for hyperpolarized magnetic resonance imaging (HP-MRI). Here we demonstrate (15N)3-azide-containing molecules exhibit long-lasting hyperpolarization lifetimes up to 9.8 min at 1 T with remarkably high polarization levels up to 11.6% in water, thus establishing (15N)3-azide as a powerful spin storage for hyperpolarization. A single (15N)-labeled azide has also been examined as an effective alternative tag with long-lived hyperpolarization. A variety of biologically important molecules are studied in this work, including choline, glucose, amino acid, and drug derivatives, demonstrating great potential of 15N-labeled azides as universal hyperpolarized tags for nuclear magnetic resonance imaging applications.
Introduction
Magnetic resonance imaging (MRI) provides an attractive non-invasive imaging method to determine structure and function with high spatial and temporal resolution. MRI has the ability to detect and monitor complex molecular systems for chemical and biochemical analysis by relying on nuclear magnetic resonance (NMR). However, its widespread applicability is limited by the inherently low sensitivity of magnetic resonance at thermal equilibrium. The hyperpolarization technique, by inducing non-equilibrium polarization, can enhance NMR signal by several orders of magnitude and has been applied successfully to increase MRI sensitivity. Hyperpolarization of heteronuclei (e.g., 13C and 15N) is particularly useful, as real-time in vivo detection of these heteronuclei allows for investigating many dynamic metabolic and physiologic processes that were previously inaccessible to imaging.1 The observable time window of a hyperpolarized molecule is primarily governed by the spin-lattice relaxation time (T1) of the nucleus within the molecules of interest. Nuclei in certain functional groups, such as 13C-carboxylic acids or quaternary 15N-amine salts, have been found to have relatively long T1 due to reduced dipole–dipole interaction without attached protons.2 The comparably long-lived properties lead to a relatively slow signal decay amenable for investigating slow processes, thus allowing translation into clinic practice.3However, it is challenging to achieve long-lived 13C or 15N centers in a target molecule that lacks a 13C-carbonyl group or quaternary 15N-amine motif. For many biologically important molecules,4 the practicality of the corresponding isotopomers is limited by their short T1 lifetimes. A short detection time window for hyperpolarized molecules presents challenges for developing effective HP-MRI agents for biomedical and clinical applications. A novel strategy to address this limitation is to incorporate a molecular tag that is capable of supporting long-lived hyperpolarization into target molecules. We have recently reported the development of 15N2-diazirines5 and 15N4-1,2,4,5-tetrazines6 as molecular tags for hyperpolarized NMR and MRI applications. Herein, we report that 15N-labeled-azide constitutes a novel class of hyperpolarized molecular tag, offering superior practicality and efficacy as well as potentially broader imaging applications (Fig. 1).
We envisioned that the (15N)3-azide group, absent of directly attached protons, is theoretically capable of affording long-lived hyperpolarization. Furthermore, the three distinct nitrogen atoms of the azide will each provide a discrete chemical shift peak on 15N-NMR that can be used for detection. Another attractive property of the azide group is that it fulfills many of the characteristics desirable in a molecular tag, featuring small size, unobtrusive electronic properties, stability in acidic or basic conditions, bioorthogonality, and synthetic ease of use.7 Numerous applications of the azides as a reaction partner in Staudinger reactions and azide–alkyne cycloaddition have been documented, representing a cornerstone in chemical biology and bioconjugation chemistry.7d,8 As the most commonly used chemical reporter, the azides have been used in the studies of nearly every class of biomolecules, including carbohydrates,9 amino acids,10 cholesterol,11 nucleosides,12 and lipids.13 Thus, the (15N)3-azide group, in addition to being a potential long-lived molecular tag for hyperpolarized NMR and MRI, can be used simultaneously in a bioorthogonal reaction to introduce other imaging readouts,7d,8,14 allowing for development of multimodal imaging agents.
In this paper, we investigated hyperpolarization of a series of (15N)3-azide-tagged molecules to examine the efficacy and potential of (15N)3-azides as a long-lasting hyperpolarized tag. We also evaluated the ability of single-labeled (15N)(14N)2-azide-tagged molecules as alternatives to offer long-lived hyperpolarization. The wide applicability and diverse examples of azide-labeled biomolecules exemplify the potentials of 15N-labeled-azides as novel and practical molecular tags for developing effective hyperpolarized agents in broad biomedical applications.
Results and discussion
Design and synthesis of (15N)3-tagged molecules
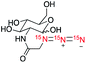
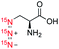
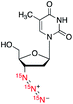
Our studies began with the design and synthesis of a series of compounds 1–6, including choline, amino acids, glucoses, and an HIV drug azidothymidine (AZT) (Table 1). They were chosen as representative examples for diverse biologically important molecules that have the potential to be developed into hyperpolarized probes and to be pursued in future biomedical applications, such as understanding the metabolic basis of related diseases as well as drug pharmacology and mode of action.2b First, sodium (15N)3-azide, the key reagent for the synthesis of all (15N)3-labeled compounds, was prepared by the oxidation of commercially available (15N)2-hydrazine monohydrate with 15N-isoamyl nitrite.15 Next, (15N)3-azide-tagged molecules 1–6 were synthesized in 3–5 steps readily from commercially available reagents.16 The locations of (15N)3-azides in the biomolecules were strategically selected so the tags have minimal effect on the bioactivity of molecules. The rapid access to (15N)3-labeled compounds 1–6 highlights the easiness and efficiency of (15N)3-azide incorporation into target molecules, as well as its good compatibility with various functional groups, allowing for the chemical labeling of (15N)3-azide in late stage of target molecule synthesis.| Cmpd | Molecular structure | ε | P/% | T 1/min | ||
|---|---|---|---|---|---|---|
| Nα | Nβ | Nγ | ||||
| a Each experiment repeated twice. Measurements performed at B = 1 T unless noted otherwise. Enhancements and polarization levels averaged over all 15N sites. T1 lifetimes derived by fitting the experimental data to a single exponential decay function and then compensating the effect of using the small flip angle pulses (see ESI for details). b T 1 lifetime measured at B = 16.4 T using thermally polarized 1 in D2O. | ||||||
| 1 |
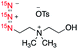
|
336![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
11.6 | (2.4 ± 0.2) | (2.9 ± 0.2) | (3.3 ± 0.2) |
| (0.36 ± 0.03)b | (0.10 ± 0.01)b | (0.25 ± 0.02)b | ||||
| 2 |
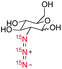
|
254![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
8.8 | (3.0 ± 0.2) | (4.0 ± 0.2) | (7.6 ± 0.3) |
| 3 |
|
322![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
11.1 | (3.0 ± 0.3) | (5.6 ± 0.3) | (8.1 ± 0.2) |
| 4 |
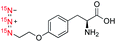
|
142![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
4.9 | (3.5 ± 0.3) | (6.2 ± 0.9) | (9.8 ± 1.7) |
| 5 |
|
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
2.8 | (1.9 ± 0.2) | (4.0 ± 0.3) | (6.8 ± 0.9) |
| 6 |
|
196![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
6.8 | (2.5 ± 0.1) | (3.6 ± 0.1) | (5.3 ± 0.1) |
Hyperpolarization of (15N)3-azide-tagged molecules using d-DNP
To investigate hyperpolarization of these (15N)3-tagged molecules, we first determined the chemical shift assignments by 15N spectroscopic analysis of thermally polarized (15N)3-azidocholine 1, including its 15N-NMR spectra with and without 1H decoupling acquired at a magnetic field of 16.4 T (Fig. 2). Based on the J-coupling analysis of JN–N-couplings and JN–H-couplings as annotated in Fig. 2, the chemical shift assignments for 15Nα, 15Nβ, and 15Nγ were as follows: 15Nα appears in the most upfield region (∼65 ppm), 15Nβ appears in the most downfield region (∼242 ppm), and 15Nγ appears in between (∼210 ppm). Because the (15N)3-azide tags in these structures are not directly adjacent to the sites of chemical changes that occur in endogenous metabolic processes, the hyperpolarized species may not necessarily generate 15N NMR shift changes sufficient for chemical shift imaging (CSI) in their respective metabolic processes. Thus, in this study, we focus on establishing efficacy and adaptability of (15N)3-azide as a remarkable tag that offers favorable long-lived hyperpolarization, paving the way for biomedical applications.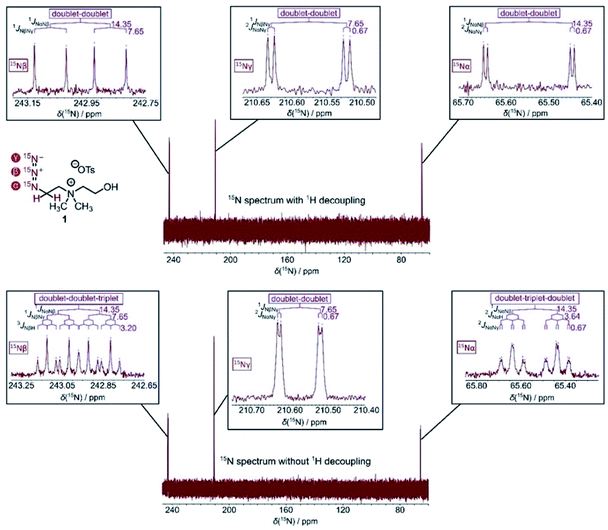 | ||
| Fig. 2 15N-NMR spectra of thermally polarized (15N)3-azide 1 (253 mM in D2O) acquired with (top) and without (bottom) 1H decoupling. The 15N spectra were measured at a magnetic field of 16.4 T. | ||
With the chemical shift assignments of 15Nα, 15Nβ, and 15Nγ established, we examined hyperpolarization efficiency of (15N)3-labeled compounds 1–6 using dissolution dynamic nuclear polarization (d-DNP). DNP is a hyperpolarization method routinely used in preclinical studies.2b,17 It is capable of polarizing almost any molecule of interest and typically uses deuterated water as the dissolution solvent, allowing for the analysis of the sample in an aqueous solution. Thus, effective hyperpolarization of (15N)3-azides by d-DNP will represent an important step toward in vivo biomedical applications. Table 1 summarize the signal enhancement (ε) and relaxation times (T1) of three individual 15N-atoms (15Nα, 15Nβ, and 15Nγ) of these (15N)3-labeled compounds measured at 1 T. Fig. 3 depicts the comparison of T1 relaxation times of (15N)3-azides 1–6 as well as the T1 differences among the three individual 15N atoms of the (15N)3-azides. As shown in Table 1, each of the (15N)3-azides 1–6 displayed highly effective 15N signal enhancements up to over 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold and long 15N relaxation lifetimes up to 9.8 min at 1 T.
000-fold and long 15N relaxation lifetimes up to 9.8 min at 1 T.
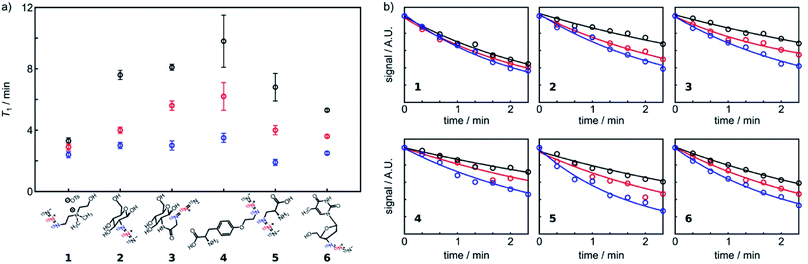 | ||
| Fig. 3 (a) Comparison of T1 relaxation times of (15N)3-azides (see Table 1 for T1 values). (b) T1 lifetime curves for (15N)3-azides. The experimental data points were fitted to an exponential decay function. The first 8 data points are shown. | ||
Our hyperpolarization experiments began with (15N)3-labeled azidoethylcholine 1. It has been shown that choline analogs, in which a methyl group is replaced with an alkyl chain of up to five carbon atoms in length, incorporate efficiently into phospholipids.18 For example, 14N-azidoethylcholine has been demonstrated to faithfully mimic endogenous choline and can be efficiently incorporated into all classes of choline phospholipid for fluorescence imaging of phospholipids in cells.19 Studies on hyperpolarization of 15N-choline demonstrated its use in imaging cellular choline uptake in vitro and in vivo.2a,20 Inspired by these earlier studies, we envision that (15N)3-azidoethylcholine 1 may serve as a potential HP-MRI probe to monitor elevated choline uptake leading to phospholipid metabolism. Hyperpolarization of (15N)3-azidoethylcholine analog 1 by d-DNP provided signal enhancement over 336![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold and T1 value up to 3.3 min. The significantly enhanced and long-lived signals are highly encouraging for its in vivo imaging applications. Among the three different 15N atoms of the (15N)3-azide, the terminal nitrogen (15Nγ) of the azide gave the longest T1 lifetime, which results from the reduced dipole–dipole interaction in the absence of neighboring protons in comparison to 15Nα and 15Nβ. The central 15Nβ showed the second longest T1 lifetime, followed by 15Nα with the shortest T1 due to the proximity to adjacent methylene protons. As shown in Table 1, T1 lifetimes of thermally polarized (15N)3-azidocholine 1 were only 21.9 s for 15Nα, 6.0 s for 15Nβ and 15.0 s for 15Nγ at a magnetic field of 16.4 T. The difference in 15N T1 lifetimes at different field strength is largely due to chemical shift anisotropy scaled linearly with B02, which suggests the advantage of imaging hyperpolarized azide tags at a low magnetic field.
000-fold and T1 value up to 3.3 min. The significantly enhanced and long-lived signals are highly encouraging for its in vivo imaging applications. Among the three different 15N atoms of the (15N)3-azide, the terminal nitrogen (15Nγ) of the azide gave the longest T1 lifetime, which results from the reduced dipole–dipole interaction in the absence of neighboring protons in comparison to 15Nα and 15Nβ. The central 15Nβ showed the second longest T1 lifetime, followed by 15Nα with the shortest T1 due to the proximity to adjacent methylene protons. As shown in Table 1, T1 lifetimes of thermally polarized (15N)3-azidocholine 1 were only 21.9 s for 15Nα, 6.0 s for 15Nβ and 15.0 s for 15Nγ at a magnetic field of 16.4 T. The difference in 15N T1 lifetimes at different field strength is largely due to chemical shift anisotropy scaled linearly with B02, which suggests the advantage of imaging hyperpolarized azide tags at a low magnetic field.
We next evaluated hyperpolarization of (15N)3-azide-tagged glucose derivatives 2 and 3 (Table 1). 13C-Labeled glucose has been developed for HP-MRI imaging of tumor glycolysis, yet it is limited by a narrow window of detection because of short polarization lifetime.21 In this study, we prepared (15N)3-labeled 2-azido-2-deoxy-glucose (2AzGlc) 2 and (15N)3-labeled azidoacetyl-glucosamine (GlcNAz) 3 as the representative analogs of (15N)3-azide-tagged monosaccharides. These two unnatural monosaccharide analogs, 2AzGlc and GlcNAz, have been reported as selective metabolic chemical reporters for glycosylation.22 Excitingly, d-DNP hyperpolarization of 2 and 3 provided ample 15N-signal enhancement over 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold, and more importantly, the T1 lifetime up to 8.1 min. The T1 lifetimes observed on the 15Nγ atoms of 2 and 3, benefiting from the reduced dipole–dipole interaction, are much longer (7.6 min and 8.1 min) than T1 lifetimes observed on the 15Nβ atoms (4 min and 5.6 min) and 15Nα (3 min). Since a hyperpolarization experiment usually records signals for 5 times the T1 relaxation time, such long T1 lifetime allows for data collection up to half an hour for (15N)3-azidoglucoses.
000-fold, and more importantly, the T1 lifetime up to 8.1 min. The T1 lifetimes observed on the 15Nγ atoms of 2 and 3, benefiting from the reduced dipole–dipole interaction, are much longer (7.6 min and 8.1 min) than T1 lifetimes observed on the 15Nβ atoms (4 min and 5.6 min) and 15Nα (3 min). Since a hyperpolarization experiment usually records signals for 5 times the T1 relaxation time, such long T1 lifetime allows for data collection up to half an hour for (15N)3-azidoglucoses.
(15N)3-Azidotyrosine 4 and (15N)3-azidoalanine 5 have also been examined, as representative examples for (15N)3-azide incorporated amino acids that can be developed into hyperpolarized metabolic imaging probes. Hyperpolarization of (15N)3-azidotyrosine 4 by d-DNP afforded 15N signal enhancement of ∼142![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold and T1 values of 3.5, 6.2 and 9.8 min for the 15Nα, 15Nβ, and 15Nγ positions, respectively. Hyperpolarization of (15N)3-azidoalanine 5 gave signal enhancement of ∼80
000-fold and T1 values of 3.5, 6.2 and 9.8 min for the 15Nα, 15Nβ, and 15Nγ positions, respectively. Hyperpolarization of (15N)3-azidoalanine 5 gave signal enhancement of ∼80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold and T1 lifetime up to 6.8 min. In comparison with other (15N)3-azide-tagged compounds (i.e., 1–3 and 6), signal enhancements of 4 and 5 were largely affected by the acidity of the compounds.23 Hyperpolarization of (15N)3-azidotyrosine 4 (ε = ∼142
000-fold and T1 lifetime up to 6.8 min. In comparison with other (15N)3-azide-tagged compounds (i.e., 1–3 and 6), signal enhancements of 4 and 5 were largely affected by the acidity of the compounds.23 Hyperpolarization of (15N)3-azidotyrosine 4 (ε = ∼142![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and (15N)3-azidoalanine 5 (ε = 80
000) and (15N)3-azidoalanine 5 (ε = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was improved significantly by adding concentrated NaOH to neutralize a solution of their conjugate acids. The favorable polarization lifetime observed on (15N)3-azidotyrosine 4 and (15N)3-azidoalanine 5 suggested that the (15N)3-azido-tagging strategy offers an innovative platform, alternative to previous 15N-labeled examples,24 to design a diverse range of hyperpolarized amino acid imaging probes.
000) was improved significantly by adding concentrated NaOH to neutralize a solution of their conjugate acids. The favorable polarization lifetime observed on (15N)3-azidotyrosine 4 and (15N)3-azidoalanine 5 suggested that the (15N)3-azido-tagging strategy offers an innovative platform, alternative to previous 15N-labeled examples,24 to design a diverse range of hyperpolarized amino acid imaging probes.
We also prepared and examined (15N)3-azidothymidine 6 to explore the applicability of (15N)3-azide tag on drug molecules for HP-MRI applications (Table 1). Azidothymidine (AZT), also known as Zidovudine, is an antiretroviral drug used for prevention and treatment of HIV/AIDS and contains an 3′-azide group in its structure.25 Thus, (15N)3-AZT 6 would make an ideal exogenous probe for imaging HIV reverse transcriptase activity in addition to its therapeutic properties. Previous studies have shown that human cells uptake AZT rapidly within 100 seconds and AZT has low toxicity at high doses.26 An earlier study determined polarization decay constants of (15N)(14N)2-AZT of 37 ± 2 s at 9.4 T and 140 ± 16 s at 50 mT for Nγ.27 In our study, hyperpolarization of (15N)3-AZT 6 by d-DNP provided signal enhancement over 196![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold and T1 values up to 5.3 min at 1 T, reinforcing 15N3-AZT 6 a promising HP-MRI contrast agent for monitoring HIV progression and drug uptake.
000-fold and T1 values up to 5.3 min at 1 T, reinforcing 15N3-AZT 6 a promising HP-MRI contrast agent for monitoring HIV progression and drug uptake.
Collectively, d-DNP hyperpolarization of (15N)3-azido compounds 1–6 all afforded high signal enhancement (ε = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–336
000–336![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and long-lived lifetimes (T1 up to 9.8 min) at 1 T. Polarization levels up to 15% and hyperpolarization lifetimes of 3–4 min have been commonly observed for 15N centers.5 We have deemed (15N)3T1 lifetimes to be satisfactory for biological studies, as 13C-isotopomers with T1 of <1 min have been applied in in vivo imaging.4 Moreover, the large chemical shift dispersion among three 15N atoms provides distinguishable 15N signals that are well-suited for identification of species arising in the spectra. These examples highlight the efficiency and general adaptability of the (15N)3-azide as a hyperpolarized tag on endogenous biomolecules and exogenous drug molecules for broad biochemical or clinical applications.
000) and long-lived lifetimes (T1 up to 9.8 min) at 1 T. Polarization levels up to 15% and hyperpolarization lifetimes of 3–4 min have been commonly observed for 15N centers.5 We have deemed (15N)3T1 lifetimes to be satisfactory for biological studies, as 13C-isotopomers with T1 of <1 min have been applied in in vivo imaging.4 Moreover, the large chemical shift dispersion among three 15N atoms provides distinguishable 15N signals that are well-suited for identification of species arising in the spectra. These examples highlight the efficiency and general adaptability of the (15N)3-azide as a hyperpolarized tag on endogenous biomolecules and exogenous drug molecules for broad biochemical or clinical applications.
Hyperpolarization of (15N)(14N)2-azide-tagged molecules using d-DNP
We next explored the potential of (15N)(14N)2-azide as an alternative hyperpolarized tag, considering the cost economic benefits of single 15N-isotopically labeled azides and inspiring results that the terminal 15N-atom of the azide group (15Nγ) consistently displayed the longest T1 lifetimes in all (15N)3-azido compounds. The longer lifetime of 15Nγ was contributed by the reduced dipole–dipole interaction in the absence of neighboring protons in comparison to 15Nα and 15Nβ.We prepared three (15N)(14N)2-azide-tagged molecules, including (15N)-azidoethylcholine 7, (15N)-azidoglucose derivative 8, and (15N)-AZT 9 (Table 2), as comparative studies with (15N)3-azido compounds 1, 3, and 6. These (15N)-azide-tagged molecules 7–9 were obtained as a mixture form of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 15Nα- and 15Nγ-labeled azide. When (15N)-azide-tagged molecules 7–9 were subjected to d-DNP hyperpolarization, we used the signals measured at the 15Nγ sites for the enhancements and polarization levels, as signal to noise ratio were too low for the Nα sites.28 Excitingly, d-DNP-hyperpolarized (15N)-azides 7–9 all afforded significantly enhanced and long-lived signals (ε = 76
1 ratio of 15Nα- and 15Nγ-labeled azide. When (15N)-azide-tagged molecules 7–9 were subjected to d-DNP hyperpolarization, we used the signals measured at the 15Nγ sites for the enhancements and polarization levels, as signal to noise ratio were too low for the Nα sites.28 Excitingly, d-DNP-hyperpolarized (15N)-azides 7–9 all afforded significantly enhanced and long-lived signals (ε = 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–121
000–121![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and T1 = 3.2–7.6 min, Table 2). These T1 values are comparable to corresponding 15Nγ sites of (15N)3-azide analogs in Table 1. For example, hyperpolarized (15N)(14N)2-azide 7 displayed a T1 lifetime of 3.2 min, analogous to 3.3 min measured at the 15Nγ atom of (15N)3-azidoethylcholine 1. Similarly, T1 lifetimes observed for hyperpolarized 8 (T1 = 7.6 min) and 9 (T1 = 5.6 min) were close to T1 values of (15N)3-glucose 3 (T1 = 8.1 min) and (15N)3-AZT 6 (T1 = 5.3 min). These comparable long T1 values observed on (15N)3- and (15N)(14N)2-azide-tagged compounds suggest that the relaxation resulting from 14N J-coupling has minimal effects in the solution phases. In comparison to the (15N)3-azido group, a single (15N)-labeled azide offers one 15N-peak for detection, yet it may function as an effective tag.
000 and T1 = 3.2–7.6 min, Table 2). These T1 values are comparable to corresponding 15Nγ sites of (15N)3-azide analogs in Table 1. For example, hyperpolarized (15N)(14N)2-azide 7 displayed a T1 lifetime of 3.2 min, analogous to 3.3 min measured at the 15Nγ atom of (15N)3-azidoethylcholine 1. Similarly, T1 lifetimes observed for hyperpolarized 8 (T1 = 7.6 min) and 9 (T1 = 5.6 min) were close to T1 values of (15N)3-glucose 3 (T1 = 8.1 min) and (15N)3-AZT 6 (T1 = 5.3 min). These comparable long T1 values observed on (15N)3- and (15N)(14N)2-azide-tagged compounds suggest that the relaxation resulting from 14N J-coupling has minimal effects in the solution phases. In comparison to the (15N)3-azido group, a single (15N)-labeled azide offers one 15N-peak for detection, yet it may function as an effective tag.
| Cmpd | Molecular structure | ε | P/% | T 1/min | ||
|---|---|---|---|---|---|---|
| Nαb | Nβ | Nγ | ||||
| a Each experiment repeated twice. Measurements performed at B = 1 T. Enhancements and polarization levels measured for 15Nγ sites. T1 lifetimes derived by fitting the experimental data to a single exponential decay function and then compensating the effect of using the small flip angle pulses (see ESI for details). b Signal to noise ratio too low for Nα. | ||||||
| 7 |
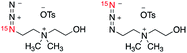
|
76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.6 | — | — | (3.2 ± 0.3) |
| 8 |
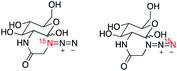
|
121![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
4.2 | — | — | (7.6 ± 0.3) |
| 9 |
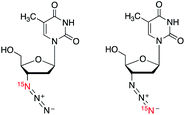
|
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.0 | — | — | (5.6 ± 0.6) |
Hyperpolarization of (15N)3-azide-tagged molecules using SABRE-SHEATH
Finally, we examined the feasibility of hyperpolarization of (15N)3-azide-tagged molecules using an alternative hyperpolarization method, SABRE in SHield Enables Alignment Transfer to Heteronuclei (SABRE-SHEATH). Compared to d-DNP, SABRE-SHEATH is experimentally simple and cost-effective, yet its biomedical application is currently limited by poor performance in water. Subjecting (15N)3-azide 3 to SABRE-SHEATH in D4-MeOH provided enhanced and long-lived 15N signals on all three individual 15Nα, 15Nβ, and 15Nγ atoms (Table 3). Compared to d-DNP hyperpolarization of (15N)3-azide 3 (Table 1), the SABRE-SHEATH hyperpolarization of (15N)3-azide 3 yielded lower polarization levels, with enhancement ranging from 3000- to 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000-fold. Long T1 lifetimes were observed, with T1 value of 2.9 min on 15Nα, 5.8 min on 15Nβ, and 9.2 min on 15Nγ. Note that the T1 values observed for (15N)3-azide 3 in two experiments cannot be compared directly, considering the variants in solvents and radical/catalyst concentrations in d-DNP and SABRE-SHEATH experiments.
000-fold. Long T1 lifetimes were observed, with T1 value of 2.9 min on 15Nα, 5.8 min on 15Nβ, and 9.2 min on 15Nγ. Note that the T1 values observed for (15N)3-azide 3 in two experiments cannot be compared directly, considering the variants in solvents and radical/catalyst concentrations in d-DNP and SABRE-SHEATH experiments.
| ε | P/% | T 1/min | ||
|---|---|---|---|---|
| a Each experiment repeated twice by SABRE-SHEATH measured at 1 T. Detailed hyperpolarization conditions in ESI. | ||||
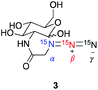
|
Nα | 3060 | 0.11 | (2.9 ± 0.9) |
| Nβ | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
0.95 | (5.8 ± 0.1) | |
| Nγ | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
0.56 | (9.2 ± 1.1) | |
Overall, this is an initial attempt to hyperpolarize (15N)3-azide 3 by SABRE-SHEATH. The result is encouraging and demonstrate the ability of (15N)3-azide tag hyperpolarization using cost-efficient and convenient SABRE-SHEATH method. A systematic study on spin dynamics, the optimized experimental conditions for efficient polarization transfer, and the scope of the substrates using SABRE will be undertaken in future studies.
Conclusion
In summary, we have investigated the incorporation of (15N)3-azide as a general hyperpolarized molecular tag into biologically relevant molecules including choline, glucose, amino acids, as well as an antiretroviral drug. Hyperpolarization of (15N)3-azide-tagged molecules by d-DNP in water afforded enhanced detection sensitivity of 15N signals up to six orders of magnitude. The three individual 15N atoms of the (15N)3-azide displayed distinguishable 15N chemical shifts and long-lasting hyperpolarization lifetimes. These favorable features of (15N)3-azide are well-suited as a practical and effective tag for developing novel HP-MRI agents. Furthermore, hyperpolarization of (15N)3-azide-tagged glucose derivative 3 has also been established using the experimentally simple and cost-effective SABRE-SHEATH method. Besides (15N)3-azide, a single (15N)(14N)2-labeled azido group is also found effective to function as a long-lived hyperpolarizable tag. Our future work will explore potentials of 15N-azide tagged agents for broad in vitro and in vivo biomedical applications.Author contributions
J. B. and H. P. conducted the synthesis and characterization of the compounds. G. Z. performed the hyperpolarization experiments. J. B. and Q. W. conceived and designed the project. W. S. W. and Q. W. supervised the overall work. All authors participated in writing and editing the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by NSF (CHE-1665090), NIH (R21EB024824), and the Camille and Henry Dreyfus Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors thank Jacob R. Lindale and Shannon L. Eriksson for useful discussion on the derivation of the Hamiltonian of the spin system and analysis of the hydride spectrum.References
- (a) J. H. Ardenkjær-Larsen, B. Fridlund, A. Gram, G. Hansson, L. Hansson, M. H. Lerche, R. Servin, M. Thaning and K. Golman, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 10158–10163 CrossRef PubMed; (b) K. Kumagai, K. Kawashima, M. Akakabe, M. Tsuda, T. Abe and M. Tsuda, Tetrahedron, 2013, 69, 3896–3900 CrossRef CAS; (c) W. Jiang, L. Lumata, W. Chen, S. R. Zhang, Z. Kovacs, A. D. Sherry and C. Khemtong, Sci. Rep., 2015, 5, 9104 CrossRef PubMed; (d) H. Nonaka, M. Hirano, Y. Imakura, Y. Takakusagi, K. Ichikawa and S. Sando, Sci. Rep., 2017, 7, 40104 CrossRef CAS PubMed.
- (a) C. Gabellieri, S. Reynolds, A. Lavie, G. S. Payne, M. O. Leach and T. R. Eykyn, J. Am. Chem. Soc., 2008, 130, 4598–4599 CrossRef CAS PubMed; (b) K. R. Keshari and D. M. Wilson, Chem. Soc. Rev., 2014, 43, 1627–1659 RSC.
- (a) K. M. Brindle, S. E. Bohndiek, F. A. Gallagher and M. I. Kettunen, Magn. Reson. Med., 2011, 66, 505–519 CrossRef PubMed; (b) E. M. Serrao and K. M. Brindle, Front. Oncol., 2016, 6, 59 Search PubMed.
- S. Meier, M. Karlsson, P. R. Jensen, M. H. Lerche and J. Ø. Duus, Mol. BioSyst., 2011, 7, 2834–2836 RSC.
- (a) T. Theis, G. X. Ortiz, A. W. J. Logan, K. E. Claytor, Y. Feng, W. P. Huhn, V. Blum, S. J. Malcolmson, E. Y. Chekmenev, Q. Wang and W. S. Warren, Sci. Adv., 2016, 2, e1501438 CrossRef PubMed; (b) J. F. P. Colell, M. Emondts, A. W. J. Logan, K. Shen, J. Bae, R. V. Shchepin, G. X. Ortiz, Jr., P. Spannring, Q. Wang, S. J. Malcolmson, E. Y. Chekmenev, M. C. Feiters, F. Rutjes, B. Blumich, T. Theis and W. S. Warren, J. Am. Chem. Soc., 2017, 139, 7761–7767 CrossRef CAS PubMed; (c) K. Shen, A. W. J. Logan, J. F. P. Colell, J. Bae, G. X. Ortiz Jr, T. Theis, W. S. Warren, S. J. Malcolmson and Q. Wang, Angew. Chem., Int. Ed., 2017, 56, 12112–12116 CrossRef CAS PubMed; (d) H. Park, G. Zhang, J. Bae, T. Theis, W. Warren and Q. Wang, Bioconjugate Chem., 2020, 31, 537–541 CrossRef CAS PubMed.
- J. N. Bae, Z. J. Zhou, T. Theis, W. S. Warren and Q. Wang, Sci. Adv., 2018, 4, eaar2978 CrossRef PubMed.
- (a) E. F. V. Scriven and K. Turnbull, Chem. Rev., 1988, 88, 297–368 CrossRef CAS; (b) S. Brase, C. Gil, K. Knepper and V. Zimmermann, Angew. Chem., Int. Ed., 2005, 44, 5188–5240 CrossRef CAS PubMed; (c) N. J. Agard, J. M. Baskin, J. A. Prescher, A. Lo and C. R. Bertozzi, ACS Chem. Biol., 2006, 1, 644–648 CrossRef CAS PubMed; (d) E. M. Sletten and C. R. Bertozzi, Acc. Chem. Res., 2011, 44, 666–676 CrossRef CAS PubMed.
- (a) C. I. Schilling, N. Jung, M. Biskup, U. Schepers and S. Brase, Chem. Soc. Rev., 2011, 40, 4840–4871 RSC; (b) S. S. van Berkel, M. B. van Eldijk and J. C. M. van Hest, Angew. Chem., Int. Ed., 2011, 50, 8806–8827 CrossRef CAS PubMed.
- (a) E. Saxon and C. R. Bertozzi, Science, 2000, 287, 2007–2010 CrossRef CAS PubMed; (b) J. M. Baskin, J. A. Prescher, S. T. Laughlin, N. J. Agard, P. V. Chang, I. A. Miller, A. Lo, J. A. Codelli and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 16793–16797 CrossRef CAS PubMed.
- (a) K. L. Kiick, E. Saxon, D. A. Tirrell and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 19–24 CrossRef CAS PubMed; (b) T. Feng, M. L. Tsao and P. G. Schultz, J. Am. Chem. Soc., 2004, 126, 15962–15963 CrossRef PubMed; (c) A. J. Link, M. K. S. Vink and D. A. Tirrell, J. Am. Chem. Soc., 2004, 126, 10598–10602 CrossRef CAS PubMed; (d) E. M. Tookmanian, E. E. Fenlon and S. H. Brewer, RSC Adv., 2015, 5, 1274–1281 RSC.
- W. P. Heal, B. Jovanovic, S. Bessin, M. H. Wright, A. I. Magee and E. W. Tate, Chem. Commun., 2011, 47, 4081–4083 RSC.
- (a) T. Pathak, Chem. Rev., 2002, 102, 1623–1667 CrossRef CAS PubMed; (b) A. B. Neef and N. W. Luedtke, Chembiochem, 2014, 15, 789–793 CrossRef CAS PubMed.
- (a) Y. Kho, S. C. Kim, C. Jiang, D. Barma, S. W. Kwon, J. K. Cheng, J. Jaunbergs, C. Weinbaum, F. Tamanoi, J. Falck and Y. M. Zhao, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 12479–12484 CrossRef CAS PubMed; (b) S. Lindner, K. Gruhle, R. Schmidt, V. M. Garamus, D. Ramsbeck, G. Hause, A. Meister, A. Sinz and S. Drescher, Langmuir, 2017, 33, 4960–4973 CrossRef CAS PubMed.
- C. P. Ramil and Q. Lin, Chem. Commun., 2013, 49, 11007–11022 RSC.
- G. Brauer, Handbook of preparative inorganic chemistry, Academic Press, Inc., New York, NY, 2nd edn, 1963 Search PubMed.
- See the detailed synthetic routes in the ESI†.
- (a) J. H. Lee, Y. Okuno and S. Cavagnero, J. Magn. Reson., 2014, 241, 18–31 CrossRef CAS PubMed; (b) M. M. Chaumeil, C. Najac and S. M. Ronen, in Methods Enzymol., ed. C. M. Metallo, Academic Press, 2015, vol. 561, pp. 1–71 Search PubMed.
- C. Y. Jao, M. Roth, R. Welti and A. Salic, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 15332–15337 CrossRef CAS PubMed.
- C. Y. Jao, M. Roth, R. Welti and A. Salic, Chembiochem, 2015, 16, 472–476 CrossRef CAS PubMed.
- C. Cudalbu, A. Comment, F. Kurdzesau, R. B. van Heeswijk, K. Uffmann, S. Jannin, V. Denisov, D. Kirik and R. Gruetter, Phys. Chem. Chem. Phys., 2010, 12, 5818–5823 RSC.
- T. B. Rodrigues, E. M. Serrao, B. W. C. Kennedy, D. E. Hu, M. I. Kettunen and K. M. Brindle, Nat. Med., 2014, 20, 93–98 CrossRef CAS PubMed.
- (a) S. T. Laughlin and C. R. Bertozzi, Nat. Protoc., 2007, 2, 2930–2944 CrossRef CAS PubMed; (b) B. W. Zaro, A. R. Batt, K. N. Chuh, M. X. Navarro and M. R. Pratt, ACS Chem. Biol., 2017, 12, 787–794 CrossRef CAS PubMed.
- C. Hundshammer, M. Grashei, A. Greiner, S. J. Glaser and F. Schilling, Chemphyschem, 2019, 20, 798–802 CrossRef CAS PubMed.
- (a) E. Chiavazza, A. Viale, M. Karlsson and S. Aime, Contrast Media Mol. Imaging, 2013, 8, 417–421 CrossRef CAS PubMed; (b) C. Hundshammer, S. Duwel, D. Ruseckas, G. Topping, P. Dzien, C. Muller, B. Feuerecker, J. B. Hovener, A. Haase, M. Schwaiger, S. J. Glaser and F. Schilling, Sensors, 2018, 18, 600 CrossRef PubMed.
- H. Mitsuya, K. J. Weinhold, P. A. Furman, M. H. St Clair, S. N. Lehrman, R. C. Gallo, D. Bolognesi, D. W. Barry and S. Broder, Proc. Natl. Acad. Sci. U. S. A., 1985, 82, 7096–7100 CrossRef CAS PubMed.
- (a) M. Hu, K. Roland, L. Ge, J. Chen, Y. Li, P. Tyle and S. Roy, J. Pharm. Sci., 1998, 87, 886–890 CrossRef CAS PubMed; (b) T. Tahara, Z. Zhang, M. Ohno, Y. Hirao, N. Hosaka, H. Doi, M. Suzuki and H. Onoe, EJNMMI Res., 2015, 5, 45 CrossRef PubMed.
- R. V. Shchepin and E. Y. Chekmenev, J. Labelled Compd. Radiopharm., 2014, 57, 621–624 CrossRef CAS PubMed.
- See the ESI† for the explanation of dependence of 14N-mediated scalar relaxation on the applied magnetic field.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis of (15N)3-azide-tagged molecules, and (15N)-azide-tagged molecules, hyperpolarization experiments, 1H, 13C and 15N-NMR spectra as well as data analysis. See DOI: 10.1039/d1sc04647k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |