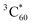Open Access Article
Open Access ArticleA supramolecular polymeric heterojunction composed of an all-carbon conjugated polymer and fullerenes†
Shengda
Wang
a,
Xingcheng
Li
 a,
Xinyu
Zhang
a,
Pingsen
Huang
a,
Pengwei
Fang
a,
Junhui
Wang
*b,
Shangfeng
Yang
a,
Xinyu
Zhang
a,
Pingsen
Huang
a,
Pengwei
Fang
a,
Junhui
Wang
*b,
Shangfeng
Yang
 *a,
Kaifeng
Wu
*a,
Kaifeng
Wu
 b and
Pingwu
Du
b and
Pingwu
Du
 *a
*a
aHefei National Laboratory of Physical Science at the Microscale, CAS Key Laboratory of Materials for Energy Conversion, Department of Materials Science and Engineering, iChEM (Collaborative Innovation Center of Chemistry for Energy Materials), University of Science and Technology of China (USTC), 96 Jinzhai Road, Hefei, Anhui Province 230026, P. R. China. E-mail: dupingwu@ustc.edu.cn; sfyang@ustc.edu.cn; Fax: +86-551-63606207
bState Key Laboratory of Molecular Reaction Dynamics, Dynamics Research Center for Energy and Environmental Materials, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, Liaoning 116023, P. R. China. E-mail: wjh@dicp.ac.cn
First published on 14th July 2021
Abstract
Herein, we design and synthesize a novel all-carbon supramolecular polymer host (SPh) containing conjugated macrocycles interconnected by a linear poly(para-phenylene) backbone. Applying the supramolecular host and fullerene C60 as the guest, we successfully construct a supramolecular polymeric heterojunction (SPh⊃C60). This carbon structure offers a means to explore the convex–concave π–π interactions between SPh and C60. The produced SPh was characterized by gel permeation chromatography, mass spectrometry, FTIR, Raman spectroscopy, and other spectroscopies. The polymeric segment can be directly viewed using a scanning tunneling microscope. Femtosecond transient absorption and fluorescence up-conversion measurements revealed femtosecond (≪300 fs) electron transfer from photoexcited SPh to C60, followed by nanosecond charge recombination to produce the C60 triplet excited state. The potential applications of SPh⊃C60 in electron- and hole-transport devices were also investigated, revealing that C60 incorporation enhances the charge transport properties of SPh. These results expand the scope of the synthesis and application of supramolecular polymeric heterojunctions.
Introduction
The design and fabrication of heterojunctions in varying dimensions have attracted much attention for many applications, such as transistors, solar cells, solid-state lasers, and diodes.1–6 It is worth noting that organic heterojunctions played very important roles in organic thin-film solar cells.7 Various organic electron-donating molecules, such as thiophenes,8 porphyrins9,10 and tetrathiafulvalene derivatives11 can construct C60-based donor–acceptor (D–A) heterojunction systems, which can effectively suppress aggregation of fullerene derivatives and facilitate energy conversion. Imahori and co-workers reported porphyrin/C60 composite clusters and discovered photocurrent generation under light irradiation.12,13 Inspired by these studies, crown-shaped small molecular heterojunctions were constructed using curved nanographene host molecules for photocurrent generation.14 Supramolecular polymers are regarded as an important source of intelligent materials and show broad applications.15–19 These supramolecular structures can be formed by various different interactions, such as metal coordination,20,21 π–π stacking,22 host–guest recognition,23–25 and hydrogen bonding.26,27 Würthner and co-workers achieved a co-assembled p–n-junction on a nanoscopic scale containing a p-type oligo(p-phenylene vinylene) donor and an n-type perylene bisimide acceptor, which are initially formed via hydrogen bonding and subsequently self-assemble into chiral stacks by π–π interactions.28 Aida and co-workers reported an unusual supramolecular polymerization reaction upon heating as well as cooling using a metalloporphyrin-based tailored monomer to investigate its stimuli-responsive applications.29 Recently, frustrated Lewis pairs were implemented into supramolecular polymers combined with π-conjugated O-bridged triphenylborane and triphenylamines by Meijer and co-workers.30 The Yang group reported novel supramolecular polymeric nanoparticles comprised of chromophoric guest molecules and disulfidebridged bispillar[5]arene to construct a light-harvesting system.31 However, the construction of highly ordered, multi-stage, multilayered polymeric supramolecular systems is still an unsolved challenge.32,33 The structural effects of charge mobilities in supramolecular polymeric heterojunction systems have been rarely explored.Conjugated polymers (CPs) have attracted great interest for various optoelectronic applications due to their semiconductivity in pristine (neutral) or doped states.34 CPs generally exhibit an efficient absorption or emission at the band edge.35 For example, excitons with bounded electron–hole pairs are created to emit strong luminescence when photons are absorbed in poly(phenylenevinylene) materials.36 The wide band-gap poly(p-phenylene) (PPP) materials have a rigid-rod character and contain very stable benzene rings.37 Therefore, much attention has also been paid to their synthesis and electronic properties.38–43 Linear or cyclic benzene rings in PPP and cyclo-para-phenylene (CPP) macrocycles can be considered as conjugated segments of graphene nanoribbons and single-walled carbon nanotubes (SWCNTs).44–49 In addition, it has been found that these macrocycles show good supramolecular interactions with fullerenes such as C60 (ref. 50 and 51) and C70.52 Their electron transfer properties between a macrocycle and fullerenes have been recently investigated by von Delius and Guldi,53,54 Martín,55 and our group.52,56,57 Thus, integrating the physical properties of macrocycle-based conjugated polymers and fullerenes to make supramolecular polymeric heterojunctions could have many interesting properties and applications in molecular electronics and materials.58
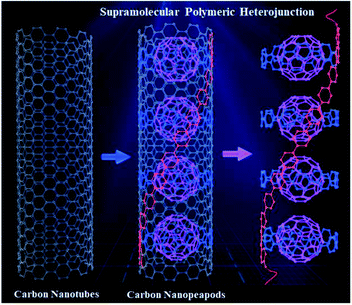
Herein, we report design and synthesis of a novel PPP-based supramolecular polymeric heterojunction containing conjugated macrocycles interconnected by a PPP backbone, which acts as a supramolecular host to form a host–guest supramolecular polymeric heterojunction with fullerene C60 (SPh⊃C60), representing the first polymeric segment of the supramolecular heterojunction of carbon nanopeapods (Fig. 1). In specific, the conjugated macrocycles mimic the curved polyphenylene-C60 peapod segment of a CNT, and the linear PPP backbone is the linear polyphenylene part along the CNT. This polymer was fully characterized by different techniques. In addition, the supramolecular interactions between SPh and fullerene C60 were investigated. Moreover, transient absorption measurements were performed to investigate the charge transport dynamics between SPh (electron donor) and C60 (electron acceptor). The charge transport properties of the supramolecular polymeric heterojunction were examined using the space charge limited current (SCLC) method, revealing that C60 incorporation enhances the charge transport properties of SPh.
Results and discussion
Molecular design of SPh
To obtain the target supramolecular polymeric heterojunction as the segment of carbon nanopeapods with desired structural features, the appropriate functional building unit for π-extension should be rationally selected. Thus, a bifunctional conjugated macrocyclic host with the reaction sites in the para position will be a good candidate for both linear polymeric π-extension and supramolecular interaction. A previous study showed that a cyclic precursor can react with the linear component containing three phenyl rings.56 Then, a tetra-substituted phenylene unit with different reaction sites can connect with the cyclic molecule by the Suzuki–Miyaura coupling reaction to achieve the bifunctional monomer and subsequently to form a long π-conjugated PPP-based polymeric segment of CNTs. Once fullerene molecules are embedded into CPP moieties of the conjugated polymer, a polymeric segment of carbon nanopeapods can be achieved.Synthesis of SPh
The synthesis procedure for SPh is summarized in Fig. 2. Compound 2′,5′-dibromo-4,4′′-dichloro-1,1′:4′,1′′-terphenyl (1) was prepared according to our previously reported procedure.56 Then, two bromo groups in 1 were transformed into 4,4,5,5-tetramethyl-1,3,2-dioxaborolane groups by Pd-catalyzed borylation to produce compound 2 in a yield of ∼60%. Subsequently, linear compound 2 was embedded into the ring-shaped structure to generate the monomer for polymerization. Under the standard conditions for a Pd-catalyzed Suzuki coupling reaction, compound 2 reacted with the curved precursor 3 to synthesize the key macrocycle intermediate 4, which was successfully converted into the targeted macrocyclic monomer 5, a light yellow solid, by an aromatization reaction. All these small molecules were fully characterized by NMR, and MS (Fig. S16–S23†). Finally, a polymerization reaction using compound 5 was carried out in dry DMF by a nickel-mediated Yamamoto homocoupling reaction, and the polymer SPh was then obtained as an intense yellow solid in a yield of ∼72%.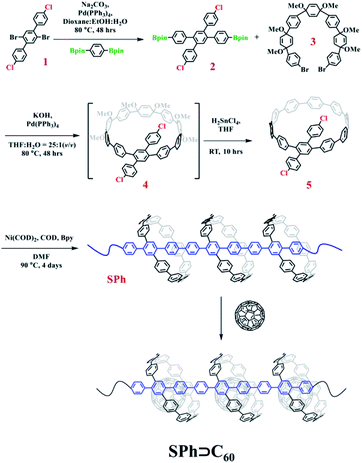 | ||
| Fig. 2 Synthesis procedure for compound 5 and the polymeric segment of carbon nanopeapods (SPh and SPh⊃C60). | ||
Characterization of the SPh and SPh⊃C60
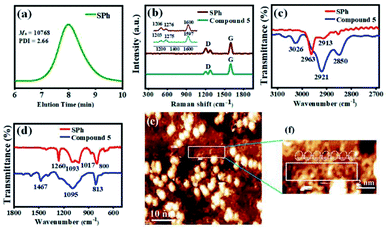
The successful synthesis of π-extended polymer SPh from compound 5 was confirmed by gel permeation chromatography (GPC), FTIR, mass spectrometry, and Raman spectroscopy. After the polymerization reaction, Soxhlet extractions with methanol and acetone were performed to remove lower molecular weight oligomers; the GPC molecular weight data for SPh are presented in Fig. 3a and S1.† The weight average molecular weight (MW), relative number-average molecular weight (Mn), and polydispersity index (PDI) of SPh were measured, and the results showed that Mn = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 768 g mol−1 using polystyrene as the standard. This weight is approximately equal to 11 repeating units. The large PDI value at 2.66 suggested a broad molecular weight distribution. The mass spectra are shown in Fig. S2 and S7,† demonstrating different lengths of the polymeric molecules with roughly similar mass distribution results before and after the chromatographic purification of the polymer. This is probably due to the high molecular weight, low solubility, and some aggregation in SPh, which make purification of each oligomer very difficult. MALDI-TOF MS spectrometry data of SPh show a series of equidistant mass peaks in the high-molecular-weight fraction with up to 17 repeating units. The m/z and interval values (Δm/z = 910.36) are consistent with the calculated values, indicating the successful polymerization of compound 5.
768 g mol−1 using polystyrene as the standard. This weight is approximately equal to 11 repeating units. The large PDI value at 2.66 suggested a broad molecular weight distribution. The mass spectra are shown in Fig. S2 and S7,† demonstrating different lengths of the polymeric molecules with roughly similar mass distribution results before and after the chromatographic purification of the polymer. This is probably due to the high molecular weight, low solubility, and some aggregation in SPh, which make purification of each oligomer very difficult. MALDI-TOF MS spectrometry data of SPh show a series of equidistant mass peaks in the high-molecular-weight fraction with up to 17 repeating units. The m/z and interval values (Δm/z = 910.36) are consistent with the calculated values, indicating the successful polymerization of compound 5.
Moreover, we compared the GPC traces of SPh⊃C60 in different solvents (Fig. S10†). Only small oligomers are present, probably due to the poor solubility of SPh⊃C60 in chloroform (CHCl3) and N,N-dimethylformamide (DMF) (Fig. S10c†). It should be noted that the concentration for GPC was approximately 5 mg mL−1 and substantial insolubilities in the concentrated solutions cannot be introduced into the mobile phase. Thus, the results only reflect smaller soluble oligomers dissolved in CHCl3 and DMF. In addition, SPh⊃C60 was further characterized by FTIR (Fig. S8†). C60 contains the C–C bond and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, and four absorption peaks are found in the FTIR spectra of SPh⊃C60 at 526, 576, 1161, and 1430 cm−1, respectively. Compared with the FTIR spectrum of SPh, the results suggest the formation of a supramolecular polymeric heterojunction (SPh⊃C60). Raman spectroscopy was also used to characterize SPh⊃C60. In Fig. S9,† the Raman dominant peak of SPh⊃C60 at 1469 cm−1, indicating the stretching mode of cages of C60, slightly shifted compared with that of C60 (1468 cm−1), which is similar to C60-β-CDP reported by Diao and co-workers.59,60 These results indicate that the formation of supramolecular complexes (SPh⊃C60) did not change the nature of C60. No appreciable Raman peaks of SPh can be recognized in SPh⊃C60, probably due to the strong fluorescence background signal of SPh.
C bond, and four absorption peaks are found in the FTIR spectra of SPh⊃C60 at 526, 576, 1161, and 1430 cm−1, respectively. Compared with the FTIR spectrum of SPh, the results suggest the formation of a supramolecular polymeric heterojunction (SPh⊃C60). Raman spectroscopy was also used to characterize SPh⊃C60. In Fig. S9,† the Raman dominant peak of SPh⊃C60 at 1469 cm−1, indicating the stretching mode of cages of C60, slightly shifted compared with that of C60 (1468 cm−1), which is similar to C60-β-CDP reported by Diao and co-workers.59,60 These results indicate that the formation of supramolecular complexes (SPh⊃C60) did not change the nature of C60. No appreciable Raman peaks of SPh can be recognized in SPh⊃C60, probably due to the strong fluorescence background signal of SPh.
Raman spectroscopy was further used to characterize SPh. Fig. 3b, S3, and S4† show the Raman spectra of SPh and monomer 5. A strong Raman G-band appears at approximately 1600 cm−1 for both SPh and compound 5, which probably arises from the symmetrical vibration caused by the collective C–C stretching modes of the benzenoid rings along the transverse tube axis. The D-band at ∼1200 cm−1 mainly originates from the in-plane C–H bending modes, and those around 1270 cm−1 correspond to ring breathing modes. In addition, the main peaks of [10]cyclo-para-phenylene were at 1206, 1265, and 1582 cm−1,61 and the signals of linear PPP are located at 1215, 1285, and 1602 cm−1.62 Some of these peaks are quite similar to signals of SPh, which further confirms the existence of cyclo-para-phenylene macrocycle and one-dimensional PPP structures. All these results suggest the polymerization of monomer 5 and the formation of SPh.
The FTIR spectra also demonstrate the successful polymerization of compound 5 into SPh (Fig. 3c, d, S5 and S6†). Fig. 3c shows the C–H stretching of 5 and SPh in the high wavenumber region. Three obvious peaks (3026 cm−1, 2921 cm−1, and 2850 cm−1) were observed for compound 5. In sharp contrast, only the peak located at 2913 cm−1 appears for SPh. In the FTIR spectra of the low wavenumber region (Fig. 3d), significant differences were also observed for SPh (mainly four peaks at 1260 cm−1, 1093 cm−1, 1017 cm−1, and 800 cm−1) and compound 5 (two sharp peaks at 1467 cm−1 and 813 cm−1, and one broad band maximized at 1095 cm−1). Similar peaks at 813 cm−1 for 5 and 800 cm−1 for SPh are associated with out-of-plane C–H deformation from the vibration of a para-disubstituted benzene ring. Moreover, the peak at 1467 cm−1 for compound 5 is completely gone after polymerization. A new peak at ∼1260 cm−1 appears in SPh, consistent with the FTIR spectrum of PPP polymers.62
The polymeric chains of SPh composed of a conjugated hoop-shaped structure were further confirmed by the scanning tunneling microscope (STM) imaging technique at the submolecular level. Using a drop casting method, the high-resolution STM image with a singly dispersed carbon nanohoop structure is shown in Fig. 3e. Many hoop-shaped structures with a disordered arrangement were adsorbed onto the surface, as well as a lot of aggregated particles with bright spots. To clearly observe the morphology of the polymeric segment, an enlarged image is shown in Fig. 3f. To test the composition of these tiny spots, an energy-dispersive X-ray spectroscopy (EDX) experiment was conducted and the results showed that these tiny spots should be carbon-containing moieties (see Fig. S11†). The polymeric chain containing around seven conjugated macrocycles can be identified and the hoop-shaped structure was clearly recognized, confirming the contracture of SPh.
Photophysical properties of SPh
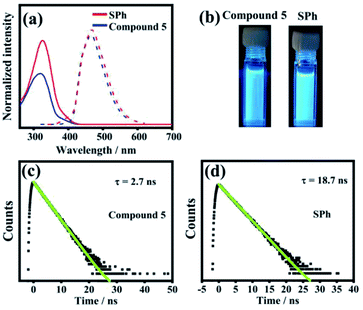
The photophysical properties of SPh and monomer 5 were investigated in solution by UV-vis absorption spectroscopy, steady-state fluorescence spectroscopy, and time-resolved fluorescence decay spectroscopy (Fig. 4). The absorption spectrum of SPh showed an intense absorption band maximized at ∼326 nm with a shoulder band in the range of 372–432 nm, which is slightly redshifted compared to 5 (maximized at ∼317 nm). Because of the same CPP skeletons, SPh and 5 show nearly the same fluorescence spectra with maximum peaks at ∼470 nm and 465 nm, respectively. The fluorescence quantum yields determined for compound 5 and SPh are ΦF = 23% for SPh and ΦF = 14% for 5 (using anthracene in ethanol as reference, ΦF = 30%). Both values are much lower than that of [10]CPP (ΦF = 65%),63 probably because the functionalized benzene rings increase the proportion of nonradiative decay of the excited states. The fluorescence colors of SPh and 5 are shown in Fig. 2b, indicating their similarity on emitting blue fluorescence.The luminescence lifetimes (τs) of SPh and 5 were further measured by the time-resolved photoluminescence (TRPL) technique using a nanosecond pulsed laser system (Fig. 4c and d). The fluorescence decay of 5 follows first-order kinetics with a lifetime τs = 2.7 ns at 469 nm when excited at ∼300 nm. SPh shows a similar single-exponential decay with a much longer fluorescence lifetime of τs = 18.7 ns. Based on the above results, the similarity of these optical properties for SPh and 5 in solution indicates that the motif of cyclo-para-phenylene plays an important role.
Supramolecular chemistry and ultrafast excited-state dynamics
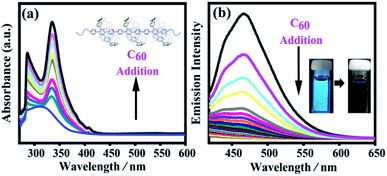
For a long time, fullerenes have been widely studied as guests in various organic host architectures owing to their unusual shape (approximately spherical) and chemical nature (nonpolar polyenes).55 These organic host architectures have specific sizes for C60 to construct various host–guest molecular systems via supramolecular interactions and weak supramolecular van der Waals interactions, such as supramolecular organic frameworks,64 cyclodextrin,65 calix[8]arene,66 crown ether,67,68 macrocyclic bis-exTTF hosts,50 and nanorings.51,69 Cyclo-para-phenylene with ten phenyl groups has an appropriate size to complex with C60, as evidenced by single crystal X-ray diffraction analysis and NMR studies.69,70 These results confirmed that such a conjugated macrocycle has the right size to selectively encapsulate C60. Recently, π-extended crown-shaped CPPs56 and porphyrin-embedded nanorings71 showed highly enhanced binding constants with fullerenes C60. Their supramolecular structures with C60 can also be directly observed by single-crystal X-ray diffraction. All these results make it possible for SPh to form a host–guest supramolecular polymeric heterojunction with fullerene C60 (SPh⊃C60).Based on its structure, SPh contains a macrocyclic unit for C60 to construct the designed carbon nanopeapod segment. We performed UV-vis experiments and fluorescence quenching experiments on the C60 titration to confirm the existence of the supramolecular interactions between SPh and C60. When C60 was added into a SPh solution in toluene, the solution color obviously changed from yellow to brown. Upon C60 titration into the SPh solution, the absorption spectra of SPh showed an obvious enhancement in the range of 280–430 nm (Fig. 5a). The absorption band maximum at ∼330 nm should be mainly due to the presence of C60. Also, the fluorescence intensity was significantly reduced under a hand-held UV lamp excited at 365 nm; measurements of its emission intensity during titration (Fig. 5b) suggest that fullerene C60 can efficiently quench the fluorescence. Monomer 5 was chosen to study the supramolecular interaction with C60 by fluorescence spectroscopy. The titrating results showed that its Ka is ∼1.02 × 105 M−1 (Fig. S12†). The interaction between monomer 5 and C60 was further observed by 1H NMR spectroscopy when C60 was added into monomer 5 in CDCl3 (Fig. S13†). The benzene signals shifted downfield by ∼0.07 ppm. These results confirmed that monomer 5 could act as a supramolecular host with C60 to form 5⊃C60, suggesting that polymer SPh can also interact with C60 to form SPh⊃C60. Mass spectrometry clearly shows the formation of the monomer 5⊃C60 complex (Fig. S14†).
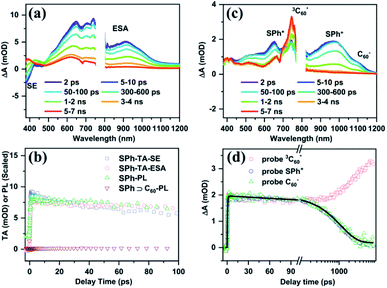
To understand the detailed quenching mechanisms between the photoexcited SPh host and C60 guest, we performed femtosecond transient absorption (TA) and fluorescence upconversion (FU) measurements (see ESI† for experimental details). In these experiments, the samples were pumped at 350 nm, which can excite both SPh and C60 species. Fig. 6a plots the TA spectra of SPh at the indicated pump–probe delays, displaying a negative feature within 400–450 nm and broad-band absorptive features extending from 500 nm to the near-infrared (NIR) region. We assign the negative feature to the stimulated emission (SE) of photoexcited SPh and the positive features to its excited state absorption (ESA). This assignment was established by comparing the TA kinetics probed for SE and ESA to the FU kinetics measured for SPh (Fig. 6a). The close agreement between these three kinetic traces suggests that they all track the decay process of photoexcited SPh.
The TA spectra of the SPh⊃C60 complex in the presence of encapsulated C60 (SPh⊃C60) are presented in Fig. 6c. In this case, the negative SE feature of SPh disappeared, indicating immediate depletion of the SPh excited state by C60. This ultrafast process was further confirmed by the FU experiment. As shown in Fig. 6b, when measured under the same conditions as pure SPh, SPh⊃C60 displayed negligible photon counts even near time zero, suggesting that the excited SPh is depleted on a timescale much faster than the instrument response (≪300 fs). Based on the energy levels of SPh and C60, we can identify this ultrafast interaction as an electron transfer from photoexcited SPh to C60. Indeed, in the TA spectra in Fig. 6c, we observe the instantaneous formation of a positive shoulder peak at ∼1080 nm that is consistent with the anion radical peak of C60 (C60−).53 Meanwhile, a positive peak at ∼650 nm and a broad positive band centered at ∼950 nm also formed instantaneously, which can be assigned to the cation radical of SPh (SPh+).72 Clearly, C60− and SPh+ are products of electron transfer from photoexcited SPh to C60, and their nearly-instantaneous formation further confirmed that this electron transfer process occurred on a femtosecond timescale. This ultrafast electron transfer is likely enabled by a strong electronic coupling between SPh and C60, which are in intimate contact due to supramolecular interactions.
The charge-separated state (SPh+–C60−) is not very long-lived, as reflected by the decay of C60− and SPh+ features on the ns timescale. Interestingly, accompanying this decay is the gradual formation of a positive peak at ∼750 nm that coincides with the spectroscopic features of the C60 triplet excited state 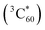 .73 Thus, the recombination of SPh+–C60− does not regenerate the ground state SPh–C60 but rather produces
.73 Thus, the recombination of SPh+–C60− does not regenerate the ground state SPh–C60 but rather produces 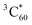 . This observation is consistent with the spin–orbit charge-transfer intersystem crossing (SOCT-ISC) phenomenon that has been well documented in the literature for organic donor–acceptor systems.74 Specifically, the orbital angular momentum change of the electron during charge recombination can induce spin-flip and populates a triplet state. A related process is also reported for semiconductor nanocrystal–organic molecule hybrid donor–acceptor systems.75 By fitting the kinetics of SPh+ and C60− plotted in Fig. 6d, the charge recombination (triplet formation) time was determined as ∼1.1 ns. The resulting
. This observation is consistent with the spin–orbit charge-transfer intersystem crossing (SOCT-ISC) phenomenon that has been well documented in the literature for organic donor–acceptor systems.74 Specifically, the orbital angular momentum change of the electron during charge recombination can induce spin-flip and populates a triplet state. A related process is also reported for semiconductor nanocrystal–organic molecule hybrid donor–acceptor systems.75 By fitting the kinetics of SPh+ and C60− plotted in Fig. 6d, the charge recombination (triplet formation) time was determined as ∼1.1 ns. The resulting 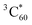 is long-lived, showing no decay within 8 ns.
is long-lived, showing no decay within 8 ns.
The measurements for compound 5 showed similar results to SPh (Fig. S15†). Thus, these time-resolved studies generally reveal femtosecond electron transfer from photoexcited compound 5 and SPh to encapsulated C60, followed by nanosecond charge recombination to produce 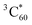 . This offers a novel approach to sensitize
. This offers a novel approach to sensitize  that can be used to, for example, generate singlet oxygen for photocatalysis and photodynamic therapy. However, the triplet formation pathway might be detrimental to optoelectronic devices utilizing SPh donors and C60 acceptors, similar to the issue reported in organic solar cells.76,77 Nonetheless, if the rate of charge sweeping out exceeds that of charge recombination in operating devices, triplet formation can be effectively suppressed.78
that can be used to, for example, generate singlet oxygen for photocatalysis and photodynamic therapy. However, the triplet formation pathway might be detrimental to optoelectronic devices utilizing SPh donors and C60 acceptors, similar to the issue reported in organic solar cells.76,77 Nonetheless, if the rate of charge sweeping out exceeds that of charge recombination in operating devices, triplet formation can be effectively suppressed.78
Charge transport properties of SPh and SPh⊃C60
Owing to the interesting photophysical properties of the polymeric structure of SPh and its host–guest interaction with C60, we further investigated the potential applications of SPh and SPh⊃C60 as the electron- and hole-transport layers for optoelectronic devices.79,80 The electron and hole mobilities (μe and μh) were measured based on the SCLC method (Fig. 7a).81 When using only SPh as the electron transport layer, an electron-only device was fabricated as ITO/ZnO/SPh/Ca/Al. The result demonstrates that the μe value of SPh is ∼4.87 × 10−5 cm2 V−1 s−1 using the Mott–Gurney equation, a value which is ∼2.5 times higher than that of PS1 which we reported previously as an electron-transport layer.44 By incorporating fullerene C60 into the SPh polymer, an electron-only ITO/ZnO/SPh⊃C60/Ca/Al device was fabricated and the electron mobility was enhanced to ∼8.06 × 10−5 cm2 V−1 s−1, which is higher than those of both SPh layer and pure C60 (∼4.87 × 10−5 cm2 V−1 s−1 for SPh and ∼6.13 × 10−5 cm2 V−1 s−1 for C60).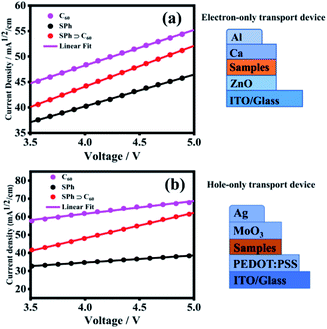 | ||
| Fig. 7 J 1/2–V plots and their corresponding fitting curves of the (a) electron-only ITO/ZnO/active layer/Ca/Al device and (b) hole-only ITO/PEDOT:PSS/active layer/MoO3/Ag device. | ||
To investigate the influence of C60 incorporation on the hole mobility of SPh, we also fabricated hole-only devices with the structure of ITO/PEDOT:PSS/active layer/MoO3/Ag, where the active layer is SPh, SPh⊃C60 or C60, and PEDOT:PSS is poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (Fig. 7b) The hole mobility of SPh was measured to be ∼2.86 × 10−5 cm2 V−1 s−1 in the SCLC region. Interestingly, upon incorporating C60 into the SPh polymer, the hole mobility is dramatically enhanced by more than ten times to ∼3.64 × 10−4 cm2 V−1 s−1, which is even higher than that of pure C60 (∼9.05 × 10−5 cm2 V−1 s−1). Based on these results, we conclude that C60 incorporation enhances te charge transport properties of SPh, promising for applications of SPh and SPh⊃C60 in optoelectronic devices.
Conclusions
In conclusion, we successfully synthesized the first supramolecular polymeric heterojunction as the segment of carbon nanopeapods by incorporating the C60 guest into the segment of carbon nanotubes (SPh) containing conjugated macrocycles as the host interconnected by a PPP backbone. In the presence of C60, the fluorescence intensity of SPh was significantly quenched. Transient absorption measurements demonstrated that, upon photoirradiation, C60− and SPh+ are generated due to femtosecond (≪300 fs) electron transfer from the photoexcited SPh donor to the C60 acceptor, followed by nanosecond charge recombination to produce the C60 triplet excited state. Furthermore, compared to the host SPh and the guest C60, the SPh⊃C60 showed enhanced electron- and hole-mobilities, promising for applications of SPh and SPh⊃C60 in optoelectronic devices. This study opens a door for bottom-up synthesis of carbon nanopeapod segments.Data availability
All analytical data is provided in the ESI. All details about synthetic procedures, STM measurements, physical and photophysical characterizations are available in the ESI.Author contributions
P. D. conceived and designed this research. S. W. synthesized the SPh material, conducted all characterizations and photophysical studies. J. W. and K. W. did ultrafast excited-state dynamics measurements. S. Y and X. L. performed SCLC measurements. S. W., X. L., X. Z., P. H., P. F., J. W., K. W., S. Y. and P. D. co-wrote the paper, and all the authors commented on it.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2017YFA0402800), the National Natural Science Foundation of China (21971229, 51772285, U1932214), the Fundamental Research Funds for the Central Universities and the Strategic Pilot Science and Technology Project of the Chinese Academy of Sciences (XDB17010100).Notes and references
- W. Zhang, W. S. Jin, T. Fukushima, A. Saeki, S. Seki and T. Aida, Science, 2011, 334, 340–343 CrossRef CAS PubMed.
- B. Z. Tian, X. L. Zheng, T. J. Kempa, Y. Fang, N. F. Yu, G. H. Yu, J. L. Huang and C. M. Lieber, Nature, 2007, 449, 885–889 CrossRef CAS PubMed.
- A. I. Hochbaum and P. D. Yang, Chem. Rev., 2010, 110, 527–546 CrossRef CAS PubMed.
- M. T. Björk, B. J. Ohlsson, T. Sass, A. I. Persson, C. Thelander, M. H. Magnusson, K. Deppert, L. R. Wallenberg and L. Samuelson, Nano Lett., 2002, 2, 87–89 CrossRef.
- S. Banerjee and S. S. Wong, Nano Lett., 2002, 2, 195–200 CrossRef CAS.
- O. Harnack, C. Pacholski, H. Weller, A. Yasuda and J. M. Wessels, Nano Lett., 2003, 3, 1097–1101 CrossRef CAS.
- S. Günes, H. Neugebauer and N. S. Sariciftci, Chem. Rev., 2007, 107, 1324–1338 CrossRef PubMed.
- K. Matsumoto, M. Fujitsuka, T. Sato, S. Onodera and O. Ito, J. Phys. Chem. B, 2000, 104, 11632–11638 CrossRef CAS.
- H. Imahori, K. Hagiwara, T. Akiyama, M. Aoki, S. Taniguchi, T. Okada, M. Shirakawa and Y. Sakata, Chem. Phys. Lett., 1996, 263, 545–550 CrossRef CAS.
- T. D. M. Bell, T. A. Smith, K. P. Ghiggino, M. G. Ranasinghe, M. J. Shephard and M. N. Paddon-Row, Chem. Phys. Lett., 1997, 268, 223–228 CrossRef CAS.
- B. Grimm, H. Isla, E. M. Perez, N. Martín and D. M. Guldi, Chem. Commun., 2011, 47, 7449–7451 RSC.
- T. Hasobe, H. Imahori, P. V. Kamat, T. K. Ahn, S. K. Kim, D. Kim, A. Fujimoto, T. Hirakawa and S. Fukuzumi, J. Am. Chem. Soc., 2005, 127, 1216–1228 CrossRef CAS PubMed.
- H. Imahori, M. Ueda, S. Kang, H. Hayashi, S. Hayashi, H. Kaji, S. Seki, A. Saeki, S. Tagawa, T. Umeyama, Y. Matano, K. Yoshida, S. Isoda, M. Shiro, N. V. Tkachenko and H. Lemmetyinen, Chem.–Eur. J., 2007, 13, 10182–10193 CrossRef CAS PubMed.
- Q. Huang, G. L. Zhuang, H. X. Jia, M. M. Qian, S. S. Cui, S. F. Yang and P. W. Du, Angew. Chem., Int. Ed., 2019, 58, 6244–6249 CrossRef CAS PubMed.
- T. Aida, E. W. Meijer and S. I. Stupp, Science, 2012, 335, 813–817 CrossRef CAS PubMed.
- S. Burattini, B. W. Greenland, D. H. Merino, W. G. Weng, J. Seppala, H. M. Colquhoun, W. Hayes, M. E. Mackay, I. W. Hamley and S. J. Rowan, J. Am. Chem. Soc., 2010, 132, 12051–12058 CrossRef CAS PubMed.
- M. Burnworth, L. M. Tang, J. R. Kumpfer, A. J. Duncan, F. L. Beyer, G. L. Fiore, S. J. Rowan and C. Weder, Nature, 2011, 472, 334–337 CrossRef CAS PubMed.
- T. F. A. de Greef and E. W. Meijer, Nature, 2008, 453, 171–173 CrossRef CAS PubMed.
- R. J. Dong, Y. F. Zhou, X. H. Huang, X. Y. Zhu, Y. F. Lu and J. Shen, Adv. Mater., 2015, 27, 498–526 CrossRef CAS.
- J. B. Beck and S. J. Rowan, J. Am. Chem. Soc., 2003, 125, 13922–13923 CrossRef CAS PubMed.
- F. Wang, J. Q. Zhang, X. Ding, S. Y. Dong, M. Liu, B. Zheng, S. J. Li, L. Wu, Y. H. Yu, H. W. Gibson and F. H. Huang, Angew. Chem., Int. Ed., 2010, 49, 1090–1094 CrossRef CAS PubMed.
- S. Burattini, B. W. Greenland, D. H. Merino, W. G. Weng, J. Seppala, H. M. Colquhoun, W. Hayes, M. E. Mackay, I. W. Hamley and S. J. Rowan, J. Am. Chem. Soc., 2010, 132, 12051–12058 CrossRef CAS PubMed.
- S. Y. Dong, Y. Luo, X. Z. Yan, B. Zheng, X. Ding, Y. H. Yu, Z. Ma, Q. L. Zhao and F. H. Huang, Angew. Chem., Int. Ed., 2011, 50, 1905–1909 CrossRef CAS PubMed.
- B. Zheng, F. Wang, S. Y. Dong and F. H. Huang, Chem. Soc. Rev., 2012, 41, 1621–1636 RSC.
- M. M. Zhang, X. Z. Yan, F. H. Huang, Z. B. Niu and H. W. Gibson, Acc. Chem. Res., 2014, 47, 346 Search PubMed.
- J. V. Barth, J. Weckesser, C. Z. Cai, P. Günter, L. Bürgi, O. Jeandupeux and K. Kern, Angew. Chem., Int. Ed., 2000, 39, 1230–1234 CrossRef CAS PubMed.
- D. C. Sherrington and K. A. Taskinen, Chem. Soc. Rev., 2001, 30, 83–93 RSC.
- F. Würthner, Z. J. Chen, F. J. M. Hoeben, P. Osswald, C. C. You, P. Jonkheijm, J. von Herrikhuyzen, A. P. H. J. Schenning, P. P. A. M. van der Schoot, E. W. Meijer, E. H. A. Beckers, S. C. J. Meskers and R. A. J. Janssen, J. Am. Chem. Soc., 2004, 126, 10611–10618 CrossRef PubMed.
- K. V. Rao, D. Miyajima, A. Nihonyanagi and T. Aida, Nat. Chem., 2017, 9, 1133–1139 CrossRef PubMed.
- B. Adelizzi, P. Chidchob, N. Tanaka, B. A. G. Lamers, S. C. J. Meskers, S. Ogi, A. R. A. Palmans, S. Yamaguchi and E. W. Meijer, J. Am. Chem. Soc., 2020, 142, 16681–16689 CrossRef CAS PubMed.
- C. L. Sun, H. Q. Peng, L. Y. Niu, Y. Z. Chen, L. Z. Wu, C. H. Tung and Q. Z. Yang, Chem. Commun., 2018, 54, 1117–1120 RSC.
- X. Zhang, L. Y. Wang, J. F. Xu, D. Y. Chen, L. Q. Shi, Y. F. Zhou and Z. H. Shen, Gaofenzi Xuebao, 2019, 50, 973–987 RSC.
- T. Aida and E. W. Meijer, Isr. J. Chem., 2020, 60, 33–47 CrossRef CAS.
- I. D. W. Samuel, G. Rumbles, C. J. Collison, R. H. Friend, S. C. Moratti and A. B. Holmes, Synth. Met., 1997, 84, 497–500 CrossRef CAS.
- D. T. McQuade, A. E. Pullen and T. M. Swager, Chem. Rev., 2000, 100, 2537–2574 CrossRef CAS PubMed.
- G. D. Scholes and G. Rumbles, Nat. Mater., 2006, 5, 920 CrossRef CAS.
- A. C. Grimsdale and K. Müllen, Adv. Polym. Sci., 2006, 199, 1–82 CrossRef CAS.
- D. L. Gin, V. P. Conticello and R. H. Grubbs, J. Am. Chem. Soc., 1994, 116, 10507–10519 CrossRef CAS.
- M. Rehahn, A. D. Schluter, G. Wegner and W. J. Feast, Polymer, 1989, 30, 1054–1059 CrossRef CAS.
- Z. J. Qiu, B. A. G. Hammer and K. Müllen, Prog. Polym. Sci., 2020, 100, 101179 CrossRef CAS.
- F. N. Lu and T. Nakanishi, Sci. Technol. Adv. Mater., 2015, 16, 014805 CrossRef PubMed.
- G. Grem, G. Leditzky, B. Ullrich and G. Leising, Adv. Mater., 1992, 4, 36–37 CrossRef CAS.
- K. P. Strunk, A. Abdulkarim, S. Beck, T. Marszalek, J. Bernhardt, S. Koser, W. Pisula, D. Jansch, J. Freudenberg, A. Pucci, U. H. F. Bunz, C. Melzer and K. Müllen, ACS Appl. Mater. Interfaces, 2019, 11, 19481–19488 CrossRef CAS PubMed.
- Q. Huang, G. L. Zhuang, M. M. Zhang, J. Y. Wang, S. D. Wang, Y. Y. Wu, S. F. Yang and P. W. Du, J. Am. Chem. Soc., 2019, 141, 18938–18943 CrossRef CAS.
- A. Basagni, F. Sedona, C. A. Pignedoli, M. Cattelan, L. Nicolas, M. Casarin and M. Sambi, J. Am. Chem. Soc., 2015, 137, 1802–1808 CrossRef CAS PubMed.
- R. Jasti, J. Bhattacharjee, J. B. Neaton and C. R. Bertozzi, J. Am. Chem. Soc., 2008, 130, 17646–17647 CrossRef CAS PubMed.
- C. Camacho, T. A. Niehaus, K. Itami and S. Irle, Chem. Sci., 2013, 4, 187–195 RSC.
- K. Y. Cheung, S. J. Gui, C. F. Deng, H. F. Liang, Z. M. Xia, Z. Liu, L. F. Chi and Q. Miao, Chem, 2019, 5, 838–847 CAS.
- W. Xu, X. D. Yang, X. B. Fan, X. Wang, C. H. Tung, L. Z. Wu and H. Cong, Angew. Chem., Int. Ed., 2019, 58, 3943–3947 CrossRef CAS PubMed.
- D. Canevet, M. Gallego, H. Isla, A. de Juan, E. M. Perez and N. Martin, J. Am. Chem. Soc., 2011, 133, 3184–3190 CrossRef CAS PubMed.
- T. Iwamoto, Y. Watanabe, T. Sadahiro, T. Haino and S. Yamago, Angew. Chem., Int. Ed., 2011, 50, 8342–8344 CrossRef CAS PubMed.
- S. S. Cui, G. L. Zhuang, D. P. Lu, Q. Huang, H. X. Jia, Y. Wang, S. F. Yang and P. W. Du, Angew. Chem., Int. Ed., 2018, 57, 9330–9335 CrossRef CAS PubMed.
- Y. Z. Xu, B. Z. Wang, R. Kaur, M. B. Minameyer, M. Bothe, T. Drewello, D. M. Guldi and M. von Delius, Angew. Chem., Int. Ed., 2018, 57, 11549–11553 CrossRef CAS PubMed.
- Y. Z. Xu and M. von Delius, Angew. Chem., Int. Ed., 2020, 59, 559–573 CrossRef CAS PubMed.
- D. Canevet, E. M. Perez and N. Martín, Angew. Chem., Int. Ed., 2011, 50, 9248–9259 CrossRef CAS PubMed.
- Q. Huang, G. L. Zhuang, H. X. Jia, M. M. Qian, S. S. Cui, S. F. Yang and P. W. Du, Angew. Chem., Int. Ed., 2019, 58, 6244–6249 CrossRef CAS PubMed.
- C. Zhao, H. B. Meng, M. Z. Nie, Q. Huang, P. W. Du, C. R. Wang and T. S. Wang, Chem. Commun., 2019, 55, 11511–11514 RSC.
- I. Khan, K. Saeed and I. Khan, Arabian J. Chem., 2019, 12, 908–931 CrossRef CAS.
- W. Zhang, M. Chen, X. D. Gong and G. W. Diao, Carbon, 2013, 61, 154–163 CrossRef CAS.
- W. Zhang, X. D. Gong, C. Liu, Y. Z. Piao, Y. Sun and G. W. Diao, J. Mater. Chem. B, 2014, 2, 5107–5115 RSC.
- H. Chen, M. R. Golder, F. Wang, R. Jasti and A. K. Swan, Carbon, 2014, 67, 203–213 CrossRef CAS.
- A. Abdulkarim, F. Hinkel, D. Jansch, J. Freudenberg, F. E. Golling and K. Müllen, J. Am. Chem. Soc., 2016, 138, 16208–16211 CrossRef CAS PubMed.
- E. R. Darzi, T. J. Sisto and R. Jasti, J. Org. Chem., 2012, 77, 6624–6628 CrossRef CAS PubMed.
- E. Fernandez-Bartolome, J. Santos, A. Gamonal, S. Khodabakhshi, L. J. McCormick, S. J. Teat, E. C. Sañudo, J. S. Costa and N. Martín, Angew. Chem., Int. Ed., 2019, 58, 2310–2315 CrossRef CAS PubMed.
- T. Andersson, K. Nilsson, M. Sundahl, G. Westman and O. Wennerstrom, J. Chem. Soc., Chem. Commun., 1992, 604–606 RSC.
- J. L. Atwood, G. A. Koutsantonis and C. L. Raston, Nature, 1994, 368, 229–231 CrossRef CAS.
- L. Moreira, J. Calbo, R. M. K. Calderon, J. Santos, B. M. Illescas, J. Arago, J. F. Nierengarten, D. M. Guldi, E. Orti and N. Martín, Chem. Sci., 2015, 6, 4426–4432 RSC.
- L. Moreira, B. M. Illescas and N. Martín, J. Org. Chem., 2017, 82, 3347–3358 CrossRef CAS PubMed.
- J. Xia, J. W. Bacon and R. Jasti, Chem. Sci., 2012, 3, 3018–3021 RSC.
- T. Iwamoto, Y. Watanabe, T. Sadahiro, T. Haino and S. Yamago, Angew. Chem., Int. Ed., 2011, 50, 8342–8344 CrossRef CAS PubMed.
- Y. Z. Xu, S. Gsanger, M. B. Minameyer, I. Imaz, D. Maspoch, O. Shyshov, F. Schwer, X. Ribas, T. Drewello, B. Meyer and M. von Delius, J. Am. Chem. Soc., 2019, 141, 18500–18507 CrossRef CAS PubMed.
- E. Kayahara, T. Kouyama, T. Kato and S. Yamago, J. Am. Chem. Soc., 2016, 138, 338–344 CrossRef CAS PubMed.
- G. Sauve, N. M. Dimitrijevic and P. V. Kamat, J. Phys. Chem., 1995, 99, 1199–1203 CrossRef CAS.
- Y. Dong, A. A. Sukhanov, J. Z. Zhao, A. Elmali, X. L. Li, B. Dick, A. Karatay and V. K. Voronkova, J. Phys. Chem. C, 2019, 123, 22793–22811 CrossRef CAS.
- X. Luo, Y. Y. Han, Z. W. Chen, Y. L. Li, G. J. Liang, X. Liu, T. Ding, C. M. Nie, M. Wang, F. N. Castellano and K. F. Wu, Nat. Commun., 2020, 11, 7528 Search PubMed.
- L. Q. Qin, X. Z. Liu, X. Zhang, J. W. Yu, L. Yang, F. G. Zhao, M. F. Huang, K. W. Wang, X. X. Wu, Y. H. Li, H. Chen, K. Wang, J. L. Xia, X. H. Lu, F. Gao, Y. P. Yi and H. Huang, Angew. Chem., Int. Ed., 2020, 59, 15043–15049 CrossRef CAS PubMed.
- A. Rao, P. C. Y. Chow, S. Gélinas, C. W. Schlenker, C.-Z. Li, H.-L. Yip, A. K.-Y. Jen, D. S. Ginger and R. H. Friend, Nature, 2013, 500, 435–439 CrossRef CAS PubMed.
- Y. Q. Hou, X. Zhang, K. P. Chen, D. Y. Liu, Z. J. Wang, Q. Y. Liu, J. Z. Zhao and A. Barbon, J. Mater. Chem. C, 2019, 7, 12048–12074 RSC.
- E. Kayahara, L. S. Sun, H. Onishi, K. Suzuki, T. Fukushima, A. Sawada, H. Kaji and S. Yamago, J. Am. Chem. Soc., 2017, 139, 18480–18483 CrossRef CAS PubMed.
- A. R. Tameev, L. Y. Pereshivko and A. V. Vannikov, Mol. Cryst. Liq. Cryst., 2008, 497, 333–338 Search PubMed.
- P. W. M. Blom, M. J. M. deJong and J. J. M. Vleggaar, Appl. Phys. Lett., 1996, 68, 3308–3310 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc03410c |
| This journal is © The Royal Society of Chemistry 2021 |