Open Access Article
Open Access ArticleSelective functionalization of the 1H-imidazo[1,2-b]pyrazole scaffold. A new potential non-classical isostere of indole and a precursor of push–pull dyes†
Kuno
Schwärzer
a,
Saroj K.
Rout
a,
Derya
Bessinger
a,
Fabio
Lima
b,
Cara E.
Brocklehurst
b,
Konstantin
Karaghiosoff
 a,
Thomas
Bein
a,
Thomas
Bein
 a and
Paul
Knochel
a and
Paul
Knochel
 *a
*a
aDepartment Chemie, Ludwig-Maximilians-Universität München, Munich 81377, Germany. E-mail: Paul.Knochel@cup.uni-muenchen.de
bGlobal Discovery Chemistry, Novartis Institutes of BioMedical Research, Basel 4057, Switzerland
First published on 30th August 2021
Abstract
We report the selective functionalization of the 1H-imidazo[1,2-b]pyrazole scaffold using a Br/Mg-exchange, as well as regioselective magnesiations and zincations with TMP-bases (TMP = 2,2,6,6-tetramethylpiperidyl), followed by trapping reactions with various electrophiles. In addition, we report a fragmentation of the pyrazole ring, giving access to push–pull dyes with a proaromatic (1,3-dihydro-2H-imidazol-2-ylidene)malononitrile core. These functionalization methods were used in the synthesis of an isostere of the indolyl drug pruvanserin. Comparative assays between the original drug and the isostere showed that a substitution of the indole ring with a 1H-imidazo[1,2-b]pyrazole results in a significantly improved solubility in aqueous media.
Introduction
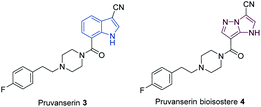
N-heterocyclic scaffolds are key structural units for pharmaceutical, agrochemical and material science applications.1,2 The study of less common heterocyclic ring systems is of special interest, since new physicochemical and medicinal properties may be expected from such classes of molecules.3 Condensed five membered N-heterocycles such as 1H-imidazo[1,2-b]pyrazoles of type 1 recently attracted much attention due to the diverse and very useful bioactivities (antimicrobial,4,5 anticancer,6,7 anti-inflammatory8) of such molecules (Fig. 1).Furthermore, the scaffold 1 can also be considered as a potential non-classical isostere of indole (2). The search for new indole replacements is mainly motivated by their often low solubility and metabolic stability.9 The promising indolyl drug pruvanserin (3, selective 5-HT2A serotonin receptor antagonist)10–12 has been discontinued in phase II clinical trials as a drug for the treatment of insomnia.13 The corresponding 1H-imidazo[1,2-b]pyrazole isostere 4 was predicted to display improved solubility in physiological media. We therefore have developed a toolbox allowing the selective functionalization of the 1H-imidazo[1,2-b]pyrazole skeleton, which enabled the preparation of the pruvanserin isostere 4 in order to compare the physicochemical properties of the matched pair 3 and 4 (Fig. 2).
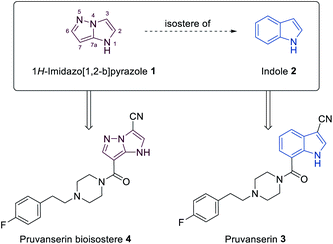 | ||
| Fig. 2 1H-Imidazo[1,2-b]pyrazole (1) as a potential replacement of indole (2) as shown for the 5-HT2A serotonin receptor antagonist pruvanserin (3). | ||
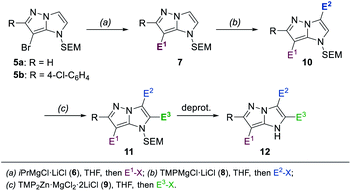
Previously reported protocols for the preparation of 1H-imidazo[1,2-b]pyrazoles require the synthesis of new starting materials for every functionalized derivative, as the ring fusion is only achieved in the final steps.14–17 To avoid this issue, we have chosen a synthetic approach involving a successive and selective functionalization of the readily available 1H-imidazo[1,2-b]pyrazole scaffold. Therefore, we envisioned to employ a Br/Mg-exchange as well as selective magnesiations and zincations using metal amides. Previously, we have reported a range of powerful Br/Mg-exchange reagents18,19 as well as kinetically highly active, sterically hindered TMP-bases (TMP = 2,2,6,6-tetramethylpiperidyl).21,22 These organometallic reagents have been used successfully in the selective functionalization of various N-heterocycles, including 1,3,4-oxadiazoles and 1,2,4-triazoles,22 and other unsaturated substrates.23
Herein, we report such a selective functionalization sequence starting with the two readily available 7-brominated, SEM-protected 1H-imidazo[1,2-b]pyrazoles 5a and 5b15 (Scheme 1). First, a Br/Mg-exchange with iPrMgCl·LiCl (6),18 followed by trapping reactions with various electrophiles, yielded mono-substituted 1H-imidazo[1,2-b]pyrazoles of type 7. Two further functionalizations in the 3- and 2-positions were achieved through consecutive metalations with TMPMgCl·LiCl (8),20 and TMP2Zn·MgCl2·2LiCl (9).21 Subsequent quenching reactions with various electrophiles then gave access to the increasingly functionalized 1H-imidazo[1,2-b]pyrazoles of type 10 and 11 respectively. After deprotection of the SEM-group, a N-heterocyclic compound of type 12 was obtained.
 | ||
| Scheme 1 Functionalization of SEM-protected 1H-imidazo[1,2-b]pyrazoles of type 5via a sequence consisting of a Br/Mg-exchange and two consecutive metalations, each followed by electrophile trapping. | ||
In addition, we report a mild fragmentation of the pyrazole ring24–27 in functionalized 1H-imidazo[1,2-b]pyrazoles of type 11 induced by metalation at the 6-position with TMP2Zn·MgCl2·2LiCl (9) (Scheme 2). This reaction proceeded via zincated intermediates of type 13 and led to a series of (1,3-dihydro-2H-imidazol-2-ylidene)malononitriles of type 14. While some (1,3-dihydro-2H-imidazol-2-ylidene)malononitriles were already reported,28,29 this fragmentation provided an entry to a variety of newly functionalized derivatives of type 14. This functional group diversity was essential for tuning the fluorescent properties of the push–pull dyes 14.30
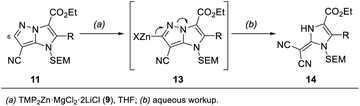 | ||
| Scheme 2 Fragmentation of functionalized 1H-imidazo[1,2-b]pyrazoles of type 11 leading to fluorescent push–pull dyes of type 14. | ||
Finally, we report a concise synthesis of the 1H-imidazo[1,2-b]pyrazole isostere 4 of pruvanserin as well as an experimental evaluation of its physicochemical properties in comparison to the original drug (3).
Results and discussion
Functionalization of the heterocyclic scaffold
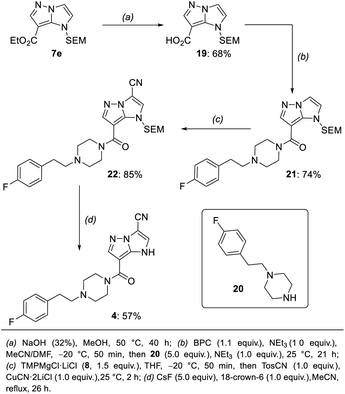
In order to differentiate all of the positions in the SEM-protected31–33 1H-imidazo[1,2-b]pyrazole 15a, we performed a selective bromination with N-bromosuccinimide (NBS, 1.0 equiv.) in acetonitrile (25 °C, 8 min, Scheme 3), providing the 7-bromide 5a in 98% yield.The prefunctionalization of the position 7 considerably facilitated further selective metalations of the 1H-imidazo[1,2-b]pyrazole scaffold. Furthermore, when the brominated 1H-imidazo[1,2-b]pyrazole 5a was treated with iPrMgCl·LiCl (6, 2.1 equiv., 0 °C to 25 °C, 1 h) in THF, the magnesiated 1H-imidazo[1,2-b]pyrazole 16 was obtained and after quenching with various electrophiles a range of products of type 7 was obtained (Scheme 4). This included the reactions with S-methyl sulfonothioate,34 tosyl cyanide and TESCl leading to the products 7a–7c in 50–96% yield. The addition of CuCN·2LiCl35 allowed an allylation in 94% yield (7d) and the formation of the ethyl ester 7e with ethyl cyanoformate in 50% yield. Additional reactions included an acylation with benzoyl chloride catalyzed by Pd(PPh3)4 (7f) in 60% yield and a range of Kumada-type cross-couplings with electron-deficient (7g, 7h) and electron-rich (7i) iodides catalyzed by PEPPSI-iPr36 in 68–88% yield.
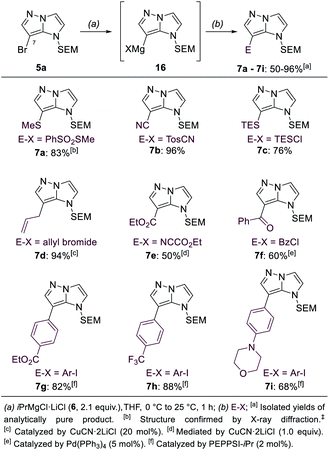 | ||
| Scheme 4 Selective functionalization of the brominated 1H-imidazo[1,2-b]pyrazole 5avia Br/Mg-exchange leading to 7-functionalized 1H-imidazo[1,2-b]pyrazoles of type 7. | ||
The mono-functionalized products of type 7 were then submitted to a selective magnesiation at the 3-position using TMPMgCl·LiCl (8, 1.5 equiv., −20 °C, 2 h) in THF (Scheme 5). Thus, the cyano-substituted 1H-imidazo[1,2-b]pyrazole 7b was magnesiated to generate the metalated intermediate 17, which was then successfully reacted with a variety of electrophiles in 57–89% yield (10a–10j). This included a copper-catalyzed allylation in 65% yield (10a), a thiolation with S-phenyl sulfonothioate in 69% yield (10b) and the reaction with ethyl cyanoformate in 65% yield (8c). A transmetalation with ZnCl2 allowed a series of Negishi-type cross-couplings affording the arylated products 10d–10j in 57–89% yield. When electron-rich iodides were used (10d, 10e), a mixture of 5 mol% Pd(OAc)2 and 10 mol% SPhos37 gave the best results. However, for electron-deficient and heteroarylic halides (10f–10i) the NHC catalyst PEPPSI-iPr36 (2 mol%) performed best. By increasing the reaction temperature from 40 °C to 60 °C, the cross-coupling could be conducted using less reactive bromides instead of iodides (10i). By using 3 mol% of the more active catalyst PEPPSI-iPent38 at 60 °C, it was possible to react a highly functionalized iodide containing an α,β-unsaturated amide, providing the polyfunctional product 10j in 57% yield.
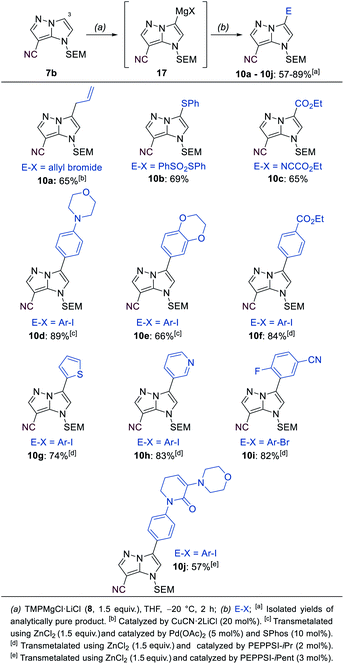 | ||
| Scheme 5 Selective metalation of the 1H-imidazo[1,2-b]pyrazole 7b using TMPMgCl·LiCl (8) followed by electrophile trapping leading to 3-substituted 1H-imidazo[1,2-b]pyrazoles of type 10. | ||
A third functionalization was achieved using the 3-ester substituted N-heterocycle 10c (Scheme 6). In this metalation, the bis-base TMP2Zn·MgCl2·2LiCl (9, 0.55–0.65 equiv.), prepared by adding MgCl2 (1.0 equiv.) and ZnCl2 (1.0 equiv.) solutions to TMPLi (2.0 equiv.) in THF, yielded the best results. The metalation proceeded selectively in the position 2 and was completed after 30 min at 0 °C, providing the bis-zinc species 18. This heterocyclic organometallic was then allylated with allyl bromide in the presence of 20 mol% CuCN·2LiCl to generate the product 11a in 72% yield. In addition, a series of copper-catalyzed acylations with aromatic, aliphatic and heteroaromatic acyl chlorides was conducted to generate the trisubstituted heterocycles 11b–11e in 61–81% yield. Finally, a range of Negishi-type cross-couplings catalyzed by 5 mol% Pd(PPh3)4 gave access to the arylated products 11f–11k in 50–69% yield. The scope of possible coupling partners included electron-deficient (11f–11h), electron-rich (11i, 11j) and heterocyclic (11k) iodides. The high chemoselectivity of the intermediate zinc species allowed the use of electrophiles containing sensitive functional groups such as an ester (11f) or a nitro group (11c, 11h).
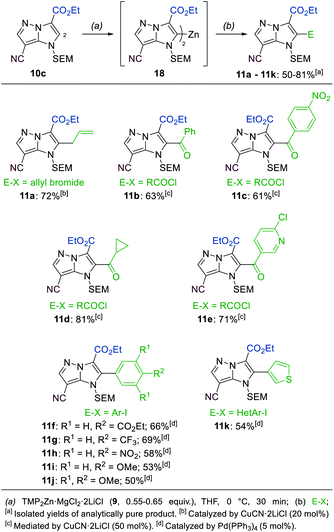 | ||
| Scheme 6 Selective metalation of the 1H-imidazo[1,2-b]pyrazole 10c using TMP2Zn·MgCl2·2LiCl (9) followed by electrophile trapping leading to 2-substituted 1H-imidazo[1,2-b]pyrazoles of type 11. | ||
Synthesis and characterization of push–pull dyes of type 14
Further metalation of the functionalized 1H-imidazo[1,2-b]pyrazoles of type 11 at the 6-position with TMP2Zn·MgCl2·2LiCl (9, 0.65 equiv., 0 °C, 30–150 min) resulted in a fragmentation of the pyrazole ring (Scheme 7). This reaction presumably proceeded via a zincated intermediate of type 13. The shift of an electron pair to the bridgehead nitrogen then led to the formation of a second nitrile. After an aqueous work-up a series of push–pull dyes of type 14 containing a (1,3-dihydro-2H-imidazol-2-ylidene)malononitrile core were isolated in 67–96% yield. The reaction was successfully performed with a range of different functionalized aryl (14a–14c), a 3-thienyl (14d) and a benzoyl substituent (14e) in the 2-position of the 1H-imidazo[1,2-b]pyrazole scaffold.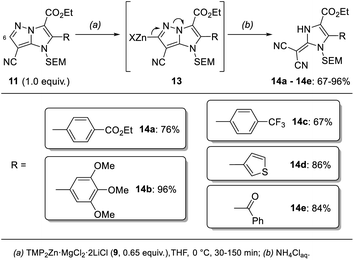 | ||
| Scheme 7 Fragmentation of 1H-imidazo[1,2-b]pyrazoles of type 11 after treatment with TMP2Zn·MgCl2·2LiCl (9) leading to push–pull dyes of type 14. | ||
In contrast to previously reported (1,3-dihydro-2H-imidazol-2-ylidene)malononitriles, for which no special optical properties were described,28,29 the compounds of type 14 displayed a distinct fluorescence in solution when irradiated with UV-light. These compounds can be classified as push–pull dyes, as they contain electron donor and electron acceptor groups connected via an organic π-system.30 The optoelectronic properties in these dyes result from an intramolecular charge-transfer (ICT), which leads to the formation of a new low-energy molecular orbital. The band gap between such a charge-transferred state and the neutral ground state is significantly lower and thus an excitation of electrons between them can often be achieved using lower energy visible light. Therefore, push–pull dyes have become highly sought after for applications in devices such as organic field-effect transistors (OFET),39 organic light-emitting diodes (OLED)40–42 and organic photovoltaic cells (OPVC).43 In addition, some push–pull compounds found application in metal-free photoredox-catalysis.44,45
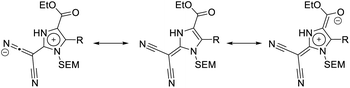
The main donor–acceptor (D–A) interaction in the compounds of type 14 is presumably happening between the malononitrile group, which is widely considered one of the strongest natural electron-withdrawing groups in organic chemistry,46,47 and the proaromatic electron-donor 2-methylene-2,3-dihydro-1H-imidazole. This group is comparable to the widely explored 1,3-dithiol-2-ylidene (dithiafulvene). The strong donor properties of these heterocycles can be attributed to the fact that the ICT in these molecules results in the formation of a resonance stabilized 6π-aromatic system.48–51 The ester substituent can also function as a second, albeit weaker acceptor group. The ICT between these groups can be described using several resonance structures (Fig. 3). Overall, the (1,3-dihydro-2H-imidazol-2-ylidene)malononitriles of type 14 can be characterized as quadrupolar donor–acceptor systems (A–π–D–π–A), as they contain a donor group, which is connected to two acceptor groups via a π-system.30
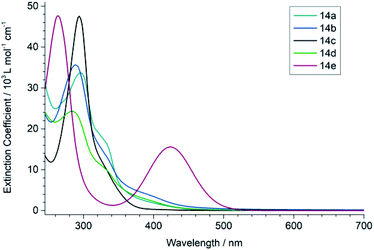
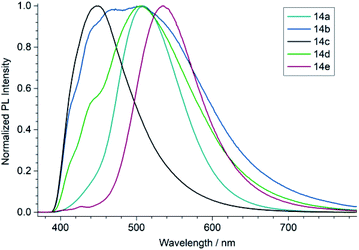
To characterize the optical properties of the (1,3-dihydro-2H-imidazol-2-ylidene)malononitriles of type 14 we measured their UV/vis (Fig. 4) as well as photoluminescence (PL) spectra (Fig. 5) in 50 μM solutions. These measurements revealed that the compound 14e with a benzoyl substituent on the heterocycle differs significantly from the compounds 14a–14d. While the latter mainly absorb in the UV range and only show a weak absorption up to approximately 450 nm, the former possesses a very pronounced second absorption band in the high-energy part of the visible spectral region with a peak absorption at 430 nm, accompanied by an overall red shift of the absorption onset. This is consistent with the colour of the compounds: 14a–14d only exhibit a very slight yellow to orange colour, while 14e is intensely yellow. A similar effect can also be seen in the PL spectrum, where the photoluminescence of 14e is significantly red shifted compared to the other compounds. A possible explanation for these observations lies within the strong acceptor properties of the benzoyl group, leading to a stronger D–A character. Thus, the (1,3-dihydro-2H-imidazol-2-ylidene)malononitrile 14e can be seen as an octupolar ((A–π)3–D), instead of a quadrupolar push–pull system.30
Functionalization of the substituted heterocycle 5b
Since the fragmentation of the pyrazole ring prevented a full functionalization of the 1H-imidazo[1,2-b]pyrazole scaffold via metalation, we have prepared a new starting material with a substituent in the 6-position following a literature procedure.15 A SEM-protection and bromination with NBS resulted in the formation of the compound 5b, which was then submitted to the previously optimized functionalization sequence (Scheme 8). The Br/Mg-exchange of 5b followed by the tosylation proceeded smoothly and provided the heterocyclic nitrile 7j in 77% yield. The first metalation with TMPMgCl·LiCl (8) then gave access to the ester 10k in 42% yield after trapping with ethyl cyanoformate. The second metalation with TMP2Zn·MgCl2·2LiCl (9) was followed by a Negishi-type cross-coupling, as well as an acylation to generate the products 11l and 11m in 66–79% yield. Finally, the SEM-deprotection of 11l was achieved using TBAF (6.0 equiv.) in THF, leading to the tetra-functionalized 1H-imidazo[1,2-b]pyrazole 12a in 81% yield.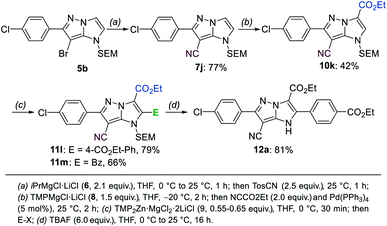 | ||
| Scheme 8 Full functionalization of the 1H-imidazo[1,2-b]pyrazole 5b followed by a SEM-deprotection leading to the tetra-substituted product 12a. | ||
Synthesis and assays of the pruvanserin isostere 4
With these methods in hand, we have performed a synthesis of the pruvanserin isostere 4 (Scheme 9). In a first step, the ester 7e (Scheme 4) was saponified with aqueous NaOH in MeOH to generate the free acid 19 in 68% yield. This was followed by an amide coupling with the amine 20 using bis(pentafluorophenyl) carbonate (BPC) as a coupling reagent,52 affording the amide 21 in 74% yield. The previously optimized conditions for the metalation of the 1H-imidazo[1,2-b]pyrazole scaffold in the 3-position (TMPMgCl·LiCl (8, 1.5 equiv.), −20 °C, 2 h) allowed the formation of the nitrile 22 in 85% yield. Finally, the SEM-group was deprotected using a combination of caesium fluoride (5.0 equiv.) and the phase-transfer catalyst 18-crown-6 (1.0 equiv.) in acetonitrile to generate the pruvanserin isostere 4 in 57% yield.‡Following the synthesis of pruvanserin (3)53 and the 1H-imidazo[1,2-b]pyrazole analogue 4, we analysed the physicochemical properties of the matched pair in order to understand the impact of incorporating an indole replacement (Table 1). Interestingly, the 1H-imidazo[1,2-b]pyrazole analogue 4 showed a lowering in the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D, or lipophilicity, which translated into a significant improvement in aqueous solubility compared to pruvanserin (3). The pKa measured at 6.4 for pruvanserin (3) corresponds to protonation of the piperazine tertiary amine, whereas the pKa measured at 7.3 for the 1H-imidazo[1,2-b]pyrazolo analogue 4 likely corresponds to the deprotonation of the core NH, which is considerably lower than the expected pKa for an indole NH. Overall, the results indicated that 1H-imidazo[1,2-b]pyrazoles could be promising core morphs worth further investigation in light of their enhanced solubility compared to indoles. Such investigations could include direct bioassay studies in order to compare the biological activity of the analogues and the original indolyl drugs. In particular, deprotonation of the 1H-imidazo[1,2-b]pyrazole in physiological medium might lead to a change in receptor interactions and cell membrane permeability. Additionally, studies regarding cytochrome P450 oxidation would be necessary in order to determine the metabolic stability of the analogues.
D, or lipophilicity, which translated into a significant improvement in aqueous solubility compared to pruvanserin (3). The pKa measured at 6.4 for pruvanserin (3) corresponds to protonation of the piperazine tertiary amine, whereas the pKa measured at 7.3 for the 1H-imidazo[1,2-b]pyrazolo analogue 4 likely corresponds to the deprotonation of the core NH, which is considerably lower than the expected pKa for an indole NH. Overall, the results indicated that 1H-imidazo[1,2-b]pyrazoles could be promising core morphs worth further investigation in light of their enhanced solubility compared to indoles. Such investigations could include direct bioassay studies in order to compare the biological activity of the analogues and the original indolyl drugs. In particular, deprotonation of the 1H-imidazo[1,2-b]pyrazole in physiological medium might lead to a change in receptor interactions and cell membrane permeability. Additionally, studies regarding cytochrome P450 oxidation would be necessary in order to determine the metabolic stability of the analogues.
Conclusions
In summary, we developed a sequence for the selective functionalization of the 1H-imidazo[1,2-b]pyrazole scaffold starting from SEM-protected and brominated compounds of type 5. The functionalizations were achieved using various magnesiated and zincated organometallics, which were generated either via a Br/Mg-exchange or via regioselective metalations using TMP-bases. A range of different trapping reactions were possible, including cross-couplings, allylations, acylations, cyanations and carboxylations. A final deprotection of the SEM-group allowed the isolation of tetra-functionalized N-heterocycles of type 12.In addition, we reported a fragmentation of the pyrazole ring in 1H-imidazo[1,2-b]pyrazoles of type 11, which was induced by a metalation at the 6-position. This gave access to push–pull dyes of type 14 containing a proaromatic (1,3-dihydro-2H-imidazol-2-ylidene)malononitrile core. The optical properties of these dyes were explored and it was found that a benzoyl substituent resulted in a significant red shift of both the absorption as well as the photoluminescence.
Finally, we have prepared a non-classical isostere (4) of the indolyl drug pruvanserin (3) in a concise manner using the previously established methodologies. The physicochemical properties of this new isostere were compared to those of the original drug and it was found that a substitution of the indole ring with a 1H-imidazo[1,2-b]pyrazole led to a significant decrease in the lipophilicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D). This translated into an increased solubility in aqueous media. Thus, further investigations of 1H-imidazo[1,2-b]pyrazoles as potential replacements of indoles in drug molecules might lead to compounds with a higher bioavailability.
D). This translated into an increased solubility in aqueous media. Thus, further investigations of 1H-imidazo[1,2-b]pyrazoles as potential replacements of indoles in drug molecules might lead to compounds with a higher bioavailability.
Data availability
The datasets supporting this article have been uploaded as part of the ESI.† Crystallographic data for 7a has been deposited at the CCDC under 2097280 and can be obtained from http://www.ccdc.cam.ac.uk.Author contributions
K. S. and P. K. conceived the project and designed the synthetical experiments. D. B. and T. B. designed the experiments for the optical characterization. F. L. and C. E. B. designed the physico-chemical assays. K. S. and S. K. R. conducted the synthetical experiments. D. B. conducted the experiments for the optical characterization. K. K. conducted the X-ray crystallography. K. S., S. K. R., D. B., C. E. B. and K. K. analysed the data. K. S. and P. K. wrote the paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the LMU Munich, the Cluster of Excellence e-conversion and the DFG for financial support. We thank Albemarle (Hoechst, Germany) for the generous gift of chemicals. We acknowledge the skilled support of Dominik Rufle, Daniel Gosling, Stephane Rodde, Guillaume Ngo and Damien Hubert (Novartis, Basel) in the final purification and profiling of pruvanserin and its isostere.References
- R. D. Taylor, M. MacCoss and A. D. G. Lawson, J. Med. Chem., 2014, 57, 5845 CrossRef CAS PubMed.
- E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257 CrossRef CAS PubMed.
- W. R. Pitt, D. M. Parry, B. G. Perry and C. R. Groom, J. Med. Chem., 2009, 52, 2952 CrossRef CAS PubMed.
- A. O. Abdelhamid, K. A. Abdelall and Y. H. Zaki, J. Heterocycl. Chem., 2010, 47, 477 CAS.
- M. V. Murlykina, M. N. Kornet, S. M. Desenko, S. V. Shishkina, O. V. Shishkin, A. A. Brazhko, V. I. Musatov, E. V. Van der Eycken and V. A. Chebanov, Beilstein J. Org. Chem., 2017, 13, 1050 CrossRef CAS PubMed.
- A. T. Baviskar, C. Madaan, R. Preet, P. Mohapatra, V. Jain, A. Agarwal, S. K. Guchhait, C. N. Kundu, U. C. Banerjee and P. V. Bharatam, J. Med. Chem., 2011, 54, 5013 CrossRef CAS PubMed.
- S. Grosse, V. Mathieu, C. Pillard, S. Massip, M. Marchivie, C. Jarry, P. Bernard, R. Kiss and G. Guillaumet, Eur. J. Med. Chem., 2014, 84, 718 CrossRef CAS PubMed.
- A. H. Shridhar, G. Banuprakash, H. H. Joy and Y. T. Vijaykumar, Int. Res. J. Pharm., 2017, 8, 25 CAS.
- T. Wang, Z. Yin, Z. Zhang, J. A. Bender, Z. Yang, G. Johnson, Z. Yang, L. M. Zadhura, C. J. D`Arienzo, D. D. Parker, C. Gesenberg, G. A. Yamanaka, Y.-F. Gong, H.-T. Ho, H. Fang, N. Zhou, B. V. McAuliffe, B. J. Eggers, L. Fan, B. Nowicka-Sans, I. B. Dicker, Q. Gao, R. J. Colonno, P.-F. Lin, N. A. Meanwell and J. F. Kadow, J. Med. Chem., 2009, 52, 7778 CrossRef CAS PubMed.
- G. D. Bartoszyk, C. van Amsterdam, H. Böttcher and C. A. Seyfried, Eur. J. Pharmacol., 2003, 473, 229 CrossRef CAS PubMed.
- R. Adamec, K. Creamer, G. D. Bartoszyk and P. Burton, Eur. J. Pharmacol., 2004, 504, 79 CrossRef CAS PubMed.
- B. R. Teegarden, H. Al Shamma and Y. Xiong, Curr. Top. Med. Chem., 2008, 8, 969 CrossRef CAS PubMed.
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). Identifier NCT00259311, Efficacy Study of LY2422347 to treat Insomnia. Nov 29, 2005; https://clinicaltrials.gov/ct2/show/NCT00259311 (accessed June 21, 2021).
- E. Vanotti, F. Fiorentini and M. Villa, J. Heterocycl. Chem., 1994, 31, 737 CrossRef CAS.
- P. Seneci, M. Nicola, M. Inglesi, E. Vanotti and G. Resnati, Synth. Commun., 1999, 29, 311 CrossRef CAS.
- J. Khalafy, A. P. Marjani and F. Salami, Tetrahedron Lett., 2014, 55, 6671 CrossRef CAS.
- N. N. Kolos, B. V. Kibkalo, L. L. Zamigaylo, I. V. Omel'chenko and O. V. Shishin, Russ. Chem. Bull., 2015, 64, 864 CrossRef CAS.
- A. Krasovskiy and P. Knochel, Angew. Chem. Int. Ed., 2004, 43, 3333 ( Angew. Chem. , 2004 , 116 , 3396 ) CrossRef CAS PubMed.
- D. S. Ziegler, B. Wei and P. Knochel, Chem. – Eur. J., 2019, 25, 2695 CrossRef CAS PubMed.
- A. Krasovskiy, V. Krasovskaya and P. Knochel, Angew. Chem. Int. Ed., 2006, 45, 2958 ( Angew. Chem. , 2006 , 118 , 3024 ) CrossRef CAS PubMed.
- S. H. Wunderlich and P. Knochel, Angew. Chem. Int. Ed., 2007, 46, 7685 ( Angew. Chem. , 2007 , 119 , 7829 ) CrossRef CAS PubMed.
- K. Schwärzer, C. P. Tüllmann, S. Graßl, B. Górski, C. E. Brocklehurst and P. Knochel, Org. Lett., 2020, 22, 1899 CrossRef PubMed.
- A. Kremsmair, J. H. Harenberg, K. Schwärzer, A. Hess and P. Knochel, Chem. Sci., 2021, 12, 6011 RSC.
- M. Takahashi, T. Mamiya, H. Hasegawa, T. Nagai and H. Wakita, J. Heterocycl. Chem., 1986, 23, 1363 CrossRef CAS.
- M. Schlosser, J.-N. Volle, F. Leroux and K. Schenk, Eur. J. Org. Chem., 2002, 2913 CrossRef CAS.
- A. Bunnell, C. O'Yang, A. Petrica and M. J. Soth, Synth. Commun., 2006, 36, 285 CrossRef CAS.
- V. L. Blair, D. C. Blakemore, D. Hay, E. Hevia and D. C. Pryde, Tetrahedron Lett., 2011, 52, 4590 CrossRef CAS.
- G. Mlostoń, M. Jasiński, A. Linden and H. Heimgartner, Helv. Chim. Acta, 2006, 89, 1304 CrossRef.
- A. V. Kutasevich, A. S. Efimova, M. N. Sizonenko, V. P. Perevalov, L. G. Kuz'mina and V. S. Mityanov, Synlett, 2020, 31, 179 CrossRef CAS.
- F. Bureš, RSC Adv., 2014, 4, 58826 RSC.
- J. P. Whitten, D. P. Matthews and J. R. McCarthy, J. Org. Chem., 1986, 51, 1891 CrossRef CAS.
- C. Despotopoulou, L. Klier and P. Knochel, Org. Lett., 2009, 11, 3326 CrossRef CAS PubMed.
- N. Fugina, W. Holzer and M. Wasicky, Heterocycles, 1992, 34, 303 CrossRef CAS.
- K. Fujiki, N. Tanifuji, Y. Sasaki and T. Yokoyama, Synthesis, 2002, 3, 343 CrossRef.
- P. Knochel, M. C. P. Yeh, S. C. Berk and J. Talbert, J. Org. Chem., 1988, 53, 2390 CrossRef CAS.
- M. G. Organ, M. Abdel-Hadi, S. Avola, N. Hadei, J. Nasielski, C. J. O'Brien and C. Valente, Chem. – Eur. J., 2006, 13, 150 CrossRef PubMed.
- T. E. Barder, S. D. Walker, J. R. Martinelli and S. L. Buchwald, J. Am. Chem. Soc., 2005, 127, 4685 CrossRef CAS PubMed.
- M. G. Organ, S. Çalimsiz, M. Sayah, K. H. Hoi and A. J. Lough, Angew. Chem. Int. Ed., 2009, 48, 2383 ( Angew. Chem. , 2009 , 121 , 2419 ) CrossRef CAS PubMed.
- P. Devibala, R. Dheepika, P. Vadivelu and S. Nagarjan, ChemistrySelect, 2019, 4, 2339 CrossRef CAS.
- S. Gong, Y. Chen, J. Luo, C. Yang, C. Zhong, J. Qin and D. Ma, Adv. Funct. Mater., 2011, 21, 1168 CrossRef CAS.
- J. Ye, Z. Chen, M.-K. Fung, C. Zheng, X. Ou, X. Zhang, Y. Yuan and C.-S. Lee, Chem. Mater., 2013, 25, 2630 CrossRef CAS.
- W.-C. Chen, Y. Yuan, S.-F. Ni, Z.-L. Zhu, J. Zhang, Z.-Q. Jiang, L.-S. Liao, F.-L. Wong and C.-S. Lee, ACS Appl. Mater. Interfaces, 2017, 9, 7331 CrossRef CAS PubMed.
- A. W. Hains, Z. Liang, M. A. Woodhouse and B. A. Gregg, Chem. Rev., 2010, 110, 6689 CrossRef CAS PubMed.
- Y. Zhao, C. Zhang, K. F. Chin, O. Pytela, G. Wei, H. Liu, F. Bureš and Z. Jiang, RSC Adv., 2014, 4, 30062 RSC.
- Z. Hloušková, M. Klikar, O. Pytela, N. Almonasy, A. Růžička, V. Jandová and F. Bureš, RSC Adv., 2019, 9, 23797 RSC.
- M. C. Zerner, C. Reidlinger, W. M. F. Fabian and H. Junek, J. Mol. Struct.: THEOCHEM, 2001, 543, 129 CrossRef CAS.
- M. Hornum, J. Kongsted and P. Reinholdt, Phys. Chem. Chem. Phys., 2021, 23, 9139 RSC.
- R. Andreu, M. J. Blesa, L. Carrasquer, J. Garín, J. Orduna, B. Villacampa, R. Alcalá, J. Casado, M. C. Ruiz Delgado, J. T. López Navarrete and M. Allain, J. Am. Chem. Soc., 2005, 127, 8835 CrossRef CAS PubMed.
- S. Alías, R. Andreu, M. J. Blesa, S. Franco, J. Garín, A. Gargera, J. Orduna, P. Romero, B. Villacampa and M. Allain, J. Org. Chem., 2007, 72, 6440 CrossRef PubMed.
- R. Andreu, M. A. Cerdán, S. Franco, J. Garín, A. B. Marco, J. Orduna, D. Palomas, B. Villacampa, R. Alicante and M. Allain, Org. Lett., 2008, 10, 4963 CrossRef CAS PubMed.
- R. Andreu, E. Galán, J. Orduna, B. Villacampa, R. Alicante, J. T. López Navarrete, J. Casado and J. Garín, Chem. – Eur. J., 2011, 17, 826 CrossRef CAS PubMed.
- K. Lombar, U. Grošelj, G. Dahmann, B. Stanovnik and J. Svete, Synthesis, 2015, 47, 497 CAS.
- A. Bathe, H. Böttcher, H. Crassier, U. Eckert and S. Emmert, Ger. Pat., 2002, DE10102944A1 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Deposition number 2097280 (7a) contains the supplementary crystallographic data for this paper. CCDC 2097280. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc04155j |
| ‡ A deprotection with TBAF in THF was also successful; however it proved difficult to separate the product 4 from the tetra-n-butylammonium salts. |
| This journal is © The Royal Society of Chemistry 2021 |