Open Access Article
Open Access ArticleRing-opening and ring-expansion reactions of carborane-fused borirane†
Hanqiang
Wang
,
Jie
Zhang
and
Zuowei
Xie
 *
*
Department of Chemistry, State Key Laboratory of Synthetic Chemistry, The Chinese University of Hong Kong, Shatin, N. T., Hong Kong, China. E-mail: zxie@cuhk.edu.hk
First published on 9th September 2021
Abstract
Though the reaction chemistry of three-membered ring molecules such as cyclopropanes and their heteroatom-containing analogues has been extensively studied, the chemical properties of their boron analogues, boriranes, are little known thus far. This work describes the diverse reactivity patterns of carborane-fused borirane 2. This borirane engages in ring-opening reactions with different types of Lewis acids, such as BBr3, GeCl2, GaCl3, BH3(SMe2) and HBpin, affording a series of ring-opening products, in which M–X or B–H bonds add across the B–C(cage) bond of the three-membered ring in 2. On the other hand, borirane 2 can undergo ring-expansion reactions with unsaturated molecules such as PhCHO, CO2 and PhCN to give ring-expansion products, five-membered boracycles, via a concerted reaction mechanism as supported by DFT calculations. The results of this work not only enrich the reaction chemistry of boriranes, but also offer new routes to boron-containing compounds and heterocycles.
Introduction
Compounds of highly strained three-membered ring systems have been an indispensable subject of considerable interest. Among them, cyclopropanes are well-studied molecules due to their numerous applications in synthetic chemistry.1–3 Likewise, heteroatom-containing analogues of cyclopropanes such as oxiranes (X = O),4–8 aziridines (X = NR),9–12 silacyclopropanes (X = SiR2),13–15 phosphiranes (X = PR)16 and thiiranes (X = S),17 in the form of cyclo-XC2R4, are also widely used as building blocks in synthesis. These three-membered ring systems possess high reactivity due to their inherent ring strain, which allows them to engage in ring-opening reactions with nucleophiles and ring-expansion transformations with π-conjugated compounds. In contrast, the chemistry of three-membered boron compounds (boriranes) is much less known.The only known base-free boriranes of type I (Scheme 1) were synthesized by Berndt's group through [2 + 2] cycloaddition of acetone and diphenylacetylene with methyleneborane.18–20 Denmark and coworkers reported the first example of Lewis base-stabilized boriranes of type II (Scheme 1) via irradiating diphenyl-(E)-2-phenylethenyl-borane under UV light.21 Later on, several base-stabilized boriranes were disclosed by the groups of Wang,22–26 Braunschweig27,28 and Curran29–31via photochemistry or salt metathesis or double hydroboration. Carborane-fused Lewis base-stabilized boriranes III and IV (Scheme 1) were prepared in our laboratory via irradiating carborane-fused azaborole under Xe light32 and in the Ye group via salt elimination between Li2-1,2-C2B10H10 and amino(dichloro)borane,33 respectively.
Though Lewis base-stabilized boriranes are isoelectronic with cyclopropanes, their reaction chemistry is little known. The only reported reactions of Lewis-base stabilized boriranes are the ring-opening reaction with HCl or CuCl, giving the B–C bond cleavage products, and the ring expansion reaction with the sulfur element.31,32 It has been documented that Lewis-base stabilized boriranes are very stable towards water, air, photolysis and heating, and did not undergo B–C bond oxidative addition reaction with Pt(PEt3)3.28 Such a remarkable stability could be ascribed to the quaternization of the boron center by a Lewis base, which may outweigh the destabilizing ring strain in three-membered boriranes. In view of the very rich reaction chemistry of cyclopropanes, aziridines and oxiranes, we would like to explore the reactivity of NHC stabilized carborane-fused boriranes as the electron-withdrawing nature of carborane and the long cage C–C bond might enhance the reactivity of such a three-membered BC2 ring system. In fact, we found that carborane-fused borirane can not only undergo ring-opening reactions with various Lewis acids, but also ring-expansion reactions with unsaturated molecules. These findings are reported in this article.
Results and discussion
Synthesis of carborane-fused borirane
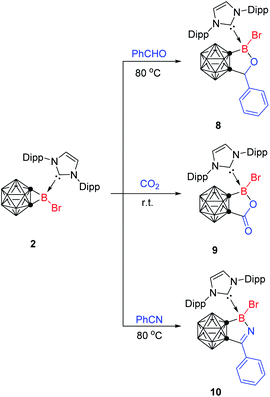
Following our previous procedures,34 reaction of 1-Li-o-carborane with BBr3 in the presence of 1,3-bis-(2,6-diisopropylphenyl)imidazole-2-ylidene (Idipp) afforded a carbene-coordinated carboranyl dibromoborane (1) in 54% isolated yield. Treatment of 1 with 1 equiv. of LiN(TMS)2 in toluene at 80 °C gave carborane-fused borirane 2 in 70% isolated yield (Scheme 2). This reaction was monitored by the 11B NMR spectrum as 2 exhibited a distinguished singlet for exo-boron at −13.5 ppm.Both 1 and 2 were structurally characterized as shown in Fig. 1. The exo-B(13) atom in 2 features a highly distorted tetrahedral geometry with binding to two cage-carbon atoms, one bromine, and one carbene carbon. In the B(13)C(1)C(2) three-membered ring, the cage C–B bond distances (1.629(4) and 1.662(4) Å) fall into the range of the reported ones in carborane-fused boriranes (1.567(4)–1.667(3) Å)32,33 and those (1.60–1.69 Å) observed in Lewis-base stabilized boriranes.25,27–29 The cage C(1)–C(2) distance of 1.580(4) Å in 2 is comparable to those (1.578(3)–1.640(3) Å) in carborane-fused boriranes,32,33 but somewhat longer than those (1.52–1.56 Å) observed in carbene-stabilized boriranes.25,27–29 These measured distances are considerably shorter than the 1.671(7) Å in 1, suggesting a large ring strain. The C(1)–B(13)–C(2) angle of 57.4(2)° in 2 compares to those observed in carborane-fused boriranes (56.6(1)° and 62.9(2)°)32,33 and Lewis-base stabilized boriranes (55.8–57.9°).25,27–29
Ring-opening reaction
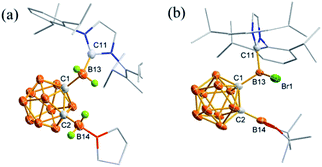
Only two examples of the heterocleavage of the C–B bond in Lewis base-stabilized boriranes were reported using CuCl or HCl as a reagent.31,32 To further explore the reactivity of boriranes, the reactions of 2 with various Lewis acids were explored.Reaction of 2 with 1 equivalent of BBr3 in toluene at room temperature afforded 3 as colorless crystals in 75% isolated yield (Scheme 3). NHC-coordinated BBr2 and BBr2 were observed at 3.9 and −0.1 ppm, respectively, in its 11B NMR spectrum. Single crystal X-ray analyses show that each cage-carbon is bonded to one exo-boron atom that is bridged by a bromine atom, confirming the cleavage of the cage C–B single bond by Lewis acid BBr3 (Fig. 2a). As expected, the Br(2)–B(13)/B(14) distances of 2.116(3)/2.205(4) Å are considerably longer than those of terminal B–Br bonds (1.985(4)–1.997(4) Å).
In the same manner, treatment of 2 with 1 equiv. of GeCl2·dioxane or GaCl3 in toluene at room temperature resulted in the isolation of 4 or 5 in 68% or 67% isolated yield (Scheme 3). The exo-boron atom was observed at 5.2 ppm in 4 and 5.3 ppm in 5 in their 11B NMR spectra, which was significantly shifted downfield compared to the −13.5 ppm observed in 2. The solid-state structure of 4 was further confirmed by single crystal X-ray analyses (Fig. 2b). The structure shows the presence of a μ2-Cl bridge and exchange of halides where the Br atom migrates from exo-B to the Ge center probably owing to the formation of the stronger B–Cl bond. The C(1)–C(2)/C(1)–B(13) distances of 1.680(5)/1.639(5) Å in 4 are close to the 1.701(6)/1.627(5) Å in 3.
The activation of the B–H bond was also achieved by 2. Treatment of 2 with two equivalents of BH3·SMe2 in toluene at 80 °C afforded, after recrystallization from THF, a ring-opening product, 1-[BH2(Idipp)]-2-BH2(THF)-1,2-C2B10H10 (6), in 91% isolated yield with H2BBr·SMe2 as a byproduct as confirmed by the 11B NMR spectrum (Scheme 4). The carbene stabilized boron was observed at −23.0 ppm as a triplet and THF-coordinated boron at 4.0 ppm as a very broad peak in its 11B NMR spectrum. Single-crystal X-ray analyses, as shown in Fig. 3a, confirm that the bromine and hydrogen exchange takes place to form a BH2(Idipp) unit. Both exo-B atoms are four-coordinated. The C(1)–C(2) distance of 1.738(6) Å is slightly longer than the 1.699(4) Å in 2. The C(cage)–B bond lengths of 1.631(6) Å and 1.618 Å in 6 are comparable to the 1.627(5) Å and 1.598(5) Å in 2.
On the other hand, treatment of 2 with 1 equivalent of pinacolborane (HBpin) at 80 °C in toluene afforded a ring-opening product, 1-[BHBr(Idipp)]-2-Bpin-1,2-C2B10H10 (7), as colorless crystals in 82% isolated yield (Scheme 4). The boron chemical shift of Bpin was observed at 30.2 ppm as a singlet in its 11B NMR spectrum. The molecular structure of 7 is shown in Fig. 3b. The C(1)–C(2)/C(1)–B(13)/C(2)–B(14) bond distances of 1.694(3)/1.640(3)/1.577(4) Å are similar to those observed in 6.
Ring-expansion reaction
The ring-expansion reactions of cyclopropanes have long been a staple of organic chemistry. In sharp contrast, the only known ring-expansion reaction of boriranes is its reaction with the sulfur element.32 We wondered if unsaturated molecules would insert into the B–C bond to give ring-expansion products.Surprisingly, 2 rapidly underwent a facile ring expansion reaction with 1 equiv. of benzaldehyde (PhCHO) in toluene at 80 °C to afford an insertion product (8) in 72% isolated yield (Scheme 5). The CHO proton was observed as a singlet at 4.79 ppm in the 1H NMR spectrum. The NHC-stabilized boron atom was observed at 3.3 ppm as a singlet in its 11B NMR spectrum. Single crystal X-ray analyses show that the B(13) atom is bonded to one bromine, one cage-carbon, one oxygen atom and one carbene carbon in a tetrahedral geometry (Fig. 4a). The C(1)–B(13)/C(11)–B(13)/B(13)–Br(1) distances of 1.640(4)/1.651(4)/2.090(3) Å are comparable to those observed in 2. The B(13)–O(1) distance of 1.429(3) Å falls in the range of the reported B–O single bond distances (1.35–1.52 Å).34–36 It was noted that only one diastereomer of 8 was obtained as confirmed by the NMR data. This can be ascribed to steric reasons since the NHC and Ph groups are in the trans positions in the structure with respect to the C(1)C(2)C(38)O(1)B(13) five-membered ring. Thus, it is suggested that such a ring expansion reaction proceeds via a kinetically and thermodynamically favorable process.
On the other hand, the insertion of CO2 into the B–C(cage) bond in 2 at room temperature afforded a boralactone, 1,2-[BBr(Idipp)OCO]-1,2-C2B10H10 (9), in 87% isolated yield (Scheme 5). The exo-boron was observed at 0.1 ppm in the 11B NMR spectrum, compared to the 3.3 ppm in 8. The molecular structure of 9 was confirmed by single-crystal X-ray analyses (Fig. 4b). The most notable feature of the structure is that the five atoms (C(1), C(2), B(13), O(1), and C(38)) are co-planar with the sum of the internal pentagon angles being 540.0°. The C(1)–C(2)/C(1)–B(13) distances of 1.638(4)/1.657(4) Å in 9 are similar to the corresponding values in 2 and 8.
The ring expansion pathway leading to 9via the addition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O across a B–C(cage) bond in 2 was investigated by DFT calculations, in which the dipp of NHC was replaced by a methyl group for simplicity. As shown in Fig. 5, this is a concerted reaction via a four-membered transition state (TS), resulting in the formation of a thermodynamically very stable addition product, 9-Me. As the reaction proceeds from 2-Me to TS, the most significant change in the structure is reflected in the largely elongated C(2)–B(13) bond, 2.568 Å in TSvs. 1.624 Å in 2-Me. As a result, the calculated natural charge on the B(13) is found to be much more positive (0.744) in TS than in 2-Me (0.373) by natural bond orbital (NBO) analyses, suggesting a borenium nature of B(13). These data indicate that the C(2)–B(13) bond becomes polarized in the transition state and behaves as a borenium and carbanion.
O across a B–C(cage) bond in 2 was investigated by DFT calculations, in which the dipp of NHC was replaced by a methyl group for simplicity. As shown in Fig. 5, this is a concerted reaction via a four-membered transition state (TS), resulting in the formation of a thermodynamically very stable addition product, 9-Me. As the reaction proceeds from 2-Me to TS, the most significant change in the structure is reflected in the largely elongated C(2)–B(13) bond, 2.568 Å in TSvs. 1.624 Å in 2-Me. As a result, the calculated natural charge on the B(13) is found to be much more positive (0.744) in TS than in 2-Me (0.373) by natural bond orbital (NBO) analyses, suggesting a borenium nature of B(13). These data indicate that the C(2)–B(13) bond becomes polarized in the transition state and behaves as a borenium and carbanion.
 | ||
| Fig. 5 DFT-calculated ground-state reaction pathway for the formation of 9-Me at the B3LYP/6-31+G(d,p) level of theory. The relative free energies (calculated at 298 K) are given in kcal mol−1. | ||
In a similar manner, reaction of 2 with benzonitrile in toluene at 80 °C gave a C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N insertion product, a five-membered boracycle (10), in 83% isolated yield (Scheme 5). The exo-boron was observed at −0.6 ppm in its 11B NMR spectrum, compared to the 3.3 ppm in 8 and 0.1 ppm in 9. Single X-ray crystal crystallography reveals the insertion of the CN moiety into the cage C–B bond to give a five membered planar C3BN heterocycle. The key structural parameters in 10 are close to those observed in 8 and 9. It was noted that the above five-membered boracycles did not react further with unsaturated molecules.
N insertion product, a five-membered boracycle (10), in 83% isolated yield (Scheme 5). The exo-boron was observed at −0.6 ppm in its 11B NMR spectrum, compared to the 3.3 ppm in 8 and 0.1 ppm in 9. Single X-ray crystal crystallography reveals the insertion of the CN moiety into the cage C–B bond to give a five membered planar C3BN heterocycle. The key structural parameters in 10 are close to those observed in 8 and 9. It was noted that the above five-membered boracycles did not react further with unsaturated molecules.
Conclusions
The ring-opening and ring-expansion reactions of carborane-fused borirane 2 have been explored, enriching largely the reaction chemistry of boriranes. Our results show that 2 can undergo facile ring-opening reactions with different types of Lewis acids to afford a new class of unsymmetrical cage-C substituted o-carboranes. On the other hand, 2 can react with various types of unsaturated substrates to give a range of ring-expansion products, five-membered boracycles, that are otherwise inaccessible by other means.DFT calculations suggest that the ring-expansion reactions proceed via a concerted pathway with a four-membered transition state and are highly thermodynamically favored processes. It is anticipated that the ring-opening reactions occur via a similar four-membered transition state (σ-bond metathesis) to generate thermodynamically more stable σ-bond metathesis products. This work not only shows the diverse reactivity patterns of boriranes, but also offers new routes to boron-containing compounds and heterocycles.
Data availability
The experimental procedures, NMR spectra, and computational details are available in the ESI. Details of the crystal structures were deposited in the Cambridge Crystallographic Data Centre with CCDC 2102172–2102180 for 1·THF, 2·THF, 3, 4·toluene, 6, 7·toluene, 8, 9 and 10·0.5 THF, respectively.†Author contributions
Z. X. conceived the project. H. W. carried out all the experiments. H. W. and J. Z. performed the DFT calculations. All authors contributed to writing the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from the Research Grants Council of HKSAR (Project No. 14305117 and 14306519) and NSFC/RGC Joint Research Scheme (Project No. N_CUHK402/18), as well as the CUHK Impact Postdoctoral Fellowship Scheme (IPDFS to J. Z.).Notes and references
- H. N. C. Wong, M. Y. Hon, C. W. TSE and Y. C. Yip, Chem. Rev., 1989, 89, 165–198 CrossRef CAS.
- C. J. Thibodeaux, W. C. Chang and H. W. Liu, Chem. Rev., 2012, 112, 1681–1709 CrossRef CAS PubMed.
- T. F. Schneider, J. Kaschel and D. B. Werz, Angew. Chem., Int. Ed., 2014, 53, 5504–5523 CrossRef CAS PubMed.
- N. G. Gaylord and E. I. Becker, Chem. Rev., 1951, 49, 413–533 CrossRef CAS.
- R. E. Parker and N. S. Isaacs, Chem. Rev., 1959, 59, 737–799 CrossRef CAS.
- J. G. Smith, Synthesis, 1984, 629–656 CrossRef CAS.
- M. V. Gil, M. J. Arévalo and Ó. López, Synthesis, 2007, 1589–1620 CrossRef CAS.
- V. Capriati, S. Florio and R. Luisi, Chem. Rev., 2008, 108, 1918–1942 CrossRef CAS PubMed.
- D. Tanner, Angew. Chem., Int. Ed., 1994, 33, 599–619 CrossRef.
- J. B. Sweeney, Chem. Soc. Rev., 2002, 31, 247–258 RSC.
- X. E. Hu, Tetrahedron, 2004, 60, 2701–2743 CrossRef CAS.
- I. D. G. Watson and A. K. Yudin, J. Org. Chem., 2003, 68, 5160–5167 CrossRef CAS PubMed.
- P. S. Skell and E. J. Goldstein, J. Am. Chem. Soc., 1964, 86, 1442 Search PubMed.
- D. Seyferth and D. C. Annarelli, J. Am. Chem. Soc., 1975, 97, 2273–2275 CrossRef CAS.
- K. Franz and K. A. Woerpel, Acc. Chem. Res., 2000, 33, 813–820 CrossRef PubMed.
- F. Mathey, Chem. Rev., 1990, 90, 997–1025 CrossRef CAS.
- W. Chew and D. N. Harpp, Sulfur Rep., 1993, 15, 1–39 CrossRef CAS.
- H. Klusik and A. Berndt, Angew. Chem., Int. Ed., 1983, 22, 877–878 CrossRef.
- P. P. Willerhausen, G. S. Lukasch, C. Kybart, J. Allwohn, W. Massa, M. L. McKee, P. V. R. Schleyer and A. Berndt, Angew. Chem., Int. Ed., 1992, 31, 1384–1386 CrossRef.
- C. C. Balzereit, C. C. Kybart, H. J. Winkler, W. Massa and A. Berndt, Angew. Chem., Int. Ed., 1994, 33, 1487–1489 CrossRef.
- S. E. Denmark, K. Nishide and A. M. Faucher, J. Am. Chem. Soc., 1991, 113, 6675–6676 CrossRef CAS.
- Y. L. Rao, H. Amarne, S. B. Zhao, T. M. McCormick, S. Martić, Y. Sun, R. Y. Wang and S. Wang, J. Am. Chem. Soc., 2008, 130, 12898–12900 CrossRef CAS PubMed.
- C. Baik, Z. M. Hudson, H. Amarne and S. Wang, J. Am. Chem. Soc., 2009, 131, 14549–14559 CrossRef CAS PubMed.
- C. Baik, S. K. Murphy and S. Wang, Angew. Chem., Int. Ed., 2010, 49, 8224–8227 CrossRef CAS PubMed.
- Y. L. Rao, L. D. Chen, N. J. Mosey and S. Wang, J. Am. Chem. Soc., 2012, 134, 11026–11034 CrossRef CAS PubMed.
- S. K. Mellerup, C. Li, T. Peng and S. Wang, Angew. Chem., Int. Ed., 2017, 56, 6093–6097 CrossRef CAS PubMed.
- P. Bissinger, H. Braunschweig, K. Kraft and T. Kupfer, Angew. Chem., Int. Ed., 2011, 50, 4704–4707 CrossRef CAS PubMed.
- H. Braunschweig, C. Claes, A. Damme, A. Deißenberger, R. D. Dewhurst, C. Hörl and T. Kramer, Chem. Commun., 2015, 51, 1627–1630 RSC.
- T. R. McFadden, C. Fang, S. J. Geib, E. Merling, P. Liu and D. P. Curran, J. Am. Chem. Soc., 2017, 139, 1726–1729 CrossRef CAS PubMed.
- J. C. Walton, T. R. McFadden and D. P. Curran, J. Am. Chem. Soc., 2017, 139, 16514–16517 CrossRef CAS PubMed.
- W. Dai, S. J. Geib and D. P. Curran, J. Am. Chem. Soc., 2019, 141, 3623–3629 CrossRef CAS PubMed.
- H. Wang, J. Zhang and Z. Xie, Angew. Chem., Int. Ed., 2017, 56, 9198–9201 CrossRef CAS PubMed.
- H. Zhang, J. Wang, W. Yang, L. Xiang, W. Sun, W. Ming, Y. Li, Z. Lin and Q. Ye, J. Am. Chem. Soc., 2020, 142, 17243–17249 CrossRef CAS PubMed.
- H. Wang, J. Zhang, J. Yang and Z. Xie, Angew. Chem., Int. Ed., 2021, 60, 19008–19012 CrossRef CAS PubMed.
- D. Vidovic, J. A. Moore, J. N. Jones and A. H. Cowley, J. Am. Chem. Soc., 2005, 127, 4566–4567 CrossRef CAS PubMed.
- Y. K. Loh, L. Ying, M. Á. Fuentes, D. C. H. Do and S. Aldridge, Angew. Chem., Int. Ed., 2019, 58, 4847–4851 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures, complete characterization data, and NMR spectra. CCDC 2102172–2102180 (1–4 and 6–10). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc04453b |
| This journal is © The Royal Society of Chemistry 2021 |