Open Access Article
Open Access ArticleNovel dual methylation of cytidines in the RNA of mammals†
Ming-Yu
Cheng
a,
Xue-Jiao
You
a,
Jiang-Hui
Ding
a,
Yi
Dai
a,
Meng-Yuan
Chen
a,
Bi-Feng
Yuan
 *ab and
Yu-Qi
Feng
*ab and
Yu-Qi
Feng
 ab
ab
aSauvage Center for Molecular Sciences, Department of Chemistry, Wuhan University, Wuhan 430072, China. E-mail: bfyuan@whu.edu.cn
bSchool of Health Sciences, Wuhan University, Wuhan 430071, China
First published on 7th May 2021
Abstract
RNA modifications play critical roles in regulating a variety of physiological processes. Methylation is the most prevalent modification occurring in RNA. Three isomeric cytidine methylation modifications have been reported in RNA, including 3-methylcytidine (m3C), N4-methylcytidine (m4C), and 5-methylcytidine (m5C), in mammals. Aside from the single methylation on the nucleobase of cytidines, dual methylation modifications occurring in both the 2′ hydroxyl of ribose and the nucleobase of cytidines also have been reported, including N4,2′-O-dimethylcytidine (m4Cm) and 5,2′-O-dimethylcytidine (m5Cm). m4Cm has been found in the 16S rRNA of E. coli, while m5Cm has been found in the tRNA of terminal thermophilic archaea and mammals. However, unlike m4Cm and m5Cm, the presumed dual methylation of 3,2′-O-dimethylcytidine (m3Cm) has never been discovered in living organisms. Thus, the presence of m3Cm in RNA remains an open question. In the current study, we synthesized m3Cm and established a liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) method to determine the dimethylation of cytidines, m3Cm, m4Cm and m5Cm. Under optimized analytical conditions, m3Cm, m4Cm and m5Cm can be clearly distinguished. Using the method, we discovered the existence of m3Cm in the RNA of mammals. The identified m3Cm is a novel modification that hasn't been reported in the three-domain system, including archaea, bacteria, and eukaryotes. We confirmed that m3Cm mainly existed in the small RNA (<200 nt) of mammals. In addition, we identified, for the first time, the presence of m4Cm in the 18S rRNA of mammalian cells. The stable isotope tracing monitored by mass spectrometry demonstrated that S-adenosyl-L-methionine was a methyl donor for all three dimethylations of cytidines in RNA. The discovery of m3Cm broadens the diversity of RNA modifications in living organisms. In addition, the discovery of m3Cm and m4Cm in mammals opens new directions in understanding RNA modification-mediated RNA processing and gene expression regulation.
Introduction
DNA cytosine methylation (5-methylcytosine, 5 mC) is the most important epigenetic modification that can regulate gene expression in genomes of higher eukaryotes.1–3 Similar to the 5 mC on DNA, RNA modifications recently were also discovered to have critical roles in regulating a variety of physiological processes.4,5 Up to date, over 150 structurally distinct modified nucleosides have been identified in various RNA species in all three kingdoms of life.6Methylation is the most prevalent modification occurring in RNA.6 Three isomeric cytidine methylation modifications have been reported in RNA, including 3-methylcytidine (m3C), N4-methylcytidine (m4C), and 5-methylcytidine (m5C).6 m3C was initially discovered in yeast RNA and later on was identified to be present in both the rRNA and tRNA of eukaryotes.7 Recently, m3C has also been found in the mRNA of mammals. m3C carries positive charges and could affect the secondary structure of RNA, change protein–RNA interactions and affect translation.8,9 m4C is mainly located in the decoding center of mammalian mitochondrial 12S rRNA and is critical for mitochondrial protein synthesis and respiratory function.10–12 m5C has long been known to be present in rRNA, mRNA, tRNA and snRNA in eukaryotes.13,14 It was reported that the distribution and level of m5C in mRNA were critical for mRNA stability and protein translation.15,16 m5C in rRNA has been recognized to be involved in ribosome biogenesis17 and translational regulation.18
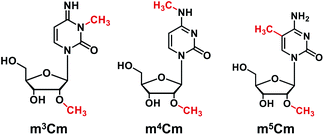
Aside from the single methylation on the nucleobase of cytidines (m3C, m4C and m5C), some dual methylation modifications occurring in both the 2′ hydroxyl of ribose and the nucleobase of cytidines also have been reported. For example, 5,2′-O-dimethylcytidine (m5Cm) has been found in the tRNA of terminal thermophilic archaea and mammalian cells.19,20N4,2′-O-Dimethylcytidine (m4Cm) has been found at position 1402 of the 16S rRNA of E. coli and participates in the fine-tuning of the local structure of the P site and the correct identification of the start codon, thus playing an important role in modulating the fidelity of decoding.21,22 However, unlike m4Cm and m5Cm, their counterpart of the dual methylation of 3,2′-O-dimethylcytidine (m3Cm) has never been discovered in living organisms in the three-domain system, including archaea, bacteria, and eukaryotes.6 Thus, the presence of m3Cm in RNA remains an open question.
RNA modifications generally have low in vivo abundance. Uncovering the presence and potential roles of dimethylated cytidines needs appropriate qualitative and quantitative methods. It was reported that the level of m5Cm in the total RNA of mammalian cells was approximately several modifications per 106 nucleosides.23 Although m4Cm was identified to be present in the 16S rRNA of E. coli, it hasn't been reported in the RNA of mammals. Moreover, m3Cm, m4Cm and m5Cm are structurally isomers and have exactly the same molecular weights, which causes the distinct determination of these dimethylated cytidines a challenging task.
Some methods have been developed for determining RNA modifications, such as two-dimensional cellulose thin layer chromatography (2D-TLC),24 liquid chromatography (LC) coupled with ultraviolet25 or mass spectrometry (MS) detection.26–29 Capillary electrophoresis with ultraviolet30 or MS detection31,32 also has been frequently employed in detecting RNA modifications. Because of the high detection sensitivity and good capability for deciphering structures, LC-MS recently has become a more widely used platform for analyzing low-abundant nucleic acid modifications.26,33,34
In the current study, we established a liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) method to determine the dimethylation of cytidines, m3Cm, m4Cm and m5Cm. Under optimized analytical conditions, m3Cm, m4Cm and m5Cm can be clearly distinguished. Using the method, we identified, for the first time, the existence of m3Cm in small RNA (<200 nt) and m4Cm in the 18S rRNA of mammalian cells. Furthermore, stable isotope tracing experiments indicated that S-adenosyl-L-methionine (SAM) was a methyl donor for all three dimethylations of cytidines in RNA.
Experimental section
Chemicals and reagents
The nucleosides and modified nucleosides, including cytidine (C), adenosine (A), uridine (U), guanosine (G), N4,2′-O-dimethylcytidine (m4Cm), 5,2′-O-dimethylcytidine (m5Cm), 2′-O-methylcytidine (Cm), and cytidine-13C5 (rC-13C5), were purchased from various commercial sources. The detailed information (CAS numbers, molecular formulas, and molecular weights) of these nucleoside standards can be found in Table S1 in the ESI.† 3,2′-O-dimethylcytidine (m3Cm) was synthesized in the current study. The chemical structures of m3Cm, m4Cm, and m5Cm are shown in Fig. 1.CIAP (calf intestinal alkaline phosphatase) and S1 nuclease were obtained from Takara Biotechnology (Dalian, China). RPMI-1640 medium, Dulbecco's Modified Eagle medium (DMEM) and fetal bovine serum were obtained from Thermo Fisher Scientific (Beijing, China). L-Methionine-(methyl-D3) (D3-Met) was purchased from Aladdin Industrial Inc. (Shanghai, China). Dimethyl sulfate (DMS) was purchased from Macklin Biochemical Technology Co., Ltd (Shanghai, China). LCMS grade methanol (MeOH) was purchased from FTSCI Co., Ltd (Wuhan, China). Analytical grade formic acid (FA), ammonium bicarbonate and ammonia hydroxide were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China).
Synthesis and characterization of 3,2′-O-dimethylcytidine
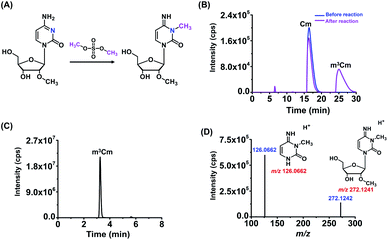
m3Cm was synthesized from 2′-O-methylcytidine (Cm) according to a previous report.35 The synthesis route is shown in Fig. 2A. Briefly, 2 mg of Cm in 800 μL of NaAc–HAc buffer (pH 4.3) reacted with 200 μL of DMS at 37 °C for 1 h. Then 1 mL of trichloromethane was added to the mixture to extract DMS. The generated m3Cm was separated and purified by HPLC. A self-made C18-T reversed-phase column (10.0 × 250 mm) was used for the separation. The mobile phases consisted of H2O (solvent A) and MeOH (solvent B). An isocratic gradient of 95% A and 5% B was utilized at a flow rate of 2.0 mL min−1. The synthesized m3Cm was characterized by high-resolution mass spectrometry and nuclear magnetic resonance (NMR) analysis.Biological samples
The human cervical carcinoma cell line (HeLa), human hepatic cell line (HL7702), human liver carcinoma cell line (HepG2) and human breast cancer cell line (MCF-7) were obtained from the China Center for Type Culture Collection. HeLa, HepG2 and MCF-7 cells were grown in DMEM medium, and HL-7702 cells were cultured in RPMI-1640 medium at 37 °C in a 5% CO2 atmosphere. The DMEM and RPMI-1640 media were supplemented with 10% fetal bovine serum, 100 U mL−1 penicillin, and 100 μg mL−1 streptomycin.Purification of different RNA species
Total RNA was extracted using TRIzol reagent (Sigma Aldrich, Beijing, China) according to the manufacturer's recommended procedure. Small RNA (<200 nt) was purified using an E.Z.N.A. MiRNA kit (Omega Bio-Tek Inc., Norcross, GA, USA). For the separation of 18S rRNA and 28S rRNA, the obtained total RNA was further processed by agarose gel electrophoresis-based purification to remove the potential trace level of contamination of the 16S rRNA of E. coli. 18S rRNA and 28S rRNA were separated and purified with 1.0% agarose gel (low melting point). The agarose gel was stained with GelRed (Invitrogen) and visualized by using a gel documentation system (Tanon, Shanghai, China). 18S rRNA and 28S rRNA were excised from the agarose gel and recovered using a Zymoclean Gel RNA Recovery kit (Zymo Research). The total RNA of E. coli was extracted using a Bacteria Total RNA Isolation kit (Sangon Biotech, Shanghai, China). The 16S rRNA of E. coli was separated and purified with 1.0% agarose gel (low melting point) in a similar way to that for the purification of the 18S rRNA of mammalian cells.Evaluation of the purity of 18S rRNA
The purity of the isolated 18S rRNA from mammalian cells was evaluated by real-time quantitative PCR. The reverse transcription of 18S rRNA from mammalian cells and 16S rRNA of E. coli was carried out using a PrimeScript™ RT reagent kit with gDNA Eraser (Takara Biotechnology) according to the manufacturer's recommended procedure.The real-time quantitative PCR mixture includes 12.5 μL of TB Green Premixe Ex Taq (Takara Biotechnology), 10 μM forward primer (1 μL), 10 μM reverse primer (1 μL), 8.5 μL of H2O, and 2 μL of cDNA product. The PCR amplification was performed on a CFX Connect real-time system (Bio-Rad Laboratories, Hercules, USA). The program includes denaturation at 95 °C for 10 s, annealing at 57 °C for 30 s, and elongation at 72 °C for 60 s for 40 cycles. The primer sequences include 16S rRNA forward primer (5′-TCAAATGAATTGACGGGGGC-3′), 16S rRNA reverse primer (5′-AGGCCCGGGAACGTATTCAC-3′), 18S rRNA forward primer (5′-GTAACCCGTTGAACCCCATT-3′), and 18S rRNA reverse primer (5′-CCATCCAATCGGTAGTAGCG-3′).
Enzymatic digestion of RNA
RNA was digested by the neutral enzymatic digestion method according to a previous study.36 The detailed procedure can be found in the ESI.†LC-ESI-MS/MS analysis
The LC-ESI-MS/MS analysis was performed on a Shimadzu 8045 mass spectrometer (Kyoto, Japan) equipped with an electrospray ionization (ESI) source (Turbo Ionspray) and a Shimadzu LC-30AD UPLC system (Tokyo, Japan). The chromatographic conditions were optimized to achieve the baseline separation of m3Cm, m4Cm and m5Cm (detailed optimization procedure can be found in the ESI†). The native nucleosides and dimethylated cytidines were detected by multiple reaction monitoring (MRM) under positive mode. The MRM mass spectrometric parameters were optimized, and the optimized parameters are listed in Table S2 in the ESI.†High-resolution mass spectrometry analysis
The dimethylated cytidines were enriched using a LC20AT HPLC system. A Hisep C18-T reversed-phase column (5 μm, 4.6 × 250 mm, Weltech Co., Ltd, Wuhan, China) was employed for the separation. 0.05% FA/H2O (pH 2.8, solvent A) and MeOH (solvent B) were used as the mobile phases. Gradients of 0–5 min, 5% B; 5–40 min, 5–45% B; 40–45 min, 45% B; 45–47 min, 45–5% B; 47–55 min, 5% B were used. The flow rate was set at 0.8 mL min−1.The HPLC-enriched dimethylated cytidines were characterized by using a high-resolution LTQ-Orbitrap Elite mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA), which was equipped with an ESI source and a Dionex Ultimate 3000 UPLC system (Thermo Fisher Scientific, Waltham, MA, USA). The MS analysis was performed in positive-ion mode with full scan detection (m/z 70–350) at a resolution of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000. The collision energy of collision induced dissociation (CID) was 15 eV. The source and ion transfer parameters applied were as follows: capillary temperature, 350 °C; heater temperature, 300 °C; auxiliary gas flow, 15 arbitrary units; sheath gas flow, 35 arbitrary units; capillary voltage, 35 V; spray voltage, 3.5 kV; the S-lens RF level, 60%. Data analysis was performed using Xcalibur v3.0.63 (Thermo Fisher Scientific, Waltham, MA, USA).
000. The collision energy of collision induced dissociation (CID) was 15 eV. The source and ion transfer parameters applied were as follows: capillary temperature, 350 °C; heater temperature, 300 °C; auxiliary gas flow, 15 arbitrary units; sheath gas flow, 35 arbitrary units; capillary voltage, 35 V; spray voltage, 3.5 kV; the S-lens RF level, 60%. Data analysis was performed using Xcalibur v3.0.63 (Thermo Fisher Scientific, Waltham, MA, USA).
Stable isotope labelling tracing
As for stable isotope tracing experiments by mass spectrometry, HeLa cells were cultured in DMEM medium which was supplemented with 0.3 mM of D3-Met to label RNA with the CD3 group. HeLa cells were harvested after culturing for 72 h. Small RNA (<200 nt) and 18S rRNA were extracted followed by enzymatical digestion and LC-ESI-MS/MS analysis.Results and discussion
Characterization of the synthesized m3Cm
In this study, we aimed to investigate the existence status of three dimethylations of cytidines, including m3Cm, m4Cm and m5Cm, in mammals. m3Cm has never been found in living organisms in the three-domain system, including archaea, bacteria, and eukaryotes. m4Cm was previously reported to exist in the 16S rRNA of E. coli, but hasn't been reported to be present in mammalian cells.21 m5Cm exists in the tRNA of eukaryotes.19 Here, we systematically investigate the presence of three dimethylated cytidines of m3Cm, m4Cm and m5Cm in mammalian cells.Because there haven't been any previous reports regarding m3Cm and m3Cm standard is not commercially available, it is essential to synthesize an m3Cm standard for the confident determination of its potential presence in living organisms. The chemical reaction for the synthesis of m3Cm is shown in Fig. 2A. Cytidine can react with DMS to produce different products under different pH conditions. We found that the methylation mainly occurred at the N3 position of the nucleobase at pH 4.3; but at pH 8.0–10.0, the methylation occurred at both the N3 and N4 positions of the nucleobase. Thus, the synthesis of m3Cm was carried out in NaAc–HAc buffer under pH 4.3.
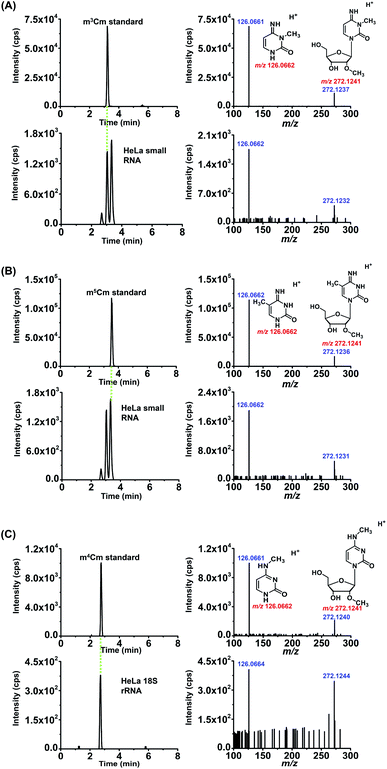
HPLC was employed to separate and purify m3Cm. In addition to the substrate of Cm (16.4 min), we observed a new peak that occurred at 25.0 min after the chemical reaction (Fig. 2B). The new peak was then collected and analyzed by high-resolution mass spectrometry. The results showed that the precursor ion (m/z 272.1242) and fragment ions (m/z 126.0662) of the synthesized product were identical to the theoretical m/z of m3Cm (m/z 272.1241 and 126.0662, Fig. 2C and D), indicating that the compound should be the desired m3Cm. The chromatographic retention time of this synthesized compound (3.2 min) is different from those of the m4Cm standard (2.7 min) and m5Cm standard (3.5 min) (see the next section). In addition, NMR analysis further confirmed the synthesized m3Cm (Fig. S1 and S2 in the ESI†). Collectively, these results demonstrated that the desired compound of m3Cm was successfully synthesized.
Separation of the isomers of m3Cm, m4Cm and m5Cm
m3Cm, m4Cm and m5Cm are structurally isomers and have exactly the same molecular weights. Their MRM transitions are also the same during LC-ESI-MS/MS analysis (Table S2 in the ESI†). Thus, complete chromatographic separation of m3Cm, m4Cm and m5Cm is essential and important to distinguish these isomers. In this respect, we optimized the separation conditions for these isomers, including the mobile phases and separation gradients.We first used 0.05% FA in H2O with different pH (2.8, 3.0, 4.0 and 5.0) as solvent A and MeOH as solvent B for the chromatographic separation. The gradient 1 was first employed for the separation at a flow rate of 0.3 mL min−1. The results showed that 0.05% FA in H2O (pH 2.8) offered the best separation for m3Cm, m4Cm and m5Cm (Fig. S3A–D in the ESI†). In addition, we also examined 2 mM NH4HCO3 as solvent A and MeOH as solvent B for the separation. However, m3Cm and m4Cm cannot be separated under these conditions (Fig. S3E in the ESI†). Thus, 0.05% FA in H2O (pH 2.8) (solvent A) and MeOH (solvent B) were employed as the mobile phases. We next evaluated the gradients in the separation of m3Cm, m4Cm and m5Cm. The results showed that gradient 2 offered better separation resolution towards m3Cm, m4Cm and m5Cm than gradient 1 (comparing Fig. S3A and S3F in the ESI†). In addition, we also examined the effect of flow rate on the separation of these isomers. The results demonstrated that the peaks of these isomers were narrower at a flow rate of 0.3 mL min−1 than those at 0.2 mL min−1 (comparing Fig. S3F and S3G in the ESI†).
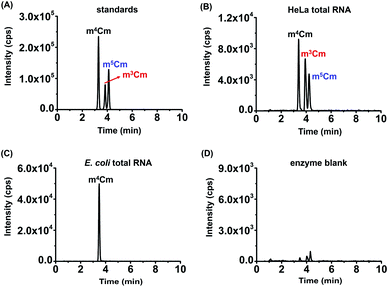
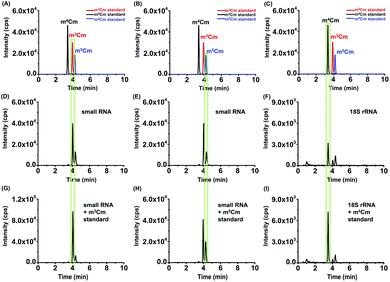
Collectively, the optimal chromatographic separation conditions for m3Cm, m4Cm and m5Cm were 0.05% FA in H2O (pH 2.8) (solvent A) and MeOH (solvent B) as the mobile phases with using the gradient 2 at a flow rate of 0.3 mL min−1. Under the optimized separation conditions, m3Cm, m4Cm and m5Cm can be well separated (Fig. 3A), which provides a good fundamental to readily determine these isomers.
Determination of m3Cm, m4Cm and m5Cm in the RNA of mammalian cells
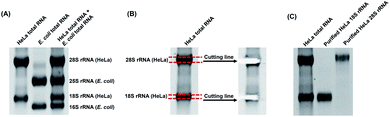
With the m3Cm, m4Cm and m5Cm standards, we next detected these dimethylated cytidines in mammalian RNA. We initially analyzed these dimethylated cytidines in the total RNA of HeLa cells and E. coli. The preliminary results showed that all three dimethylated cytidines can be detected in the total RNA of HeLa cells (Fig. 3B); however, only m4Cm was detected in E. coli (Fig. 3C). It should be noted that in the sample with only adding enzymes and omitting the RNA sample, we observed tiny peaks whose retention times were similar to those of m3Cm, m4Cm and m5Cm standards (Fig. 3D), indicating that the enzymes might contain trace levels of these modifications. However, the intensities of these peaks were much lower than those detected from total RNA, which should not affect the determination of these dimethylated cytidines.We next detected m3Cm, m4Cm and m5Cm in different RNA species from HeLa cells. The overall results showed that m3Cm and m5Cm were mainly present in small RNA (<200 nt), while m4Cm was mainly present in 18S rRNA. m4Cm has been reported to be present in the 16S rRNA of E. coli.21 In this study, we aimed to investigate the presence of m3Cm, m4Cm and m5Cm in mammalian cells. It is possible that the cultured mammalian cells contain trace levels of bacterial contamination. In this respect, it is critical to obtain highly pure rRNA for the determination of m4Cm in mammalian cells. To exclude the potential contamination of the rRNA of E. coli in the isolated rRNA of mammalian cells, we employed agarose gel electrophoresis to separate and purify the 18S and 28S rRNA. The results showed that both the 18S rRNA and 28S rRNA of HeLa cells can be well differentiated from the 16S rRNA and 25S rRNA of E. coli (Fig. 4A). The bands of the 18S rRNA and 28S rRNA of HeLa cells were then cut and purified (Fig. 4B and C). The real-time quantitative PCR results showed that the purity of the isolated 18S rRNA of HeLa cells was higher that 99.999% (Fig. S4 in the ESI†).
The retention times of two compounds detected in the small RNA (<200 nt) of HeLa cells were similar to that of m3Cm and m5Cm standards (Fig. 5A, B, D and E); the retention time of one compound detected in the 18S rRNA of HeLa cells was similar to that of the m4Cm standard (Fig. 5C and F), indicating the existence of these dimethylated cytidines in different RNA species. Furthermore, we separately added the standards of m3Cm, m4Cm and m5Cm to the digested nucleosides from the small RNA (<200 nt) or 18S rRNA of HeLa cells. It can be seen that the spiked standards had the same retention times as those detected in HeLa cells and increased the peak intensities (Fig. 5G–I), suggesting that the detected compounds should be m3Cm, m4Cm and m5Cm.
We also confirmed these dimethylated cytidines from HeLa cells by high-resolution MS analysis. The enzymatically digested cytidine modifications from the small RNA (<200 nt) or 18S rRNA of HeLa cells were first enriched by offline HPLC before high-resolution MS analysis (Fig. S5 in the ESI†). The MS analysis showed that the precursor ions and product ions (m/z shown in blue) of the detected compounds in the RNA of HeLa cells were identical to their corresponding theoretical values (m/z shown in red) as well as to those of the standards (Fig. 6), further confirming the detected compounds of m3Cm, m4Cm and m5Cm. Taken together, the results suggested that m3Cm and m5Cm were present in the small RNA (<200 nt) of mammals and m4Cm was present in the 18S rRNA of mammals.
Metabolic labeling of cells
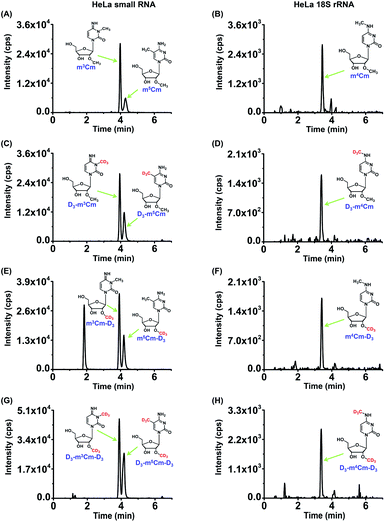
To further confirm the presence of m3Cm, m4Cm and m5Cm in the RNA of mammals, we conducted stable isotope tracing experiments by mass spectrometry. ATP and L-methionine (Met) could be converted into S-adenosyl-L-methionine (SAM) by methionine adenosyltransferase and SAM is a methyl group donor for the methylation of nucleic acids.37 If these dimethylated cytidines exist in mammalian cells, then the culturing of cells in DMEM medium containing isotopically labeled L-methionine (D3-Met) would lead to the transfer of the CD3 group from D3-Met to m3Cm, m4Cm and m5Cm. Theoretically, single and dual CD3 might be added to these dimethylated cytidines.As for m3Cm, three compounds, D3-m3Cm (CD3 labeled nucleobase), m3Cm-D3 (CD3 labeled ribose), D3-m3Cm-D3 (CD3 labeled both nucleobase and ribose), can be theoretically formed in the RNA of cells that were cultured in a medium containing D3-Met (Fig. S6 in the ESI†). We cultured human HeLa cells in DMEM medium supplied with 0.3 mM D3-Met. Then 18S rRNA and small RNA (<200 nt) were isolated and analyzed by our developed method. The results showed that, in addition to m3Cm, all three compounds, D3-m3Cm, m3Cm-D3 and D3-m3Cm-D3, were distinctly detected (Fig. 7). The concentration of unlabeled methionine in the DMEM medium is 0.2 mM. Thus, the theoretical percentage of D3-Met in the medium is approximately 60% of the total methionine. It can be seen that approximately 60% of the measured m3Cm carried the CD3 nucleobase group and CD3 ribose group (Table S3 in the ESI†), which is equivalent to the theoretical percentage of D3-Met in total methionine in the medium. Similar to m3Cm, CD3 labeled m4Cm and m5Cm were both clearly detected (Fig. 7). Collectively, the results further confirmed the presence of these three dimethylated cytidines in mammalian cells and indicated that SAM was the methyl donor for the dual methylation of cytidines in RNA.
Quantification of m3Cm, m4Cm and m5Cm in RNA
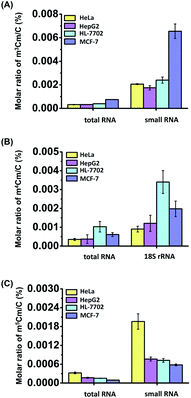
Calibration curves were constructed to quantitatively measure the dimethylated cytidines in RNA. A mixture of cytidine standards with a series of amounts ranging from 0.05 nmol to 5 nmol (for cytidine) or from 0.5 fmol to 500 fmol (for m3Cm, m4Cm, and m5Cm) and fixed amounts of isotope internal standards (rC-13C5) were prepared. The calibration curves were constructed by plotting the peak area ratios (analytes/IS) against the amounts of analytes with triplicate measurements. The results showed that good linearities were obtained with the coefficients of determination (R2) being greater than 0.99 (Table S4 in the ESI†). The accuracy and reproducibility of the method were evaluated with the relative errors (REs) and intra- and inter-day relative standard deviations (RSDs) being less than 13.7% and 14.9%, respectively (Tables S4 and S5 in the ESI†), demonstrating that good accuracy and reproducibility were achieved.In addition to HeLa cells, these modifications of m3Cm, m4Cm and m5Cm were also detected and quantified in other human cells, including human HL-7702 hepatic cells, human HepG2 hepatocellular carcinoma and human MCF-7 breast adenocarcinoma cells. The quantification results showed that the measured m3Cm ranged from 0.0003% to 0.0008%, m4Cm ranged from 0.0004% to 0.001%, and m5Cm ranged from 0.0001% to 0.0003% in total RNA (Fig. 8 and Table S6 in the ESI†). In small RNA (<200 nt), the m3Cm ranged from 0.002% to 0.007%, and m5Cm ranged from 0.0006% to 0.002% (Fig. 8 and Table S6 in the ESI†). The measured levels of m5Cm in total RNA and small RNA (<200 nt) are comparable with those in previous studies.23 In 18S rRNA, the m4Cm ranged from 0.0009% to 0.003% (Fig. 8 and Table S6 in the ESI†).
The results clearly indicated that m3Cm and m5Cm are mainly present in small RNA (<200 nt) and m4Cm is mainly present in 18S rRNA. Previous studies demonstrated that tRNA accounts for 90% of small RNA (<200 nt).38 Therefore, the m3Cm detected in small RNA (<200 nt) most likely comes from tRNA. The identified m3Cm is a totally new modification that has never been found in living organisms. As for m4Cm, it is firstly identified to be present in the RNA of mammals in the current study. Although the potential roles of m3Cm and m4Cm in RNA are still elusive, we speculate that m3Cm and m4Cm could enhance the structural stability of tRNA and 18S rRNA. It was reported that the methyltransferase-like protein 6 (METTL6) could catalyze the formation of m3C in serine tRNA.39 FtsJ RNA 2′-O-methyltransferase 1 (FTSJ1) is involved in the 2′-O-methylation of cytidine in the anti-codon region of tRNA.40 Thus, we speculate that METTL6 may methylate cytidine in tRNA to form m3C, which is then further methylated by FTSJ1 to form m3Cm. Future study on identifying enzymes responsible for the formation and removal of the methyl groups of m3Cm and m4Cm will promote uncovering the functions of these dual methylations of cytidines in RNA.
Conclusions
In summary, using the developed LC-ESI-MS/MS method, we identified the existence of a novel modification of m3Cm in the RNA of mammals. We confirmed that m3Cm mainly exists in the small RNA (<200 nt) of mammals. The identified m3Cm is a totally new modification that has never been found in living organisms. In addition, we identified, for the first time, the presence of m4Cm in 18S rRNA of mammalian cells. The stable isotope tracing monitored by mass spectrometry demonstrated that SAM was the methyl donor for these dimethylations of cytidines in RNA. The discovery of m3Cm broadens the diversity of RNA modifications in living organisms. Moreover, the discovery of m3Cm and m4Cm in mammals indicates the new layer of RNA epigenetic modifications. Future functional investigating of these dual methylations of cytidines in RNA will unveil the complexity in the regulation of biological processes through RNA modifications.Author contributions
M. Y. C. and B. F. Y. designed the experiments and interpreted the data. M. Y. C., X. J. Y., and J. H. D. performed the synthesis and purification of m3Cm and carried out the metabolic labelling and mass spectrometry analysis. Y. D. and M. Y. C. performed the cell culturing and RNA isolation. Y. Q. F. interpreted the mass spectrometry data. M. Y. C. and B. F. Y. wrote the manuscript.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
The work is supported by the National Natural Science Foundation of China (22074110, 21635006, and 21721005), and the Fundamental Research Funds for the Central Universities (2042021kf0212).References
- C. Luo, P. Hajkova and J. R. Ecker, Science, 2018, 361, 1336–1340 CrossRef CAS PubMed.
- T. Liu, C. J. Ma, B. F. Yuan and Y. Q. Feng, Sci. China: Chem., 2018, 61, 381–392 CrossRef CAS.
- Y. Feng, N. B. Xie, W. B. Tao, J. H. Ding, X. J. You, C. J. Ma, X. Zhang, C. Yi, X. Zhou, B. F. Yuan and Y. Q. Feng, CCS Chem., 2020, 2, 994–1008 Search PubMed.
- K. Chen, B. S. Zhao and C. He, Cell Chem. Biol., 2016, 23, 74–85 CrossRef CAS PubMed.
- X. Li, X. Xiong and C. Yi, Nat. Methods, 2016, 14, 23–31 CrossRef PubMed.
- P. Boccaletto, M. A. Machnicka, E. Purta, P. Piatkowski, B. Baginski, T. K. Wirecki, V. de Crecy-Lagard, R. Ross, P. A. Limbach, A. Kotter, M. Helm and J. M. Bujnicki, Nucleic Acids Res., 2018, 46, D303–D307 CrossRef CAS PubMed.
- A. Noma, S. Yi, T. Katoh, Y. Takai, T. Suzuki and T. Suzuki, RNA, 2011, 17, 1111–1119 CrossRef CAS PubMed.
- F. Liu and C. He, J. Biol. Chem., 2017, 292, 14704–14705 CrossRef CAS PubMed.
- C. J. Ma, J. H. Ding, T. T. Ye, B. F. Yuan and Y. Q. Feng, ACS Chem. Biol., 2019, 14, 1418–1425 CrossRef CAS PubMed.
- I. Laptev, E. Shvetsova, S. Levitskii, M. Serebryakova, M. Rubtsova, V. Zgoda, A. Bogdanov, P. Kamenski, P. Sergiev and O. Dontsova, Nucleic Acids Res., 2020, 48, 8022–8034 CrossRef CAS PubMed.
- H. Chen, Z. Shi, J. Guo, K. J. Chang, Q. Chen, C. H. Yao, M. C. Haigis and Y. Shi, J. Biol. Chem., 2020, 295, 8505–8513 CrossRef CAS PubMed.
- L. Van Haute, A. G. Hendrick, A. R. D'Souza, C. A. Powell, P. Rebelo-Guiomar, M. E. Harbour, S. Ding, I. M. Fearnley, B. Andrews and M. Minczuk, Nucleic Acids Res., 2019, 47, 10267–10281 CrossRef CAS PubMed.
- L. Trixl and A. Lusser, Wiley Interdiscip. Rev.: RNA, 2019, 10, e1510 CrossRef PubMed.
- L. Fu, C. R. Guerrero, N. Zhong, N. J. Amato, Y. Liu, S. Liu, Q. Cai, D. Ji, S. G. Jin, L. J. Niedernhofer, G. P. Pfeifer, G. L. Xu and Y. Wang, J. Am. Chem. Soc., 2014, 136, 11582–11585 CrossRef CAS PubMed.
- J. E. Squires, H. R. Patel, M. Nousch, T. Sibbritt, D. T. Humphreys, B. J. Parker, C. M. Suter and T. Preiss, Nucleic Acids Res., 2012, 40, 5023–5033 CrossRef CAS PubMed.
- Y. Yang, L. Wang, X. Han, W. L. Yang, M. Zhang, H. L. Ma, B. F. Sun, A. Li, J. Xia, J. Chen, J. Heng, B. Wu, Y. S. Chen, J. W. Xu, X. Yang, H. Yao, J. Sun, C. Lyu, H. L. Wang, Y. Huang, Y. P. Sun, Y. L. Zhao, A. Meng, J. Ma, F. Liu and Y. G. Yang, Mol. Cell, 2019, 75, 1188–1202 CrossRef CAS PubMed e1111.
- M. Schosserer, N. Minois, T. B. Angerer, M. Amring, H. Dellago, E. Harreither, A. Calle-Perez, A. Pircher, M. P. Gerstl, S. Pfeifenberger, C. Brandl, M. Sonntagbauer, A. Kriegner, A. Linder, A. Weinhausel, T. Mohr, M. Steiger, D. Mattanovich, M. Rinnerthaler, T. Karl, S. Sharma, K. D. Entian, M. Kos, M. Breitenbach, I. B. Wilson, N. Polacek, R. Grillari-Voglauer, L. Breitenbach-Koller and J. Grillari, Nat. Commun., 2015, 6, 6158 CrossRef CAS PubMed.
- S. Blanco and M. Frye, Curr. Opin. Cell Biol., 2014, 31, 1–7 CrossRef CAS PubMed.
- S. M. Huber, P. van Delft, A. Tanpure, E. A. Miska and S. Balasubramanian, J. Am. Chem. Soc., 2017, 139, 1766–1769 CrossRef CAS PubMed.
- C. G. Edmonds, P. F. Crain, T. Hashizume, R. Gupta, K. O. Stetter and J. A. McCloskey, J. Chem. Soc., Chem. Commun., 1987, 909–910 RSC.
- S. Kimura and T. Suzuki, Nucleic Acids Res., 2010, 38, 1341–1352 CrossRef CAS PubMed.
- J. L. Nichols and B. G. Lane, Biochim. Biophys. Acta, 1966, 119, 649–651 CrossRef CAS.
- Y. Feng, C. J. Ma, J. H. Ding, C. B. Qi, X. J. Xu, B. F. Yuan and Y. Q. Feng, Anal. Chim. Acta, 2020, 1098, 56–65 CrossRef CAS PubMed.
- H. Grosjean, G. Keith and L. Droogmans, Methods Mol. Biol., 2004, 265, 357–391 CAS.
- H. M. Liebich, S. Muller-Hagedorn, M. Bacher, H. G. Scheel-Walter, X. Lu, A. Frickenschmidt, B. Kammerer, K. R. Kim and H. Gerard, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2005, 814, 275–283 CrossRef CAS PubMed.
- B. Chen, B. F. Yuan and Y. Q. Feng, Anal. Chem., 2019, 91, 743–756 CrossRef CAS PubMed.
- C. B. Qi, H. P. Jiang, J. Xiong, B. F. Yuan and Y. Q. Feng, Chin. Chem. Lett., 2019, 30, 553–557 CrossRef CAS.
- C. B. Qi, J. H. Ding, B. F. Yuan and Y. Q. Feng, Chin. Chem. Lett., 2019, 30, 1618–1626 CrossRef CAS.
- X. J. You, T. Liu, C. J. Ma, C. B. Qi, Y. Tong, X. Zhao, B. F. Yuan and Y. Q. Feng, Anal. Chem., 2019, 91, 10477–10483 CrossRef CAS PubMed.
- K. Petru, J. Siroka, L. Bydzovska, L. Krcmova and M. Polasek, Electrophoresis, 2014, 35, 2546–2549 CrossRef CAS PubMed.
- F. Yuan, X. H. Zhang, J. Nie, H. X. Chen, Y. L. Zhou and X. X. Zhang, Chem. Commun., 2016, 52, 2698–2700 RSC.
- Y. Yu, S. H. Zhu, F. Yuan, X. H. Zhang, Y. Y. Lu, Y. L. Zhou and X. X. Zhang, Chem. Commun., 2019, 55, 7595–7598 RSC.
- W. Y. Lai, J. Z. Mo, J. F. Yin, C. Lyu and H. L. Wang, TrAC, Trends Anal. Chem., 2019, 110, 173–182 CrossRef CAS.
- M. D. Lan, B. F. Yuan and Y. Q. Feng, Chin. Chem. Lett., 2019, 30, 1–6 CrossRef CAS.
- H. Bredereck, H. Haas and A. Martini, Chem. Ber., 1948, 81, 307–313 CrossRef CAS.
- Q. Y. Cheng, J. Xiong, C. J. Ma, Y. Dai, J. H. Ding, F. L. Liu, B. F. Yuan and Y. Q. Feng, Chem. Sci., 2020, 11, 1878–1891 RSC.
- B. J. Landgraf, E. L. McCarthy and S. J. Booker, Annu. Rev. Biochem., 2016, 85, 485–514 CrossRef CAS PubMed.
- D. Su, C. T. Chan, C. Gu, K. S. Lim, Y. H. Chionh, M. E. McBee, B. S. Russell, I. R. Babu, T. J. Begley and P. C. Dedon, Nat. Protoc., 2014, 9, 828–841 CrossRef CAS PubMed.
- V. V. Ignatova, S. Kaiser, J. S. Y. Ho, X. Bing, P. Stolz, Y. X. Tan, C. L. Lee, F. P. H. Gay, P. R. Lastres, R. Gerlini, B. Rathkolb, A. Aguilar-Pimentel, A. Sanz-Moreno, T. Klein-Rodewald, J. Calzada-Wack, E. Ibragimov, M. Valenta, S. Lukauskas, A. Pavesi, S. Marschall, S. Leuchtenberger, H. Fuchs, V. Gailus-Durner, M. H. de Angelis, S. Bultmann, O. J. Rando, E. Guccione, S. M. Kellner and R. Schneider, Sci. Adv., 2020, 6, eaaz4551 CrossRef CAS PubMed.
- M. P. Guy, M. Shaw, C. L. Weiner, L. Hobson, Z. Stark, K. Rose, V. M. Kalscheuer, J. Gecz and E. M. Phizicky, Hum. Mutat., 2015, 36, 1176–1187 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Evaluation of the purity of isolated 18S rRNA by real-time quantitative PCR; enzymatic digestion of RNA; Tables S1–S6; Fig. S1–S6. See DOI: 10.1039/d1sc01972d |
| This journal is © The Royal Society of Chemistry 2021 |