Open Access Article
Open Access ArticleCopper-catalyzed [3 + 1] cyclization of cyclopropenes/diazo compounds and bromodifluoroacetamides: facile synthesis of α,α-difluoro-β-lactam derivatives†
Mengru
Zhang
a,
Hexin
Li
a,
Jinbo
Zhao
 ac,
Yan
Li
ac,
Yan
Li
 *a and
Qian
Zhang
*a and
Qian
Zhang
 *ab
*ab
aDepartment of Chemistry, Jilin Province Key Laboratory of Organic Functional Molecular Design & Synthesis, Northeast Normal University, Changchun 130024, China. E-mail: liy078@nenu.edu.cn; zhangq651@nenu.edu.cn
bState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, China
cDepartment of Chemistry, Jilin Provincial Key Laboratory of Carbon Fiber Development and Application, College of Chemistry and Life Science, Changchun University of Technology, Changchun 130012, China
First published on 29th July 2021
Abstract
We have developed a novel copper-catalyzed cyclization of cyclopropenes/diazo compounds and bromodifluoroacetamides, efficiently synthesizing a series of α,α-difluoro-β-lactams in moderate to excellent yields under mild reaction conditions. This reaction represents the first example of [3 + 1] cyclization for the synthesis of β-lactams utilizing a metal carbene intermediate as the C1 synthon.
Introduction
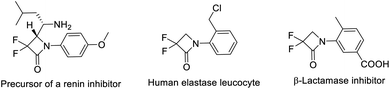
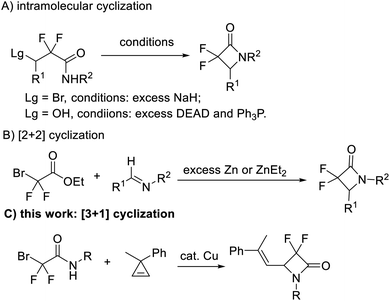
Metal carbenes nowadays have been recognized as important active intermediates for many organic reactions, such as cyclopropanations, X–H (X = C, Si, O, S, N, etc.) insertions, 1,2-migrations, Buchner reactions, [2,3]-sigmatropic rearrangements, ylide formations and others.1 In these reactions, a metal carbene can be used as a C1 synthon to form diverse carbon- and hetero-ring compounds.2 However, it is very rare to utilize a metal carbene intermediate as a C1 synthon to construct four-membered cyclic compounds via a [3 + 1] cyclization process. In 2009, Barluenga and co-workers reported the first example of [3 + 1] cyclization by using a copper carbene intermediate derived from simple diazo compounds, vinyldiazo esters as the C1 synthon to synthesize cyclobutene derivatives.3 Recently, Schomaker et al. successfully developed a [3 + 1] cyclization of rhodium carbene with bicyclic methylene aziridines to produce highly substituted methylene azetidines with excellent stereo- and regio-selectivity.3b,c To the best of our knowledge, using in situ generated metal carbene as the C1 synthon to form β-lactams via the [3 + 1] cyclization reaction has never been reported.β-Lactams have been recognized as one of the most acclaimed classes of aza-ring compounds since the structure elucidation of penicillin in 1945.4 Diverse β-lactam derivatives have shown important antibacterial, antimicrobial, anticancer, antiviral, antihyperglycemic and other biological activities.5 The incorporation of the difluoromethylene (CF2) group into organic compounds can usually substantially alter the physical and biological properties of the compounds, resulting in useful biological and pharmacological effects.6 In fact, α,α-difluoro-β-lactams have been disclosed to be effective in the inhibition of human leukocyte elastase (Scheme 1).7 Several methods have been established to synthesize these compounds.7–10 Among them, intramolecular ring closure of 3-functionalized-2,2-difluoroamides8 (Scheme 2A) and [2 + 2] cyclization of halodifluoroacetates with imines9 (Scheme 2B) were common methods. While the former often needs multistep synthesized substrates and excess sodium hydride or phosphine,8 the latter requires excess zinc powder or organozinc reagent9 and sometimes provides a mixture of the α,α-difluoro-β-amino ester and α,α-difluoro-β-lactam.9b Recently, our group realized copper-catalyzed [3 + 2] cyclization of α-bromodifluoroacetamides with alkenes/alkynes to synthesize α,α-difluoro-γ-lactam derivatives, where α-bromodifluoroacetamides might be recognized as a three-atom synthon and acted as both the difluoromethylene group (CF2) and amido group source.11 We envision that a [3 + 1] cyclization of α-bromodifluoroacetamides might be realized by choosing an appropriate C1 synthon. Herein, we report the first example of copper-catalyzed [3 + 1] cyclization of α-bromodifluoroacetamides with cyclopropenes for facile access to a series of α,α-difluoro-β-lactams (Scheme 2c).
Results and discussion
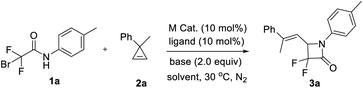
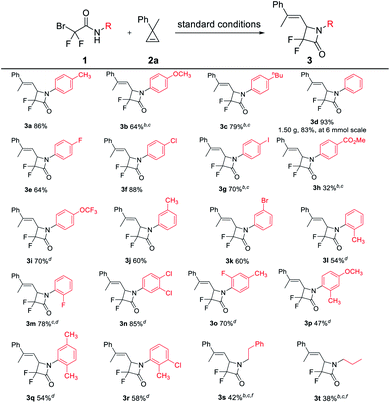
Given that cyclopropenes can usually serve as carbene precusors12 and considering our recent work on the copper-catalyzed ring-opening coupling reaction of cyclopropene and phosphite via a possible copper vinyl carbene species,13 we chose cyclopropene (2a) for the initial test of the [3 + 1] cyclization. The reaction of α-bromodifluoroacetamide 1a (0.2 mmol) and 2a (0.24 mmol, 1.2 equiv.) was performed in the presence of CuI (10 mol%), phen (L1, 10 mol%) and K2CO3 (2.0 equiv.) in CH3CN (2 mL) under a nitrogen atmosphere at 30 °C. After 24 h, we were pleased to find that the expected [3 + 1] cyclization product α,α-difluoro-β-lactam 3a was obtained in 60% yield (entry 1). When the ligand was absent, the yield of 3a decreased to 51% (entry 2), which showed that the ligand played a minor role through the coordination with the active Cu species.14 Without the catalyst or the base, no reaction occurred (entries 3 and 4). Other metal catalysts, such as Pd, Rh and Ag, which are usually used in the formation of metal carbenes, were ineffective for this [3 + 1] cyclization (for details, see ESI Table S2†). Further copper catalyst screening found that CuI was the superior choice (entries 5–7). Other ligands L2–L4 did not improve the yield of 3a (entries 8–10; for details, see ESI Table S1†). Scanning the base (entries 11 and 12; for details, see ESI Table S3†) showcased that K2CO3 was the best one. Other solvents were also tested (entries 13 and 14; for details, see ESI Table S4†), and no better results were obtained. When the reaction was performed at an elevated temperature (40 °C), the yield of 3a was increased to 65% (entry 15). To our delight, 3a was obtained in 86% yield when cyclopropene was added via a syringe15 for 30 min (entry 16). Decreasing or increasing the amount of K2CO3 could not improve the yield of 3a (entries 17 and 18). During these reactions, only E-α,α-difluoro-β-lactam 3a was obtained and the corresponding Z-isomer was not observed.With the optimized reaction conditions (Table 1, entry 16), we set to investigate the scope of α-bromodifluoroacetamides 1 (Table 2). N-Aryl-α-bromodifluoroacetamides bearing either electron-donating or -withdrawing groups at the para/meta/ortho positions of the aromatic rings, such as 1a–1m, worked well and afforded the desired α,α-difluoro-β-lactams 3a–3m in moderate to excellent yields, but for the reaction of 1b, 1c, 1g, 1h, 1l and 1m, a relatively larger amount of 2a or a higher temperature was required. Disubstituted N-aryl-α-bromodifluoroacetamides 1n–1r also easily underwent the [3 + 1] cyclization, giving the desired products 3n–3r in 47–85% yields. These results showed that the electronic effect was inconsequential during the transformation. Compared with other α-bromodifluoroacetamides 1, ortho-substituted amides 1l, 1m and 1o–1r gave the corresponding [3 + 1] cyclization products 3l, 3m and 3o–3r in reasonable yields, which showed that the steric hindrance had no clear effect on the reactivity profile. N-Alkyl-α-bromodifluoroacetamides 1s and 1t were also examined and the corresponding α,α-difluoro-β-lactams 3s and 3t were obtained in acceptable yields. Furthermore, other amides instead of 1 were tested. The decomposition of α-bromo-α,α-difluoroacetamide was observed under the optimal conditions, without the formation of the desired product. No reaction occurred for α-bromo-N-phenylacetamide, and almost quantitative feedstock was recovered. Pleasingly, a gram-scale reaction (6 mmol of 1d) can be readily implemented under the standard conditions with only slightly diminished reactivity (1.50 g, 83% yield).
| Entry | M cat. | Ligand | Base | Solvent | Yield (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol, 1.0 equiv.), 2a (0.24 mmol, 1.2 equiv.), solvent (2 mL), catalyst (10 mol%), ligand (10 mol%), base (2.0 equiv.), 24 h. Yields of isolated 3a were given. N.R. = no reaction. b Cu cat. = Cu(CH3CN)4PF6. c The reaction was performed at 40 °C. d The solution of 1a in 2 mL CH3CN was added via a syringe for 30 minutes. e 1 equiv. K2CO3 was used. f 3 equiv. K2CO3 was used. | |||||
| 1 | CuI | L1 | K2CO3 | CH3CN | 60 |
| 2 | CuI | None | K2CO3 | CH3CN | 51 |
| 3 | None | L1 | K2CO3 | CH3CN | N.R. |
| 4 | CuI | L1 | None | CH3CN | N.R. |
| 5 | CuCl | L1 | K2CO3 | CH3CN | 3 |
| 6 | CuBr | L1 | K2CO3 | CH3CN | 35 |
| 7 | Cu cat.b | L1 | K2CO3 | CH3CN | N.R. |
| 8 | CuI | L2 | K2CO3 | CH3CN | 59 |
| 9 | CuI | L3 | K2CO3 | CH3CN | 60 |
| 10 | CuI | L4 | K2CO3 | CH3CN | 45 |
| 11 | CuI | L1 | KOtBu | CH3CN | N.R. |
| 12 | CuI | L1 | Cs2CO3 | CH3CN | Trace |
| 13 | CuI | L1 | K2CO3 | THF | 28 |
| 14 | CuI | L1 | K2CO3 | DCE | <20 |
| 15c | CuI | L1 | K2CO3 | CH3CN | 65 |
| 16c,d | CuI | L1 | K2CO3 | CH3CN | 86 |
| 17c,d,e | CuI | L1 | K2CO3 | CH3CN | 46 |
| 18c,d,f | CuI | L1 | K2CO3 | CH3CN | 57 |
|
|||||
| a Reactions conditions: 1 (0.2 mmol, 1.0 equiv.), 2a (0.24 mmol, 1.2 equiv.), CuI (10 mol%), L1 (10 mol%), K2CO3 (2.0 equiv.), CH3CN (4 mL), 40 °C, N2, 24 h; isolated yields. b 2a (0.40 mmol, 2.0 equiv.). c Performed at 50 °C. d 2a (0.30 mmol, 1.5 equiv.). e Performed at 70 °C. f 1.0 equiv. K2CO3 was used. |
|---|
|
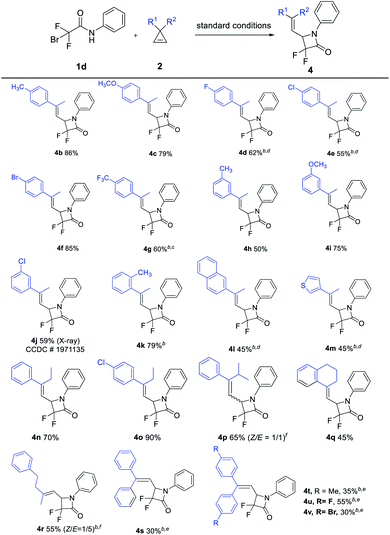
Subsequently, we surveyed the scope of cyclopropenes 2 (Table 3). The aryl methyl cyclopropenes 2b–2k, with either electron-donating or -withdrawing functional groups at the para, meta, or ortho positions on the aromatic rings could be efficiently converted into the desired α,α-difluoro-β-lactams 4b–4k in moderate to good yields. The structure of the [3 + 1] cyclization product was further determined by a single-crystal diffraction experiment of 4j. Naphthyl- or thienyl-containing cyclopropenes 2l and 2m could undergo the [3 + 1] cyclization smoothly, resulting in the corresponding products 4l and 4m in moderate yields. Next, other alkyl groups connected to cyclopropenes were examined. When ethyl group substituted aryl cyclopropenes 2n and 2o were used as the substrates, the desired [3 + 1] cyclization proceeded very smoothly and stereoselectively formed E-α,α-difluoro-β-lactams 4n and 4o in excellent yields. The reaction of i-propyl-substituted aryl cyclopropene 2p could produce [3 + 1] cyclization product 4p in a moderate yield (50% total yield), albeit with a low selectivity (Z/E = 1/1). For the tetrahydronaphthyl substituted substrate 2q, E-α,α-difluoro-β-lactam 4q could be generated in 45% yield with specific selectivity. Dialkyl cyclopropene 2r and diaryl cyclopropenes 2s–2v were also suitable substrates, and the desired products 4s–4v were obtained in acceptable yields. Finally, a multisubstituted cyclopropene, namely 1,3-dimethyl-3-phenyl cyclopropene, was tested. Nevertheless, the reaction was very complicated and no desired product was observed.
| a Reactions conditions: 1a (0.2 mmol, 1.0 equiv.), 2 (0.30 mmol, 1.5 equiv.), CuI (10 mol%), L1 (10 mol%), K2CO3 (2.0 equiv.), CH3CN (4 mL), 40 °C, N2, 24 h; isolated yields. b 2 (0.40 mmol, 2.0 equiv.). c Performed at 50 °C. d Performed at 60 °C. e Performed at 70 °C. f Z/E ratio was determined by 1H NMR spectroscopy. |
|---|
|
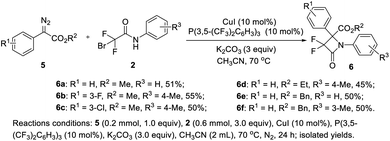
In addition, diazoacetates, as the most commonly used metal carbene precursors,1 were tested for the novel [3 + 1] cyclization (Scheme 3). Gratifyingly, under slightly modified conditions, diazoacetates could undergo the desired [3 + 1] cyclization. As shown in Scheme 3, methyl 2-diazo-2-phenylacetates 5a–5c afforded α,α-difluoro-β-lactams 6a–6c in 51–55% isolated yields. Ethyl and benzylic diazos 5d and 5e were suitable carbene precursors for the reaction and gave α,α-difluoro-β-lactams 6d–6f in acceptable yields. Nevertheless, the vinyldiazo compound, such as methyl (E)-2-diazopent-3-enoate, could not produce the desired lactam, only giving a complex mixture.
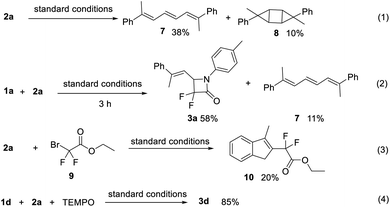
To gain insight into the mechanism of this novel [3 + 1] cyclization, some mechanistic experiments were carried out. In the absence of α-bromodifluoroacetamide 1, cyclopropene 2a could undergo dimerization to form conjugated triene 7 and cyclobutane 8 under standard conditions (Scheme 4, eqn (1)). Furthermore, when the model reaction was quenched after 3 h under standard conditions, 3a and 7 were obtained in 58% and 11% yields, respectively (Scheme 4, eqn (2)). These results suggested that a copper vinyl carbene might be the reaction intermediate.16 Additionally, given that α-bromo-α,α-difluoroacetamide 1 could produce a carbon radical in the presence of a copper catalyst,11,17 α-Bromodifluoroacete 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 instead of 1a, was employed to react with cyclopropene 2a. As a result, 20% of indene 10 was obtained (Scheme 4 eqn (3)),18 which was not detected in the reaction of α-bromodifluoroacetamide 1a and cyclopropene 2a. Moreover, a radical inhibitor experiment was also performed. In the presence of 5.0 equivalents of TEMPO ((2,2,6,6-tetramethylpiperidin-1-yl)oxyl), the yield of 3d slightly decreased to 85% (Scheme 4 eqn (4)). Collectively, these experimental results signified that radical species might not be involved in this [3 + 1] cyclization.
17 instead of 1a, was employed to react with cyclopropene 2a. As a result, 20% of indene 10 was obtained (Scheme 4 eqn (3)),18 which was not detected in the reaction of α-bromodifluoroacetamide 1a and cyclopropene 2a. Moreover, a radical inhibitor experiment was also performed. In the presence of 5.0 equivalents of TEMPO ((2,2,6,6-tetramethylpiperidin-1-yl)oxyl), the yield of 3d slightly decreased to 85% (Scheme 4 eqn (4)). Collectively, these experimental results signified that radical species might not be involved in this [3 + 1] cyclization.
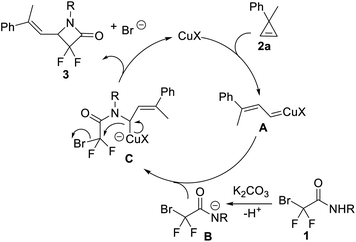
On the basis of the experimental results, as well as previous studies,13,19,20 we proposed a possible reaction mechanism (Scheme 5). The Cu(I)-complex reacted with cyclopropene 2a to form a ring-opened vinyl copper carbene intermediate A.20 Compared with normally accepted copper carbene inserting into the N–H bond of aniline derivatives,21 α-bromo-α,α-difluoroacetamide 1 with a relatively high acidity and low nucleophilicty22 might be favoured to undergo a deprotonation in the presence of K2CO3 to form nitrogen anion species B. Subsequently, the nitrogen anion species B attacked the copper carbene A, generating Cu(I) species C, followed by an intramolecular nucleophilic substitution reaction to yield the expected product 3 and release the Cu(I) catalyst.23 It should be noted that different from previous reports using a vinyl carbene intermediate as a C3 synthon,19 herein the copper vinyl carbene species acted as an interesting C1 synthon.
Conclusions
In conclusion, we have developed a facile and efficient copper-catalyzed [3 + 1] cyclization of cyclopropenes/diazo compounds and bromodifluoroacetamides and therefore furnished a straightforward and efficient method for synthesizing a wide range of valuable α,α-difluoro-β-lactams under mild conditions. This is the first example of employing an in situ generated metal carbene as the C1 synthon in [3 + 1] cyclization for the synthesis of α,α-difluoro-β-lactams. This novel methodology might provide a new pathway for the preparation of cyclic compounds by employing in situ generated metal carbenes.Author contributions
M. Z., Y. L. and Q. Z. conceived the idea. M. Z. performed all experiments including condition optimizations, exploring the scope and investigating the mechanism. Y. L. and Q. Z. supervised the project. H. L. and J. Z. supported other authors to perform the project well. All the authors discussed the results and commented on the manuscript.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
We thank the NSFC (21831002) and the Ten Thousand Talents Program for generous financial support.References
- (a) Y. Xia, D. Qiu and J. Wang, Chem. Rev., 2017, 117, 13810–13889 CrossRef CAS PubMed; (b) M. P. Doyle, M. A. McKervey and T. Ye, Modern Catalytic Methods for Organic Synthesis with Diazo Compounds: From Cyclopropanes to Ylides, Wiley, New York, 1998 Search PubMed; (c) M. P. Doyle and D. C. Forbes, Chem. Rev., 1998, 98, 911–935 CrossRef CAS; (d) Z. Zhang and J. Wang, Tetrahedron, 2008, 64, 6577–6605 CrossRef CAS; (e) M. P. Doyle, R. Duffy, M. Ratnikov and L. Zhou, Chem. Rev., 2010, 110, 704–724 CrossRef CAS; (f) A. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire and M. A. McKervey, Chem. Rev., 2015, 115, 9981–10080 CrossRef CAS.
- (a) S. Chanthamath and S. Iwasa, Acc. Chem. Res., 2016, 49, 2080–2090 CrossRef CAS PubMed; (b) J.-R. Chen, X.-Q. Hu, L.-Q. Lu and W.-J. Xiao, Chem. Rev., 2015, 115, 5301–5365 CrossRef CAS PubMed.
- (a) J. Barluenga, L. Riesgo, L. A. Lopez, E. Rubio and M. Tomas, Angew. Chem., Int. Ed., 2009, 48, 7569–7572 CrossRef CAS; (b) S. C. Schmid, I. A. Guzei and J. M. Schomaker, Angew. Chem., Int. Ed., 2017, 56, 12229–12233 CrossRef CAS; (c) S. C. Schmid, I. A. Guzei, I. Fernández and J. M. Schomaker, ACS Catal., 2018, 8, 7907–7914 CrossRef CAS PubMed.
- D. Crowfoot, C. W. Bunn, B. W. Rogers-Low and A. Turner-Jones, in, Chemistry of Penicillin, ed H. T. Clarke, J. R. Johnson and R. Robinson, Princeton University Press, Princeton, NJ, 1949 Search PubMed.
- For selected examples, see: (a) E. Ebrahimi, A. Jarrahpour, N. Heidari, V. Sinou, V. Sinou, J. M. Brunel, A. R. Zolghadr and E. Turos, Med. Chem. Res., 2016, 25, 247–262 CrossRef CAS; (b) A. Jarrahpour, P. Shirvani, V. Sinou, C. Latour and J. M. Brunel, Med. Chem. Res., 2016, 25, 149–162 CrossRef CAS; (c) M. W. Majewski, P. A. Miller, A. G. Oliver and M. J. Miller, J. Org. Chem., 2017, 82, 737–744 CrossRef CAS; (d) K.-W. Yang, Y. Zhou, Y. Ge and Y. Zhang, Chem. Commun., 2017, 53, 8014–8017 RSC; (e) S. Thaisrivongs, H. J. Schostarez, D. T. Pals and S. R. Turner, J. Med. Chem., 1987, 30, 1837–1842 CrossRef CAS PubMed; (f) J.-L. Maillard, C. Favreau, M. Reboud-Ravaux, R. Kobaiter, R. Joyeau and M. Wakselman, Eur. J. Cell Biol., 1990, 52, 213–218 CAS; (g) R. Joyeau, H. Molines, R. Labia and M. Wakselman, J. Med. Chem., 1988, 31, 370–374 CrossRef CAS PubMed.
- (a) E. P. Gillis, K. J. Eastman, M. D. Hill, D. J. Donnelly and N. A. Meanwell, J. Med. Chem., 2015, 58, 8315–8359 CrossRef CAS PubMed; (b) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC; (c) W. K. Hagmann, J. Med. Chem., 2008, 51, 4359–4369 CrossRef CAS PubMed.
- R. Joyeau, A. Felk, S. Guilaume, M. Wakselman, I. Vergely, C. Doucet, N. Boggetto and M. Reboud-Ravaux, J. Pharm. Pharmacol., 1996, 48, 1218–1230 CrossRef CAS.
- (a) M. Bordeau, F. Frébault, M. Gobet and J.-P. Picard, Eur. J. Org. Chem., 2006, 4147–4154 CrossRef CAS; (b) A. Otaka, J. Watanabe, A. Yukimasa, Y. Sasaki, H. Watanabe, T. Kinoshita, S. Oishi, H. Tamamura and N. Fujii, J. Org. Chem., 2004, 69, 1634–1645 CrossRef CAS PubMed; (c) A. Otaka, H. Watanabe, E. Mitsuyama, A. Yukimasa, H. Tamamura and N. Fujii, Tetrahedron Lett., 2001, 42, 285–287 CrossRef CAS; (d) K. Nakayama, H. C. Kawato, H. Inagaki, R. Nakajima, A. Kitamura, K. Someya and T. Ohta, Org. Lett., 2000, 2, 977–980 CrossRef CAS PubMed; (e) K. Uoto, S. Ohsuki, H. Takenoshita, T. Ishiyama, S. Iimura, Y. Hirota, I. Mrrsui, H. Terasawa and T. Soga, Chem. Pharm. Bull., 1997, 45, 1793–1804 CrossRef CAS; (f) S. Thaisrivongs, H. J. Schostarez, D. T. Pals and S. R. Turner, J. Med. Chem., 1987, 30, 1837–1842 CrossRef CAS PubMed.
- (a) A. Tarui, T. Ikebata, K. Sato, M. Omote and A. Ando, Org. Biomol. Chem., 2014, 12, 6484–6489 RSC; (b) N. Boyer, P. Gloanec, G. De Nanteuil, P. Jubault and J.-C. Quirion, Eur. J. Org. Chem., 2008, 4277–4295 CrossRef CAS; (c) K. Sato, A. Tarui, S. Matsuda, M. Omote, A. Ando and I. Kumadaki, Tetrahedron Lett., 2005, 46, 7679–7681 CrossRef CAS; (d) S. Marcotte, X. Pannecoucke, C. Feasson and J.-C. Quirion, J. Org. Chem., 1999, 64, 8461–8464 CrossRef CAS; (e) M. R. Angelastro, P. Bey, S. Hehdi and N. P. Peet, Bioorg. Med. Chem. Lett., 1992, 2, 1235–1238 CrossRef; (f) J. E. Baldwin, G. P. Lynch and C. J. Schofield, J. Chem. Soc., Chem. Commun., 1991, 736–738 RSC; (g) T. Taguchi, O. Kitagawa, Y. Suds, S. Ohkawa, A. Hashimoto, Y. Iitaka and Y. Kobayashi, Tetrahedron Lett., 1988, 41, 5291–5294 CrossRef.
- (a) R. Joyeau, H. Molines, R. Labia and M. Wakselman, J. Med. Chem., 1988, 31, 370–374 CrossRef CAS; (b) S. Fustero, B. Fernández, P. Bello, C. del Pozo, S. Arimitsu and G. B. Hammond, Org. Lett., 2007, 9, 4251–4253 CrossRef CAS PubMed.
- (a) Y. Lv, W. Pu, Q. Chen, Q. Wang, J. Niu and Q. Zhang, J. Org. Chem., 2017, 82, 8282–8289 CrossRef CAS; (b) Y. Lv, W. Pu, Q. Wang, Q. Chen, J. Niu and Q. Zhang, Adv. Synth. Catal., 2017, 359, 3114–3119 CrossRef CAS.
- For a recent review, see: R. Vicente, Chem. Rev., 2021, 121, 162–226 CrossRef CAS.
- Z. Li, G. Peng, J. Zhao and Q. Zhang, Org. Lett., 2016, 18, 4840–4843 CrossRef CAS.
- (a) F. Hu and X. Lei, ChemCatChem, 2015, 7, 1539–1542 CrossRef CAS; (b) M. Ohashi, N. Ishida, K. Ando, Y. Hashimoto, A. Shigaki, K. Kikushima and S. Ogoshi, Chem. Eur. J., 2018, 24, 9794–9798 CrossRef CAS.
- Upon slowly syringing the solution of 1a, 3a was obtained in 86% yield, and only a trace amount of 5 was observed. While 1a was added in one portion to the vial, 3a and 5 were obtained in 65% and 15% yields, respectively. Slowly syringing 1a might decease the concentration of vinyl carbene copper species, thus efficiently avoiding the formation of 5.
- Y. Zhou, B. G. Trewyn, R. J. Angelici and L. K. Woo, J. Am. Chem. Soc., 2009, 131, 11734–11743 CrossRef CAS.
- A. Prieto, R. Melot, D. Bouyssi and N. Monteiro, ACS Catal., 2016, 6, 1093–1096 CrossRef CAS.
- A radical pathway was favored for the formation of 10. For details, see ESI Scheme S7.†.
- For a selected review, see: K. O. Marichev and M. P. Doyle, Org. Biomol. Chem., 2019, 17, 4183–4195 RSC.
- (a) S. Chuprakov and V. Gevorgyan, Org. Lett., 2007, 9, 4463–4466 CrossRef CAS; (b) J. Chen and S. Ma, Chem.–Asian J., 2010, 5, 2415–2421 CrossRef CAS PubMed; (c) P.-H. Li, S. Yang, T.-G. Hao, Q. Xu and M. Shi, Org. Lett., 2019, 21, 3162–3166 CrossRef CAS PubMed.
- For selected reviews, see: (a) S.-F. Zhu and Q.-L. Zhou, Acc. Chem. Res., 2012, 45(8), 1365–1377 CrossRef CAS PubMed; (b) X. Guo and W. Hu, Acc. Chem. Res., 2013, 46(11), 2427–2440 CrossRef CAS.
- The gem-difluoro unit endowed the amide compounds with a relatively low nucleophilicity and high acidity, see: (a) R. D. Trepka, J. W. Belisle and J. K. Harrington, J.Org. Chem., 1974, 39, 1094–1098 CrossRef CAS; (b) C.-P. Zhang, Z.-L. Wang, Q.-Y. Chen, C.-T. Zhang, Y.-C. Gu and J.-C. Xiao, J. Fluorine Chem., 2010, 131, 761–766 CrossRef CAS.
- (a) S. Mori, E. Nakamura and K. Morokuma, J. Am. Chem. Soc., 2000, 122, 7294–7307 CrossRef CAS; (b) N. Yoshikai and E. Nakamura, Chem. Rev., 2012, 112, 2339–2372 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1971135. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d1sc02930d |
| This journal is © The Royal Society of Chemistry 2021 |