Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Graphene nanoribbon-based supramolecular ensembles with dual-receptor targeting function for targeted photothermal tumor therapy†
Wei-Tao
Dou‡
 a,
Fugui
Xu‡
b,
Chen-Xi
Xu
ac,
Jie
Gao
ac,
Hong-Bo
Ru
c,
Xiangfeng
Luan
a,
Fugui
Xu‡
b,
Chen-Xi
Xu
ac,
Jie
Gao
ac,
Hong-Bo
Ru
c,
Xiangfeng
Luan
 b,
Jiacheng
Zhang
b,
Jiacheng
Zhang
 b,
Ling
Zhu
ac,
Adam C.
Sedgwick
b,
Ling
Zhu
ac,
Adam C.
Sedgwick
 d,
Guo-Rong
Chen
a,
Yi
Zang
c,
Tony D.
James
d,
Guo-Rong
Chen
a,
Yi
Zang
c,
Tony D.
James
 ef,
He
Tian
ef,
He
Tian
 a,
Jia
Li
*c,
Yiyong
Mai
a,
Jia
Li
*c,
Yiyong
Mai
 *b and
Xiao-Peng
He
*b and
Xiao-Peng
He
 *a
*a
aKey Laboratory for Advanced Materials and Joint International Research Laboratory of Precision Chemistry and Molecular Engineering, Feringa Nobel Prize Scientist Joint Research Center, School of Chemistry and Molecular Engineering, Frontiers Center for Materiobiology and Dynamic Chemistry, East China University of Science and Technology, 130 Meilong RD, Shanghai 200237, P. R. China. E-mail: xphe@ecust.edu.cn
bSchool of Chemistry and Chemical Engineering, Frontiers Science Center for Transformative Molecules, Shanghai Key Laboratory of Electrical Insulation and Thermal Ageing, Shanghai Jiao Tong University, 800 Dongchuan RD, Shanghai 200240, P. R. China. E-mail: mai@sjtu.edu.cn
cNational Center for Drug Screening, State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 189 Guo Shoujing Rd., Shanghai 201203, P. R. China. E-mail: jli@simm.ac.cn
dDepartment of Chemistry, The University of Texas at Austin, Austin, Texas 78712-1224, USA
eDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK
fSchool of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, China
First published on 15th July 2021
Abstract
Triple negative breast cancer (TNBC) is one of the most malignant subtypes of breast cancer. Here, we report the construction of graphene nanoribbon (GNR)-based supramolecular ensembles with dual-receptor (mannose and αvβ3 integrin receptors) targeting function, denoted as GNR-Man/PRGD, for targeted photothermal treatment (PTT) of TNBC. The GNR-Man/PRGD ensembles were constructed through the solution-based self-assembly of mannose-grafted GNRs (GNR-Man) with a pyrene-tagged αvβ3 integrin ligand (PRGD). Enhanced PTT efficacies were achieved both in vitro and in vivo compared to that of the non-targeting equivalents. Tumor-bearing live mice were administered (tail vein) with GNR-Man/PRGD and then each mice group was subjected to PTT. Remarkably, GNR-Man/PRGD induced complete ablation of the solid tumors, and no tumor regrowth was observed over a period of 15 days. This study demonstrates a new and promising platform for the development of photothermal nanomaterials for targeted tumor therapy.
Introduction
Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer associated with poor patient prognosis. This is due to its high tumor heterogeneity, rapid proliferation and early metastasis.1,2 TNBC lacks the expression of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2),3,4 which eliminates the possibility of using targeted therapies. This necessitates the use of traditional chemotherapy, radiotherapy, and surgical intervention.5–8 Therefore, new and effective therapeutic strategies are needed to overcome the limitations/challenges (non-invasive and non-toxic) for current treatments of TNBC.9,10In recent years, photothermal therapy (PTT) has emerged as an attractive light-based therapeutic approach for the treatment of diseases.11–15 In brief, PTT involves the light irradiation of an optical absorber (PTT agent) that is localised at the diseased location. This results in the conversion of light energy to heat, which then induces cell death.16–18 In comparison to traditional therapeutics, the use of light affords a non-invasive strategy with high specificity.19–23 To date, several platforms have been developed for PTT and were demonstrated in a range of disease models.24–28 Graphene nanoribbons (GNRs) have displayed significant photothermal conversion efficiencies (PCE) for potential use as PTT.29–36 However, targeted biocompatible platforms are needed for in vivo applications to limit off-target toxicities and to improve therapeutic outcomes.
Throughout nature, glycoconjugates are known to selectively bind to carbohydrate receptors and modulate a variety of physiological and pathological processes. Such examples include cell–cell recognition,37 signal transmission,38 virus invasion39 and bacterial infection.40 Previous studies have reported the overexpression of mannose receptors (MRs) on the cell surface of tumor-associated macrophages (TAMs). TAMs have been associated with the regulation of immunity, cancer metastasis and progression of ovarian and breast cancer.41 In recent years, researchers have exploited the high affinity of mannose for MRs to develop targeted imaging and therapeutic agents.42,43 These efforts inspired our group to develop glycoconjugate-based materials (abbreviated as glycomaterials) for targeted PTT of TNBC both in vitro and in vivo.
Results and discussion
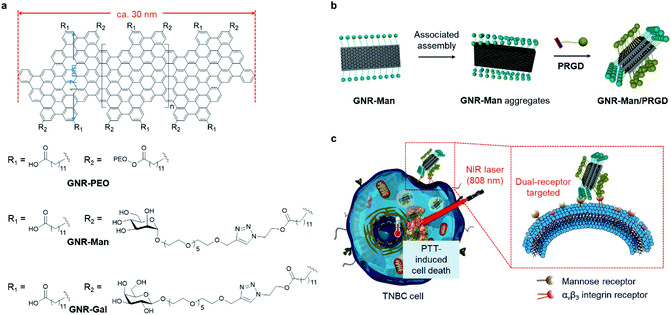
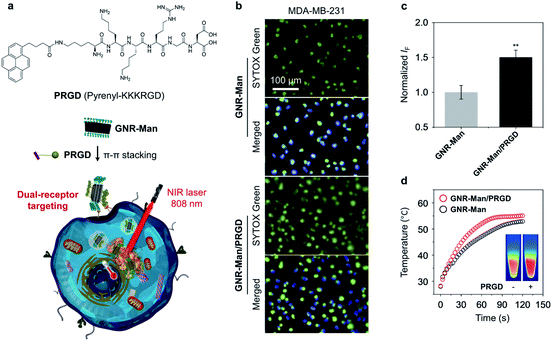
Here, we report the construction of two glycomaterials (GNR-Man and GNR-Gal), utilizing our previously reported carboxyl-functionalized GNR (GNR-COOH) precursor (Scheme 1a and S2†).44,45GNR-Man and GNR-Gal were synthesized by grafting mannose (grafting percentage 72%) and galactose (grafting percentage 59%) at the edge of GNRs for use as receptor-targeting agents, respectively. Both GNR-Man and GNR-Gal glycomaterials achieved targeted PTT of TNBC (MDA-MB-231) and hepatoma (Hep-G2) cell lines, which overexpress MRs and asialoglycoprotein receptors (ASGPr – galactose selective), respectively (Scheme 1c). We then explored, the supramolecular self-assembly between GNR-Man and a pyrene-tagged peptide ligand, PRGD (pyrene-KKKRGD, a well-known αvβ3 integrin-targeting ligand),46 to generate GNR-based supramolecular ensembles with dual-receptor targeting function (denoted as GNR-Man/PRGD) (Scheme 1b), which were employed for the precision-enhanced PTT of MDA-MB-231 (a TNBC cell line)-xenograft mice (tail-vein injection).To the best of our knowledge, this study represents the first example of structurally defined GNRs for targeted tumor therapy. Previously, GNRs have been shown to have a superior photothermal-conversion efficiency exceeding those of many photothermal materials commonly used for PTT.29,30 However, these systems were not designed for biological application. In this work, to avoid off-target toxicity and realize tumor specificity, we have aimed to modify structurally defined GNRs with both carbohydrate and peptide ligands to produce dual-targeting GNR-Man/PRGD supramolecular ensembles for the PTT treatment of tumors. Using GNR-Man/PRGD as an in vivo PTT agent, complete ablation of TNBC-xenograft solid tumors was achieved, with no tumor regrowth over a period of 15 days. In addition, immunohistochemical assays suggested good biocompatibility of the GNR-Man/PRGD ensembles.
Carboxyl-functionalized GNRs (GNR-COOH) and poly(ethylene oxide) (PEO)-functionalized (molecular weight = 2000 g mol−1) GNR (GNR-PEO, grafting percentage 42%) were synthesized according to previously reported protocols;44,45GNR-PEO was used as a control sample. GNRs functionalized with azido units (GNR-N3) were synthesized through the esterification of GNR-COOH with 2-azidoethanol. Subsequently, the Cu(I)-catalyzed azide–alkyne “click reaction” between GNR-N3 and O-alkynyl-galactoside/O-alkynyl-mannoside afforded GNR-Gal and GNR-Man, respectively (Schemes S1 and S2†). All functionalized GNRs were characterized by Fourier transform infrared (FTIR) spectroscopy (Fig. S2†), and the grafting degrees of GNR-Gal and GNR-Man were determined using thermal gravimetric analysis (TGA) (Fig. S1†). Based on the weight loss at 600 °C in the TGA curves, the grafting percentages of GNR-Gal and GNR-Man were estimated to be 59% and 72%, respectively.
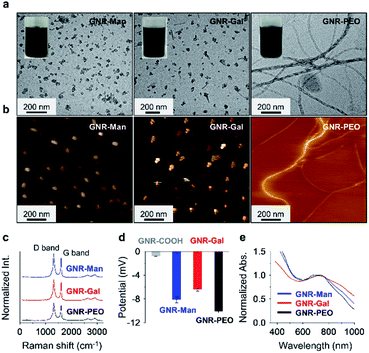
The sonication of GNR-Gal, GNR-Man or GNR-PEO in a phosphate buffer saline (PBS) solution (0.01 M, pH 7.40, 0.5% Triton X-100 (TX-100), v/v) afforded their stable homogeneous dispersions without visible precipitation (Fig. 1a). Transmission electron microscopy (TEM) and atomic force microscopic (AFM) images indicated that GNR-Man and GNR-Gal formed ensembles with an average diameter of ca. 35 nm and 40 nm, respectively (Fig. 1a and b). In contrast, GNR-PEO formed spring-like nanowires with an average diameter of 20 nm and a length up to micrometer scale (Fig. 1a and b).29 The hydrodynamic diameters of GNR-Man and GNR-Gal were determined to be 61 nm and 86 nm by dynamic light scattering (DLS), respectively (Fig. S3†). The larger particle diameters determined by DLS than those by TEM are attributed to stretching of the alkyl and oligo(ethylene oxide) chains in liquid phase. Typical Raman signals corresponding to the D band (disordered band) at 1325 cm−1 and the G band (graphite band) at 1603 cm−1 were consistently observed for GNR-PEO, GNR-Gal and GNR-Man.47 This suggests that the introduction of the glycoligands did not affect the conjugated GNR backbone (Fig. 1c).30 In addition, the modification with hydrophilic glycosyl and PEO groups significantly improved water solubility of the GNRs with respect to GNR-COOH, which is supported by zeta potential analysis (Fig. 1d). The good aqueous dispersibility of the GNRs is reflected in a broad-band UV-vis-NIR adsorption ranging from 400 to 1000 nm (Fig. 1e).
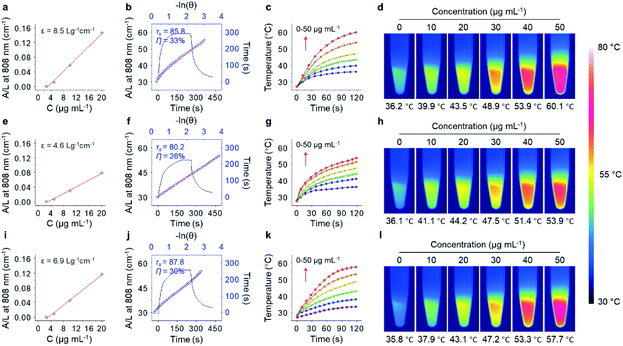
With the excellent water dispersibility and inherent near-infrared (NIR) absorption characteristics (Fig. S4†), the mass extinction coefficient and PCE of the three GNRs under NIR light irradiation (808 nm, 1.0 W cm−2) was determined. The extinction coefficient of GNR-Man (Fig. 2a), GNR-Gal (Fig. 2e) and GNR-PEO (Fig. 2i) at 808 nm was measured to be 8.5 L g−1 cm−1, 4.6 L g−1 cm−1 and 6.9 L g−1 cm−1, respectively. The PCE of GNR-Man (Fig. 2b), GNR-Gal (Fig. 2f) and GNR-PEO (Fig. 2j) at 808 nm was determined to be 33%, 26% and 30%, respectively.48 The photothermal performance of the GNRs in PBS at increasing concentrations (0, 10, 20, 30, 40 and 50 μg mL−1) were evaluated (808 nm, 1.0 W cm−2, 2 min), which demonstrated a concentration-dependent photothermal performance (Fig. 2c, g and k for GNR-Man, GNR-Gal and GNR-PEO, respectively). Photothermal imaging indicated that the highest temperature reached was 60.1 °C for a GNR-Man containing solution (50 μg mL−1) (Fig. 2d). While, the highest temperature reached was 53.9 °C and 57.7 °C for GNR-Gal (Fig. 2h) and GNR-PEO (Fig. 2i), respectively. No obvious change in the photothermal performance was observed for GNR-Man after ten cycles (Fig. S5†). The above results confirmed the potential of the GNRs as PTT agents.
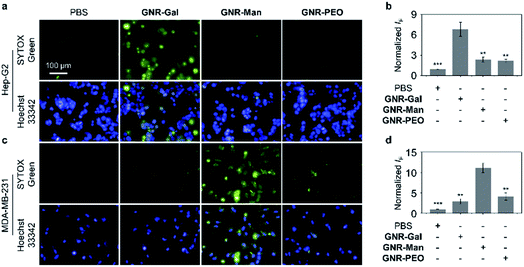
The targeting ability of the glycosylated GNRs were subsequently evaluated using a cellular assay. Hep-G2 (human hepatoma) and MDA-MB-231 (TNBC) cell lines were used as they overexpress ASGPrs (galactose-selective receptor) and MRs (mannose-selective receptor), respectively. The corresponding receptor expression was confirmed by real-time quantitative polymerase chain reaction (Fig. S6†). Cell viability study without light irradiation was performed for each GNR sample, in which minimal cytotoxicity for materials ranging from 0 to 40 μg mL−1 was observed. (Fig. S7†). To illustrate the PTT efficacy, a concentration of 40 μg mL−1 was used for cellular experiments. The targeting ability of each GNR after light irradiation (808 nm, 1.0 W cm−2, 3 min) was evaluated by quantifying the number of dead cells using fluorescence imaging with SYTOX Green, as shown in Fig. 3, GNR-PEO displayed a minimal phototoxicity for each cell line. In contrast, treatment of the appropriate cell lines with GNR-Gal and GNR-Man (GNR-Gal – Hep-G2; GNR-Man – MDA-MB-231) followed by light irradiation led to a 3.1-fold and 2.7-fold increase in dead cells, respectively, in comparison to that of GNR-PEO (Fig. 3a–d). Minimal dead cells were observed for GNR-Gal treated MDA-MB-231 cells and GNR-Man treated Hep-G2 cells after light irradiation. These results illustrate the potential cellular targeting ability of the glycosylated GNRs.
The photothermal effect for both GNR-Gal and GNR-Man was dependent on the GNR concentration (Fig. S8†) and the laser power (Fig. S9†). The receptor-dependent targeting for the GNR-based glycomaterials was demonstrated via a competition assay. The pre-incubation of free galactose with Hep-G2 and the pre-incubation of free mannose with MDA-MB-231 reduced the photothermal therapeutic effect (decreased quantity of dead cells) of GNR-Gal and GNR-Man in a concentration-dependent manner, respectively (Fig. S10†). This further suggests that the targeted PTT effect observed is dependent on the glycoligand-receptor recognition. These preliminary results illustrate the success of our glycosylation strategy for enhancing the selective internalization of GNRs in a target cancer cell line. Given our focus was on developing a therapeutic platform for TNBC, GNR-Man was the main focus for the remainder of this study.
To further enhance the targeting capability of the GNRs, we incorporated a peptide ligand onto GNR-Man (pyrenyl-KKKRGD, denoted as PRGD). This ligand is selective for integrin αvβ3 receptors, which are expressed on the surface of a range of cancer cells including breast cancer.49 This chosen peptide ligand was modified with a pyrene moiety to enable its association with the GNRs through π–π stacking; the chemical structure of the pyrene-functionalized peptide PRGD is shown in Fig. 4a. The supramolecular assembly between GNR-Man and PRGD was achieved through their mixing in aqueous solution, followed by sonication for 15 min. The resultant mannose/PRGD functionalized supramolecular assemblies (GNR-Man/PRGD) were designed to target both MR and αvβ3 receptors on the surface of TNBC cells (Fig. 4a).
The supramolecular ensemble GNR-Man/PRGD was characterised by TEM, DLS and Raman spectroscopy. Particle-like structures were observed for GNR-Man/PRGD (Fig. S11a†), which are morphologically similar to that of GNR-Man. However, an obvious increase in the sizes of the particles was observed in comparison to that of the GNR-Man counterpart (Fig. S11b†). In the Raman spectra, the ID/IG ratio of GNR-Man (1.2) increased to 1.3 after self-assembly with PRGD, supporting the association of the GNR with the peptide ligand (Fig. S12†).50 The PCE of GNR-Man/PRGD did not change compared to that of GNR-Man only (Fig. 4d). DLS analysis revealed smaller PDI values for GNR-Man/PRGD and GNR-Gal/PRGD when compared to GNR-Man and GNR-Gal (Fig. S13a†), which is indicative of an enhanced aqueous dispersibility. The absolute zeta potential values for GNR-Man/PRGD and GNR-Gal/PRGD were larger than that of GNR-Man and GNR-Gal (Fig. S13b†), which indicates an improved aqueous stability. Additional DLS analysis showed no changes to the hydrodynamic diameter of GNR-Man/PRGD and GNR-Gal/PRGD over the course of 7 days (Figs. S13c and d†). In contrast, the hydrodynamic diameters of GNR-Man and GNR-Gal were shown to increase gradually over time, suggesting their slow aggregation. Overall, this data suggests an improved aqueous stability and solubility for GNRs/PRGD assemblies.
With these ideal solution-based properties observed for GNR-Man/PRGD, we subsequently evaluated its PTT performance against MDA-MB-231 cells. Indeed, more dead cells were observed (determined via SYTOX green staining) for GNR-Man/PRGD treatment when compared to cells treated with GNR-Man only (Fig. 4b and c). These selective properties of GNR-Man/PRGD for MDA-MB-321 cells was further confirmed through its evaluation with additional cancer cell lines. These include Hela cells (human cervical cell line),51 MCF-7 cells (human breast cancer cell line)52 and Hep-G2 cells (human hepatoma cell line),53 all of which are reported to have a lower expression of the integrin αvβ3 receptor. Both GNR-Man/PRGD and GNR-Man were shown to have minimal phototoxicities for these chosen cell lines, which further supports the good selectivity of GNR-Man/PRGD and GNR-Man for MDA-MB-321 cells (Fig. S14†). While, no dark cytotoxicity was observed for GNR-Man/PRGD (Fig. S15†).
To illustrate the importance of the RGD targeting unit, PTT experiments were performed using GNR-PEO/PRGD and GNR-PEO. These GNRs lacked the mannose functionality, which enabled the targeting ability of the RGD unit to be solely evaluated. The light irradiation of MDA-MB-321 cells using GNR-PEO/PRGD led to the death of more cells when compared to the use of GNR-PEO. The light irradiation of HeLa cells using GNR-PEO/PRGD and GNR-PEO led to no differences in the number of dead cells being observed (Fig. S16†). This observation supports the targeting ability of PRGD on GNRs for MDA-MB-231 cells. No cytotoxicity was observed for the use of PRGD only (Fig. S16c†).
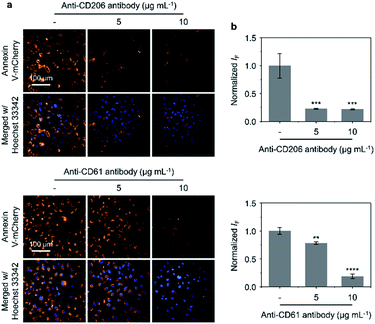
Antibody blockade assays were subsequently carried out to evaluate the importance of the targeting units on GNR-Man/PRGD (mannose and PRGD) for their corresponding receptors (CD206 and CD61) on MDA-MB-231 cells. Annexin V-mCherry was used to visualise apoptotic cells.54 As shown in Fig. 5a and b, blocking receptors CD206 and CD61 with their corresponding antibodies (MRC1 and ITGB3) resulted in the reduction of the number of apoptotic cells being observed (after PTT treatment with GNR-Man/PRGD). This antibody-mediated reduction was shown in a concentration-dependent manner, which provides support that both MR and αvβ3 receptors are targets for GNR-Man/PRGD. Moreover, increasing the PRGD concentrations on GNR-Man led to an increased number of apoptotic cells being observed after PTT. Finally, we noticed fewer apoptotic cells being produced when cells were incubated at 4 °C with GNR-Man/PRGD prior to light irradiation (Fig. S18†). This gives evidence that the cell internalization of GNR-Man/PRGD is kinetically controlled via a receptor-mediated endocytic process.55
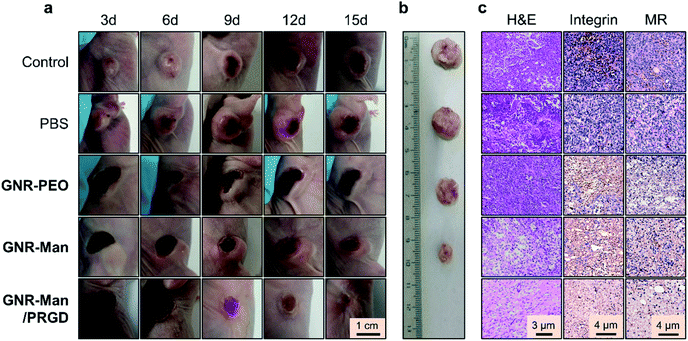
With the excellent dual-receptor targeting ability of GNR-Man/PRGD confirmed, the PTT performance of the GNR-based agents were evaluated using tumor bearing MDA-MB-231 subcutaneous xenografts of live mice. The tumor-bearing mice (n = 3) were systematically administrated with PBS (0.01 M, pH 7.40), GNR-PEO (0.5 mg mL−1), GNR-Man (0.5 mg mL−1) and GNR-Man/PRGD (0.5 mg mL−1/0.25 mM) using tail-vein injection, respectively. After 24 hours post-injection, tumors were irradiated (808 nm, 1.5 W cm−2) for 5 min. The corresponding photothermal images were taken (Fig. 6a and S19†), and the time-dependent local temperature increase was recorded (Fig. 6b). In addition, the tumor volume (Fig. 6c) and body weight (Fig. 6d) of the mice were measured every 3 days within 15 days. The administration of each GNR did not affect the body weight of the mice (Fig. 6d). Interestingly, minimal temperature increases (42.1 °C) were observed for the control groups, PBS and GNR-PEO. In contrast, the local temperature at the tumor after 2 min irradiation reached 54.4 °C and 67.2 °C for GNR-Man and GNR-Man/PRGD, respectively. The greater local temperature at the tumor site for the GNR-Man/PRGD-treated mice suggested an enhanced accumulation of the GNRs in the tumor. Gratifyingly, for the GNR-Man/PRGD treatment group the volume of the tumor decreased to zero in three days, and the tumor did not regrow even after 15 days. In contrast, GNR-Man resulted in reduced shrinkage of tumor volume and regrowth of the tumor was observed after 6 days (Fig. 7a). It is important to note, that GNR-Man displayed a statistically significant therapeutic effect against the non-targeted GNR-PEO. This highlights the importance of mannose as a targeting unit.
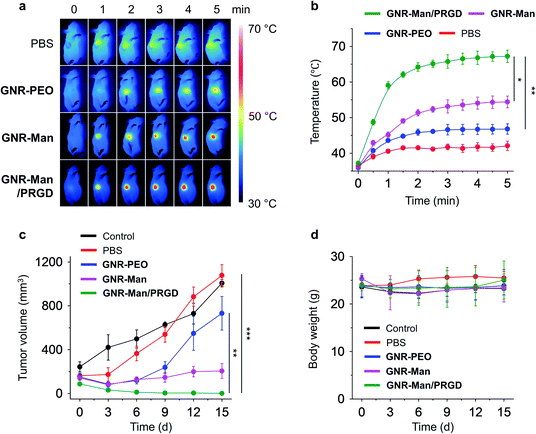 | ||
| Fig. 6 (a) Photothermal images and (b) time-dependent temperature increase at the tumor site of tumor bearing live mice (MDA-MB-231-xenograft) under 808 nm light irradiation (1.5 W cm−2, 5 min) (n = 3; for original images of all three groups, see Fig. S13†). Light irradiation was applied to the tumor site of all mice 24 h after a systemic administration of PBS (0.01 M, pH 7.40), GNR-PEO (0.5 mg mL−1), GNR-Man (0.5 mg mL−1) and GNR-Man/PRGD (0.5 mg mL−1/0.25 mM) through tail-vein injection into mice (n = 3); the injection volume was 5 mg kg−1. (c) Relative tumor volume and (d) body weight of living mice bearing MDA-MB-231-xenografts without (control) and with PTT in different groups for 15 days. *P < 0.05, **P < 0.01, ***P < 0.001. S. D. means standard deviation (n = 3). | ||
To confirm the significant therapeutic efficacy of GNR-Man/PRGD, we removed the tumors from the mice after 15 days. No solid tumor could be isolated from the mice treated using GNR-Man/PRGD due to the disappearance of the tumor, whereas tumors of different sizes were obtained from the mice treated with GNR-PEO or GNR-Man (Fig. 7b). Hematoxylin-eosin (H&E) staining was performed on each isolated tumor and major organs. Typical TNBC cells were seen in the control, PBS, GNR-PEO and GNR-Man groups. In stark contrast, no TNBC cells were observed in the slice of the tissue removed from the tumor region of the mice treated with GNR-Man/PRGD on the fifteenth day (Fig. 7c). Subsequent immunochemical staining corroborated the absence of both integrin and MR receptors for GNR-Man/PRGD treated tissue slice group compared to those treated from the other groups (Fig. 7c). In addition, no obvious morphological changes were observed for the stained organ slices (Fig. S20†), suggesting the biocompatibility of GNR-Man/PRGD. However, before further development extensive pharmacological evaluations are required to determine the full biocompatibility of the GNRs for live subjects.
Conclusions
In summary, we have synthesized GNR-based glycomaterials, GNR-Gal and GNR-Man, by covalently grafting glycoligands to structurally defined GNRs. GNR-Man/PRGD supramolecular ensembles with dual-receptor targeting function were further developed using the supramolecular self-assembly between GNR-Man and a pyrene-tagged RGD ligand (PRGD). The successful targeting of GNR-Man/PRGD towards both MR and αvβ3 integrin receptors resulted in a highly effective PTT for TNBC in vitro and in vivo, evidenced through the nearly complete tumor ablation and no tumor regrowth being observed 15 days after PTT. These results clearly demonstrate the potential of dual-receptor targeting for effective cancer therapy. In addition, this study illustrates the potential application of structurally well-defined GNRs as photothermal agents for tumor therapy.Ethical statement
All animal experiments were conducted under the principles of the Regulations of Experimental Animal Administration issued by the State Committee of Science and Technology of the People's Republic of China and followed the guidelines of the Animal Care and Use Committee of Shanghai Institute of Materia Medica, Chinese Academy of Sciences.Data availability
All experimental data, procedures for data analysis and pertinent data including NMR of compounds used are provided in the ESI.Author contributions
W.-T. D. and F. X. contributed equally to this research project. J. L., Y. M. and X.-P. H. conceived the project. W.-T. D., F. X., C. X. X., J. G., H. B. R., L. Z., X. L. and J. Z. performed the experiments. G.-R. C., Y. Z., J. L., Y. M., X.-P. H. and H. T. supervised the research. W.-T. D., F. X., A. C. S., T. D. J., Y. M. and X.-P. H. wrote, and revised the paper. All the authors discussed the results and contributed to the preparation of the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the National Natural Science Foundation of China (No. 21788102, 91853201, 21774076 and 21776078), The National Key Sci-Tech Special Projects of Infection Diseases of China (2018ZX10732202), the Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX03), the International Cooperation Program of Shanghai Science and Technology Committee (No. 17520750100), the Program of Shanghai Academic Research Leader (19XD1421700), the Fundamental Research Funds for the Central Universities (222201717003), National Postdoctoral Program for Innovative Talents (BX20190115), Shanghai Post-doctoral Excellence Program (2019044) and China Postdoctoral Science Foundation (2020M681206) for financial support. The Instrumental Analysis Center of Shanghai Jiao Tong University is also acknowledged for some measurements. TDJ wishes to thank the Royal Society for a Wolfson Research Merit Award and the Open Research Fund of the School of Chemistry and Chemical Engineering, Henan Normal University for support (2020ZD01).Notes and references
- R. Dent, M. Trudeau, K. I. Pritchard, W. M. Hanna, H. K. Kahn, C. A. Sawka, L. A. Lickley, E. Rawlinson, P. Sun and S. A. Narod, Clin. Cancer Res., 2007, 13, 4429–4434 CrossRef PubMed.
- S. P. Shah, A. Roth, R. Goya, A. Oloumi, G. Ha, Y. Zhao, G. Turashvili, J. Ding, K. Tse, G. Haffari, A. Bashashati, L. M. Prentice, J. Khattra, A. Burleigh, D. Yap, V. Bernard, A. McPherson, K. Shumansky, A. Crisan, R. Giuliany, A. Heravi-Moussavi, J. Rosner, D. Lai, I. Birol, R. Varhol, A. Tam, N. Dhalla, T. Zeng, K. Ma, S. K. Chan, M. Griffith, A. Moradian, S.-W. G. Cheng, G. B. Morin, P. Watson, K. Gelmon, S. Chia, S. Chin, C. Curtis, O. M. Rueda, P. D. Pharoah, S. Damaraju, J. Mackey, K. Hoon, T. Harkins, V. Tadigotla, M. Sigaroudinia, P. Gascard, T. Tlsty, J. F. Costello, I. M. Meyer, C. J. Eaves, W. W. Wasserman, S. Jones, D. Huntsman, M. Hirst, C. Caldas, M. A. Marra and S. Aparicio, Nature, 2012, 486, 395–399 CrossRef CAS PubMed.
- L. A. Carey, E. C. Dees, L. Sawyer, L. Gatti, D. T. Moore, F. Collichio, D. W. Ollila, C. I. Sartor, M. L. Graham and C. M. Perou, Clin. Cancer Res., 2007, 13, 2329–2334 CrossRef CAS PubMed.
- B. D. Lehmann, J. A. Bauer, X. Chen, M. E. Sanders, A. B. Chakravarthy, Y. Shyr and J. A. Pietenpol, J. Clin. Invest., 2011, 121, 2750–2767 CrossRef CAS PubMed.
- W. D. Foulkes, I. E. Smith and J. S. Reis-Filho, N. Engl. J. Med., 2010, 363, 1938–1948 CrossRef CAS PubMed.
- C. Belli, B. A. Duso, E. Ferraro and G. Curigliano, Breast, 2019, 45, 15–21 CrossRef PubMed.
- C. Denkert, C. Liedtke, A. Tutt and G. von Minckwitz, Lancet, 2017, 389, 2430–2442 CrossRef CAS.
- E. L. Mayer and H. J. Burstein, J. Clin. Oncol., 2016, 34, 3369–3371 CrossRef CAS PubMed.
- J.-R. Jhan and E. R. Andrechek, Pharmacogenomics, 2017, 18, 1595–1609 CrossRef CAS PubMed.
- J. Xu, Y. Liu, Y. Li, H. Wang, S. Stewart, K. V. d. Jeught, P. Agarwal, Y. Zhang, S. Liu, G. Zhao, J. Wan, X. Lu and X. He, Nat. Nanotechnol., 2019, 14, 388–397 CrossRef CAS PubMed.
- H. S. Jung, P. Verwilst, A. Sharma, J. Shin, J. L. Sessler and J. S. Kim, Chem. Soc. Rev., 2018, 47, 2280–2297 RSC.
- C. Chen, Z. Wu, P. Ding, N. Sun, H. Liu, Y. Chen, Z. Wang and R. Pei, Adv. Fiber Mater., 2020, 2, 186–193 CrossRef.
- J. Zhao and W. Cui, Adv. Fiber Mater., 2020, 2, 229–245 CrossRef.
- B. Guo, Z. Sheng, D. Hu, C. Liu, H. Zheng and B. Liu, Adv. Mater., 2018, 30, 1802591 CrossRef PubMed.
- J. Li and K. Pu, Chem. Soc. Rev., 2019, 48, 38–71 RSC.
- Y. Wang, N. Gong, Y. Li, Q. Lu, X. Wang and J. Li, J. Am. Chem. Soc., 2020, 142, 1735–1739 CrossRef CAS PubMed.
- W. Sang, Z. Zhang, Y. Dai and X. Chen, Chem. Soc. Rev., 2019, 48, 3771–3810 RSC.
- G. Baffou, F. Cichos and R. Quidant, Nat. Mater., 2020, 19, 946–958 CrossRef CAS PubMed.
- C. Zhang and K. Pu, Chem. Soc. Rev., 2020, 49, 4234–4253 RSC.
- L. Cheng, C. Wang, L. Feng, K. Yang and Z. Liu, Chem. Rev., 2014, 114, 10869–10939 CrossRef CAS PubMed.
- Y. Liu, K. Ai, J. Liu, M. Deng, Y. He and L. Lu, Adv. Mater., 2013, 25, 1353–1359 CrossRef CAS PubMed.
- Z. Sun, H. Xie, S. Tang, X. F. Yu, Z. Guo, J. Shao, H. Zhang, H. Huang, H. Wang and P. K. Chu, Angew. Chem., Int. Ed., 2015, 54, 11526–11530 CrossRef CAS PubMed.
- Y. Liu, P. Bhattarai, Z. Dai and X. Chen, Chem. Soc. Rev., 2019, 48, 2053–2108 RSC.
- H. S. Jung, P. Verwilst, A. Sharma, J. Shin, J. L. Sessler and J. S. Kim, Chem. Soc. Rev., 2018, 47, 2280–2297 RSC.
- Z. Mi, P. Yang, R. Wang, J. Unruangsri, W. Yang, C. Wang and J. Guo, J. Am. Chem. Soc., 2019, 141, 14433–14442 CrossRef CAS PubMed.
- X. Zhen, J. Zhang, J. Huang, C. Xie, Q. Miao and K. Pu, Angew. Chem., Int. Ed., 2018, 57, 7804–7808 CrossRef CAS PubMed.
- X. Zhang, J. Du, Z. Guo, J. Yu, Q. Gao, W. Yin, S. Zhu, Z. Gu and Y. Zhao, Adv. Sci., 2019, 6, 1801122 CrossRef PubMed.
- H. Ji, K. Dong, Z. Yan, C. Ding, Z. Chen, J. Ren and X. Qu, Small, 2016, 12, 6200–6206 CrossRef CAS PubMed.
- Y. Huang, W.-T. Dou, F. Xu, H.-B. Ru, Q. Gong, D. Wu, D. Yan, H. Tian, X.-P. He, Y. Mai and X. Feng, Angew. Chem., Int. Ed., 2018, 57, 3366–3371 CrossRef CAS PubMed.
- Z.-H. Yu, X. Li, F. Xu, X.-L. Hu, J. Yan, N. Kwon, G.-R. Chen, T. Tang, X. Dong, Y. Mai, D. Chen, J. Yoon, X.-P. He and H. Tian, Angew. Chem., Int. Ed., 2020, 59, 3658–3664 CrossRef CAS PubMed.
- A. Narita, X.-Y. Wang, X. Feng and K. Müllen, Chem. Soc. Rev., 2015, 44, 6616–6643 RSC.
- S. Li, Y. Chen, H. Liu, Y. Wang, L. Liu, F. Lv, Y. Li and S. Wang, Chem. Mater., 2017, 29, 6087–6094 CrossRef CAS.
- R. Kurapati, K. Kostarelos, M. Prato and A. Bianco, Adv. Mater., 2016, 28, 6052–6074 CrossRef CAS PubMed.
- K. Yang, L. Feng and Z. Liu, Adv. Drug Delivery Rev., 2016, 105, 228–241 CrossRef CAS PubMed.
- J. T. Robinson, S. M. Tabakman, Y. Liang, H. Wang, H. S. Casalongue, D. Vinh and H. J. Dai, J. Am. Chem. Soc., 2011, 133, 6825–6831 CrossRef CAS PubMed.
- G. Reina, J. M. González-Domínguez, A. Criado, E. Vázquez and A. Bianco, Chem. Soc. Rev., 2017, 46, 4400–4416 RSC.
- N. C. Reichardt, M. Martín-lomas and S. Penadés, Chem. Soc. Rev., 2013, 42, 4358–4376 RSC.
- L. L. Kiessling and J. C. Grim, Chem. Soc. Rev., 2013, 42, 4476–4491 RSC.
- W.-T. Dou, Z.-Y. Qin, J. Li, D.-M. Zhou and X.-P. He, Sci. Bull., 2019, 64, 1902–1909 CrossRef CAS.
- C. R. Bertozzi and L. L. Kiessling, Science, 2001, 291, 2357–2364 CrossRef CAS PubMed.
- B. Thomas, K.-C. Yan, X.-L. Hu, M. Donnier-Maréchal, G.-R. Chen, X.-P. He and S. Vidal, Chem. Soc. Rev., 2020, 49, 593–641 RSC.
- J. M. Irache, H. H. Salman, C. Gamazo and S. Espuelas, Expet Opin. Drug Deliv., 2008, 5, 703–724 CrossRef CAS PubMed.
- C. Jiang, H. Cai, X. Peng, P. Zhang, X. Wu and R. Tian, Mol. Imag., 2017, 16, 1–10 CrossRef CAS PubMed.
- Y. Huang, Y. Mai, U. Beser, J. Teyssandier, G. Velpula, H. Gorp, L. A. Straasø, M. R. Hansen, D. Rizzo, C. Casiraghi, R. Yang, G. Zhang, D. Wu, F. Zhang, D. Yan, S. D. Feyter, K. Müllen and X. Feng, J. Am. Chem. Soc., 2016, 138, 10136–10139 CrossRef CAS PubMed.
- F. Xu, C. Yu, A. Tries, H. Zhang, M. Kläui, K. Basse, M. R. Hansen, N. Bilbao, M. Bonn, H. I. Wang and Y. Mai, J. Am. Chem. Soc., 2019, 141, 10972–10977 CrossRef CAS PubMed.
- X. Han, K. Cheng, Y. Xu, Y. Wang, H. Min, Y. Zhang, X. Zhao, R. Zhao, G. J. Anderson, L. Ren, G. Nie and Y. Li, J. Am. Chem. Soc., 2020, 142, 2490–2496 CrossRef CAS PubMed.
- A. Narita, X. Feng, Y. Hernandez, S. A. Jensen, M. Bonn, H. Yang, I. A. Verzhbitskiy, C. Casiraghi, M. R. Hansen, A. H. R. Koch, G. Fytas, O. Ivasenko, B. Li, K. S. Mali, T. Balandina, S. Mahesh, S. D. Feyter and K. Müllen, Nat. Chem., 2014, 6, 126–132 CrossRef CAS PubMed.
- H. Lin, S. Gao, C. Dai, Y. Chen and J. Shi, J. Am. Chem. Soc., 2017, 139, 16235–16247 CrossRef CAS PubMed.
- L. F. Kadem, K. G. Suana, M. Holz, W. Wang, H. Westerhaus, R. Herges and C. Selhuber-Unkel, Angew. Chem., Int. Ed., 2017, 56, 225–229 CrossRef CAS PubMed.
- D.-K. Ji, G.-R. Chen, X.-P. He and H. Tian, Adv. Funct. Mater., 2015, 25, 3483–3487 CrossRef CAS.
- S. Wu, F. Su, H. Y. Magee, D. R. Meldrum and Y. Tian, RSC Adv., 2019, 9, 34235–34243 RSC.
- Y. Gai, Y. Jiang, Y. Long, L. Sun, Q. Liu, C. Qin, Y. Zhang, D. Zeng and X. Lan, Mol. Pharm., 2020, 17, 349–358 CrossRef CAS PubMed.
- J. J. H. Chu and M. L. Ng, J. Biol. Chem., 2004, 279, 54533–54541 CrossRef CAS PubMed.
- M. Li, J. Xia, R. Tian, J. Wang, J. Fan, J. Du, S. Long, X. Song, J. W. Foley and X. Peng, J. Am. Chem. Soc., 2018, 140, 14851–14859 CrossRef CAS PubMed.
- M. Sousa de Almeida, E. Susnik, B. Drasler, P. Taladriz-Blanco, A. Petri-Fink and B. Rothen-Rutishauser, Chem. Soc. Rev., 2021, 50, 5397–5434 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1sc02154k |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2021 |