Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Direct Cu-mediated aromatic 18F-labeling of highly reactive tetrazines for pretargeted bioorthogonal PET imaging†
Rocío
García-Vázquez‡
ac,
Umberto M.
Battisti‡
a,
Jesper T.
Jørgensen
bc,
Vladimir
Shalgunov
abc,
Lars
Hvass
bc,
Daniel L.
Stares
a,
Ida N.
Petersen
b,
François
Crestey
a,
Andreas
Löffler
d,
Dennis
Svatunek
 d,
Jesper L.
Kristensen
d,
Jesper L.
Kristensen
 a,
Hannes
Mikula
a,
Hannes
Mikula
 d,
Andreas
Kjaer
d,
Andreas
Kjaer
 *bc and
Matthias M.
Herth
*bc and
Matthias M.
Herth
 *ac
*ac
aDepartment of Drug Design and Pharmacology, Faculty of Health and Medical Sciences, University of Copenhagen, Jagtvej 160, 2100, Copenhagen, Denmark. E-mail: matthias.herth@sund.ku.dk
bCluster for Molecular Imaging, Department of Biomedical Sciences, University of Copenhagen, Blegdamsvej 9, 2100, Copenhagen, Denmark
cDepartment of Clinical Physiology, Nuclear Medicine & PET, Rigshospitalet, Blegdamsvej 9, 2100, Copenhagen, Denmark
dInstitute of Applied Synthetic Chemistry, Technische Universität Wien (TU Wien), Getreidemarkt 9, 1060, Vienna, Austria
First published on 28th July 2021
Abstract
Pretargeted imaging can be used to visualize and quantify slow-accumulating targeting vectors with short-lived radionuclides such as fluorine-18 – the most popular clinically applied Positron Emission Tomography (PET) radionuclide. Pretargeting results in higher target-to-background ratios compared to conventional imaging approaches using long-lived radionuclides. Currently, the tetrazine ligation is the most popular bioorthogonal reaction for pretargeted imaging, but a direct 18F-labeling strategy for highly reactive tetrazines, which would be highly beneficial if not essential for clinical translation, has thus far not been reported. In this work, a simple, scalable and reliable direct 18F-labeling procedure has been developed. We initially studied the applicability of different leaving groups and labeling methods to develop this procedure. The copper-mediated 18F-labeling exploiting stannane precursors showed the most promising results. This approach was then successfully applied to a set of tetrazines, including highly reactive H-tetrazines, suitable for pretargeted PET imaging. The labeling succeeded in radiochemical yields (RCYs) of up to approx. 25%. The new procedure was then applied to develop a pretargeting tetrazine-based imaging agent. The tracer was synthesized in a satisfactory RCY of ca. 10%, with a molar activity of 134 ± 22 GBq μmol−1 and a radiochemical purity of >99%. Further evaluation showed that the tracer displayed favorable characteristics (target-to-background ratios and clearance) that may qualify it for future clinical translation.
Introduction
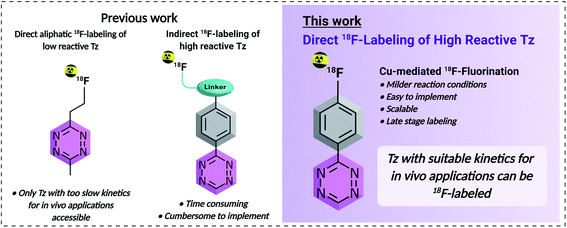
Positron Emission Tomography (PET) is a powerful, non-invasive and routinely used imaging tool in precision medicine or drug development.1–3 Its high sensitivity (the level of detection approaches 10−12 M of tracer), isotropism and quantitativity are in combination unmatched compared to any other in vivo molecular imaging technique.4,5 Fluorine-18 (18F) is considered as the best suited PET radionuclide for clinical applications as it provides almost ideal physical characteristics for molecular imaging. These include a relatively short positron range (2.4 mm max. range in water), a good branching ratio (96.7% positron decay) and a half-life of approx. 110 min, which enables the distribution of 18F-labeled tracers within a several hundred kilometers range.6–8 Recently, bioorthogonal chemistry has emerged as a versatile tool for pretargeted nuclear imaging of slow-accumulating targeting vectors such as monoclonal antibodies (mAbs) or other nanomedicines.9–13 Improved imaging contrast (up to 100-fold) and lower radiation burden to healthy tissue can be achieved using pretargeting compared to conventional imaging strategies.14 These improved imaging characteristics are a result of the temporal separation of the slow targeting process of nanomedicines from the actual imaging step. Consequently, the exceptional target specificity of nanomedicines as well as the optimal pharmacokinetics of small molecules for molecular imaging, e.g. selective target accumulation and rapid clearance from blood, can be exploited using pretargeted imaging.15,16 So far, the most prominent reaction for pretargeted imaging is the tetrazine (Tz) ligation.11,17 Excellent chemoselectivity, metabolic stability and high reactivity make the Tz ligation as exceptional as the biotin–(strept)avidin interaction for pretargeting strategies.18–21 The Tz ligation is driven by the Inverse-Electron-Demand Diels–Alder (IEDDA) cycloaddition between an electron-deficient Tz and a strained trans-cyclooctene (TCO) derivative, followed by a retro-Diels–Alder elimination of nitrogen.10,22–24 Despite efforts focused on TCO-based click imaging agents,25,26 the use of radiolabeled Tz has gradually emerged in recent literature.10Throughout the last decade, the labeling of Tzs was mostly limited to chelation of radiometals such as 64Cu, 89Zr, 44Sc or 68Ga.27–31 In 2013, the first successful attempt to label a Tz moiety with a covalently bound PET radionuclide, i.e. with carbon-11, was reported by our group.32 Despite significant progress in the field, until recently all reported 18F-Tzs had electron-donating alkyl substituents at the Tz ring and thus had low reactivity towards TCOs.21 The reason for this is that highly reactive mono- or bis-(hetero)aryl-substituted Tzs decompose under the harsh conditions used for standard nucleophilic 18F-fluorination (SN2 or SNAr) approaches.14,21,33 Only relatively base insensitive and less reactive Tzs could be radiolabeled, via an 18F-aliphatic substitution (SN2) strategy. Radiochemical yields (RCYs) up to 18% were achieved.21 More recently, the preparation of a highly reactive 18F-labeled glycosylated Tz by Keinänen and co-workers and an [18F]AlF-NOTA-labeled Tz radioligand by Meyer and co-workers were reported.31–33 The latest strategy added to this portfolio is the synthesis of 18F-radiolabeled tetrazines via the copper-catalyzed azide–alkyne cycloaddition.14,21
Within this study, we aimed to develop a simple, scalable and reliable direct aromatic radiofluorination procedure that can be applied to access highly reactive 18F-labeled Tzs (Fig. 1). Direct aromatic [18F]fluorinations are in general fast and efficient and the corresponding fluoroarenes are more stable towards defluorination than their aliphatic counterparts.34 For these reasons, the synthesis of 18F-fluorinated aryls has found widespread application within the last decade.8,35–38 Typically, nucleophilic aromatic substitution (SNAr) is the method of choice to radiolabel fluoroarenes. However, they require relatively strong basic conditions and high temperature, and as such, the SNAr is not ideally suited to 18F-label structures containing highly reactive Tz moieties which are known to be base-sensitive.21,39 Recently, several mild aromatic 18F-labeling strategies have been reported that proceed at lower temperatures and with short reaction time, while using less basic reaction conditions. In particular, Cu-mediated oxidative fluorinations of tin and boronic esters or acids allow fluorination of electron-rich substrates under mild conditions.40–44 In this work, several SNAr and oxidative fluorinations were screened in order to label highly reactive Tzs. Cu-mediated fluorinations of stannane precursors succeeded in moderate RCYs (d.c.) of 10–24% at the end of the synthesis (EOS). Based on these results, a new Tz, compound 21, that possesses the necessary lipophilicity (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4 < −3) and high rate constant (>50
D7.4 < −3) and high rate constant (>50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 s−1) for in vivo pretargeting experiments was designed.14 [18F]21 was radiolabeled in a RCY (d.c.) of 11 ± 3%, with an Am of 134 ± 22 GBq μmol−1 (d.c.) and a RCP of ≥99%.35,37,40 Pretargeted in vivo PET imaging in tumor-bearing mice showed a mean tumor uptake of [18F]21 of 0.99 ± 0.14% ID per g (mean ± S.E.M.) after only 1 hour with a high mean tumor-to-muscle ratio of 10. We believe that the developed tracer shows pharmacokinetic properties warranting in depth preclinical evaluation in the near future and that the developed labeling method will pave the way for developing 18F-Tz based pretargeted imaging agents with favorable reaction kinetics, good metabolic stability and a pharmacokinetic profile required for bioorthogonal in vivo chemistry.
000 M−1 s−1) for in vivo pretargeting experiments was designed.14 [18F]21 was radiolabeled in a RCY (d.c.) of 11 ± 3%, with an Am of 134 ± 22 GBq μmol−1 (d.c.) and a RCP of ≥99%.35,37,40 Pretargeted in vivo PET imaging in tumor-bearing mice showed a mean tumor uptake of [18F]21 of 0.99 ± 0.14% ID per g (mean ± S.E.M.) after only 1 hour with a high mean tumor-to-muscle ratio of 10. We believe that the developed tracer shows pharmacokinetic properties warranting in depth preclinical evaluation in the near future and that the developed labeling method will pave the way for developing 18F-Tz based pretargeted imaging agents with favorable reaction kinetics, good metabolic stability and a pharmacokinetic profile required for bioorthogonal in vivo chemistry.
 | ||
| Fig. 1 Comparison of previously reported 18F-labeling strategies of tetrazines vs. the direct aromatic 18F-labeling approach developed in this work. | ||
Results and discussion
Preparation of tetrazine precursors
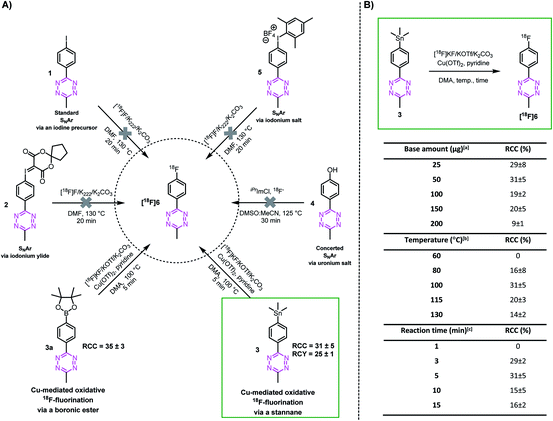
In order to explore whether highly reactive Tzs can be directly 18F-labeled, we investigate different nucleophilic 18F-labeling strategies, such as concerted nucleophilic aromatic substitution of uronium or iodonium salts,36,45–47 hypervalent iodonium based precursors,48–50 minimalistic labeling strategies35,37 and Cu-mediated reactions. Tz 6 was initially selected as a simple model as it is readily accessible and displays moderate stability against strong bases. This allows us to first study the suitability of these types of reaction before attempting the most promising strategy with base-sensitive Tz-scaffolds. Precursors 1–5 and reference compound 6 were synthesized similarly to reported procedures (ESI, section S2†).51,52 In our hands, 18F-labeling strategies including SNAr approaches resulted in decomposition of the product. In contrast, the Cu-mediated 18F-fluorination starting from the stannane (3) and the boronic ester (3a) precursor resulted in the radiolabeling of the model compound [18F]6. The radiochemical conversion (RCC) was approximately 14% at the first attempt (Fig. 2A).53 However, only the stannane precursors of more reactive Tzs could be synthesized. Boronic ester precursors decomposed (ESI, section S2†). Consequently, further optimization of temperature, reaction time and amount of base at the start was only performed with precursor 3 and led to an improvement of approx. 30% RCC (Fig. 2B).Synthesis and radiolabeling of tetrazines with increased reaction kinetics
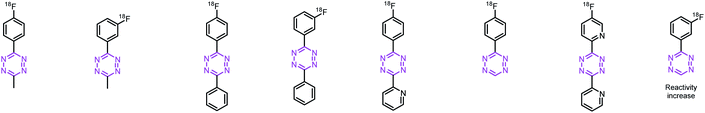
With these encouraging results, we decided to study whether more reactive Tzs could also be labeled using this strategy. Tzs with stepwise increased reactivity were selected to investigate the scope of our radiofluorination method (Table 1). Precursors and reference compounds were synthesized using known procedures (ESI, section S2†)51,52,54,55 and radiolabeling was conducted using the best conditions identified labeling our model compound [18F]6. Moderate RCCs (12–31%) as well as sufficient decay-corrected (d.c.) RCYs (10–24%) were observed at the end of synthesis (EOS) for methyl-, phenyl- and H-Tzs (Table 1). The automated synthesis including [18F]fluoride concentration and drying, labeling, high-performance liquid chromatography (HPLC) separation and formulation was carried out within 90 minutes (ESI, section S3†). Radiochemical purity (RCP) was >99% for all prepared 18F-fluorinated tetrazines, and the molar activity (Am) was 190 ± 10 GBq μmol−1 (d.c) (n = 3) for [18F]6, which is in line with the results obtained for other tracers on the used module and the same starting activity. The typical activity yield was 2.5–3 GBq starting from ∼12 GBq fluoride-18. Pyridyl structures could not be labeled using this labeling strategy, most likely due to a chelation of the copper ion with the respective pyridyl moieties of the Tz.56 As expected, the most reactive Tz resulted in the lowest RCY. However, the observed RCYs are in the range of many clinically applied PET tracers.41,42,57| Compound | [18F]6 | [18F]7 | [18F]8 | [18F]9 | [18F]10 | [18F]11 | [18F]12 | [18F]13 |
|---|---|---|---|---|---|---|---|---|
| a Radiochemical conversion (RCC) and radiochemical purity (RCP) were determined by radio-HPLC and radio-TLC (n = 3). b Radiochemical yield (RCY) was decay corrected to the starting amount of radioactivity received from the cyclotron and the isolated product without a formulation step (n = 3). c Relative IEDDA reactivity was calculated based on second order rate constants determined by stopped-flow measurements of the respective reference compound (19F-Tz) with trans-cyclooctene at 25 °C in 1,4-dioxane or acetonitrile (see the ESI). d No product could be isolated. | ||||||||
| RCCa [%] | 30 ± 5 | 28 ± 1 | 30 ± 5 | 31 ± 2 | —d | 18 ± 4 | —d | 12 ± 1 |
| RCYb [%] | 23 ± 1 | 26 ± 2 | 23 ± 2 | 24 ± 3 | —d | 15 ± 3 | —d | 11 ± 3 |
| Rel. reactivityc | 1.0 | 1.4 | 1.8 | 3.0 | 10 | 70 | 91 | 96 |
| RCPa [%] | ≥99 | ≥99 | ≥99 | ≥99 | —d | 99 | —d | 99 |
Effect of synthesis and radiolabeling of H-Tz upon substitution in the aryl ring
To study the effect of different substituents at the aryl ring, [18F]13 was selected for further analysis since it displayed the highest relative IEDDA reactivity. The IEDDA reactivity is one of the most crucial factors for pretargeted in vivo applications.14 Electron-donating and electron-withdrawing substituents were introduced on the phenyl moiety at different positions, and the substitution pattern was correlated with its synthetic accessibility and RCCs (used as a surrogate for RCYs, RCC correlated with RCY in our study) (Table 2). While all 5-substituted stannane precursors were successfully synthesized from respective iodo-Tz intermediates, only the methyl and/or methoxy derivatives among 4- and 6-substituted stannanes could be prepared – most likely due to steric hindrance.40–42 During 18F-fluorinations, only 3,5-disubstituted stannane precursors provided useful RCCs in the order of 14–31%. No or only minimal product formation could be observed with a different substitution profile (Table 2). Hence, the 3,5-disubstitution pattern was identified to be best suited for Cu-mediated oxidative 18F-fluorinations.| R | Compound | Position | ||
|---|---|---|---|---|
| (-p, -m, -o) | 4 (-p) | 5 (-m) | 6 (-o) | |
| a Stannane precursor could not be synthesized. b RCCs were determined by radio-HPLC and radio-TLC (n = 3). c Decomposed during the Cu-mediated 18F-fluorination. d Iodo-Tz intermediate could not be synthesized. | ||||
| –CH3 | [18F]14 | —a | 14 ± 3b | —c |
| –OCH3 | [18F]15 | 4 ± 1 | 17 ± 3b | —c |
| –NHCOCH3 | [18F]16 | —a | 31 ± 3b | —d |
| –CONH2 | [18F]17 | —a | 24 ± 2b | —d |
| –CONHCH3 | [18F]18 | —a | 20 ± 3b | —d |
Design of the promising tetrazine
Recently, our group has demonstrated that the performance of Tz-derivatives and probes for pretargeted in vivo ligation strongly depends on the lipophilicity and the IEDDA reactivity of the Tz agent. Low polarity (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4 < −3) and rate constants > 50
D7.4 < −3) and rate constants > 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 s−1 for the click reaction with axially configured TCO tags (Dulbecco's PBS, 37 °C) resulted in the best target-to-background ratios.14 In this respect, we designed two highly reactive Tzs, which contained polar groups and allowed for direct 18F-labeling. Tz 19 possesses a clog
000 M−1 s−1 for the click reaction with axially configured TCO tags (Dulbecco's PBS, 37 °C) resulted in the best target-to-background ratios.14 In this respect, we designed two highly reactive Tzs, which contained polar groups and allowed for direct 18F-labeling. Tz 19 possesses a clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4 of −3.09 and a rate constant of 91
D7.4 of −3.09 and a rate constant of 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 s−1, and Tz 21 possesses a clog
000 M−1 s−1, and Tz 21 possesses a clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4 of −6.93 and a rate constant of 82
D7.4 of −6.93 and a rate constant of 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 s−1 (ESI, section S5†). Both compounds were synthesized in sufficient yields via a Pinner-like synthesis (ESI, section S2†) and evaluated in an in vivo assay recently described by our group (Fig. 3A).14 This assay, inspired by traditional receptor blocking studies, applies anti-TAG72 mAb CC49 modified with axially configured TCO tags (CC49-TCO) and [111In]DOTA-Tz (22), which has previously successfully been used for pretargeted imaging in (TAG72 expressing) LS174T tumors.28 In short, tumor-bearing mice are injected with a CC49-TCO, 72 h before the non-labeled Tz is to be tested. Subsequently, [111In]DOTA-Tz (22) is injected after 1 h and a biodistribution is performed 22 h later (ESI, section S5†).14,28 The assay evaluates the blocking ability of the non-labeled Tz, and therefore allows estimation of the in vivo ligation performance of this compound. Higher blocking capacity is correlated with better in vivo performance of the respective Tz.14 As expected – based on our previous data – we found a correlation between clog
000 M−1 s−1 (ESI, section S5†). Both compounds were synthesized in sufficient yields via a Pinner-like synthesis (ESI, section S2†) and evaluated in an in vivo assay recently described by our group (Fig. 3A).14 This assay, inspired by traditional receptor blocking studies, applies anti-TAG72 mAb CC49 modified with axially configured TCO tags (CC49-TCO) and [111In]DOTA-Tz (22), which has previously successfully been used for pretargeted imaging in (TAG72 expressing) LS174T tumors.28 In short, tumor-bearing mice are injected with a CC49-TCO, 72 h before the non-labeled Tz is to be tested. Subsequently, [111In]DOTA-Tz (22) is injected after 1 h and a biodistribution is performed 22 h later (ESI, section S5†).14,28 The assay evaluates the blocking ability of the non-labeled Tz, and therefore allows estimation of the in vivo ligation performance of this compound. Higher blocking capacity is correlated with better in vivo performance of the respective Tz.14 As expected – based on our previous data – we found a correlation between clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4 and in vivo blocking of the Tzs tested in the assay (Pearson's r = 0.89, p <0.01) and the most polar Tz 21 (clog
D7.4 and in vivo blocking of the Tzs tested in the assay (Pearson's r = 0.89, p <0.01) and the most polar Tz 21 (clog![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D7.4 = −6.93) resulting in the best blocking effect (90%) (Fig. 3B) was selected for further development.
D7.4 = −6.93) resulting in the best blocking effect (90%) (Fig. 3B) was selected for further development.
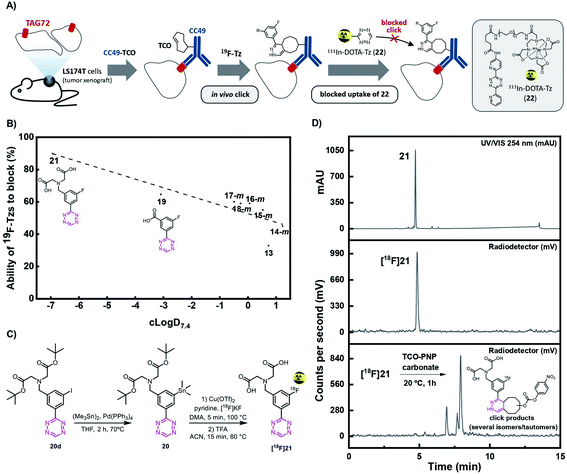 | ||
| Fig. 3 (A) Visualization of the blocking assay. Tumor-bearing mice were first injected with CC49-TCO, 72 h before administration of the non-radioactive Tz. After 1 h, 111In-labeled Tz ([111In]DOTA-Tz, (22), was injected and an ex vivo biodistribution was carried out 22 h p.i. in order to determine the blocking effect of the non-radioactive Tz. (B) Ability of 19F-Tzs (13, 14–18-m, 19, and 21) to block 111In-DOTA-Tz (22) in the in vivo screening assay described in (A) (n = 3) (ESI, section S5†). (C) Synthesis and radiolabeling of [18F]21. (D) Analytical-HPLC of reference compound 21 (UV/Vis, 254 nm) (upper panel), and radio-HPLC of the purified [18F]21 (middle panel) and ligation product after click reaction with the TCO-PNP carbonate (23), one hour post-injection (lower panel). Analytical HPLC conditions: Luna 5 μm C18(2) 100 Å, 150 mm × 4.6 mm; eluents: A, H2O with 0.1% TFA; B, MeCN with 0.1% TFA; gradient from 100% A to 100% B over 12 min, back to 100% A over 3 min, flow rate 2 mL min−1. | ||
Synthesis, radiolabeling and stability of final compound [18F]21
The shelf stability of Tz 21 was assessed in phosphate-buffered saline (PBS) by analytical-HPLC. Compound 21 did not show degradation in PBS after 12 h at 37 °C at a concentration of 2 nmol mL−1 (98%). Consequently, the stannane precursor 20 was synthesized in 4 steps (ESI, section S2†). Radiolabeling succeeded in a one-pot, two-step sequence with a RCY (d.c.) of 11 ± 3% (n = 4) and an overall synthesis time of ca. 90 minutes including synthesis, separation and formulation. [18F]21 was obtained with an Am of 134 ± 22 GBq μmol−1 (d.c.), a RCP of ≥99% (n = 4) and an activity yield of 600–700 MBq (EOS) starting from ∼12 GBq fluoride-18 (Fig. 3C and D). [18F]21 was stable in PBS at room temperature for minimum 4 h and rapidly reacted with TCO-PNP carbonate (23) as confirmed by radio-HPLC (Fig. 3D and ESI, section S3†). Residual amounts of Cu and Sn in the final formulated solution were analyzed by ICP-MS and found to be well below the allowed limits specified in the ICH Guidelines (41–60 and 2.3–3.0 μg L−1vs. 300 and 600 μg per day, respectively).41,58–60Pretargeted PET in vivo imaging
Next, we evaluated the performance of [18F]21 in pretargeted PET imaging (Fig. 4A). Balb/c nude mice bearing LS174T tumor xenografts (n = 3 per group) were injected i.v. with either CC49-TCO (100 μg, 3.9 nmol, ∼7 TCOs per mAb) or non-modified CC49 (control). After 72 h, [18F]21 (2.86 ± 0.99 MBq/100 μL) was administered and the mice were PET/CT scanned after 1 h. Image-derived uptake in tumor, heart (surrogate for blood) and muscle tissue was quantified as percentage injected dose per gram (mean %ID per g) (Fig. 4B–E). After completion of the scan, mice were euthanized and ex vivo biodistribution was performed (ESI, section S6†). Mice pretreated with CC49-TCO demonstrated a mean tumor uptake of [18F]21 of 0.99 ± 0.14% ID per g (mean ± S.E.M.). The tracer displayed good target-to-background ratios with muscle uptake < 0.15% ID per g for all animals (Table S9†). This was also evident from PET/CT images, where tumor uptake in the CC49-TCO group was clearly visible (Fig. 4E). The mean tumor-to-blood ratio was 0.9, and thereby the specific uptake is similar to what was previously reported for other pretargeted imaging agents in the same tumor model.14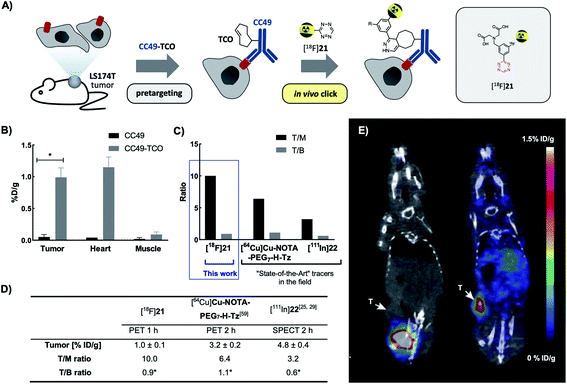 | ||
| Fig. 4 PET/CT scan of CC49-TCO pretargeted [18F]21 in LS174T tumor xenograft bearing mice. (A) General pretargeted imaging approach. (B) PET-image derived mean %ID per g in tumor, heart and muscle tissue 1 h p.i. of [18F]21. Data are shown as mean ± S.E.M; n = 3 per group. *p < 0.05 (Welch's t-test). (C and D) Image-derived tumor uptake (mean %ID per g), tumor-to-muscle (T/M) and tumor-to-blood ratio (T/B) of [18F]21 in comparison with the “state-of-the-art” applied Tz imaging agents [64Cu]Cu-NOTA-PEG7-H-Tz (PET 2 h p.i., n = 4) and [111In]22 (SPECT 2 h p.i., n = 4). Tumor uptake and ratios of [64Cu]Cu-NOTA-PEG7-H-Tz and [111In]22 2 h p.i. in nude BALB/c mice bearing subcutaneous LS174T tumor xenografts pretreated with CC40-TCO (100 μg) have recently been published.28,60 Data are shown as mean ± standard error of mean (SEM). *Image-derived uptake in heart from SPECT and PET images used as a surrogate for blood.28,60 (E) Representative images from PET/CT-scans 1 h p.i. of [18F]21. Mice were administered with either non-modified CC49 (left) or CC49-TCO (right), 72 h prior to [18F]21 injection. Arrows indicate LS174T tumor xenografts. Scale bar indicates mean %ID per g. | ||
In contrast, a mean tumor-to-muscle ratio of 10 was detected which in fact is significantly higher compared to what has previously been found for the “state-of-the-art” Tz-based imaging agents [18F]22 and [64Cu]Cu-NOTA-PEG7-H-Tz in a similar pretargeting set-up (LS174T bearing mice, using CC49-TCO 72 h prior to tracer injection, similar imaging timeframes) (Fig. 4C and D).29,61 However, [18F]21 showed a 3 to 5-fold lower tumor uptake compared to those imaging agents (Fig. 4E).29,61 All tissues including tumors showed low 18F-uptake in control animals (CC49) (tumor uptake of 0.05 ± 0.04% ID per g). The findings from the imaging experiment were confirmed by ex vivo biodistribution data (Table S10†). Except for the tumor, the only tissue where the tracer uptake was significant was blood. This accumulation is likely caused by the in vivo ligation of [18F]21 to CC49-TCO still circulating in the bloodstream, an observation that has been reported before for other pretargeting pairs.10 If residual mAbs are removed from the blood pool by e.g. a clearing agent, subsequent injection of [18F]21 will likely result in an improved tumor-to-blood ratio.10
Conclusion
In conclusion, this work enabled the first direct 18F-labeling of highly reactive Tzs starting from stannane precursors via a Cu-mediated approach. Applying this strategy, we have successfully prepared a new 18F-Tz, [18F]21, with highly favorable characteristics for pretargeted in vivo imaging. The developed procedure is simple, short, reproducible and scalable. Therefore, it is more suitable for clinical applications than previously used multistep 18F-labeling strategies. We are thus convinced that our method for the direct radiofluorination of highly reactive tetrazines will improve and accelerate the clinical translation of pretargeted imaging based in vivo click chemistries.Data availability
All data needed for this article is published within this article or in its ESI.†Author contributions
The organic synthesis was carried out through contributions of RGV, UMB, DLS, INP, and FC. The radiolabeling experiments were performed by RGV, VS, and INP. In vivo studies were performed by LH, JTJ, and AK. DS, AL and HM evaluated the Tz reaction kinetics. The study was designed by MMH, AK, RGV and UMB. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
The authors declare no competing financial interest. All animal experiments in this study were approved by national animal welfare committees in Austria and Denmark, and the experiments were performed in accordance with European guidelines.Acknowledgements
All animal experiments were performed under a protocol approved by the Animal Research Committee of the Danish Ministry of Environment and Food (license no.: 2016-15-0201-00920) and the Animal Ethics Committee of the University of Copenhagen, and in compliance with the guidelines in Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. This project has received funding from the European Union's EU Framework Programme for Research and Innovation Horizon 2020, under grant agreement no. 668532 and from the European Union's Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No. 813528. HM, AK and MMH have received funding from the European Union's EU Framework Programme for Research and Innovation Horizon 2020 (grant agreement no. 670261). VS was supported by BRIDGE – Translational Excellence Programme at the Faculty of Health and Medical Sciences, University of Copenhagen, funded by the Novo Nordisk Foundation (grant agreement no. NNF18SA0034956). The Lundbeck Foundation, the Novo Nordisk Foundation, the Innovation Fund Denmark, and the Research Council for Independent Research are further acknowledged. The modified antibody used in this study was kindly provided by Tagworks Pharmaceuticals.References
- J. L. Kristensen and M. M. Herth, Textbook of drug design and discovery, CRC press, London and New York, 5th edn, 2017 Search PubMed.
- M. Piel, I. Vernaleken and F. Rosch, J. Med. Chem., 2014, 57, 9232–9258 CrossRef CAS PubMed.
- B. Theek, L. Y. Rizzo, J. Ehling, F. Kiessling and T. Lammers, Clin. Transl. Imaging, 2014, 2, 66–76 Search PubMed.
- M. M. Herth, M. Barz, D. Moderegger, M. Allmeroth, M. Jahn, O. Thews, R. Zentel and F. Rosch, Biomacromolecules, 2009, 10, 1697–1703 CrossRef CAS.
- S. M. Ametamey, M. Honer and P. A. Schubiger, Chem. Rev., 2008, 108, 1501–1516 CrossRef CAS.
- D. Le Bars, J. Fluorine Chem., 2006, 127, 1488–1493 CrossRef CAS.
- X. Deng, J. Rong, L. Wang, N. Vasdev, L. Zhang, L. Josephson and S. H. Liang, Angew. Chem., Int. Ed., 2019, 58, 2580–2605 CrossRef CAS PubMed.
- P. E. Edem and E. J. L. Stéen, Late-Stage Fluorination of Bioactive Molecules and Biologically-Relevant Substrates, Elsevier, Copenhagen, 1st edn, 2018 Search PubMed.
- J. M. Baskin, J. A. Prescher, S. T. Laughlin, N. J. Agard, P. V. Chang, I. A. Miller, A. Lo, J. A. Codelli and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 16793–16797 CrossRef CAS.
- E. J. L. Stéen, P. E. Edem, K. Nørregaard, J. T. Jørgensen, V. Shalgunov, A. Kjaer and M. M. Herth, Biomaterials, 2018, 179, 209–245 CrossRef PubMed.
- N. K. Devaraj, R. Weissleder and S. A. Hilderbrand, Bioconjugate Chem., 2008, 19, 2297–2299 CrossRef CAS.
- N. K. Devaraj, G. M. Thurber, E. J. Keliher, B. Marinelli and R. Weissleder, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 4762–4767 CrossRef CAS.
- E. J. L. Stéen, J. T. Jørgensen, K. Johann, K. Nørregaard, B. Sohr, D. Svatunek, A. Birke, V. Shalgunov, P. E. Edem, R. Rossin, C. Seidl, F. Schmid, M. S. Robillard, J. L. Kristensen, H. Mikula, M. Barz, A. Kjær and M. M. Herth, ACS Nano, 2020, 14, 568–584 CrossRef.
- E. J. L. Stéen, J. T. Jørgensen, C. Denk, U. M. Battisti, K. Nørregaard, P. E. Edem, K. Bratteby, V. Shalgunov, M. Wilkovitsch, D. Svatunek, C. B. M. Poulie, L. Hvass, M. Simón, T. Wanek, R. Rossin, M. Robillard, J. L. Kristensen, H. Mikula, A. Kjaer and M. M. Herth, ACS Pharmacol. Transl. Sci., 2021, 4, 824–833 CrossRef.
- M. Patra, K. Zarschler, H. J. Pietzsch, H. Stephan and G. Gasser, Chem. Soc. Rev., 2016, 45, 6415–6431 RSC.
- D. M. Goldenberg, R. M. Sharkey, G. Paganelli, J. Barbet and J.-F. Chatal, J. Clin. Oncol., 2006, 24, 816 CrossRef.
- N. K. Devaraj, R. Upadhyay, J. B. Haun, S. A. Hilderbrand and R. Weissleder, Angew. Chem., Int. Ed. Engl., 2009, 48, 7013–7016 CrossRef CAS.
- N. K. Devaraj and R. Weissleder, Acc. Chem. Res., 2011, 44, 816–827 CrossRef CAS PubMed.
- L. Carroll, H. L. Evans, E. O. Aboagye and A. C. Spivey, Org. Biomol. Chem., 2013, 11, 5772–5781 RSC.
- M. T. Taylor, M. L. Blackman, O. Dmitrenko and J. M. Fox, J. Am. Chem. Soc., 2011, 133, 9646–9649 CrossRef CAS.
- C. Denk, D. Svatunek, T. Filip, T. Wanek, D. Lumpi, J. Fröhlich, C. Kuntner and H. Mikula, Angew. Chem., Int. Ed. Engl., 2014, 53, 9655–9659 CrossRef CAS PubMed.
- B. L. Oliveira, Z. Guo and G. J. L. Bernardes, Chem. Soc. Rev., 2017, 46, 4895–4950 RSC.
- M. L. Blackman, M. Royzen and J. M. Fox, J. Am. Chem. Soc., 2008, 130, 13518–13519 CrossRef CAS.
- E. J. L. Stéen, V. Shalgunov, C. Denk, H. Mikula, A. Kjær, J. L. Kristensen and M. M. Herth, Eur. J. Org. Chem., 2019, 2019, 1722–1725 CrossRef.
- L. Wyffels, D. Thomae, A. M. Waldron, J. Fissers, S. Dedeurwaerdere, P. Van der Veken, J. Joossens, S. Stroobants, K. Augustyns and S. Staelens, Nucl. Med. Biol., 2014, 41, 513–523 CrossRef CAS.
- E. M. F. Billaud, S. Belderbos, F. Cleeren, W. Maes, M. Van de Wouwer, M. Koole, A. Verbruggen, U. Himmelreich, N. Geukens and G. Bormans, Bioconjugate Chem., 2017, 28, 2915–2920 CrossRef CAS PubMed.
- M. R. Lewis, M. Wang, D. B. Axworthy, L. J. Theodore, R. W. Mallet, A. R. Fritzberg, M. J. Welch and C. J. Anderson, J. Nucl. Med., 2003, 44, 1284–1292 CAS.
- R. Rossin, P. Renart Verkerk, S. M. van den Bosch, R. C. Vulders, I. Verel, J. Lub and M. S. Robillard, Angew. Chem., 2010, 122, 3447–3450 CrossRef.
- P. E. Edem, J. T. Jørgensen, K. Nørregaard, R. Rossin, A. Yazdani, J. F. Valliant, M. Robillard, M. M. Herth and A. Kjaer, Molecules, 2020, 25 Search PubMed.
- P. E. Edem, J. P. Sinnes, S. Pektor, N. Bausbacher, R. Rossin, A. Yazdani, M. Miederer, A. Kjær, J. F. Valliant, M. S. Robillard, F. Rösch and M. M. Herth, EJNMMI Res., 2019, 9, 49 CrossRef PubMed.
- J. P. Meyer, J. L. Houghton, P. Kozlowski, D. Abdel-Atti, T. Reiner, N. V. Pillarsetty, W. W. Scholz, B. M. Zeglis and J. S. Lewis, Bioconjugate Chem., 2016, 27, 298–301 CrossRef CAS PubMed.
- M. M. Herth, V. L. Andersen, S. Lehel, J. Madsen, G. M. Knudsen and J. L. Kristensen, Chem. Commun., 2013, 49, 3805–3807 RSC.
- O. Keinänen, X. G. Li, N. K. Chenna, D. Lumen, J. Ott, C. F. Molthoff, M. Sarparanta, K. Helariutta, T. Vuorinen, A. D. Windhorst and A. J. Airaksinen, ACS Med. Chem. Lett., 2016, 7, 62–66 CrossRef.
- M. Kuchar and C. Mamat, Molecules, 2015, 20, 16186–16220 CrossRef CAS PubMed.
- M. Tredwell and V. Gouverneur, Angew. Chem., Int. Ed., 2012, 51, 11426–11437 CrossRef CAS.
- C. N. Neumann, J. M. Hooker and T. Ritter, Nature, 2016, 534, 369–373 CrossRef CAS.
- S. Preshlock, M. Tredwell and V. Gouverneur, Chem. Rev., 2016, 116, 719–766 CrossRef CAS.
- H. Teare, E. G. Robins, A. Kirjavainen, S. Forsback, G. Sandford, O. Solin, S. K. Luthra and V. Gouverneur, Angew. Chem., Int. Ed. Engl., 2010, 49, 6821–6824 CrossRef CAS PubMed.
- Z. Li, H. Cai, M. Hassink, M. L. Blackman, R. C. Brown, P. S. Conti and J. M. Fox, Chem. Commun., 2010, 46, 8043–8045 RSC.
- M. Tredwell, S. M. Preshlock, N. J. Taylor, S. Gruber, M. Huiban, J. Passchier, J. Mercier, C. Génicot and V. Gouverneur, Angew. Chem., Int. Ed. Engl., 2014, 53, 7751–7755 CrossRef CAS PubMed.
- K. J. Makaravage, A. F. Brooks, A. V. Mossine, M. S. Sanford and P. J. H. Scott, Org. Lett., 2016, 18, 5440–5443 CrossRef CAS PubMed.
- S. Preshlock, S. Calderwood, S. Verhoog, M. Tredwell, M. Huiban, A. Hienzsch, S. Gruber, T. C. Wilson, N. J. Taylor, T. Cailly, M. Schedler, T. L. Collier, J. Passchier, R. Smits, J. Mollitor, A. Hoepping, M. Mueller, C. Genicot, J. Mercier and V. Gouverneur, Chem. Commun., 2016, 52, 8361–8364 RSC.
- J. Zischler, N. Kolks, D. Modemann, B. Neumaier and B. D. Zlatopolskiy, Chemistry, 2017, 23, 3251–3256 CrossRef CAS PubMed.
- N. J. Taylor, E. Emer, S. Preshlock, M. Schedler, M. Tredwell, S. Verhoog, J. Mercier, C. Genicot and V. Gouverneur, J. Am. Chem. Soc., 2017, 139, 8267–8276 CrossRef CAS.
- T. L. Ross, J. Ermert, C. Hocke and H. H. Coenen, J. Am. Chem. Soc., 2007, 129, 8018–8025 CrossRef CAS.
- M. H. Beyzavi, D. Mandal, M. G. Strebl, C. N. Neumann, E. M. D'Amato, J. Chen, J. M. Hooker and T. Ritter, ACS Cent. Sci., 2017, 3, 944–948 CrossRef CAS PubMed.
- Y. D. Kwon, J. Son and J. H. Chun, J. Org. Chem., 2019, 84, 3678–3686 CrossRef CAS.
- I. N. Petersen, J. Villadsen, H. D. Hansen, J. Madsen, A. A. Jensen, N. Gillings, S. Lehel, M. M. Herth, G. M. Knudsen and J. L. Kristensen, Org. Biomol. Chem., 2017, 15, 4351–4358 RSC.
- I. N. Petersen, J. L. Kristensen and M. M. Herth, Eur. J. Org. Chem., 2017, 2017, 453–458 CrossRef CAS.
- I. Nymann Petersen, J. Madsen, C. Bernard Matthijs Poulie, A. Kjær and M. Manfred Herth, Molecules, 2019, 24 Search PubMed.
- J. W. McIntee, C. Sundararajan, A. C. Donovan, M. S. Kovacs, A. Capretta and J. F. Valliant, J. Org. Chem., 2008, 73, 8236–8243 CrossRef CAS.
- J. Yang, M. R. Karver, W. Li, S. Sahu and N. K. Devaraj, Angew. Chem., Int. Ed. Engl., 2012, 51, 5222–5225 CrossRef CAS PubMed.
- M. M. Herth, S. Ametamey, D. Antuganov, A. Bauman, M. Berndt, A. F. Brooks, G. Bormans, Y. S. Choe, N. Gillings, U. O. Häfeli, M. L. James, K. Kopka, V. Kramer, R. Krasikova, J. Madsen, L. Mu, B. Neumaier, M. Piel, F. Rösch, T. Ross, R. Schibli, P. J. H. Scott, V. Shalgunov, N. Vasdev, W. Wadsak and B. M. Zeglis, Nucl. Med. Biol., 2021, 93, 19–21 CrossRef CAS PubMed.
- Y. Qu, F. X. Sauvage, G. Clavier, F. Miomandre and P. Audebert, Angew. Chem., Int. Ed. Engl., 2018, 57, 12057–12061 CrossRef CAS PubMed.
- S. A. Albu, S. A. Al-Karmi, A. Vito, J. P. Dzandzi, A. Zlitni, D. Beckford-Vera, M. Blacker, N. Janzen, R. M. Patel, A. Capretta and J. F. Valliant, Bioconjugate Chem., 2016, 27, 207–216 CrossRef CAS PubMed.
- H. T. Chifotides, I. D. Giles and K. R. Dunbar, J. Am. Chem. Soc., 2013, 135, 3039–3055 CrossRef CAS.
- J. Q. Wang, W. Tueckmantel, A. Zhu, D. Pellegrino and A. L. Brownell, Synapse, 2007, 61, 951–961 CrossRef CAS PubMed.
- P. Borman and D. Elder, Journal, 2018, 39–40 Search PubMed.
- M. S. Sanford and P. J. Scott, ACS Cent. Sci., 2016, 2, 128–130 CrossRef CAS.
- F. Zarrad, B. D. Zlatopolskiy, P. Krapf, J. Zischler and B. Neumaier, Molecules, 2017, 22 Search PubMed.
- C. B. M. Poulie, J. T. Jørgensen, V. Shalgunov, G. Kougioumtzoglou, T. E. Jeppesen, A. Kjaer and M. M. Herth, Molecules, 2021, 26 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc02789a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2021 |